Abstract
Aesculus hippocastanum L., also known as horse chestnut, is an ornamental tree whose seeds are mostly discarded in landfills in the regions where they are grown. However, recent studies have shown that these seeds can be a source of interesting compounds for several industries. This work aimed to chemically characterize horse chestnut seeds at the level of compounds recognized for their wide bioactivity, i.e., organic acids, including phenolic compounds, using chromatographic methodologies (UFLC-DAD and LC-DAD-ESI/MSn). In addition, the bioactivity of these seeds was evaluated by in vitro methodologies, seeking to relate the respective (bio)activity to the compounds present in the endocarp (husk), seed coat (skin), and peeled seed (pulp). The antioxidant activity (lipid peroxidation inhibition and oxidative haemolysis inhibition), antibacterial potential (against Gram-positive and Gram-negative bacteria) and cytotoxicity (in human tumour cell lines and porcine liver primary cells) were evaluated. Kaempferol-O-pentoside-O-hexoside-O-hexoside was the main phenolic identified in the pulp. At the same time, (-)-epicatechin and β-type (epi)catechin dimer were the major phenolics present in husk and skin, respectively. In general, A. hippocastanum extracts presented antioxidant and antibacterial potential, without toxicity up to the maximal tested dose. Overall, these findings anticipate potential applications of A. hippocastanum seeds in food- or pharmaceutical-related uses.
1. Introduction
More than 50,000 species of plants have been used as co-adjuvants and inclusively as therapeutic promoters by the pharmaceutical, cosmetic, and food industries. Different parts of the plants have been used as medicinal agents, including seeds, roots, leaves, fruits, skin, flowers, or even the entire plant, due to the presence of bioactive molecules responsible for their therapeutic effects [1,2]. For this reason, more and more species are being explored for their bioactive components to identify the most active chemical compounds, establish adequate amounts for their incorporation into drugs or nutraceuticals, and determine their side effects [3]. In fact, in recent years, nutritional therapy and herbal medicine have become a trend in health support. There are now strong recommendations to use herbal nutraceuticals that are becoming increasingly popular to improve health and prevent and treat diseases [4].
Horse chestnut (Aesculus hippocastanum L.), a native plant from Southeastern Europe, is nowadays widely cultivated in temperate zones as an ornamental and landscape tree, due to its beauty and excellent resistance to the environmental conditions [5,6]. This species has historically been used as animal feed for farm animals, and some Native American people have included it in their diet. In recent years, A. hippocastanum has been investigated for its therapeutic properties, presenting itself as a species of medical and/or clinical interest. Although its fruit is considered toxic due to the presence of certain molecules (e.g., aescin and esculin), horse chestnut extracts, namely seed extracts, have been traditionally used in the treatment of coronary disease and therapy for chronic venous insufficiency such as pain, heaviness, and tension in the leg [7,8]. The husk and leaves of A. hippocastanum have been employed as an astringent to treat diarrhoea and haemorrhoids [8]. The medicinal properties of this plant have been associated with aescin, a mixture of saponins present in its chemical composition, and with anti-inflammatory, vasoconstrictor, and vasoprotective effects. However, and since some extracts free of aescin also revealed anti-inflammatory activity, it is assumed that there are other compounds with similar properties in this plant [7,9].
Nowadays, most of the seeds of A. hippocastanum are disposed of in landfills. Therefore, the present work intended to provide more information about horse chestnut, specifically on the chemical characterization and bioactive molecules, mainly phenolic compounds, and related bioactivity of its botanical parts. For this purpose, chromatographic techniques such as UFLC-DAD and LC-DAD-ESI/MSn were employed, as well as widely used and validated in vitro assays to certify the bioactivities.
2. Materials and Methods
2.1. Sampling of Aesculus hippocastanum L. Fruits
The fruits of Aesculus hippocastanum were collected in the orchards of the Polytechnic Institute of Bragança, Portugal. Samples were taken from 10–15 trees used as ornamental elements in the Polytechnic Institute’s orchards. All plants were sown in the same season, so they were similar in age and maturation. As an integral part of a higher education orchard, the health of the crops is attested by agronomists and institution employees. In this way, issues inherent to the use of healthy fruits were ensured.
The fruits were expected to reach maximum maturity and fell to the ground for collection and subsequent use. Approximately 5 kg were harvested. After harvest, the pericarp and endocarp were separated from the pulp, through mechanical methods, and then all samples were frozen, lyophilized (FreeZone 4.5 model 7750031, Labconco, Kansas City, MO, USA), reduced to a fine dried powder (~20 mesh), and stored in a cold place protected from light.
2.2. Bioactive Compounds of Aesculus hippocastanum L. Fruits
Organic acids. The organic acid profile of the different parts (pulp, skin, and husk) of the fruits of A. hippocastanum was determined following the method optimized by Barros et al. (2013a) [10]. Briefly, the powdered lyophilized samples (∼2 g) were extracted by maceration using meta-phosphoric acid (25 mL, 25 °C at 150 rpm). After 45 min of extraction, the samples were filtered through Whatman No. 4 paper and 0.2 μm nylon filters for chromatographic analysis (into autosampler vials). The analysis was performed using a Shimadzu 20A series UFLC (Shimadzu Corporation, Kyoto, Japan). The separation of the compounds was accomplished on a SphereClone (Phenomenex, Torrance, CA, USA) reverse-phase C18 column (5 µm, 250 mm × 4.6 mm i.d) thermostatted at 35 °C, and all the conditions were maintained likewise. The detection was carried out in a DAD using 215 nm and 245 nm (for ascorbic acid) as preferred wavelengths. The organic acids found in the samples were quantified by comparison of the area of their peaks recorded at the previously mentioned wavelengths with calibration curves obtained from commercial standards. The results were expressed in g/100 g of dry weight.
Phenolic acids and related compounds. The phenolic acid determination was performed in the three studied parts of horse chestnut (pulp, skin, and husk) in hydroalcoholic extracts [11]. To prepare the hydroethanolic extracts, 1 g of each powdered sample was submitted to extraction with an ethanol:water mixture (80:20, v/v; 30 mL) at 25 °C and 150 rpm over 1 h, followed by filtration through a Whatman filter paper No. 4. Thereafter, the residue was re-extracted with one additional portion of the hydroethanolic mixture, and the combined extracts were evaporated under reduced pressure (rotary evaporator Büchi R-210, Flawil, Switzerland). The obtained aqueous extract was frozen and then lyophilized. Finally, a hydroethanolic extract was prepared (10 mg/mL; EtOH:H2O, 80:20 v/v) and filtered through 0.2 μm nylon filters for chromatographic analysis. The analytical system was a Dionex Ultimate 3000 UPLC instrument (Thermo Scientific, San Jose, CA, USA) equipped with a DAD coupled to a mass detector (LC-DAD-ESI/MSn). All the chromatographic conditions were maintained as previously developed by the abovementioned authors. Data acquisition was carried out with an Xcalibur® data system (Thermo Finnigan, San Jose, CA, USA) and the phenolic compounds were identified by comparing their retention times, UV-vis, and mass spectra with those obtained with standard compounds, when available. Otherwise, compounds were tentatively identified by comparing the obtained information with available data reported in the literature. For quantitative analysis, a calibration curve for each available phenolic standard (caffeic, chlorogenic and p-coumaric acid, catechin, quercetin-3-O-glucoside, and kaempferol-3-O-rutinoside) was constructed based on the UV-Vis signal. For the identified phenolic compounds for which a commercial standard was not available, the quantification was performed through the calibration curve of another compound from the same phenolic group [11]. The results were expressed as mg/g of extract.
2.3. Bioactivity Evaluation of Aesculus hippocastanum L. Fruits
2.3.1. Extract Preparation
For each part of the fruit (pulp, skin, and husk), 1 g of each lyophilized powdered sample was extracted with 30 mL of ethanol under magnetic stirring for 1 h at room temperature. Then, the residue was re-extracted maintaining the same operational conditions. The combined extracts were evaporated at 40 °C in the previously mentioned rotary evaporator to remove the alcohol. Afterwards, the samples were frozen and further lyophilized. The resulting lyophilized extracts were used to evaluate the bioactive properties of A. hippocastanum. Therefore, the lyophilized samples were redissolved in distilled water to test the antioxidant potential (stock solution 10 mg/mL) and the cytotoxic and anti-inflammatory properties (stock solution 8 mg/mL for both assays). To evaluate the antibacterial properties of the extracts under study, a stock solution of 20 mg/mL was prepared using the lyophilized samples dissolved in culture medium with 5% DMSO. Subsequently, these stock solutions were successively diluted to obtain the various concentrations necessary to perform the experimental work.
2.3.2. Antioxidant Properties
TBARS formation inhibition. The inhibition of lipid peroxidation using a thiobarbituric acid reactive substances (TBARS) assay was performed as previously reported by Barreira et al. (2013) [12]. The assay tested the hydroalcoholic extracts in porcine (Sus domesticus Erxleben) brain and the inhibition ratio was calculated after a spectrophotometric analysis and measurement of the absorbance of the control and sample solutions at 532 nm. The concentration providing 50% of antioxidant activity (EC50) was calculated by interpolation from the graph of TBARS formation inhibition percentage against the sample concentration.
Oxidative haemolysis inhibition assay (OxHLIA). The antihaemolytic activity of the extracts was evaluated as previously described by Lockowandt et al. (2019) [13]. The procedure followed was the one described by Takebayashi et al. (2012) [14] with some modifications. A solution of erythrocytes at 2.8% (v/v) was prepared and resuspended in PBS. In a 48-well microplate, the erythrocyte solution was mixed with the PBS solution (control), the studied samples dissolved in PBS, or water (for complete haemolysis). The optical density was measured at 690 nm, and after that the microplate was incubated under the same conditions and the optical density was measured every 10 min at the same wavelength for approximately 400 min. The percentage of the erythrocyte population that remained intact was calculated and the results were expressed as haemolysis delay time (Δt). The Δt values were then correlated to the antioxidant sample concentrations and, from the correlation obtained, the extract concentration able to promote a Δt haemolysis delay was calculated. The results were presented as EC50 values (μg/mL) at Δt 60 min (extract concentration required to keep 50% of the erythrocyte population intact for 60 min).
2.3.3. Antibacterial Properties
The microorganisms used to test the antibacterial potential of A. hippocastanum extracts were clinical isolates from patients hospitalized in various departments of the Local Health Unit of Bragança and Hospital Center of Trás-os-Montes and Alto-Douro Vila Real, Northeast of Portugal, and the assay was performed as reported by Pires et al. (2018) [11]. Five Gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, Morganella morganii, Proteus mirabilis, Pseudomonas aeruginosa, isolated from urine and expectoration) and three Gram-positive bacteria (MRSA (methicillin-resistant Staphylococcus aureus), Listeria monocytogenes and Enterococcus faecalis) were used to screen the antibacterial activity. To calculate the minimum inhibitory concentrations (MIC) the microdilution method and a rapid p-iodonitrotetrazolium chloride (INT) colorimetric assay were used as proposed by Kuete et al. (2011a,b) [15,16] with some amendments [17]. The antibiotic susceptibility profile had been previously obtained for all the tested bacteria [17]. MIC was defined as the lowest concentration that inhibits visible bacterial growth. Besides the MIC, the minimum bactericidal concentration (MBC) was also calculated.
2.3.4. Cytotoxic and Anti-Inflammatory Properties
The A. hippocastanum extracts were tested against four human tumour cell lines, namely HeLa (cervical adenocarcinoma), HepG2 (hepatocellular carcinoma), MCF-7 (breast adenocarcinoma), and NCI-H460 (non-small cell lung cancer). Each of the cell lines were plated in a 96-well plate at an appropriate density, and a sulphorhodamine B (SRB) assay was performed as previously reported by Reis et al. (2014) [18]. The cell growth inhibition was calculated after measurement of the absorbance of the control and the sample solutions at 540 nm. The results were expressed as GI50 values (sample concentration that inhibited 50% of the net cell growth). For hepatotoxicity evaluation, a primary culture cell, designated as PLP2, was used [19]. The extracts were co-cultured with porcine liver cells and the same procedure described above for the SRB assay was performed for the growth inhibition. The results were also expressed as GI50 values.
The anti-inflammatory activity was evaluated in a RAW 264.7 cell line, according to Moro et al. (2012) [20] and García-Lafuente et al. (2014) [21] with some modifications. The assay was performed following a procedure described by Taofiq et al. (2015) [22]. The nitric oxide produced by the cells in the presence or absence of the tested extracts was determined by measuring the absorbance at 540 nm and comparing with the standard calibration curve.
2.4. Statistical Analysis
The results obtained throughout the different evaluation studies were analysed by applying different statistical tools, selected according to the degree of complexity of the results and considering the defined research purposes. All statistical tests were performed at a 5% significance level using IBM SPSS Statistics for Windows, version 25 (IBM Corporation, New York, NY, USA). Three samples were used for each preparation and all the assays were carried out in triplicate. The results were expressed as mean values ± standard deviation (SD). Whenever possible, an analysis of variance (ANOVA) was applied to compare differences among different A. hippocastanum fruit parts. The typical requirements, homoscedasticity by the Levene test and normal distribution by the Shapiro Wilk’s test, were preliminarily performed. The Welch test was applied to verify the existence of statistically significant differences. The ANOVA results were classified using the Tukey HSD test or Tamhane’s T2, when homoscedasticity was verified or not, respectively. When a specific factor was studied using only two levels, a simple Student’s t-test was used to classify the results.
3. Results
3.1. Bioactive Compounds of Aesculus hippocastanum L. Fruits
The available literature on Aesculus hippocastanum suggests the application of its extracts for therapeutic purposes, which can be included in pharmaceutical/nutraceutical formulations. Therefore, compounds commonly referred to as potentially bioactive (i.e., organic acids and phenolic compounds) were surveyed and identified in detail.
Organic Acids
Organic acids are biomolecules which are indispensable for the human body since they are essential intermediates in cell metabolism [23]. Generally, in all analysed samples, seven organic acids were identified (Table 1), specifically oxalic, quinic, malic, citric, ascorbic, shikimic, and fumaric (only in trace amounts) acids.

Table 1.
Organic acid profile (g/100 g dw) of pulp, skin, and husk of Aesculus hippocastanum L. fruits (mean ± SD).
The botanical part with the highest concentration of organic acids was, by far, the husk, mainly due to its content of quinic acid (7.6 g/100 g dw), which was not detected in the pulp or the skin. Likewise, shikimic acid was only detected in the husk (1.06 g/100 g dw), which also showed the highest quantities of malic (2.0 g/100 g dw) and oxalic (0.20 g/100 g dw) acid. In turn, citric acid and ascorbic acid were only detected in the peeled seed (pulp). According to these results, A. hippocastanum husks, a waste product considered to have no value, might be considered a source of organic acids, mainly quinic acid, a compound known for its bioactive properties [24].
3.2. Phenolic Profile of Aesculus hippocastanum L. Fruits
Table 2 presents the peak characteristics (retention time, λmax in the visible region, mass spectral data), tentative identifications, and quantification of phenolic compounds in the hydroalcoholic extracts of pulp, husk, and skin of A. hippocastanum. Overall, forty compounds were identified: 18 in pulp, 13 in skin, and 15 in husk, with all fruit parts presenting a distinct chemical profile. The phenolic profile of horse chestnut has been previously described by other authors, mainly in the pulp [25,26,27] and husk [28,29].

Table 2.
Retention time (Rt), wavelengths of maximum absorption in the visible region (λmax), mass spectral data, tentative identification, and quantification (mg/g extract) of phenolic compounds in pulp, skin, and husk of Aesculus hippocastanum L. fruits.
The main family of phenolic compounds found in horse chestnut skin and husk were (epi)catechin derivatives, being the common compounds between both samples at peaks 2, 11, 12, 17, 21, and 24. Peaks 7, 26, and 29 presented a pseudomolecular ion at m/z 577 and MS2 fragments at m/z 451 (−126 u), 425 (−152 u), and 407 (−152−18 u), and also m/z 289 and 287, consistent with the loss of two (epi)catechin units, being for that matter tentatively identified as β-type (epi)catechin dimers. Similarly, peaks 3, 12, 15, 21, and 22 ([M-H]− at m/z 865); peaks 2, 16, 17, 19, 24, and 25 ([M-H]− at m/z 1153); and peak 30 ([M-H]− at m/z 1441) were assigned as β-type (epi)catechin trimers, tetramers, and pentamers, respectively [31,40,41]. Meanwhile, peak 11 was positively identified as (-)-epicatechin in comparison with the commercial standard and was the major compound found in husk and skin samples (2.7 ± 0.1 and 3.3 ± 0.1 mg/g extract, respectively). The skin sample presented a benzophenone (peak 5; [M-H]− at m/z 879) assigned as maclurin tri-O-galloyl-glucoside, considering the previously reported fragmentation pattern reported by Berardini et al. (2004) [34] in peels of Mangifera indica. Thus, to the best of our knowledge this compound was not previously reported in horse chestnut. The pulp presented ten flavonoid derivatives (quercetin, kaempferol, and isorhamnetin glycoside derivatives), six phenolic acids (caffeic and coumaric acid derivatives), one coumarin derivative and an unknown compound. The major family of compounds found in horse chestnut pulp samples were flavonol glycoside derivatives, mainly quercetin derivatives. Nevertheless, husk samples also presented two quercetin glycoside derivatives (peaks 27 and 32), having one in common with the pulp; however, skin samples did not reveal this type of molecule in their profile. Peaks 18, 20, and 23 ([M-H]− at m/z 757); 27 ([M-H]− at m/z 946); 28 ([M-H]− at m/z 862); 32 ([M-H]− at m/z 593); 36 ([M-H]− at m/z 979); 37 ([M-H]− at m/z 930); and 39 ([M-H]− at m/z 921) all presented an MS2 fragment at m/z 301 and UV-Vis spectra around 350 nm, being tentatively assigned as quercetin glycoside derivatives, and all were previously reported in chestnut horse by Hübner et al. (1999) and Kapusta et al. (2007) [25,26]. Peaks 31 ([M-H]− at m/z 741) and 34 ([M-H]− at m/z 739); 33 ([M-H]− at m/z 771); and 40 ([M-H]− at m/z 623) presented unique MS2 fragments at m/z 285 and 315, respectively, being tentatively identified as kaempferol and isorhamnetin glycoside derivatives, respectively, as previously reported by Kapusta et al. (2007) [26] in horse chestnut seeds and powdered waste water by-products. Regarding the phenolic acid derivatives, caffeic and p-coumaric acid derivatives were the only compounds present in pulp (peaks 1, 6, 13, and 14) and husk (8, 35, and 38) samples, whereas skin samples did not reveal these types of compounds. Nevertheless, both fruit parts did not reveal any common compound. Peak 1 ([M-H]− at m/z 341) was identified as caffeic acid hexoside. Peaks 6, 13, and 14 were identified as tris-caffeoyl-spermidine and di-caffeoyl-spermidine according to their UV spectra and pseudomolecular ions, being previously identified by Kang et al. (2016) [38], and previously reported in horse chestnut samples by Martin-Tanguy et al. (1978) [42]. Peak 1 ([M-H]− at m/z 341) corresponded to a caffeic acid derivative, releasing an MS2 fragment at m/z 179 with the loss of a hexosyl moiety [M-H-162]−, being tentatively assigned as caffeic acid hexoside. In addition, compound 8 ([M-H]− at m/z 471) corresponded to a coumaric acid derivative, releasing an MS2 fragment at m/z 163 with the loss of a deoxyhexosyl-hexosyl moiety [M-H-308]−, being tentatively assigned as p-coumaric acid deoxyhexosyl-hexoside. Peaks 35 and 38 ([M-H]− at m/z 633) presented a fragment at m/z 163 and UV spectra similar to those of coumaric acid, and thus no further identification could be performed; these were tentatively identified as coumaric acid derivatives. Two coumarins were found in husk (peak 4) and pulp (peak 9) and were tentatively identified as 7,8-dihydroxycoumarin-8-glucoside and dihydro-coumaric acid-O-hexoside taking into account their fragmentation pattern previously reported by Ieri et al. (2012) [33] in Prunus mahaleb L. and by Oszmiański et al. (2015) [43] who studied the leaves of Aesculus glabra.
A representative chromatogram of the pulp is presented in Figure 1.
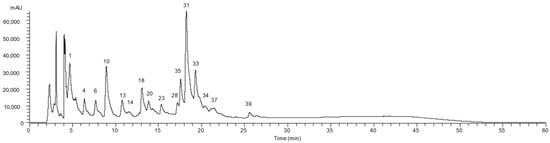
Figure 1.
Phenolic profile of the pulp of Aesculus hippocastanum recorded at 280 nm. Peak numbering is indicated as defined in Table 2.
3.3. Bioactivity of Aesculus hippocastanum L. Fruits
According to the results obtained for the individual compounds, it would be expected that at least the husk (higher quantities of organic acids) and skin (higher amounts of phenolic compounds) would reveal antioxidant properties. The results obtained for the TBARS formation inhibition and OxHLIA corroborate this hypothesis since the EC50 values obtained for skins and husks were strikingly lower. Comparing skin and husk, it could be concluded that the highest activity was measured in the husk for both assays (Table 3), which also agrees with the higher contents in organic acids that were quantified in A. hippocastanum husk (Table 1). These results are a strong indicator of the potential use of the extracts of A. hippocastanum in food, pharmaceutical, or cosmetic applications.

Table 3.
Antioxidant activity of pulp, skin, and husk of Aesculus hippocastanum fruits (EC50 values; µg/mL; mean ± SD).
Regarding the antibacterial activity (Table 4), none of the assayed parts showed bactericidal activity up to the maximum tested concentration (20 mg/mL). Nonetheless, the same extracts inhibit bacterial growth, especially among Gram-positive bacteria. Within this group, MRSA showed the highest sensitivity, whereas Listeria monocytogenes turned out to be the most resistant. Escherichia coli was the most sensitive species among Gram-negative bacteria, whereas Proteus mirabilis and Pseudomonas aeruginosa stood out as the most resistant. Considering the assayed parts, the seed coat (skin) showed the highest antimicrobial activity, which agrees with its higher content in phenolic compounds. However, this correlation was not observed for all assayed parts (MIC values for husk extracts were closer to the ones from pulp than those from skin), which indicates that other compounds besides phenolics contributed to the antibacterial activity.

Table 4.
Antimicrobial activity of the ethanolic extracts of pulp, skin, and husk of Aesculus hippocastanum fruits.
The potential cytotoxicity (Table 5) of the hydroethanolic extracts of different A. hippocastanum parts was evaluated using four human tumour cell lines and a non-tumour culture isolated from porcine liver. As observed for antioxidant and antimicrobial activities, skins and husks showed higher activity, as evidenced by the obtained GI50 values, which once again validated the higher activity of husk extracts compared with skin extracts. HepG2 was the most sensitive tumour cell line, closely followed by HeLa and MCF7, whereas NCI-H460 showed slightly higher resistance. The effect on the non-tumour cells was less pronounced.

Table 5.
Cytotoxicity of pulp, skin, and husk of A. hippocastanum fruits (values are presented as GI50 values in µg/mL of extract)).
In addition, the anti-inflammatory activity was evaluated in a RAW 264.7 cell line (data not shown). Pulp extracts showed no activity up to the maximum assayed concentration (400 µg/mL of extract), whereas the highest activity was again obtained with husk extracts (husk GI50 = 98 ± 2 µg/mL of extract; skin GI50 = 125 ± 5 µg/mL). Considering both activity types, these results indicate the potential use of skin and husk extracts in pharmaceutical applications.
4. Discussion
To the best of our knowledge, there is no detailed description in the literature about the organic acids in horse chestnut seeds. Organic acids are essential intermediates in cellular metabolism, produced in central energy pathways, detoxification mechanisms, neurotransmitter breakdown, or microbial metabolism. Therefore, they are considered biomarkers, providing an overview of the human physiological state and possible metabolic disorders [23,44]. As shown in Table 1, the botanical part with the highest concentration of organic acids was found to be the husk, mainly due to its content of quinic acid (7.6 g/100 g dw). In addition to their importance in metabolism, organic acids have recognized bioactivities. Quinic acid is known for its biological potential, including potential antioxidant, antidiabetic, anticancer, antimicrobial, antiviral, antiaging, protective, anti-nociceptive, and analgesic effects [24]. It is essential to highlight that the studied seeds also revealed the presence of oxalic, malic, citric, ascorbic, and shikimic acid distributed among the three botanical parts. As previously mentioned, the husk was the part that effectively revealed the highest content of organic acids, which would be expected since this is the defensive barrier of the seeds and therefore is typically rich in bioactive compounds that prevent the entry of harmful organisms.
Regarding phenolic compounds, forty compounds were identified, confirming that each part of the seed has a particular profile (Table 2). The main family of phenolic compounds found in A. hippocastanum skin and husk were (epi)catechin derivatives. According to the authors of [28], horse chestnut bark is rich in esculin, esculetin, and fraxetin, all coumarin derivatives. On the other hand, the authors of [29] identified ten phenolic compounds in horse chestnut husk belonging to two chemical classes: coumarins and flavan-3-ols. The results obtained in this work are not at all different from those found in the literature since we found catechin (flavan-3-ol) derivatives and coumarins in horse chestnut skin and husk. However, in the present study, it was possible to find/identify more compounds, namely fourteen phenolic compounds in the skin and fifteen in the bark.
Regarding A. hippocastanum pulp, eighteen phenolic compounds were found, namely ten flavonoid derivatives (quercetin, kaempferol, and isorhamnetin glycoside derivatives), six phenolic acids (caffeic and coumaric acid derivatives), one coumarin derivative and an unknown compound (Figure 1 and Table 2). Other authors reported a similar profile in A. hippocastanum seed pulp [27]. The authors of [45] also identified kaempferol and procyanidin A2 in horse chestnut seeds, and the authors of [46] identified the three flavonoids quercetin, kaempferol, and rutin. Although there are some fluctuations in the profiles, the leading families of phenolic compounds described in this work are similar to those described in the literature. Some differences may be due to the employed extraction methodologies (for example, the authors of [45] used ultrasound-assisted extraction, and the authors of [45,46] used methanolic extracts) or even the edaphoclimatic conditions under which the different samples were developed.
According to the results obtained for the individual compounds, it would be expected that at least the husk (higher quantities of organic acids) and skin (higher amounts of phenolic compounds) would reveal antioxidant properties. Phenolic compounds, by and large, are widely reported for their bioactive potential, namely their antioxidant capacity. Both assays used to determine the antioxidant activity confirmed this speculation since the lowest EC50 values were effectively verified in the husk (0.7 and 0.24 µg/mL, for TBARS and OxHLIA, respectively) and skin (1.4 and 2.4 µg/mL, for TBARS and OxHLIA, respectively). Regarding the pulp, this revealed an EC50 of 865 µg/mL for the TBARS assay and no activity was detected for the OxHLIA assay (Table 3). It should be highlighted that in the field of natural products, it is well known that the antioxidant activity of phenolics is controlled by intermolecular interactions that can be either synergistic or antagonistic. Thus, there may be some fluctuations in the results, such as those verified in the present study, in which the sample with the highest content of phenolic compounds (skin) is not precisely the one that demonstrated the most increased antioxidant activity (husk). Nevertheless, both have this bioactivity (and the values obtained are of the same order of magnitude). These results are a strong indicator of the potential use of the ethanolic extracts of A. hippocastanum in food or pharmaceutical applications. A similar level of antioxidant activity was previously found in Turkish samples of the species studied herein [47]. Other studies reporting the antioxidant activity of A. hippocastanum are available in the literature but used different techniques, such as the DPPH radical scavenging assay and oxygen radical absorbance capacity (ORAC) methods [45,48], methodologies less precise than those used in this work. The authors of [47] have studied a so-called “escin mixture” obtained from the ethanol extract of A. hippocastanum seeds, administering it orally to male mice. They concluded that it increased the antioxidative defence system of the body and prevented high-fat-diet-induced lipid peroxidation in male mice.
Regarding A. hippocastanum antibacterial activity (Table 4), none of the tested ex-tracts revealed bactericidal effects on Gram-positive and Gram-negative bacteria up to the maximum assayed concentration (20 mg/mL). However, it was possible to observe an inhibitory effect mainly against Gram-positive bacteria (Enterococcus faecalis, Listeria monocytogenes, and MRSA) for all the studied extracts. MRSA showed the highest sensitivity, whereas Listeria monocytogenes turned out to be the most resistant. The extract obtained from the skin was also particularly active against the tested Gram-negative species, obtaining better MIC values compared with the pulp and husk. Given these results, it can be inferred that perhaps the phenolic compounds are the molecules responsible for the antimicrobial properties of the samples under study since the horse chestnut skin revealed the highest content of total phenols and flavonoids. However, this correlation was not observed for all the assayed parts (MIC values for bark extracts were closer to the ones from pulp than those from skin), which indicates that other compounds besides phenolics contributed to the antibacterial activity. Other authors [49] also tested an ethanolic extract from A. hippocastanum bark against E. coli, P. mirabilis, and M. morganii, recording lower MICs than those of this work, but with the same order of magnitude (1–2 mg/mL).
Analysing the results obtained for the cytotoxicity of the studied extracts against human tumour cell lines (Table 5), the same tendency observed for the antioxidant and antibacterial activities was verified. Generally, the extracts obtained from the skin and husk showed higher activity, as evidenced by the obtained GI50 values (ranging from 52 to 100 µg/mL). Higher GI50 values were shown by the pulp, ranging from 238 to >400 µg/mL. HepG2 was the most sensitive tumour cell line, closely followed by HeLa and MCF7, whereas NCI-H460 showed slightly higher resistance. The effect over the non-tumour cells was less pronounced, but this might also be related with the higher division rate of tumour cell lines. In addition, it should be considered that horse chestnut is rich in saponins that are known for their associated toxicity; hence its consumption is not recommended. Therefore, these compounds may be related to the cytotoxicity observed on tumour and non-tumour cells. Effectively, even though there is little information regarding the cytotoxic potential of A. hippocastanum, some in vitro studies were performed using different tumour models, including breast cancer, cervical cancer, and leukaemia cell lines. The studies infer that the extracts’ mechanism of action may be via DNA fragmentation, apoptosis, and suppression of the hypoxia-induced VEGF secretion [50,51,52]. However, it is important to highlight that these properties are typically associated with the saponins present in the extracts, mainly aescin, with no reference to other compounds, such as phenolics.
In addition to the previously mentioned assays, the anti-inflammatory activity of the hydroethanolic extracts under study was evaluated in a RAW 264.7 cell line (data not shown). Pulp extracts showed no activity up to the maximum assayed concentration (400 µg/mL of extract), whereas the highest activity was again obtained with husk extracts (bark GI50 = 98 ± 2 µg/mL; skin GI50 = 125 ± 5 µg/mL). These results corroborate the previous ones, suggesting the potential use of horse chestnut skin and bark extracts in therapeutic applications.
Given the lack of information regarding the chemical characterization and bioactive potential of A. hippocastanum that still exists, this work intended, through a more in-depth chemical description, to focus on bioactive compounds, go beyond the state-of-the-art, and try to relate these compounds to the bioactivity presented by the samples. Therefore, it offers new data proving the bioactivity of the matrix under investigation, making it a potential candidate for incorporation into pharmaceutical or nutraceutical formulations. Moreover, another strength of the present study is to promote the reuse of bio-residues that have no use and are mostly disposed of in landfills.
5. Conclusions
The main goal of this study was to characterize different bioactive compounds in specific parts of A. hippocastanum fruits, namely the pulp, skin, and husk, and further evaluate their bioactivity. Regarding the phenolic compound profile, kaempferol-O-pentoside-O-hexoside-O-hexoside was the main compound in pulp, whereas (-)-epicatechin and β-type (epi)catechin dimer were the major phenolic compounds in husk and skin, respectively. In all cases, flavonoids stood out as the major subgroup of phenolic compounds. Comparing the studied parts, skin proved to be the best source of these bioactive compounds. In turn, organic acids were particularly abundant in the husk, mainly due to its content of quinic acid. Regarding the bioactivity assays, the hydroethanolic extracts of A. hippocastanum husk showed the highest antioxidant activity, cytotoxicity, and anti-inflammatory activity, whereas skin revealed the strongest antibacterial potential.
Overall, A. hippocastanum fruit proved to be a valuable source of bioactive compounds, especially flavonoids and organic acids. Lastly, using a circular economy approach, the by-products of A. hippocastanum fruit (mainly husk and skin) could have interesting applications to obtain value-added compounds that are likely to be used in different sectors, such as the food, cosmetic, and pharmaceutical industries.
Future studies may test the stability of these extracts in food, cosmetic, and pharmaceutical formulations to confirm whether they remain after incorporation.
Author Contributions
Conceptualization, Project administration, Supervision, and Resources: L.B., J.C.M.B., I.C.F.R.F. and K.Z.; Methodology and Investigation: A.D., F.S.R., C.P., T.C.S.P.P. and R.C.C.; Writing—original draft: F.S.R. and J.C.M.B.; Supervision, Writing—review & editing: L.B., J.C.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the European Regional Development Fund (ERDF) through the Regional Operational Program North 2020, within the scope of the Project GreenHealth—Digital strategies in biological assets to improve well-being and promote green health, Norte-01-0145-FEDER-000042.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available.
Acknowledgments
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES (PIDDAC), CIMO (UIDB/00690/2020 and UIDP/00690/2020), and SusTEC (LA/P/0007/2020). The authors also acknowledge the national funding by FCT—Foundation for Science and Technology, through the institutional scientific employment programme contract with L. Barros, and through the individual scientific employment programme contract with J.C.M. Barreira (CEECIND/04479/2017) and F.S. Reis (2021.03728.CEECIND).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Raynor, D.K.; Dickinson, R.; Knapp, P.; Long, A.F.; Nicolson, D.J. Buyer beware? Does the information provided with herbal products available over the counter enable safe use? BMC Med. 2011, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Makuch, M.; Matławska, I. Flavonoids from the flowers of Aesculus hippocastanum. Acta Pol. Pharm. 2011, 68, 403–408. [Google Scholar] [PubMed]
- Wilczyński, S.; Podlaski, R. The effect of climate on radial growth of horse chestnut (Aesculus hippocastanum L.) in the Świętokrzyski National Park in central Poland. J. For. Res. 2007, 12, 24–33. [Google Scholar] [CrossRef]
- Busia, K. Fundamentals of Herbal Medicine: Major Plant Families, Analytical Methods, Materia Medica; Xlibris Publishing: Bloomington, UK, 2016; Volume 2. [Google Scholar]
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A handbook for Practice on a Scientific Basis, 3rd ed.; Medpharm GmbH Scientific Publishers: Stuttgart, Germany, 2004. [Google Scholar]
- de Farias, M.F.A.M.S.; Patriota, L.L.S.; Lira, C.B.S.; Aguiar, L.M.S.; Barros, B.R.S.; Paiva, P.M.G.; de Melo, C.M.L.; Santos, N.D.L.; Napoleão, T.H. Purification, characterization, and immunomodulatory activity of a lectin from the seeds of horse chestnut (Aesculus hippocastanum L.). Curr. Res. Biotechnol. 2022, 4, 203–210. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by ultra fast liquid chromatography and photodiode array detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Rodrigues, S.; Carvalho, A.M.; Ferreira, I.C.F.R. Development of hydrosoluble gels with Crataegus monogyna extracts for topical application: Evaluation of antioxidant activity of the final formulations. Ind. Crop. Prod. 2013, 42, 175–180. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crop. Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Takebayashi, J.; Iwahashi, N.; Ishimi, Y.; Tai, A. Development of a simple 96-well plate method for evaluation of antioxidant activity based on the oxidative haemolysis inhibition assay (OxHLIA). Food Chem. 2012, 134, 606–610. [Google Scholar] [CrossRef]
- Kuete, V.; Ango, P.Y.; Fotso, G.W.; Kapche, G.D.; Dzoyem, J.P.; Wouking, A.G.; Ngadjui, B.T.; Abegaz, B.M. Antimicrobial activities of the methanol extract and compounds from Artocarpus communis (Moraceae). BMC Complement. Altern. Med. 2011, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Kamga, J.; Sandjo, L.P.; Ngameni, B.; Poumale, H.M.; Ambassa, P.; Ngadjui, B.T. Antimicrobial activities of the methanol extract, fractions and compounds from Ficus polita Vahl. (Moraceae). BMC Complement. Altern. Med. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Morales, P.; Cámara, M.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Wild Fragaria vesca L. fruits: A rich source of bioactive phytochemicals. Food Funct. 2016, 7, 4523–4532. [Google Scholar] [CrossRef]
- Reis, F.S.; Barreira, J.C.M.; Calhelha, R.C.; van Griensven, L.J.; Ćirić, A.; Glamočlija, J.; Soković, M.; Ferreira, I.C.F.R. Chemical characterization of the medicinal mushroom Phellinus linteus (Berkeley & Curtis) Teng and contribution of different fractions to its bioactivity. LWT Food Sci. Technol. 2014, 58, 478–485. [Google Scholar]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno [3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; García-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Moro, C.; Manchón, N.; Gonzalo-Ruiz, A.; Villares, A.; Guillamón, E.; Rostagno, M.; Mateo-Vivaracho, L. In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chem. 2014, 161, 216–223. [Google Scholar] [CrossRef]
- Taofiq, O.; Calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Int. 2015, 76, 821–827. [Google Scholar] [CrossRef]
- Mouskeftara, T.; Virgiliou, C.; Theodoridis, G.; Gika, H. Analysis of urinary organic acids by gas chromatography tandem mass spectrometry method for metabolic profiling applications. J. Chromatogr. A 2021, 1658, 462590. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022, 1–30. [Google Scholar]
- Hübner, G.; Wray, V.; Nahrstedt, A. Flavonol oligosaccharides from the seeds of Aesculus hippocastanum. Planta Med. 1999, 65, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, I.; Janda, B.; Szajwaj, B.; Stochmal, A.; Piacente, S.; Pizza, C.; Franceschi, F.; Franz, C.; Oleszek, W. Flavonoids in horse chestnut (Aesculus hippocastanum) seeds and powdered wastewater byproducts. J. Agric. Food Chem. 2007, 55, 8485–8490. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Albiston, C.; Añibarro-Ortega, M.; Ferreira, I.C.F.R.; Pinela, J.; Barros, L. Sonoextraction of phenolic compounds and saponins from Aesculus hippocastanum seed kernels: Modeling and optimization. Ind. Crop. Prod. 2022, 185, 115142. [Google Scholar] [CrossRef]
- Coruh, N. and Özdoğan, N. Fluorescent coumarin components of the husk of Aesculus hippocastanum. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1334–1350. [Google Scholar] [CrossRef]
- Owczarek, A.; Olszewska, M.A. Development and validation of UHPLC-PDA method for simultaneous determination of bioactive polyphenols of horse-chestnut bark using numerical optimization with MS Excel Solver. J. Pharm. Biomed. Anal. 2020, 190, 113544. [Google Scholar] [CrossRef]
- Barros, L.; Duenas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of cultivated, in vitro cutured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Barros, L.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Santos, E.A.; Regis, W.C.B.; Ferreira, I.C.F.R. The powerful in vitro bioactivity of Euterpe oleracea Mart. seeds and related phenolic compounds. Ind. Crop. Prod. 2015, 76, 318–322. [Google Scholar] [CrossRef]
- Melgar, B.; Pereira, E.; Oliveira, M.B.P.P.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Extensive profiling of three varieties of Opuntia spp. fruit for innovative food ingredients. Food Res. Int. 2017, 101, 259–265. [Google Scholar] [CrossRef]
- Ieri, F.; Pinelli, P.; Romani, A. Simultaneous determination of anthocyanins, coumarins and phenolic acids in fruits, kernels and liqueur of Prunus mahaleb L. Food Chem. 2012, 135, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Berardini, N.; Carle, R.; Schieber, A. Characterization of gallotannins and benzophenone derivatives from mango (Mangifera indica L. cv. ’Tommy Atkins’) peels, pulp and kernels by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Negri, G.; Barreto, L.M.R.C.; Sper, F.L.; Carvalho, C.D.; Campos, M.D.G.R. Phytochemical analysis and botanical origin of Apis mellifera bee pollen from the municipality of Canavieiras, Bahia State, Brazil. Braz. J. Food Technol. 2018, 21, e2016176. [Google Scholar] [CrossRef]
- Riedelsberger, J.; Blatt, M.R. Roots—The hidden provider. Front. Plant Sci. 2017, 8, 1021. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Rosa rubiginosa and Fraxinus oxycarpa herbal teas: Characterization of phytochemical profiles by liquid chromatography-mass spectrometry, and evaluation of the antioxidant activity. New J. Chem. 2017, 41, 7681–7688. [Google Scholar] [CrossRef]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MS(n). Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Chen, P.; Ozcan, M.; Harnly, J.M. Chromatographic profiles and identification of new phenolic components of Ginkgo biloba leaves and selected products. J. Agric. Food Chem. 2008, 56, 6671–6679. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kalisz, S.; Aneta, W. The content of phenolic compounds in leaf tissues of white (Aesculus hippocastanum L.) and red horse chestnut (Aesculus carea H.) colonized by the horse chestnut leaf miner (Cameraria ohridella Deschka & Dimić). Molecules 2014, 19, 14625–14636. [Google Scholar]
- Paterska, M.; Bandurska, H.; Wysłouch, J.; Molińska-Glura, M.; Moliński, K. Chemical composition of horse-chestnut (Aesculus) leaves and their susceptibility to chestnut leaf miner Cameraria ohridella Deschka & Dimić. Acta Physiol. Plant. 2017, 39, 105. [Google Scholar]
- Martin-Tanguy, J.; Cabanne, F.; Perdrizet, E.; Martin, C. The distribution of hydroxycinnamic acid amides in flowering plants. Phytochem. 1978, 17, 1927–1928. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Biernat, A. The content of phenolic compounds in leaf tissues of Aesculus glabra and Aesculus parviflora Walt. Molecules 2015, 20, 2176–2189. [Google Scholar] [CrossRef]
- Hur, H.; Paik, M.J.; Xuan, Y.; Nguyen, D.T.; Ham, I.H.; Yun, J.; Cho, Y.K.; Lee, G.; Han, S.U. Quantitative measurement of organic acids in tissues from gastric cancer patients indicates increased glucose metabolism in gastric cancer. PLoS ONE 2014, 9, e98581. [Google Scholar] [CrossRef] [PubMed]
- Kędzierski, B.; Kukula-Koch, W.; Widelski, J.; Głowniak, K. Impact of harvest time of Aesculus hippocastanum seeds on the composition, antioxidant capacity and total phenolic content. Ind. Crop. Prod. 2016, 86, 68–72. [Google Scholar] [CrossRef]
- Čukanović, J.; Tešević, V.; Jadranin, M.; Ljubojević, M.; Mladenović, E.; Kostić, S. Horse chestnut (Aesculus hippocastanum L.) seed fatty acids, flavonoids and heavy metals plasticity to different urban environments. Biochem. Syst. Ecol. 2020, 89, 103980. [Google Scholar] [CrossRef]
- Küçükkurt, I.; Ince, S.; Keleş, H.; Akkol, E.K.; Avcı, G.; Yeşilada, E.; Bacak, E. Beneficial effects of Aesculus hippocastanum L. seed extract on the body’s own antioxidant defense system on subacute administration. J. Ethnopharmacol. 2010, 129, 18–22. [Google Scholar] [CrossRef]
- Makino, M.; Katsube, T.; Ohta, Y.; Schmidt, W.; Yoshino, K. Preliminary study on antioxidant properties, phenolic contents, and effects of Aesculus hippocastanum (horse chestnut) seed shell extract on in vitro cyclobutane pyrimidine dimer repair. J. Intercult. Ethnopharmacol. 2017, 6, 414–419. [Google Scholar] [CrossRef]
- Khar’kov, Y.K.; Arsene, M.M.; Aliya, M.V.; Viktorovna, P.I.; Elena, V.G.; Azova, M.M.; Amira, A.A. Assessment of Antimicrobial activity of ethanolic and aqueous extracts of Aesculus hippocastanum L. (horse chestnut) bark against bacteria isolated from urine of patients diagnosed positive to urinary tract infections. Front. Biosci. 2022, 14, 11. [Google Scholar]
- Fedotcheva, T.A.; Sheichenko, O.P.; Sheichenko, V.I.; Fedotcheva, N.I.; Shimanovskii, N.L. Preparation of a horse chestnut extract with a 50% content of escin and its actions on tumor cell proliferation and isolated mitochondria. Pharm. Chem. J. 2019, 53, 57–64. [Google Scholar] [CrossRef]
- Mojžišová, G.; Mojžiš, J.; Pilátová, M.; Varinská, L.; Ivanová, L.; Strojný, L.; Richnavský, J. Antiproliferative and antiangiogenic properties of horse chestnut extract. Phytother. Res. 2013, 27, 159–165. [Google Scholar] [CrossRef]
- Owczarek-Januszkiewicz, A.; Kicel, A.; Olszewska, M.A. Aesculus hippocastanum in the pharmaceutical industry and beyond–Phytochemistry, bioactivity, present application, and future perspectives. Ind. Crop. Prod. 2023, 193, 116187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).