Volatile and Non-Volatile Content Determination and Biological Activity Evaluation of Fresh Humulus lupulus L. (cv. Chinook) Leaves and Inflorescences
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Materials
2.3. Extraction Process
2.4. SPME Sampling
2.5. GC-MS Analysis of Fresh Hops Samples
2.6. DI-SPME-GC-MS and GC-MS Analysis ofHops Extracts
2.7. GC-MS Analysis of Derivatized Methanolic Extracts
2.8. Determination of Total Polyphenols
2.9. Determination of Total Flavonoids
2.10. Antioxidant Assays
2.10.1. DPPH (2,2-Diphenyl-picryl hydrazyl) Test
2.10.2. ABTS [2,2’-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)diammonium salt)] test
2.11. Cell Cultures and Cytotoxicity Assay
2.12. Statistical Analysis
3. Results
3.1. Chemical Composition of Fresh Inflorescences and Leaves (Unpowdered and Powdered)
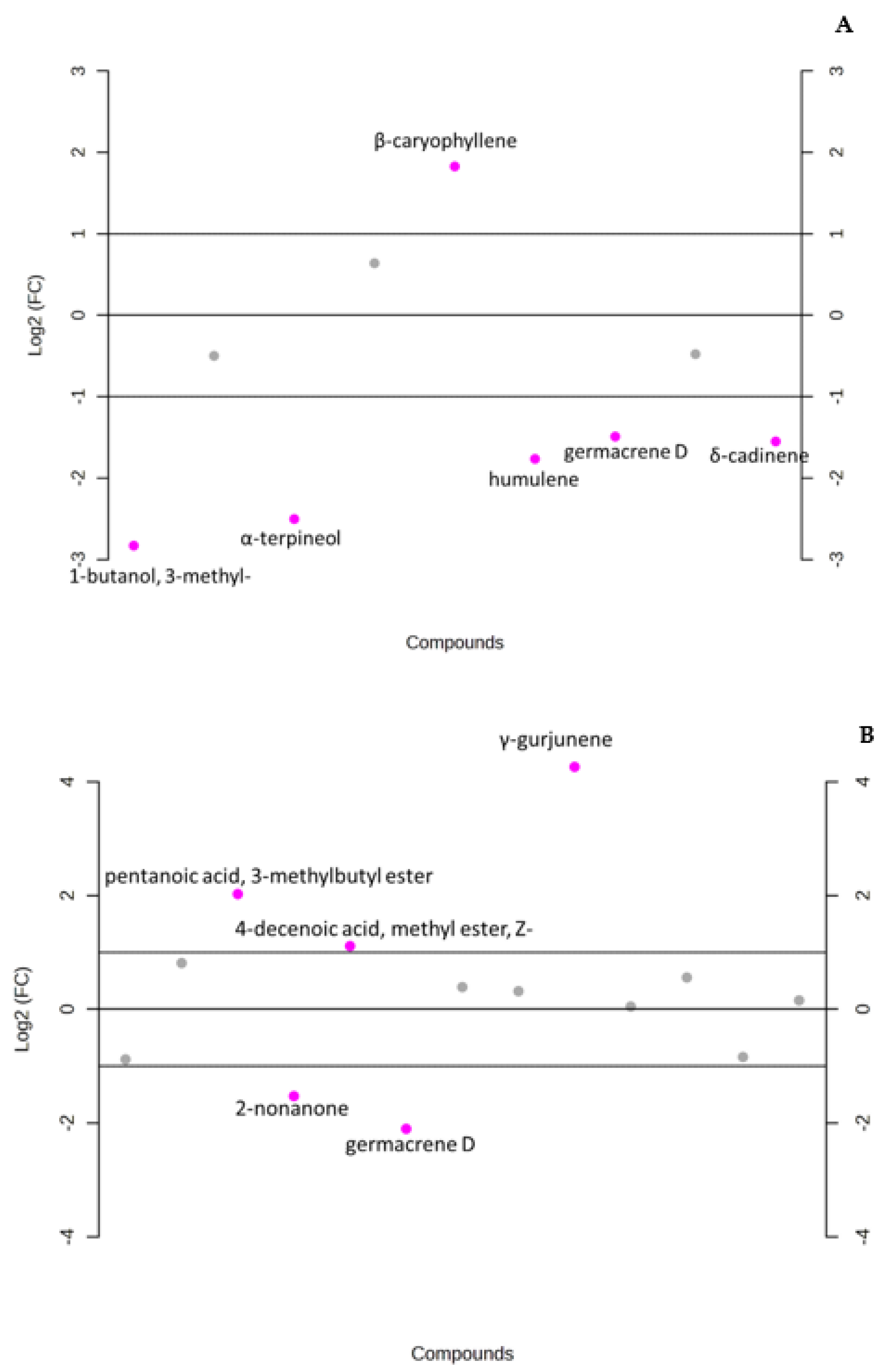
3.2. Data Analysis
3.3. Chemical Volatile Composition of Aqueous Extracts
3.4. Chemical Volatile Composition of Methanolic Extracts
3.5. Chemical Composition of Methanolic Extracts after Derivatization
3.6. Total Polyphenols and Flavonoids
3.7. Antioxidant Activity
3.8. Cytotoxic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takoi, K. “Flavor Hops” Varieties and Various Flavor Compounds Contributing to Their “Varietal Aromas”: A review. Tech. Quart. Master Brew. Assoc. Am. 2019, 56, 113–123. [Google Scholar]
- McFarland, B. World’s Beers: One Thousand Craft Brews from Cask to Glass; Sterling Publishing Company: New York, NY, USA, 2009. [Google Scholar]
- Nezi, P.; Cicaloni, V.; Tinti, L.; Salvini, L.; Iannone, M.; Vitalini, S.; Garzoli, S. Metabolomic and Proteomic Profile of Dried Hop Inflorescences (Humulus lupulus L. cv. Chinook and cv. Cascade) by SPME-GC-MS and UPLC-MS-MS. Separations 2022, 9, 204. [Google Scholar] [CrossRef]
- Motti, R.; de Falco, B. Traditional Herbal Remedies Used for Managing Anxiety and Insomnia in Italy: An Ethnopharmacological Overview. Horticulturae 2021, 7, 523. [Google Scholar] [CrossRef]
- Guimarães, B.P.; Nascimento, P.G.B.D.; Ghesti, G.F. Intellectual Property and Plant Variety Protection: Prospective Study on Hop (Humulus lupulus L.) Cultivars. World Pat. Inf. 2021, 65, 102041. [Google Scholar] [CrossRef]
- European Commission. Agriculture and Rural Development. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/hops_en (accessed on 26 October 2022).
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and Present Use, and Future Potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus L., a Very Popular Beer Ingredient and Medicinal Plant: Overview of Its Phytochemistry, Its Bioactivity, and Its Biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.S.; Lorenzo, J.M. Humulus lupulus L. as a Natural Source of Functional Biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- Ovidi, E.; Laghezza Masci, V.; Taddei, A.R.; Torresi, J.; Tomassi, W.; Iannone, M.; Tiezzi, A.; Maggi, F.; Garzoli, S. Hemp (Cannabis sativa L., Kompolti cv.) and Hop (Humulus lupulus L., Chinook cv.) Essential Oil and Hydrolate: HS-GC-MS Chemical Investigation and Apoptotic Activity Evaluation. Pharmaceuticals 2022, 15, 976. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Ovidi, E.; Laghezza Masci, V.; Tiezzi, A.; Garzoli, S. Detection of Volatiles by HS-SPME-GC/MS and Biological Effect Evaluation of Buddha’s Hand Fruit. Molecules 2022, 27, 1666. [Google Scholar] [CrossRef]
- Cicaloni, V.; Salvini, L.; Vitalini, S.; Garzoli, S. Chemical Profiling and Characterization of Different Cultivars of Cannabis sativa L. Inflorescences by SPME-GC-MS and UPLC-MS. Separations 2022, 9, 90. [Google Scholar] [CrossRef]
- Iannone, M.; Ovidi, E.; Vitalini, S.; Laghezza Masci, V.; Marianelli, A.; Iriti, M.; Tiezzi, A.; Garzoli, S. From Hops to Craft Beers: Production Process, VOCs Profile Characterization, Total Polyphenol and Flavonoid Content Determination and Antioxidant Activity Evaluation. Processes 2022, 10, 517. [Google Scholar] [CrossRef]
- William, E. Wallace “Mass Spectra” by NIST Mass Spectrometry Data Center, in NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2022; p. 20899. [Google Scholar] [CrossRef]
- Vitalini, S.; Grande, S.; Visioli, F.; Agradi, E.; Fico, G.; Tomè, F. Antioxidant Activity of Wild Plants Collected in Valsesia, an Alpine Region of Northern Italy. Phytother. Res. 2006, 20, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Iriti, M.; Vinciguerra, V.; Garzoli, S. A Comparative Study of the Chemical Composition by SPME-GC/MS and Antiradical Activity of Less Common Citrus Species. Molecules 2021, 26, 5378. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Madeo, M.; Tava, A.; Iriti, M.; Vallone, L.; Avato, P.; Cocuzza, C.E.; Simonetti, P.; Argentieri, M.P. Chemical Profile, Antioxidant and Antibacterial Activities of Achillea moschata Wulfen, an Endemic Species from the Alps. Molecules 2016, 21, 830. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr.Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yin, Y. Aroma characterization of regional Cascade and Chinook hops (Humulus lupulus L.). Food Chem. 2021, 364, 130410. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Hurley, K.; Xu, z.; Xu, Y.; Rutto, L.; O’keefe, S.; Scoggins, H.; Yin, Y. Performance of alternative drying techniques on hop (Humulus lupulus L.) aroma quality: An HS-SPME-GC-MS-O and chemometrics combined approach. Food Chem 2022, 381, 132289. [Google Scholar] [CrossRef]
- Schindler, R.; Sharrett, Z.; Perri, M.J.; Lares, M. Quantification of α-Acids in Fresh Hops by Reverse-Phase High Performance Liquid Chromatography. ACS Omega 2019, 4, 3565–3570. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleira, H.A.R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds from Medicinal Plants (Hops and Juniper Berries) and Their Antioxidant Activity. Foods 2020, 9, 7. [Google Scholar] [CrossRef]
- Gresta, F.; Calvi, A.; Santonoceto, C.; Strano, T.; Ruberto, G. Agronomic traits and essential oil profiles of Humulus lupulus L. cultivated in southern Italy. J. Essent. Oil Res. 2022. [Google Scholar] [CrossRef]
- Rutnik, k.; Hrnčič, M.K.; Košira, I.J. Hop Essential Oil: Chemical Composition, Extraction, Analysis, and Applications. Food Rev Int. 2021, 38, 529–551. [Google Scholar] [CrossRef]
- Morcol, T.B.; Wysocki, K.; Sankaran, R.P.; Matthews, P.D.; Kennelly, E.J. UPLC-QTof-MSE Metabolomics Reveals Changes in Leaf Primary and Secondary Metabolism of Hop (Humulus lupulus L.) Plants under Drought Stress. J. Agric. Food Chem. 2020, 68, 4698–14708. [Google Scholar] [CrossRef] [PubMed]
- Ceh, B.; Kac, M.; Kosir, I.J.; Bram, V. Relationships between Xanthohumol and Polyphenol Content in Hop Leaves and Hop Cones with Regard to Water Supply and Cultivar. Int. J. Mol. Sci. 2007, 8, 989–1000. [Google Scholar] [CrossRef]
- Derkanosova, A.A.; Orinicheva, A.A.; Muravev, A.S. Chemical Composition and Antioxidant Activity of Hop Products. J. Int. Acad. Refrig. 2016, 4, 19–22. [Google Scholar] [CrossRef]
- Abram, V.; Ceh, B.; Vidmar, M.; Hercezi, M.; Lazic, N.; Bicik, V.; Mozina, S.S.; Kosir, I.J.; Kac, M.; Demsar, L.; et al. A Comparison of Antioxidant and Antimicrobial Activity between Hop Leaves and Hop Cones. Ind. Crops Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Keskin, Ş.; Şirin, Y.; Çakir, H.E.; Keskin, M. An Investigation of Humulus lupulus L.: Phenolic Composition, Antioxidant Capacity and Inhibition Properties of Clinically Important Enzymes. S. Afr. J. Bot. 2019, 120, 170–174. [Google Scholar] [CrossRef]
- Dziedziński, M.; Szczepaniak, O.; Telichowska, A.; a Kobus-Cisowska, J. Antioxidant capacity and Cholinestaerase Inhibiting Properties of Dietary Infusions with Humulus Lupulus. J. Elem. 2020, 25, 657–673. [Google Scholar]
- Stanius, Ž.; Dudenas, M.; Kaškoniene, V.; Stankevĭcius, M.; Skrzydlewska, E.; Drevinskas, T.; Ragažinskiene, O.; Obelevĭcius, K.; Maruška, A. Analysis of the Leaves and Cones of Lithuanian Hops (Humulus lupulus L.) Varieties by Chromatographic and Spectrophotometric Methods. Molecules 2022, 27, 2705. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, X.H.; Tang, J.; Liu, K. Antioxidant Activities of Hops (Humulus lupulus) and Their Products. J. Am. Soc. Brew. Chem. 2007, 65, 116–121. [Google Scholar] [CrossRef]
- Tava, A.; Iriti, M.; Vitalini, S. Composition and Antioxidant Activity of the Essential Oil from Achillea moschata Wulfen Growing in Valchiavenna and Valmalenco (Italian Central Alps). Int. J. Hortic. Sci. Technol. 2020, 7, 335–341. [Google Scholar]
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts. Antioxidants 2020, 9, 19. [Google Scholar] [CrossRef]
- Avery, S.V. Molecular targets of oxidative stress. Biochem. J. 2011, 434, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, B.; Rajhi, I. Potential Antioxidant Activity of Terpenes. In Terpenes and Terpenoids; Perveen, S., Al-Taweel, A.M., Eds.; IntechOpen: London, UK, 2021. [Google Scholar]
- Elagbar, Z.A.; Naik, R.R.; Shakya, A.K.; Bardaweel, S.K. Fatty Acids Analysis, Antioxidant and Biological Activity of Fixed Oil of Annona muricata L. Seeds. J. Chem. 2016, 2016, 6948098. [Google Scholar]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I. Antioxidant activity of Linalool. J. Eng. Technol. 2018, 36, 64–67. [Google Scholar] [CrossRef]
- Gunawan, I.W.G.; Bawa Putra, A.A.; Widihati, I.A.G. The Response to oxidative stress α-Humulene Compounds Hibiscus manihot L Leaf on the Activity of 8-Hydroxy-2-Deoksiquanosin Levels Pancreatic β-Cells in diabetic Rats. Biomed. & Pharmacol. J. 2016, 9, 433–441. [Google Scholar]
- Alonso-Esteban, J.I.; Pinela, J.; Barros, L.; Ćirićc, A.; Soković, M.; Calhelha, R.C.; Torijsa-Isasa, E.; Sánchez-Mata, M.d.C.; Ferreira, I.C.F.R. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) Seeds. Ind. Crops Prod. 2019, 134, 154–159. [Google Scholar] [CrossRef]
- Farag, M.A.; Wessjohann, L.A. Cytotoxic effect of commercial Humulus lupulus L. (hop) preparations—In comparison to its metabolomic fingerprint. J. Adv. Res. 2013, 4, 417–421. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Shen, Y.-L. Linalool Exhibits Cytotoxic Effects by Activating Antitumor Immunity. Molecules 2014, 19, 6694–6706. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef]
- Byczek-Wyrosteka, A.; Kitel, R.; Rumak, K.; Skonieczna, M.; Kasprzycka, A.; Walczak, K. Simple 2(5H)-furanone derivatives with selective cytotoxicity towards non-small cell lung cancer cell line A549—Synthesis, structure-activity relationship and biological evaluation. Eur. J. Med. Chem. 2018, 150, 687–697. [Google Scholar] [CrossRef]
- Pacheco, B.S.; dos Santos, M.A.Z.; Schultze, E.; Martins, R.M.; Lund, R.G.; Seixas, F.K.; Colepicolo, P.; Collares, T.; Paula, F.R.; Pereira De Pereira, C.M. Cytotoxic Activity of Fatty Acids From Antarctic Macroalgae on the Growth of Human Breast Cancer Cells. Front Bioeng Biotechnol. 2018, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Ymashita, U.; Kurihara, H.; Fukushi, E.; Kawabta, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Antic Res. 2002, 22, 2587–2590. [Google Scholar]
- Spriha, S.E.; Abdur Rahman, S.M. A Review on Biological Activities of Sugars and Sugar Derivatives. J. Pharm. Sci. 2022, 20, 381–394. [Google Scholar] [CrossRef]

| N° | COMPONENT 1 | LRI 2 | LRI 3 | Fresh I. 4 (Unpowdered) | Fresh L. 5 (Unpowdered) | Fresh I. 6 (Powdered) | Fresh L. 7 (Powdered) |
|---|---|---|---|---|---|---|---|
| 1 | 1-hepten-3-ol | 862 | 869 | - | - | - | 0.5 ± 0.02 |
| 2 | styrene | 893 | 898 | - | 1.3 ± 0.02 | - | - |
| 3 | hexanoic acid, 5-methyl-, methyl ester | 965 | 963 | - | - | 0.1 ± 0.02 | - |
| 4 | β-pinene | 988 | 986 | - | - | 0.9 ± 0.02 | - |
| 5 | β-myrcene | 991 | 987 | 37.9 ± 0.31 | 2.7 ± 0.02 | 20.5 ± 0.02 | 1.9 ± 0.02 |
| 6 | propanoic acid, 2-methyl-, 2-methylbutylester | 993 | 989 | 0.3 ± 0.05 | - | - | - |
| 7 | propanoic acid, 2-methyl-, 3-methylbutylester | 998 | 996 | 0.5 ± 0.02 | - | 0.9 ± 0.03 | - |
| 8 | limonene | 1028 | 1030 | 0.2 ± 0.02 | - | 0.4 ± 0.03 | 2.0 ± 0.03 |
| 9 | trans-β-ocimene | 1035 | 1040 | 0.1 ± 0.02 | - | - | - |
| 10 | butanoic acid,3-methylbutyl ester | 1052 | 1056 | - | - | 1.1 ± 0.02 | - |
| 11 | 6-methylheptanoic acid, methyl ester | 1063 | 1068 | 0.2 ± 0.02 | - | 0.4 ± 0.03 | - |
| 12 | pentanoic acid, 3-methylbutyl ester | 1092 | 1090 | 0.1 ± 0.02 | - | - | - |
| 13 | 2-nonanone | 1096 | 1092 | 0.2 ± 0.02 | - | 0.1 ± 0.02 | - |
| 14 | octanoic acid, methyl ester | 1128 | 1132 | - | - | 0.3 ± 0.02 | - |
| 15 | α-terpineol | 1175 | 1170 | - | 0.7 ± 0.01 | - | 0.1 ± 0.02 |
| 16 | geranic acid methyl ester | 1305 | 1302 | 0.2 ± 0.02 | - | - | - |
| 17 | decanoic acid, methyl ester | 1312 | 1309 | - | - | 0.2 ± 0.03 | - |
| 18 | α-cubebene | 1355 | 1350 | 0.2 ± 0.02 | - | 0.4 ± 0.03 | 0.3 ± 0.02 |
| 19 | ylangene | 1380 | 1376 | 0.2 ± 0.03 | - | - | - |
| 20 | α-copaene | 1384 | 1385 | 0.9 ± 0.04 | 3.1 ± 0.02 | - | 4.8 ± 0.03 |
| 21 | β-caryophyllene | 1442 | 1440 | 12.6 ± 0.03 | 20.2 ± 0.08 | 16.4 ± 0.04 | 71.6 ± 0.11 |
| 22 | humulene | 1470 | 1465 | 28.4 ± 0.03 | 33.8 ± 0.02 | 35.3 ± 0.03 | 10.0 ± 0.10 |
| 23 | γ-muurolene | 1475 | 1471 | 2.6 ± 0.03 | 8.4 ± 0.02 | 2.7 ± 0.03 | - |
| 24 | germacrene D | 1480 | 1475 | 1.4 ± 0.02 | 2.5 ± 0.03 | 0.3 ± 0.03 | 0.9 ± 0.03 |
| 25 | γ-gurjunene | 1482 | 1477 | 0.2 ± 0.02 | - | 4.3 ± 0.03 | - |
| 26 | β-eudesmene | 1483 | 1480 | 2.9 ± 0.04 | 2.9 ± 0.02 | 1.6 ± 0.02 | - |
| 27 | α-selinene | 1492 | 1489 | 1.9 ± 0.03 | - | - | - |
| 28 | γ-cadinene | 1505 | 1509 | 1.6 ± 0.03 | - | - | - |
| 29 | valencene | 1520 | 1515 | 1.5 ± 0.04 | 6.5 ± 0.01 | - | - |
| 30 | selina-3,7(11)-diene | 1533 | 1530 | 1.4 ± 0.02 | 0.9 ± 0.02 | 1.6 ± 0.05 | - |
| 31 | α-muurolene | 1541 | 1537 | - | 3.8 ± 0.02 | 2.4 ± 0.01 | 2.8 ± 0.04 |
| 32 | guaia-1(10), 11-diene | 1555 | 1512 | - | - | 2.6 ± 0.01 | 0.8 ± 0.02 |
| 33 | δ-cadinene | 1559 | 1538 | 3.2 ± 0.05 | 10.3 ± 0.03 | 4.7 ± 0.03 | 3.4 ± 0.02 |
| 34 | caryophyllene oxide | 1591 | 1585 | - | 0.2 ± 0.03 | - | |
| SUM | 98.6 | 99.9 | 97.4 | 99.4 | |||
| Monoterpenoids | 38.2 | 3.4 | 21.8 | 4.0 | |||
| Sesquiterpenoids | 58.9 | 92.4 | 72.5 | 94.6 | |||
| Others | 1.5 | 4.1 | 3.1 | 0.8 |
| N° | COMPONENT 1 | LRI 2 | LRI 3 | IAE | LAE |
|---|---|---|---|---|---|
| 1 | pentane, 1-methoxy- | 710 | 708 | - | 17.5 ± 0.04 |
| 2 | 1-butanol, 3-methyl- | 715 | 718 | - | 16.6 ± 0.03 |
| 3 | β-myrcene | 985 | 987 | 13.1 ± 0.05 | 8.3 ± 0.02 |
| 4 | propanoic acid, 2-methyl-, 3-methylhetyl ester | 995 | 996 | 8.9 ± 0.03 | - |
| 5 | linalool | 1089 | 1092 | 6.8 ± 0.03 | 49.6 ± 0.09 |
| 6 | β-caryophyllene | 1442 | 1440 | 10.8 ± 0.05 | - |
| 7 | humulene | 1490 | 1499 | 41.6 ± 0.08 | 8.0 ± 0.03 |
| 8 | δ-cadinene | 1561 | 1538 | 4.4 ± 0.02 | - |
| 9 | humulene epoxide II | 1572 | 1570 * | 14.3 ± 0.03 | - |
| SUM | 99.9 | 100.0 | |||
| Monoterpenoids | 19.9 | 57.9 | |||
| Sesquiterpenoids | 71.1 | 8.0 | |||
| Others | 8.9 | 34.1 |
| N° | COMPONENT 1 | LRI 2 | LRI 3 | IME | LME |
|---|---|---|---|---|---|
| 1 | butanal, 3-methyl- | 648 | 651 | - | 2.5 ± 0.06 |
| 2 | propanoic acid, 2-methyl- | 762 | 765 | 3.9 ± 0.03 | - |
| 3 | 2,3-butanediol | 770 | 769 | 24.6 ± 0.04 | - |
| 4 | butanoic acid, 3-methyl | 851 | 857 | 7.1 ± 0.02 | - |
| 5 | acetic acid 2-hydroxyethyl ester | 866 | 862 | - | 2.7 ± 0.06 |
| 6 | butanoic acid, 2-methyl- | 871 | 868 | 1.2 ± 0.02 | - |
| 7 | 2(5H)-furanone, 5,5-dimethyl- | 954 | 952 | 8.7 ± 0.04 | - |
| 8 | 2-pentenoic acid, 2-methyl- | 974 | 974 * | 3.7 ± 0.03 | - |
| 10 | 2-heptanol, 2-methyl- | 990 | 920 * | 42.7 ± 0.04 | - |
| 11 | trans-arbusculone | 1080 | 1071 | 1.9 ± 0.04 | - |
| 12 | trans-nerolidol | 1571 | 1547 | 1.3 ± 0.02 | - |
| 13 | humulene epoxide II | 1572 | 1570 * | 0.8 ± 0.02 | - |
| 14 | neophytadiene | 1842 | 1836 | - | 8.8 ± 0.06 |
| 15 | palmitic acid | 1980 | 1973 | - | 23.2 ± 0.04 |
| 16 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | 2112 | 2116 | - | 11.8 ± 0.04 |
| 17 | 9,12,15-octadecatrienal | 2130 | 2109 § | - | 47.6 ± 0.05 |
| SUM | 95.9 | 96.5 | |||
| Monoterpenoids | - | - | |||
| Sesquiterpenoids | 2.1 | - | |||
| Others | 93.8 | 96.5 |
| N° | COMPONENT | LRI calc | LRI lit | IME | LME |
|---|---|---|---|---|---|
| ORGANIC ACIDS | |||||
| 1 | lactic | 1050 | 1060 | 2.8 | 0.4 |
| 2 | 4-hydroxymandelic | 1775 | 1782 | - | 0.3 |
| 3 | 2-hydroxy-3-methylvaleric | 1670 | 1680 § | tr | - |
| 4 | glycolic | 1986 | 1997 § | 0.1 | - |
| 5 | glyceric | 1355 | 1350 | 0.1 | - |
| 6 | erytronic | 1572 | 1567 | 0.3 | - |
| 7 | galacturonic | 2100 | 2096 | 0.2 | - |
| 8 | quininic | 1862 | 1854 | 1.2 | - |
| 9 | acrylic | 1110 | 1100 | 2.3 | 2.1 |
| 10 | arabinonic | 1814 | 1812 | - | 0.6 |
| SUGARS | |||||
| 11 | lyxose | 1600 | 1610 * | tr | 0.4 |
| 12 | ribose | 1660 | 1669 * | tr | - |
| 13 | xylose | 1710 | 1740 § | tr | 0.2 |
| 14 | rhamnose | 1630 | 1642 * | tr | - |
| 15 | ribofuranose | 1625 | 1637 * | - | 1.2 |
| 16 | fructofuranose | 1845 | 1857 * | 31.4 | 31.9 |
| 17 | sorbofuranose | 1782 | 1779 | 9.3 | 3.2 |
| 18 | tagatofuranose | 1807 | 1800 | - | 18.6 |
| 19 | mannopyranose | 1785 | 1793 | 15.3 | - |
| 20 | talopyranose | 1935 | 1943 * | 1.1 | - |
| 21 | glucopyranose | 1841 | 1837 | 21.6 | - |
| 22 | allofuranose | 1888 | 1896 * | 1.2 | - |
| 23 | talofuranose | 1875 | 1882 | 0.3 | - |
| 24 | galactopyranose | 1935 | 1952 § | - | 0.4 |
| 25 | methyl β-arabinofuranoside | 1820 | 1834 § | - | 0.3 |
| 26 | 2-O-glycerol-α-galactopyranoside | 2168 | 2180 § | 0.4 | - |
| SUGAR ALCOHOLS | |||||
| 27 | glycerol | 1305 | 1300 | 4.2 | 6.8 |
| 28 | ribitol | 1718 | 1727 | 0.1 | 0.3 |
| 29 | arabitol | 1770 | 1776 | - | 1.7 |
| 30 | pinitol | 1855 | 1869 * | 0.9 | 13.9 |
| 31 | myo-inositol | 2090 | 2096 | 0.9 | 1.0 |
| FATTY ACIDS | |||||
| 32 | linolenic | 2212 | 2218 | 4.6 | - |
| AMINOACIDS | |||||
| 33 | valine | 1218 | 1221 | 0.1 | - |
| OTHERS | |||||
| 34 | phytol | 2155 | 2162 | 0.8 | - |
| 35 | acetoacetic acid, ethyl ester | 938 | 944 | - | 11.9 |
| Extracts | Total Polyphenols | |
|---|---|---|
| (mg GAE/g Extract) | (mg GAE/g Fresh Hop) | |
| IAE | 203.6 ± 3.6 | 16.3 ± 0.4 |
| LAE | 295.3 ± 7.9 | 65.0 ± 2.3 |
| IME | 374.7 ± 6.9 | 40.7 ± 0.7 |
| LME | 317.5 ± 1.6 | 87.3 ± 1.5 |
| Extracts | Total Flavonoids | |
|---|---|---|
| (mg QE/g Extract) | (mg QE/g fresH Hop) | |
| IAE | 103.7 ± 6.2 | 8.3 ± 0.5 |
| LAE | 92.7 ± 5.7 | 20.0 ± 1.2 |
| IME | 85.6 ± 4.0 | 9.3 ± 0.3 |
| LME | 117.3 ± 3.2 | 32.25 ± 0.6 |
| Extracts | ABTS | |
|---|---|---|
| RSA (%) | mM TE | |
| IAE | 36.2 ± 1.5 | 0.78 ± 0.1 |
| LAE | 62.9 ± 1.2 | 1.38 ± 0.0 |
| IME | 96.3 ± 0.8 | 2.14 ± 0.2 |
| LME | 96.1 ± 1.1 | 2.13 ± 0.8 |
| Extracts | DPPH | |
|---|---|---|
| RSA (%) | mM TE | |
| IAE | 15.6 ± 1.1 | 0.05 ± 0.0 |
| LAE | 37.4 ± 0.7 | 0.13 ± 0.0 |
| IME | 84.8 ± 0.5 | 0.30 ± 0.0 |
| LME | 81.7 ± 1.0 | 0.29 ± 0.0 |
| Cell Lines | LME (IC50, μg/mL) | IME (IC50, μg/mL) |
|---|---|---|
| H1299 | 220 ± 10 | 180 ± 20 |
| A375 | 240 ± 25 | 210 ± 16 |
| MCF7 | 190 ± 30 | 190 ± 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitalini, S.; Di Martile, M.; Cicaloni, V.; Iannone, M.; Salvini, L.; Del Bufalo, D.; Iriti, M.; Garzoli, S. Volatile and Non-Volatile Content Determination and Biological Activity Evaluation of Fresh Humulus lupulus L. (cv. Chinook) Leaves and Inflorescences. Separations 2023, 10, 91. https://doi.org/10.3390/separations10020091
Vitalini S, Di Martile M, Cicaloni V, Iannone M, Salvini L, Del Bufalo D, Iriti M, Garzoli S. Volatile and Non-Volatile Content Determination and Biological Activity Evaluation of Fresh Humulus lupulus L. (cv. Chinook) Leaves and Inflorescences. Separations. 2023; 10(2):91. https://doi.org/10.3390/separations10020091
Chicago/Turabian StyleVitalini, Sara, Marta Di Martile, Vittoria Cicaloni, Matteo Iannone, Laura Salvini, Donatella Del Bufalo, Marcello Iriti, and Stefania Garzoli. 2023. "Volatile and Non-Volatile Content Determination and Biological Activity Evaluation of Fresh Humulus lupulus L. (cv. Chinook) Leaves and Inflorescences" Separations 10, no. 2: 91. https://doi.org/10.3390/separations10020091
APA StyleVitalini, S., Di Martile, M., Cicaloni, V., Iannone, M., Salvini, L., Del Bufalo, D., Iriti, M., & Garzoli, S. (2023). Volatile and Non-Volatile Content Determination and Biological Activity Evaluation of Fresh Humulus lupulus L. (cv. Chinook) Leaves and Inflorescences. Separations, 10(2), 91. https://doi.org/10.3390/separations10020091









