Removal of Ni(II) from Aqueous Solution by Novel Lycopersicon esculentum Peel and Brassica botrytis Leaves Adsorbents
Abstract
:1. Introduction
2. Experimental
2.1. Sample Collection and Adsorption Studies
2.2. Instruments and Materials
2.3. Equilibrium Adsorption Isotherms
2.4. Biosorption Kinetics
2.5. Adsorption Studies
3. Result and Discussion
3.1. Adsorption Studies
3.2. Kinetics Studies
3.3. Effect of Initial Metal Ion Concentration
3.4. Effect of Adsorbent Dosage
3.5. Effect of Temperature
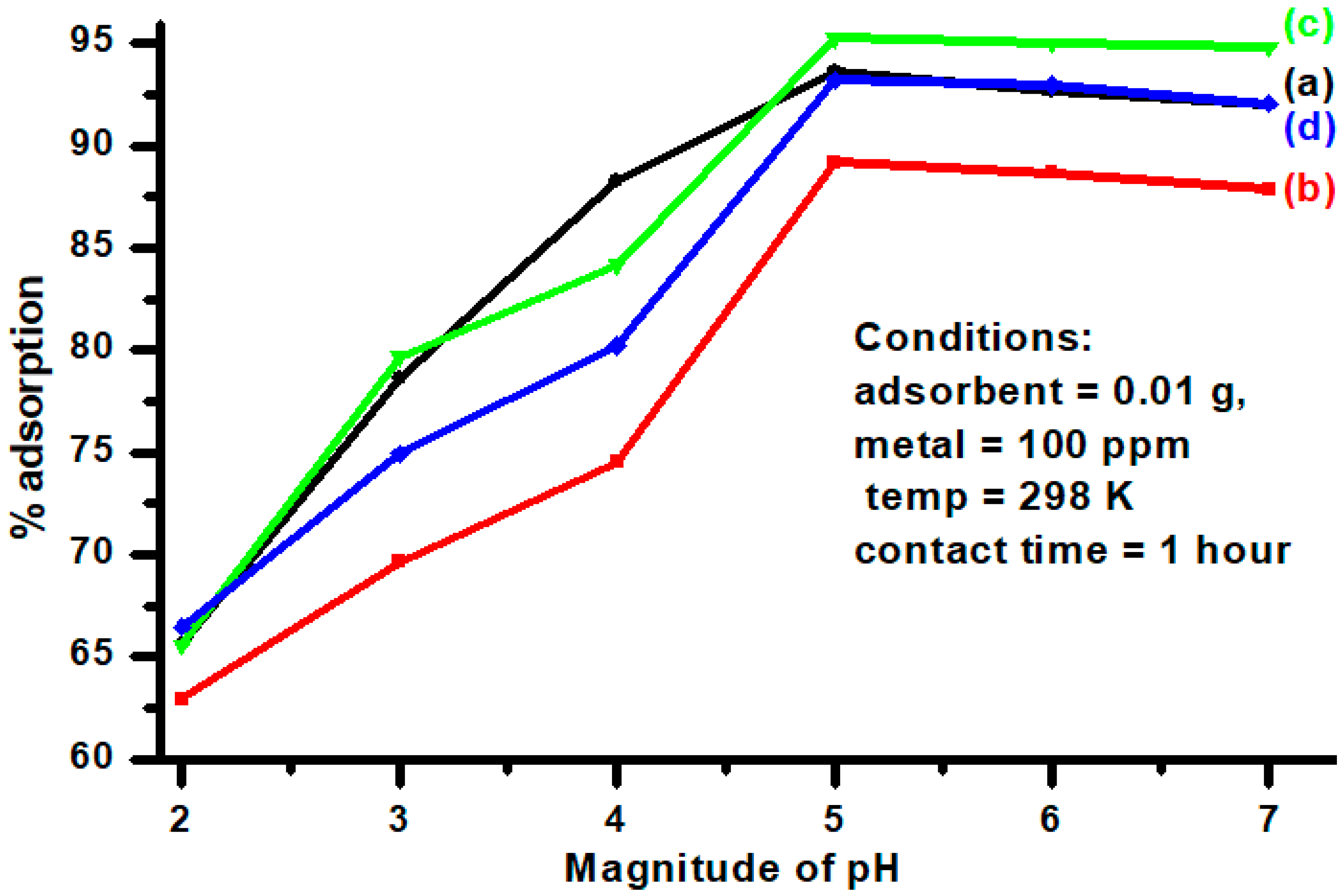
3.6. Effect of pH
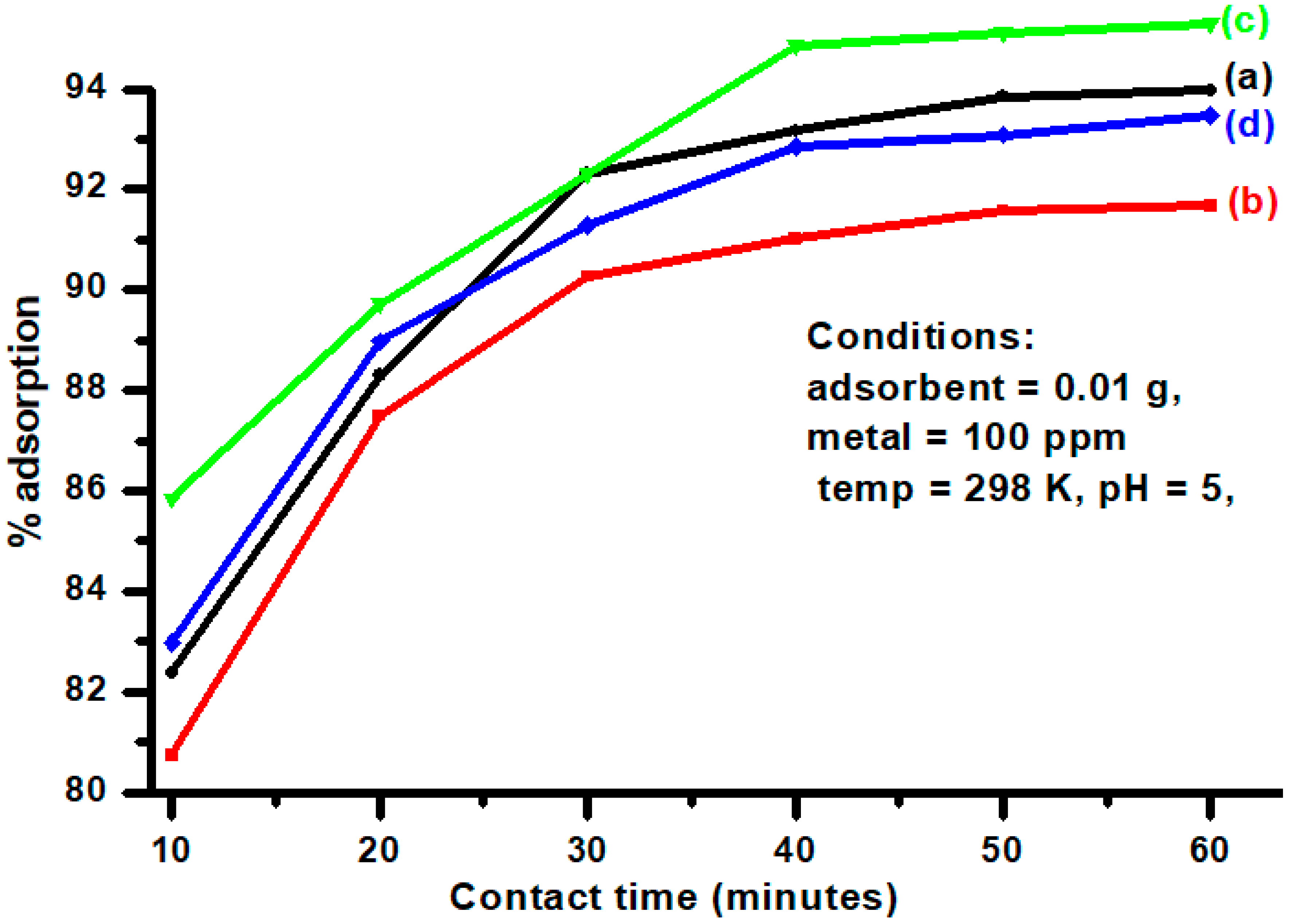
3.7. Effect of Contact Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Research Highlights
- ✓ Lycopersicon esculentum peel and Brassica botrytis leaves were used as adsorbents.
- ✓ Activated carbon was incorporated in Lycopersicon esculentum peel and Brassica botrytis leaves.
- ✓ Ni adsorption was improved by AC incorporation.
- ✓ Ni adsorption was effected by reaction parameters.
References
- Din, I.; Shaharun, M.; Naeem, A.; Alotaibi, M.; Alharthi, A.; Nasir, Q. Effect of reaction conditions on the activity of novel carbon nanofiber-based Cu/ZrO2 catalysts for CO2 hydrogenation to methanol, Comptes Rendus. Chimie 2020, 23, 57–61. [Google Scholar] [CrossRef]
- Sultana, Q.; Naeem, A.; Mahmood, T.; Din, I.; Saeed, T.; Khan, N.; Ahmad, T. Sorption Studies of Chromate by Iron Oxide from Drinking Water. Z. Phys. Chem. 2019, 235, 407–425. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Sami, U.; Al-Sehemi, A.G.; Mubashir, M.; Mukhtar, A.; Saqib, S.; Bustam, M.A.; Cheng, C.K.; Ibrahim, M.; Show, P.L. Adsorption behavior of mercury over hydrated lime: Experimental investigation and adsorption process characteristic study. Chemosphere 2021, 271, 129504. [Google Scholar]
- Babar, M.; Munir, H.M.S.; Nawaz, A.; Ramzan, N.; Azhar, U.; Sagir, M.; Tahir, M.S.; Ikhlaq, A.; Mubashir, M.; Khoo, K.S.; et al. Comparative study of ozonation and ozonation catalyzed by Fe-loaded biochar as catalyst to remove methylene blue from aqueous solution. Chemosphere 2022, 307, 135738. [Google Scholar] [CrossRef]
- Liang, T.; Goudari, S.; Chen, C. A simple and versatile nickel platform for the generation of branched high molecular weight polyolefins. Nat. Commun. 2020, 11, 372. [Google Scholar] [CrossRef]
- El-Sadaawy, M.; Abdelwahab, O. Adsorptive removal of nickel from aqueous solutions by activated carbons from doum seed (Hyphaenethebaica) coat. Alex. Eng. J. 2014, 53, 399–408. [Google Scholar] [CrossRef]
- Vieira, M.; Neto, A.; Gimenes, M.; Da Silva, M. Sorption kinetics and equilibrium for the removal of nickel ions from aqueous phase on calcined Bofe bentonite clay. J. Hazard. Mater. 2010, 177, 362–371. [Google Scholar] [CrossRef]
- Boujelben, N.; Bouzid, J.; Elouear, Z. Adsorption of nickel and copper onto natural iron oxide- coated sand from aqueous solutions: Study in single and binary systems. J. Hazard. Mater. 2009, 163, 376–382. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Haralambous, K.; Loizidou, M. Use of ultrafiltration membranes and aluminosilicate minerals for nickel removal from industrial wastewater. J. Membr. Sci. 2010, 360, 234–249. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, A. Biosorption optimization of nickel removal from water using Punica granatum peel waste. Colloids Surf. B Biointerfaces 2010, 76, 544–548. [Google Scholar] [CrossRef]
- Khosravi, Y.; Zamani, A.; Parizanganeh, A.; Yaftian, M. Assessment of spatial distribution pattern of heavy metals surrounding a lead and zinc production plant in Zanjan Province, Iran. Geoderma Reg. 2018, 12, 10–17. [Google Scholar] [CrossRef]
- Khelifi, O.; Nacef, M.; Affoune, A. Nickel (II) adsorption from aqueous solutions by physico-chemically modified sewage sludge. Iran. J. Chem. Chem. Eng. (IJCCE) 2018, 37, 73–87. [Google Scholar]
- Al-Abbad, E.A.; Al Dwairi, R.A. Removal of nickel (II) ions from water by Jordan natural zeolite as sorbent material. J. Saudi Chem. Soc. 2021, 25, 101233. [Google Scholar] [CrossRef]
- Shaker, O.; Safwat, S.; Matta, M. Nickel removal from wastewater using electrocoagulation process with zinc electrodes under various operating conditions: Performance investigation, mechanism exploration, and cost analysis. Environ. Sci. Pollut. Res. 2022, 1–13. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Motamedi, M. Adsorption of nickel, Ni(II), in aqueous solution by modified zeolite as a cation-exchange adsorbent. J. Chem. Eng. Data 2019, 65, 185–197. [Google Scholar] [CrossRef]
- Wang, S.; Kwak, J.-H.; Islam, M.; Naeth, M.; El-Din, M.G.; Chang, S. Biochar surface complexation and Ni(II), Cu(II), and Cd(II) adsorption in aqueous solutions depend on feedstock type. Sci. Total Environ. 2020, 712, 136538. [Google Scholar] [CrossRef]
- Garrafa-Galvez, H.; Nava, O.; Soto-Robles, C.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Luque, P. Green synthesis of SnO2 nanoparticle using Lycopersicon esculentum peel extract. J. Mol. Struct. 2019, 1197, 354–360. [Google Scholar] [CrossRef]
- Silva-Vera, W.; Avendaño-Muñoz, N.; Nuñez, H.; Ramírez, C.; Almonacid, S.; Simpson, R. CO2 laser drilling coupled with moderate electric fields for enhancement of the mass transfer phenomenon in a tomato (Lycopersicon esculentum) peeling process. J. Food Eng. 2020, 276, 109870. [Google Scholar] [CrossRef]
- Giuseppina, T.; Daniela, D.; Marianna, P.; Rosa, C.; Barbara, N.; Annarita, P. Natural products from tomato peels (Lycopersicon esculentum variety “Hybrid Rome”): New challenges and new opportunities of application: Chemical, biotechnological and pharmacological. J. Biotechnol. 2007, 131, S26–S27. [Google Scholar] [CrossRef]
- Bhatt, S.; Singh, B.; Gupta, M. Antioxidant and prebiotic potential of Murraya koenigii and Brassica oleracea var. botrytis leaves as food ingredient. J. Agric. Food Res. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Collado-González, J.; Piñero, M.; Otálora, G.; López-Marín, J.; del Amor, F. Exogenous spermidine modifies nutritional and bioactive constituents of cauliflower (Brassica oleracea var. botrytis L.) florets under heat stress. Sci. Hortic. 2021, 277, 109818. [Google Scholar] [CrossRef]
- Kumar, V.; Thakur, R.; Kumar, P. Assessment of heavy metals uptake by cauliflower (Brassica oleracea var. botrytis) grown in integrated industrial effluent irrigated soils: A prediction modeling study. Sci. Hortic. 2019, 257, 108682. [Google Scholar] [CrossRef]
- Din, I.; Alotaibi, M.; Alharthi, A. Green synthesis of methanol over zeolite based Cu nano-catalysts, effect of Mg promoter. Sustain. Chem. Pharm. 2020, 16, 100264. [Google Scholar] [CrossRef]
- Saeed, T.; Naeem, A.; Mahmood, T.; Ahmad, Z.; Farooq, M.; Farida; Din, I.; Khan, I. Comparative study for removal of cationic dye from aqueous solutions by manganese oxide and manganese oxide composite. Int. J. Environ. Sci. Technol. 2020, 18, 659–672. [Google Scholar] [CrossRef]
- Shah, R.K.; Naglah, A.M.; Al-Omar, M.A.; Almehizia, A.A.; AlReshaidan, S.; Subaihi, A.; Alharbi, A.; Hameed, A.M.; Alkabli, J.; Fetoh, M.E.; et al. Efficient removal of Ni(II) ions from aqueous solutions using analcime modified with dimethylglyoxime composite. Arab. J. Chem. 2021, 14, 103197. [Google Scholar] [CrossRef]
- Najim, T.; Elais, N.; Dawood, A. Adsorption of Copper and Iron Using Low Cost Material as Adsorbent. E-J. Chem. 2009, 6, 682568. [Google Scholar] [CrossRef]
- El-Sayed, G.O.; Dessouki, H.A.; Ibrahim, S.S. Biosorption Of Ni(II) And Cd (II) Ions From Aqueous Solutions Onto Rice Straw. Chem. Sci. J. 2010, 2010, CSJ-9. [Google Scholar] [CrossRef]
- Naeem, A.; Din, I.; Saeed, T.; Alotaibi, M.; Alharthi, A.; Habib, A.; Malik, T. Synthesis, characterization and adsorption studies of h-BN crystal for efficient removal of Cd2+ from aqueous solution. Ceram. Int. 2020, 47, 4749–4757. [Google Scholar]
- Gorzin, F.; Bahri Rasht Abadi, M.M. Adsorption of Cr (VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorpt. Sci. Technol. 2018, 36, 149–169. [Google Scholar] [CrossRef]
- Kubilay, Ş.; Gürkan, R.; Savran, A.; Şahan, T. Removal of Cu(II), Zn(II) and Co(II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption 2007, 13, 41–51. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Itua, A.B.; Maliki, M.; Ize-Iyamu, C.O.; Omorogbe, S.O.; Aigbodion, A.I.; Ikhuoria, E.U. The removal of nickel and lead ions from aqueous solutions using green synthesized silica microparticles. Heliyon 2020, 6, e04907. [Google Scholar] [CrossRef]
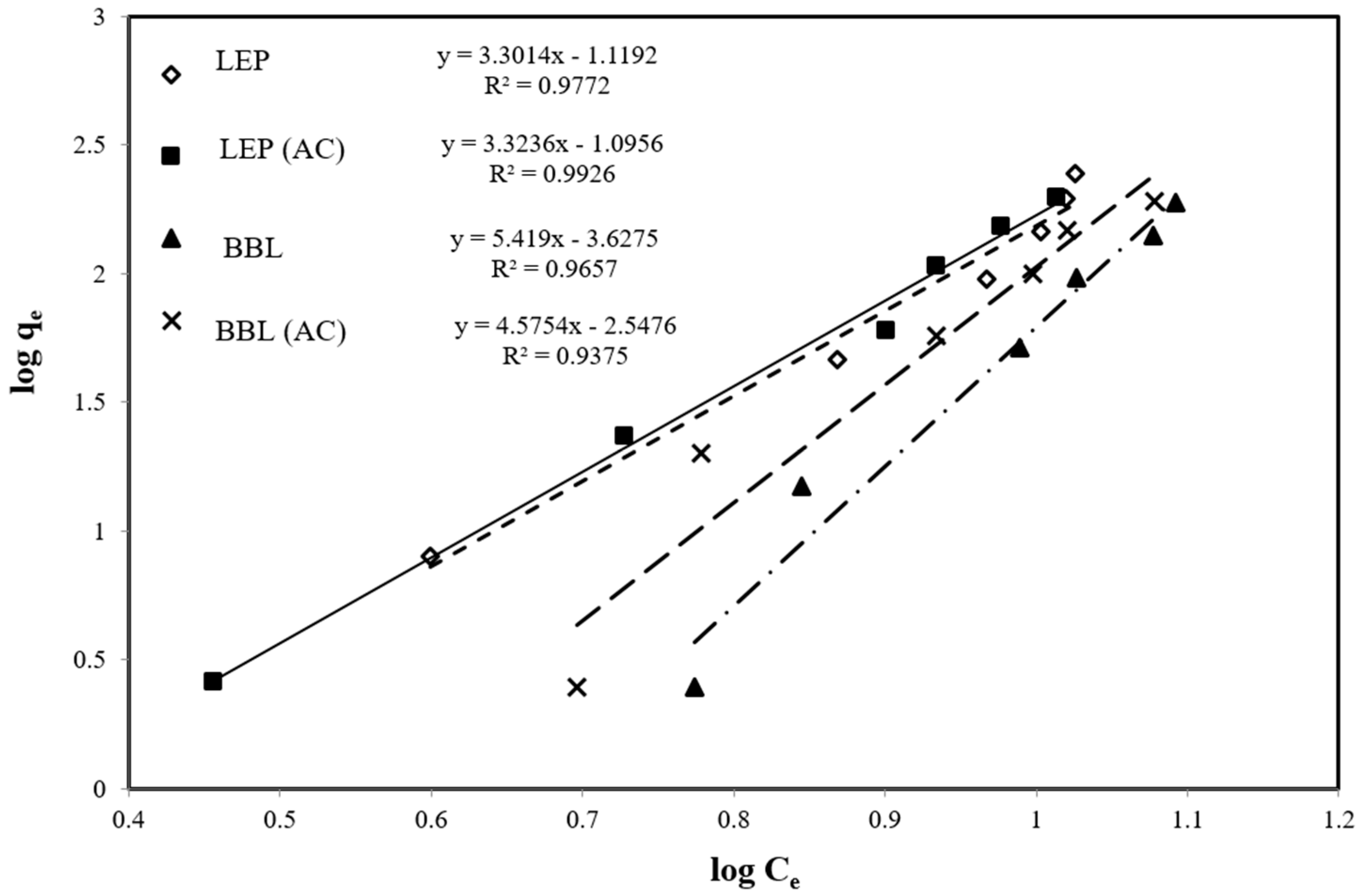
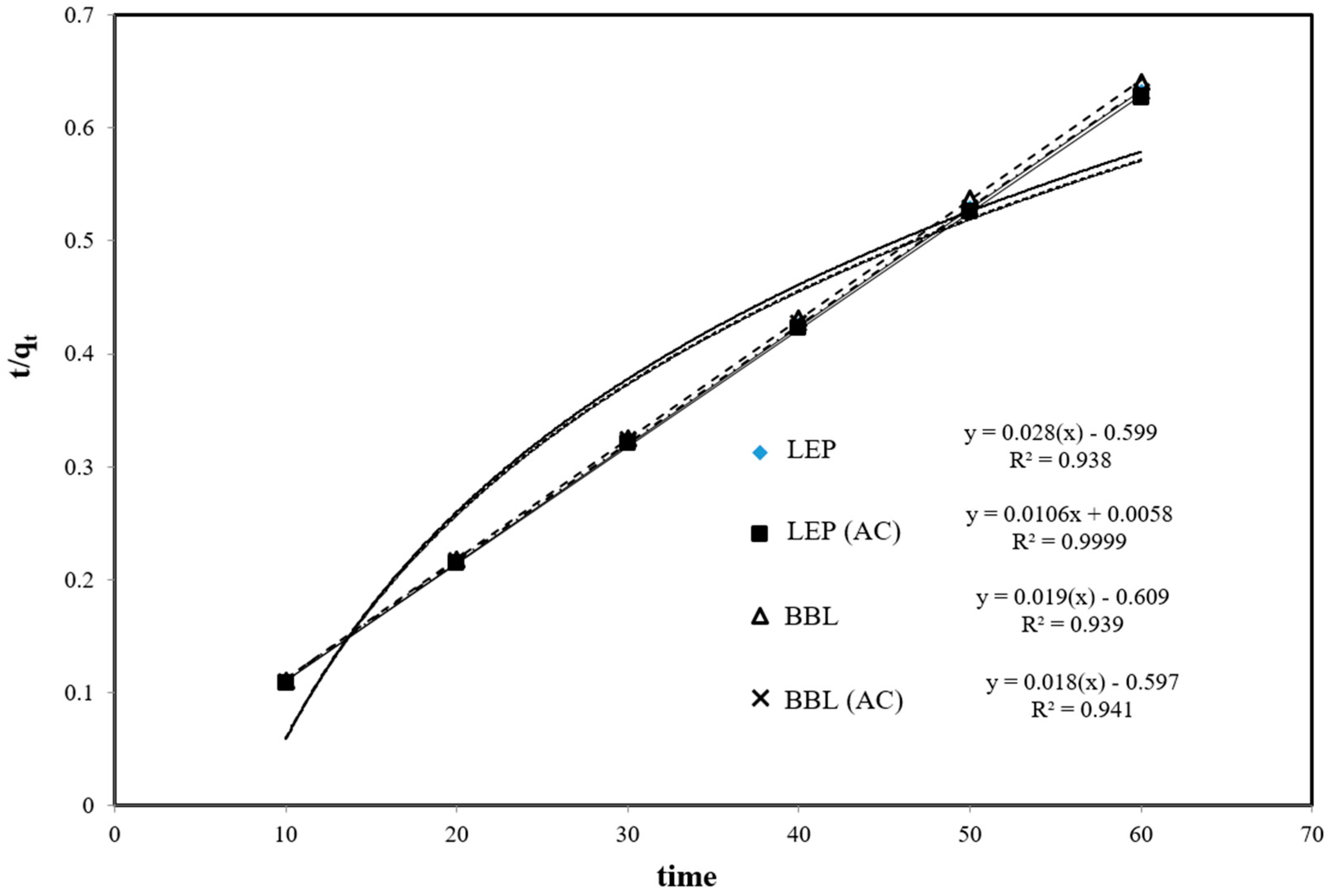
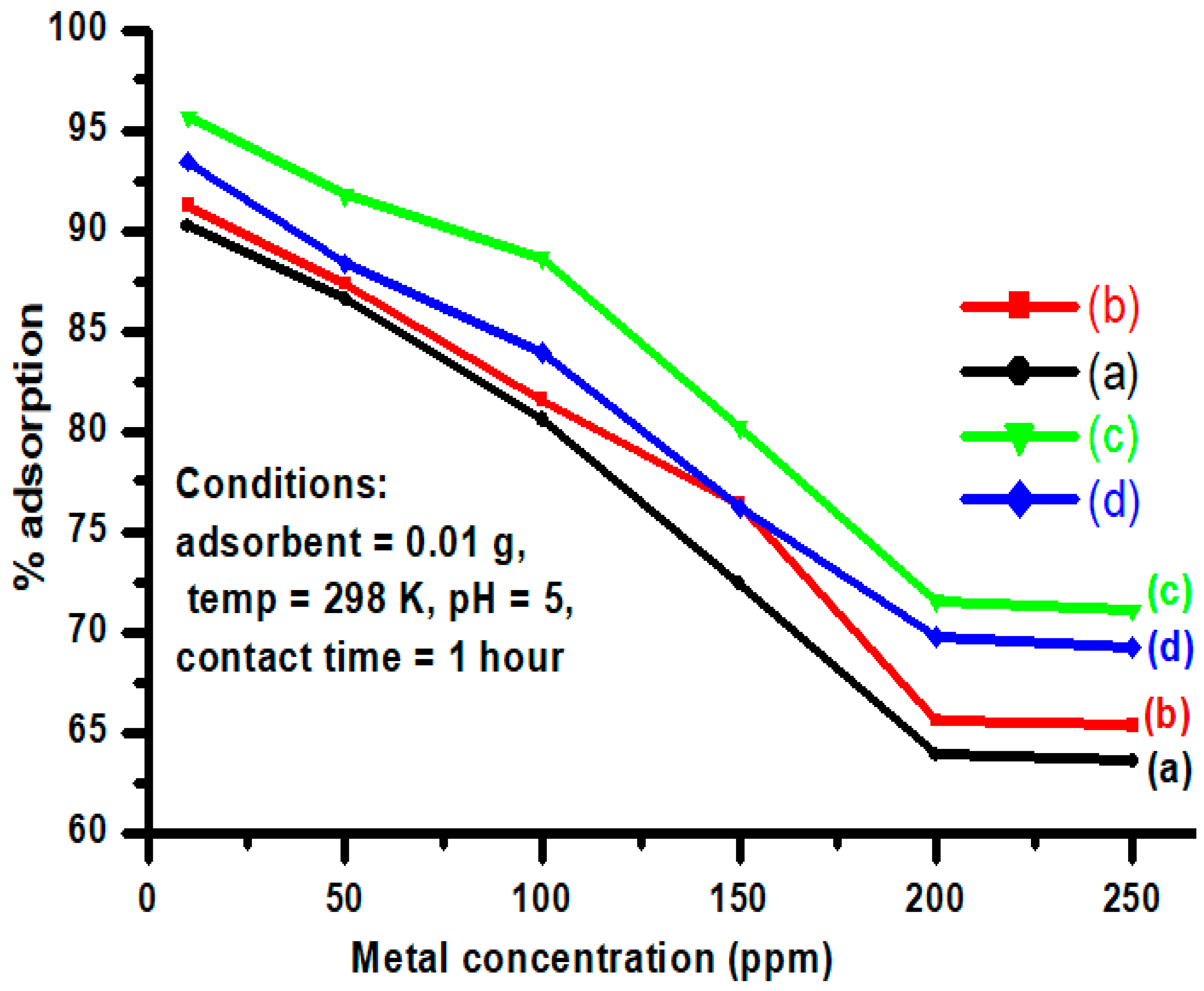

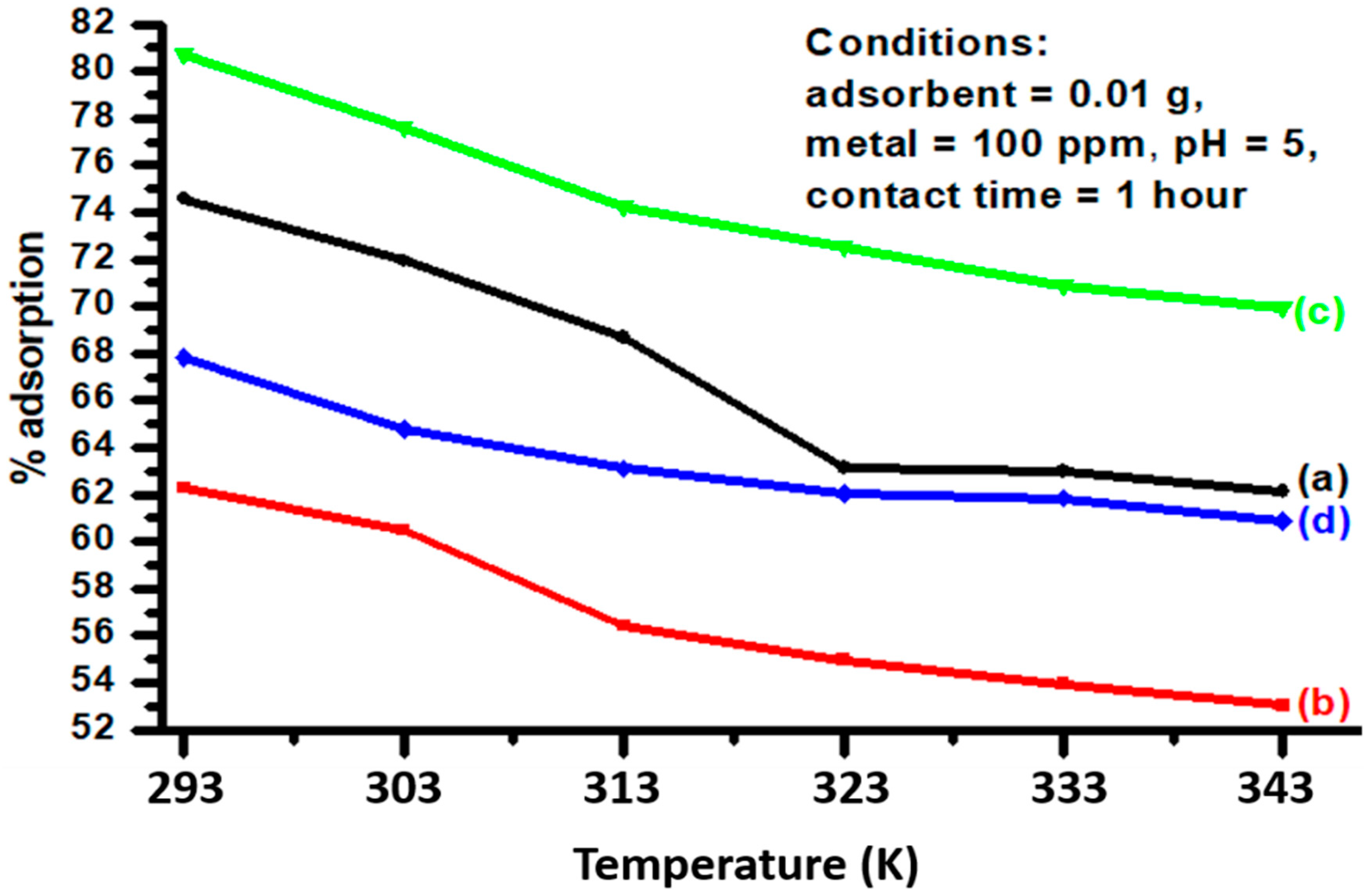
| Adsorbents | Kf (mg/g) | 1/n | n | R2 |
|---|---|---|---|---|
| LYCOPERSICON ESCULENTUM PEEL | 0.048 | 3.301 | 0.302 | 0.977 |
| LYCOPERSICON ESCULENTUM PEEL (AC) | 0.336 | 2.296 | 0.435 | 0.992 |
| BRASSICA BOTRYTIS LEAVES | 0.260 | 5.419 | 0.198 | 0.965 |
| BRASSICA BOTRYTIS LEAVES(AC) | 0.310 | 4.575 | 0.248 | 0.937 |
| Adsorbents | Ke (mg/g) | K2 g.mg−1.min−1 | R2 |
|---|---|---|---|
| LYCOPERSICON ESCULENTUM PEEL | 19.585 | 0.002 | 0.938 |
| LYCOPERSICON ESCULENTUM PEEL (AC) | 18.575 | 0.013 | 0.999 |
| BRASSICA BOTRYTIS LEAVES | 18.756 | 0.021 | 0.939 |
| BRASSICA BOTRYTIS LEAVES(AC) | 19.944 | 0.024 | 0.941 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Din, I.U.; Shah, Q.U.; Tasleem, S.; Naeem, A.; I. Alharthi, A.; Ayad Alotaibi, M. Removal of Ni(II) from Aqueous Solution by Novel Lycopersicon esculentum Peel and Brassica botrytis Leaves Adsorbents. Separations 2023, 10, 113. https://doi.org/10.3390/separations10020113
Din IU, Shah QU, Tasleem S, Naeem A, I. Alharthi A, Ayad Alotaibi M. Removal of Ni(II) from Aqueous Solution by Novel Lycopersicon esculentum Peel and Brassica botrytis Leaves Adsorbents. Separations. 2023; 10(2):113. https://doi.org/10.3390/separations10020113
Chicago/Turabian StyleDin, Israf Ud, Qadeer Ullah Shah, Syed Tasleem, Abdul Naeem, Abdulrahman I. Alharthi, and Mshari Ayad Alotaibi. 2023. "Removal of Ni(II) from Aqueous Solution by Novel Lycopersicon esculentum Peel and Brassica botrytis Leaves Adsorbents" Separations 10, no. 2: 113. https://doi.org/10.3390/separations10020113
APA StyleDin, I. U., Shah, Q. U., Tasleem, S., Naeem, A., I. Alharthi, A., & Ayad Alotaibi, M. (2023). Removal of Ni(II) from Aqueous Solution by Novel Lycopersicon esculentum Peel and Brassica botrytis Leaves Adsorbents. Separations, 10(2), 113. https://doi.org/10.3390/separations10020113








