Abstract
The chemical composition of essential oil extracted from the aerial parts of Ballota saxatilis Sieber ex C.Presl from Jordan has been elucidated by gas chromatography–mass spectrometry (GC-MS). Additionally, aqueous methanol (BsA), Butanol (BsB) and water (BsW) extracts were screened for their total phenol content (TPC), total flavonoid content (TFC), and antioxidant activities using the 2,2 Diphenyl-1-picrylhydrazyl (DPPH) and 2,2-Azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt (ABTS) methods. The most potent extracts were screened for their phenolic acids and flavonoid content using liquid the chromatography–mass spectrometry (LC-MS) technique. The results indicated that the essential oil predominantly contained cis-pinane (14.76%), β-caryophyllene (8.91%) and allo-aromadendrene epoxide (6.39%). Among the different extracts investigated, the BsB fraction had the most TPC and TFC (455.79 ± 1.03 µg gallic acid/g dry extract; 272.62 ± 8.28 µg quercetin/g dry extract, respectively) and had the best radical and radical cation scavenging activities, as determined using the DPPH and ABTS methods. Quantitative and qualitative LC-MS analyses of BsA and BsB using LC-MS revealed each of the kaempferol-3-O-rutinoside (30.29%), chrysoeriol-7-glucoside (7.93%) and luteolin 7-o-glucoside (7.76%) as the main constituents of the BsA fraction. The BsB fraction was rich in 7,4′-dimethoxy-3-hydroxyflavone (34.68%), kaempferol-3,7,4′-trimethyl ether (29.17%) and corymbosin (9.66%) and lower concentration levels of kaempferol-3-O-rutinoside (1.63%) and chrysoeriol-7-glucoside (0.51%).
1. Introduction
The genus Ballota, which belongs to the Lamiaceae family, consists of approximately 33 species [1,2,3]. The Ballota species are known to be wide distributed in the Mediterranean region, particularly in the Middle East and North Africa [2]. In Jordan, this genus is present in three species: Ballota nigra, Ballota undulata and Ballota saxatilis [1,3]. The use of the Ballota species as an herbal medicine has been reported for the treatment of many ailments, such as whooping cough. In addition, other reports show their activity as being anti-ulcer, anti-haemorrhoidal, antioxidant, antiemetic, antilisterial, and antimicrobial [4,5,6,7]. Reports have also indicated interesting antifungal, antidiabetic and hepatoprotective properties [5,7].
Several studies have been conducted to explore the essential oil and secondary metabolite components of the Ballota species [4,5,6,7,8,9,10,11,12,13,14]. The results show that plants belonging to this genus family contain flavonoids, diterpenoids, iridoids, and phenolic acids and their derivatives, such as verbascosides, verminosides, and iridoids [4,5,6,7,8,9,10,11,12,13,14]. In addition, essential oils of several of the Ballota species were found to contain germacrene D, caryophyllene, caryophyllene oxide, -cadinol, -pinene, and hexacosanol, which possess interesting anti-inflammatory and antimicrobial activity [15,16,17,18].
Ballota saxatilis Sieber ex C.Presl is one of the perennial herbs known to grow wild in Jordan along the roadsides and waste places of mountainous regions of Irbid, Jerash, Al-Salt, Amman and Al-Karak. This plant has been used as an herbal remedy for the treatment of wounds, burns, coughing and inflammations of the respiratory and nervous systems [1]. Upon reviewing the literature, we found that phytochemical studies of B. saxatilis are quite limited. Citoglu et al. reported the isolation of terpenoids and flavonoids from B. saxatilis, to which it owes antimicrobial activity [11]. In addition, Bruno et al. reported the isolation of 18-hydroxyballonigrine from the aerial parts of B. saxatilis and investigated its antifeedant activity [12]. The essential oil of B. saxatilis showed the highest percentages of oxygenated monoterpenes and sesquiterpenes hydrocarbons [15]. In previous studies, the antioxidant activities of the ethanolic extracts of Ballota species were examined for superoxide anion scavenging activity and the inhibition of lipid peroxidation. The extracts of B. saxatilis subsp. Saxatilis inhibited lipid peroxidation with IC50 values from 12 to 20 mg/mL [13].
Alcohols, phenols, aldehydes, esters, and ketones are among of the principal ingredients in essential oils, along with hydrocarbon [19] There are various techniques for extracting essential oils, but steam distillation is the most widely used method because it does not require the use of organic solvents and provides a high extraction yield. Hydrodistillation (HD), in particular, is the most widely used technique for obtaining essential oils from plants for research purposes [20,21,22,23]. This method involves packing plant components with enough water in a static compartment to boil. As an alternative, direct steam might be injected into the plant sample.
As a continuation of our previous relevant work [24,25,26,27,28,29,30], this present study aims to identify the chemical composition of essential oils obtained by hydrodistillation of the aerial parts of B. saxatilis growing in Jordan using the GC-MS technique. Furthermore, we aim to screen the extracts for their total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activities using the DPPH• and ABTS•+ assay methods. Phenolic acids and flavonoids contents in the most active extracts will be identified using LC–MS.
2. Materials and Methods
2.1. Chemicals
The 2,2 Diphenyl-1-picrylhydrazyl (DPPH, purity ≥ 97%), 2,2-Azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt (ABTS, purity ≥ 98%), Iron (II) chloride (purity 97%), Potassium persulfate (purity ≥ 99.0%), Sodium carbonate anhydrous (purity ≥ 99.5%), Aluminum chloride (purity 98%), Sodium hydroxide (purity 98%), Methanol (purity 99.6%), n-Butyl Alcohol (purity 99.9%), Petroleum ether (purity 99%), Potassium iodide (purity 99%), Iron (III) chloride (purity 99%), sulfuric acid (purity 96.9%), ammonia (purity 99%), Mercury (II) chloride (purity 99%), and hydrocarbon mixture (n-alkanes, C8-C20) were all products of Sigma-Aldrich,
2.2. Plant Material
The fresh aerial parts of B. saxatilis Sieber ex C. Presl. Were collected in July 2021, from the Burqash forest in Ajloun, northern Jordan (N 32.443450; E 35.739591). The identity of the plant was confirmed by Prof. Jamil N. Lahham at the Department of Biological Sciences, Yarmouk University, Irbid, Jordan. A voucher specimen (YU/03/LB/1003) was deposited in the herbarium of the Department of Biological Sciences at Yarmouk University, Irbid, Jordan.
2.3. Hydrodistillation of Essential Oil
The fresh aerial parts of B. saxatilis (250 g) were minced and suspended in distilled water (250 mL) and then subjected to hydrodistillation with a Clevenger apparatus for 3 h [19,20,21,22,23,24]. The obtained oil was separated by extraction with diethyl ether (2.0 mL), twice. After the evaporation of the diethyl ether, the resulting oil was dissolved in n-hexane (GC-grade), dried over anhydrous sodium sulfate, and then stored in an amber glass vial at 4–6 °C.
2.4. Extraction and Fractionation
The aerial parts of B. saxatilis were collected (2.0 kg) during the full flowering season and dried in a shadowed place for 10 days. In a Soxhlet extractor, the finely powdered air-dried aerial parts of B. saxatilis were defatted in petroleum ether for 6 h. After the defatting was completed, the plant residue was extracted in the same apparatus with methanol for 12 h. The obtained alcoholic gummy residue was then partitioned, according to the procedure described in the literature [24,25,26,27,28,29,30], between chloroform and water (1:1). Polar compounds in the water were recovered by extraction with n-butanol, thus affording the butanol (BsB) and water (BsW) fractions. The dried chloroform residue was subjected to partitioning between 10% aqueous methanol and hexane (1:1) to obtain the aqueous methanol (BsA) and hexane (BsH) fractions. The obtained different fractions were then assayed for their total phenol content (TPC), total flavonoid content (TFC), and in vitro antioxidant activities, according to the procedure listed in the literature [24,25,26,27,28,29,30].
2.5. Profiling Secondary Metabolite Classes by Chemical Methods
To determine the main classes of secondary metabolites, qualitative analysis was performed on each crude fraction obtained after the partitioning approach described in the preceding section, including butanol, aqueous methanol, and water extracts, using the method described by Siddiqui and Ali [31].
2.6. Determination of TPC and TFC
The total flavonoid content of the B. saxatilis species in various extracts was evaluated using an aluminium chloride assay, as described in the literature [24,25,26,27,28,29,30]. In a separate volumetric flask (10 mL), an aliquot (1 mL) of extracts (1 mg/mL) was obtained, then distilled water was added (4 mL), followed by sodium nitrite (0.3 mL, 5%, w/v), and the mixture was allowed to stand for 5 min. After that, aluminium chloride was added (0.3 mL, 10%, w/v) and the mixture was incubated for 6 min. Sodium Hydroxide was then added (2.0 mL, 1.0 M NaOH) and the final volume of solution was topped up to 10 mL with distilled water. After approximately 15 min, the absorbance was measured at 510 nm. The total flavonoid content (reported in mg/g quercetin equivalents) was calculated from the equation: Y = 0.0003X + 0.0392, R2 = 0.9956, where Y is the absorbance at 510 nm and X is the total flavonoid content in the extracts.
The total phenolic content was determined using the Folin–Ciocalteu technique [24,25,26,27,28,29,30]. A mixture of Folin–Ciocalteu reagent (2.5 mL, 0.2 N) and sodium carbonate (2.0 mL) was added to the extract (0.5 mL). After approximately 15 min, the absorbance of the combined solution was measured at 765 nm and compared to a blank solution of methanol. Gallic acid was used as a calibration curve standard, and the absorbance was measured at various concentrations (4–20 mg/mL). All of the measurements were reproducible within three trials. The total phenolic content (mg/g gallic acid equivalents) was calculated from the regression equation: Y = 0.0078X + 0.1042, R2 = 0.9981, where Y is the absorbance at 765 nm and X is the total phenolic content in the extracts.
2.7. Evaluation of the Antioxidant Activity
2.7.1. DPPH• Free Radical Scavenging Activity
The total radical scavenging capacity of the extracts was determined and compared to those of ascorbic acid and α-tocopherol as standards. To assess the DPPH• free radical scavenging capacity of the extract, we adopted the method described by Al-Qudah et al. [24,25,26,27,28,29,30], with slight modifications. A solution of DPPH• (0.1 mM in methanol) was added (2 mL) to the samples of the extract solution (1.0 mL each) of different concentrations (0.005, 0.01, 0.05, 0.1, 0.5 and 1.0 mg/mL). These solutions were incubated in the dark for 30 min. Then, the absorbance was measured at 517 nm against the blank samples and a standard curve was constructed versus the different concentrations of DPPH•. The ability to scavenge the DPPH• radical was calculated using the following equation:
where AC is the absorbance of the blank and AS is the absorbance of the extract solution. We observed that [DPPH•] decreases significantly upon the exposure to radical scavengers. For an improved interpretation of the results, the IC50 values were determined by the linear regression method from plots of the percent of antiradical activity against the concentration of the tested compounds.
2.7.2. ABTS•+ Radical Scavenging Assay
The total antioxidant activity was evaluated using the radical cation (ABTS•+) decolorization test, refs. [23,24,25,26]. The ABTS•+ cation radical solution was prepared by reacting similar quantities of 7 mM of ABTS and 2.4 mM of potassium per sulphate (K2S2O8) solutions for 16 h at (2–3 °C) in the dark. Before use, this solution was diluted with distilled water to obtain an absorbance of 0.75 ± 0.02 at 734 nm. The reaction mixture comprised 3 mL of ABTS•+ solution and 1 mL of extracts at different concentrations (0.005, 0.01, 0.05, 0.1, 0.5 and 1.0 mg/mL) and the absorbance was sample measured at 734 nm by using a UV-Vis spectrophotometer. The blank was run in each assay and all measurements were taken after at least 5 min. The ABTS•+ scavenging capacity of the extract was compared with those of the ascorbic acid and α- tocopherol, and the percentage of inhibition was calculated as:
where AC is the absorbance of the blank sample and AS is the absorbance in the presence of the extract. The IC50 values were calculated by the linear regression method of plots from the percent of antiradical activity against the concentration of the tested compounds.
2.8. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC-MS analysis of the hydrodistilled essential oil was performed according to the procedure described in the literature [20,21,22,23,24]. Gas chromatography-mass spectrometry (GC-MS) analysis was performed using Agilent 6890 series II–5973 mass spectrometers interfaced with the HP chemstation (Agilent Technologies, Santa Clara, CA, USA). Briefly, chromatographic analysis was performed utilizing the HP-5 MS capillary column (30 m, 0.25 mm, 0.25 mm). The oven temperature was kept at 60 °C for one minute, then ramped to 240 °C at a rate of 3 °C/min, and maintained at 240 °C for another 3 min. The injector and detector temperatures were 250 °C and 300 °C, respectively. The flow rate of the carrier gas (helium) was 0.90 mL/min and the ionization voltage was set at 70 eV. A standard hydrocarbon mixture (n-alkanes, C8-C20) was analyzed under the same chromatographic conditions. The injection volume was 1.0 μL (split ratio 1:10). The mass spectra were scanned in the range 35–500 amu, scan time 5 scans s−1.
Individual components in the essential oil were identified based on the comparison of their mass spectra with those in the NIST and Wiely-MS libraries, and by comparing their calculated retention indices (RIs) with those of the available authentic standards and the literature data [32,33]. Standard authentic samples of cis-pinane, α-terpinene, p-cymene, Linalool, β-caryophyllene and germacrene D were also analyzed for confirmation purposes.
2.9. LC-MS Analysis of Phytochemicals
To screen the secondary metabolites of interest utilizing direct injection, a Bruker Daltonik (Bremen, Germany) Impact II ESI-Q-TOF system was employed in both positive (M + H) and negative (M − H) electrospray ionization modes. High-resolution Bruker TOF MS was utilized for the identification of m/z using ion source Apollo II ion funnel electrospray. Drying temperature used was 200 °C, the capillary voltage of 2500 V, nebulizer gas flow was 8 L/min, and the nebulizer gas pressure of 2.0 bar. The mass resolution was 50,000 FSR (Full Sensitivity Resolution) with mass accuracy of <1 ppm and TOF repetition rate up to 20 kHz. A Bruker solo 2.0 C-18 UHPLC column (100 mm 2.1 mm 2.0 mm) was used for elution with a flow rate of 0.51 mL/min and a column temperature of 40 °C. The elution gradient consisted of a mobile phase A (water with 0.05% formic acid) and a mobile phase B (acetonitrile). The gradient program applied was linear gradient 5–80% B (0–27 min); 95% B (27–29 min), 5% B (29.1–35 min). Plant samples (1.0 mg) were dissolved in DMSO (2.0 mL) and solution was topped up to 50 mL with acetonitrile. Each sample was then centrifuged at 4000 rpm for 2 min and 3.0 µL of sample was injected. The composition of the samples was identified based on the comparison of their mass spectrum to a wide-range library that includes more than 60 natural compounds.
3. Results and Discussion
3.1. Essential Oil Composition
The hydro-distillation of the aerial parts of B. saxatilis afforded a yellowish colored oil (yielded 0.06% v/w). The obtained essential oil was subjected to GC-MS analysis to reveal its chemical composition, The results are shown in Table 1 and the compounds are listed according to their elution order.

Table 1.
Chemical composition and percentages of essential oil components from aerial parts of B. saxatilis from Jordan.
As shown in Table 1, a total of 62 components, which represent 80.02% of the total oil composition, were identified. The essential oil was dominated by three different classes of terpenoids, including monoterpene hydrocarbons (MH, 22.20%), sesquiterpene hydrocarbons (SH, 21.99%), and oxygenated monoterpenes (OM, 21.04%). The MH class mainly contained cis-pinane (14.76%), limonene (3.83%), and trans- pinane (1.59%). The main representatives of SH included each of the β-caryophyllene (8.91%) Germacrene B (3.51%), and γ-muurolene (3.40%). Moderate concentration levels of oxygenated monoterpenes were detected (13.16%), while aliphatic hydrocarbons and phenolic compounds were detected in low concentration levels (0.98%, 0.65%, respectively).
While looking through the relevant reports in the literature, we found one report related to the chemical composition of essential oil obtained from different parts of B. saxatilis from Jordan [15]. We compared the reported results to ours; they found both hydrocarbons and oxygenated derivatives of sesquiterpenes with equal concentration levels. In addition, the report uncovered that the essential oil was dominated by β-caryophyllene and germacrene D (7.90% and 7.60%, respectively). We hypothesize that the chemical composition variability among the reported values and our results are due to environmental factors [34].
3.2. Profiling Secondary Metabolite Classes by Chemical Methods
The results of the phytochemical screening tests on the different extracts obtained from the aerial parts of B. saxatilis are shown in Table 2. These indicate that both the BsA and BsB fractions were rich in tannins, terpenes, and flavonoids. Additionally, the BsA also contained alkaloids and saponins, while the BsB fractions showed a positive test for the presence of glycosides. However, among the different classes of secondary metabolites, the water fraction (BsW) predominantly contained tannins and flavonoids. Our results were consistent with previous studies, which show the diversity of the phytochemical components of the Ballota species, in which they contained flavonoids, phenylpropanoids, terpenoids, glycosides, and iridoids [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18].

Table 2.
Major phytochemical classes detected in B. saxatilis different extracts.
3.3. TPC, TFC and Antioxidant Activity
The results of the TPC, TFC and antioxidant activity are listed in Table 3. These results clearly indicate that the BsB fraction had the highest TPC and TFC (455.79 ± 1.03 mg/g gallic acid equivalents; 272.62 ± 8.28 mg/g quercetin equivalents, respectively). This fraction also had the highest DPPH• and ABTS•+ activity (IC50: 0.037 ± 0.001 mg/mL) when compared to the positive controls (Table 3). The results obtained are in line with the results of the TPC and TFC.

Table 3.
Results for TPC (mg/g gallic acid equivalents), TFC (mg/g quercetin equivalents), and IC50 (mg/mL) for the antioxidant activity of the different fractions of B. saxatilis from Jordan.
3.4. LC-MS Profiling of Phenolics, Flavonoids and Other Metabolites
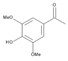
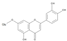
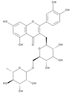
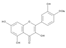
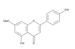
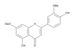
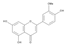
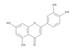
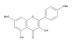
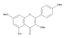
The results of the LC-MS analysis of the BsA and BsB extracts are summarized in Table 4. Both extracts were screened qualitatively and quantitatively for the presence of the selected phenolic acids, flavonoids, selected terpenoids, and fatty acids. A total of 23 compounds were detected in the BsB fraction, whilst 22 compounds were found in the BsA fraction. A careful inspection of the results indicates that 11 flavonoids can be seen in the BsB fraction, which makes up 75.92% of the total composition. This high concentration level of flavonoid derivatives explains the high antioxidant activities we observed. This fraction mostly contained 7,4′-dimethoxy-3-hydroxyflavone (34.68%), kaempferol-3,7,4′-trimethyl ether (29.17%), and corymbosin (9.66%).

Table 4.
LC-MS data for phenolics, flavonoids, selected terpenoids and acids in detected in the BsA and BsB extracts from B. saxatilis from Jordan.
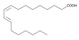
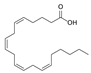
The analysis of the BsA fraction revealed significant differences in its flavonoids content compared to the BsB fraction. Interestingly, the main component detected in the BsA fraction, kaempferol-3-O-rutinoside (30.29%), was not seen in the BsB fraction. Furthermore, Kaempferol-3,7,4′-trimethyl ether was detected in much higher concentration levels in the BsA fraction (29.17%) compared to the BsB fraction (0.65%). The BsA fraction was found to be dominated by kaempferol-3-O-rutinoside (30.29%), 7-glu chrysoeriol (7.93%), and luteolin 7-O-glucoside (7.76%). Additionally, the BsA fraction was found to have higher levels of non-phenolic α-linolenic acid, 9Z, 11E-linoleic acid, (Z)-3-hydroxyoctadec-7-enoic acid, and 9-trans-palmitelaidic acid compared to the BsA fraction. Such differences could be attributed to the solubility of these compounds in the solvents used during the extraction process.
4. Conclusions
A comprehensive phytochemical analysis and antioxidant activity of B. saxatilis essential oil, obtained from the aerial parts collected in Jordan, revealed the dominance of sesquiterpene and monoerpene hydrocarbonsm, among which cis-pinane, β-caryophyllene, and allo-aromadendrene epoxide were detected as the main components. Additionally, the comparative evaluation of the BsB, BsW, and BsA fractions of B. saxatilis revealed that the BsB had the highest TPC, TFC, and antioxidant activity, as determined using the DPPH and ABTS methods. This was further confirmed by the detection of high concentration levels of several floavonoids and phenolic acid derivatives in the LC-MS analysis of the investigated BsB and BsA fractions. It could therefore be inferred that the presence of these phytochemicals, particularly phenols and flavonoids, in the crudes of B. saxatilis therefore justify the medicinal usefulness of this plant against free radicals or oxidative stress-induced ailments.
Author Contributions
Conceptualization, N.A.-B., F.K.A., S.T.A.-O. and M.A.A.-Q.; analysis of literature data, H.I.A.-J., N.A.-B., T.T.B. and M.A.A.-Q.; data curation, R.M.B., Y.A.-D., N.A.-B. and M.A.A.-Q.; writing—original draft preparation, H.I.A.-J., A.G.A., R.M.B., Y.A.-D., N.A.-B. and M.A.A.-Q.; writing—review and editing, H.I.A.-J., A.G.A., R.M.B., Y.A.-D., N.A.-B., S.T.A.-O. and M.A.A.-Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank the Deanship of Scientific Research and Graduate Studies at Yarmouk University for funding this research project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Eisawi, D.M. List of Jordan Vascular Plants. Mitt. Bot. Staatssamml. München 1982, 18, 79–182. [Google Scholar]
- Davis, P.H. Flora of Turkey and the East Aegean Islands, 7th ed.; Edinburgh University Press: Scotland, UK, 1972; pp. 156–165. [Google Scholar]
- Zaghloul, M.; Hamrick, J.; Moustafa, A. Allozymic and morphologic differentiation between three Ballota species (Labiatae) growing in Southern Sinai, Egypt. Catrina Int. J. Environ. Sci. 2014, 9, 53–64. [Google Scholar] [CrossRef]
- Çitoğlu, G.S.; Sever, B.; Antus, S.; Baitz-Gács, E.; Altanlar, N. Antifungal diterpenoids and flavonoids from Ballota inaequidens. Pharm. Biol. 2005, 42, 659–663. [Google Scholar] [CrossRef]
- Abdullah, M.; Al-Khafaji, K.A.; Mouhamad, R.; Hussein, R.R. In-vivo study of the anti-diabetic effect of Ballota saxatilis. DYSONA-Life Sci. 2020, 1, 70–75. [Google Scholar]
- Vlachou, G.; Papafotiou, M.; Bertsouklis, K.F. In vitro propagation of Ballota acetabulosa. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014), Brisbane, Australia, 22 March 2016; Volume 1113, pp. 171–174. [Google Scholar]
- Fraternale, D.; Ricci, D. Essential oil composition and antifungal activity of aerial parts of Ballota nigra ssp foetida collected at flowering and fruiting times. Nat. Prod. Commun. 2014, 9, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, T.; Bader, A.; Vassallo, A.; Braca, A.; Morelli, I.; Pizza, C.; De Tommasi, N. Secondary metabolites from Ballota undulata (Lamiaceae). Biochem. Syst. Ecol. 2005, 33, 341–351. [Google Scholar] [CrossRef]
- Meriçli, A.H.; Meriçli, F.; Tuzlaci, E. Flavonoids of Ballota acetabulosa. Acta Pharm. Turc. 2005, 30, 143–144. [Google Scholar]
- Ferreres, F.; Tomas-Barberan, F.A.; Tomas-Lorente, F. Flavonoid compounds from Ballota hirsuta. J. Nat. Prod. 1988, 49, 554–555. [Google Scholar] [CrossRef]
- Citoglu, G.; Tanker, M.; Sever, B. Note Flavonoid Aglycones from Ballota saxatilis subsp. saxatilis. Pharm. Biol. 1999, 37, 158–160. [Google Scholar] [CrossRef]
- Bruno, M.; Bondi, M.L.; Piozzi, F.; Arnold, N.A.; Simmonds, M.S. Occurrence of 18-hydroxyballonigrine in Ballota saxatilis ssp. saxatilis from Lebanon. Biochem. Syst. Ecol. 2001, 29, 429–431. [Google Scholar] [CrossRef]
- Citoğlu, G.S.; Coban, T.; Sever, B.; İşcan, M. Antioxidant properties of Ballota species growing in Turkey. J. Ethnopharmacol. 2004, 92, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Marrelli, M.; Formisano, C.; Menichini, F.; Senatore, F.; Bruno, M.; Conforti, F. Phytochemical profile of three Ballota species essential oils and evaluation of the effects on human cancer cells. Nat. Prod. Res. 2017, 31, 436–444. [Google Scholar] [CrossRef]
- Bader, A.; Caponi, C.; Cioni, P.L.; Flamini, G.; Morelli, I. Composition of the essential oil of Ballota undulata, B. nigra ssp. foetida and B. saxatilis. Flavour Fragr. J. 2003, 18, 502–504. [Google Scholar] [CrossRef]
- Fraternale, D.; Bucchini, A.; Giamperi, L.; Ricci, D. Essential oil composition and antimicrobial activity of Ballota nigra L. ssp foetida. Nat. Prod. Commun. 2009, 4, 585–588. [Google Scholar] [CrossRef]
- Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N. Antimicrobial activity of the essential oil obtained from roots and chemical composition of the volatile constituents from the roots, stems, and leaves of Ballota nigra from Serbia. J. Med. Food 2009, 12, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Couladis, M.; Chinou, I.B.; Tzakou, O.; Loukis, A. Composition and antimicrobial activity of the essential oil of Ballota pseudodictamnus L. Bentham. Phytother. Res. 2002, 16, 723–726. [Google Scholar] [CrossRef]
- Younis, A.; Riaz, A.; Khan, M.A.; Khan, A.A.; Pervez, M.A. Extraction and identification of chemical constituents of the essential oil of Rosa species. Acta Hortic. 2008, 766, 485–492. [Google Scholar] [CrossRef]
- Bicchi, C.; Joulain, D. Techniques for preparing essential oils and aromatic extracts. Flavour Fragr. J. 2018, 33, 133–134. [Google Scholar] [CrossRef]
- Dilworth, L.L.; Riley, C.K.; Stennett, D.K. Pharmacognosy: Fundamentals, Applications and Strategies. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 61–80. [Google Scholar]
- Dobreva, A.; Tintchev, F.; Heinz, V.; Schulz, H.; Toepfl, S. Effect of pulsed electric fields (PEF) on oil yield and quality during distillation of white oil-bearing rose (Rosa alba L.). Z. Für Arznei-Gewürzpflanzen 2010, 15, 127–132. [Google Scholar]
- Rassem, H.H.; Nour, A.H.; Yunus, R.M. Techniques for Extraction of Essential Oils from Plants: A Review. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Al-Qudah, M.A.; Abu Zarga, M.H. Chemical composition of essential oils from aerial parts of Sisymbrium irio from Jordan. E-J. Chem. 2010, 7, 6–10. [Google Scholar] [CrossRef]
- Al-Qudah, M.A. Antioxidant acitvity and chemical composition of essential oils of fresh and air-dried Jordanian Nepeta curviflora Boiss. J. Biol. Act. Prod. Nat. 2016, 6, 101–111. [Google Scholar]
- Al-Qudah, M.A. Chemical composition of essential oil from Jordanian Lupinus varius L. Arab. J. Chem. 2013, 6, 225–227. [Google Scholar] [CrossRef]
- Alkhatib, R.Q.; Almasarweh, A.B.; Abdo, N.M.; Mayyas, A.S.; Al-Qudah, M.A.; Abu-Orabi, S.T. Chromatographic analysis (LC-MS and GC-MS), antioxidant activity, antibacterial activity, total phenol, and total flavonoid determination of Cleome arabica L. growing in Jordan. Int. J. Food Prop. 2022, 25, 1920–1933. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Migdadi, R.S.; Mayyas, A.S.; Al-Zereini, W.A.; Al-Dalahmeh, Y.; Abu Orabi, F.M.; Abu-Orabi, S.T. Chemical Composition, Cytotoxicity and antioxidant activity of the essential oil from flower buds and leaves of the Pulicaria incisa (Lam.) DC and Pulicaria crispa (Forskel) Oliver. J. Essent. Oil Bear. Plants 2022, 25, 758–772. [Google Scholar] [CrossRef]
- Al-Dalahmeh, Y.; Al-Bataineh, N.; Al-Balawi, S.S.; Lahham, J.N.; Al-Momani, I.F.; Al-Sheraideh, M.S.; Al-Qudah, M.A. LC-MS/MS Screening, Total phenolic, flavonoid and antioxidant Contents of crude extracts from three Asclepiadaceae species growing in Jordan. Molecules 2022, 27, 859. [Google Scholar] [CrossRef]
- Abu-Orabi, S.T.; Al-Qudah, M.A.; Saleh, N.R.; Bataineh, T.T.; Obeidat, S.M.; Al-Sheraideh, M.S.; Lahham, J.N. Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius. Arab. J. Chem. 2020, 13, 6256–6266. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Ali, M. Practical Pharmaceutical Chemistry; CBS Publishers & Distributors: Delhi, India, 1997. [Google Scholar]
- Bicchi, C.; Chaintreau, A.; Joulain, D. Identification of flavour and fragrance constituents. Flavour Fragr. J. 2018, 33, 201–202. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils by Ion Trap Mass Spectroscopy; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).