Research Progress on Adsorption and Separation of Petroleum Hydrocarbon Molecules by Porous Materials
Abstract
1. Introduction
2. Introduction to Adsorption Separation Technology
2.1. Shape Selective Adsorption Materials
2.2. Complex Adsorption Materials
2.3. Polarity Selective Adsorbent
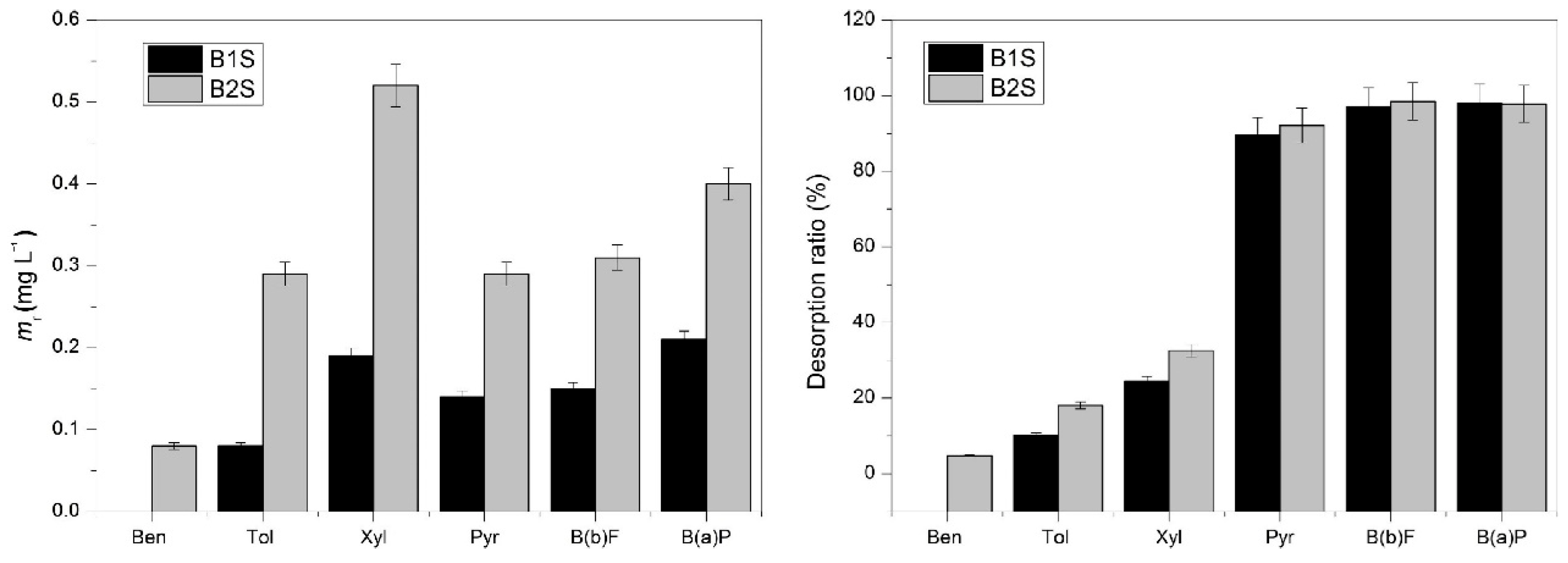
3. Research Progress on Adsorption and Separation of Hydrocarbon Molecules
3.1. n-Alkanes
| Component | n-Alkanes | Isoparaffin | Olefins | Cycloparaffin | Aromatics | Total |
|---|---|---|---|---|---|---|
| content% | 27.92 | 30.42 | 0.02 | 28.85 | 12.78 | 100 |
3.2. Aromatic Hydrocarbons
3.2.1. Carbon Materials
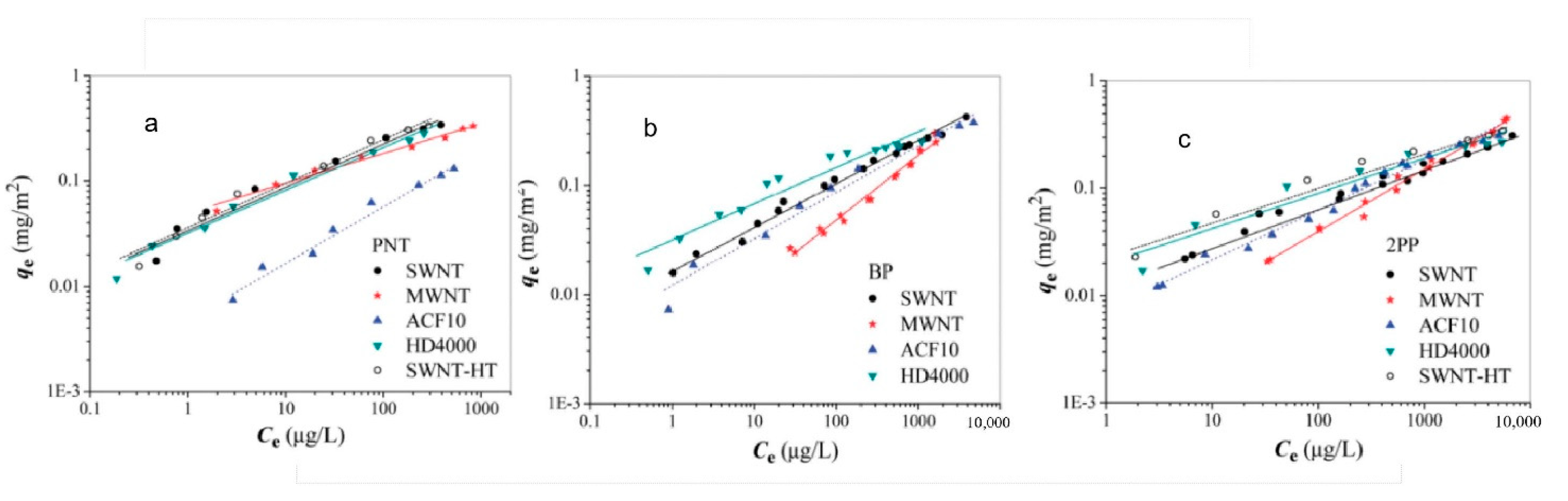
3.2.2. Silicon-Based Materials
3.2.3. Polymer Adsorption Technology
3.2.4. Metal-Organic Frameworks (MOFs)
3.2.5. Chitosan
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Dai, H.J.; Li, H.L.; Zhou, G.W.; He, Y.F.; Zhou, W.M.; Chen, W.; Shi, H. X Degradation characteristics of organic pollutants in the treatment of printing and dyeing wastewater by GC-MS. J. Zhejiang Univ. (Sci. Ed.) 2014, 41, 72–77. [Google Scholar]
- Long, R.Q.; Yang, R.T. Carbon nanotubes as superior sorbent for dioxin removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.J.; Cui, X.Y. Bioavailability of persistent organic pollutants in soil. Environ. Ecol. 2021, 3, 15–26. [Google Scholar]
- Huang, W.Q.; Tian, Q.; Zhao, S.H.; Gao, X.Q.; Lu, X.W.; Wang, Y.Y. Evaporative oil and gas adsorption separation and recovery technology. Oil Gas Storage Transp. 1998, 12, 47–50. [Google Scholar]
- Huang, W.Q.; Zhong, Q. Analysis and comparison of oil and gas recovery technology. Chem. Eng. 2005, 5, 56–59+68. [Google Scholar]
- Ding, L.S. Basic Research on the Removal of Aromatics from Simulated Catalytically Cracked Diesel Fuel; China University of Petroleum: Beijing, China, 2018. [Google Scholar]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Ni, Q.; Lai, J.B.; Peng, D.Y.; Guan, C.S.; Long, J. Research progress of ionic liquid extraction and separation of hydrocarbon compounds. Prog. Chem. Ind. 2021, 6, 1–11. [Google Scholar]
- Yin, M.F.; Tang, Z.; Zhang, R.; Liu, Z.C.; Liu, H.Y.; Xu, C.M.; Meng, X. H Separation of n-octane/o-xylene by ionic liquid liquid-liquid extraction. Chin. J. Chem. Eng. 2021, 72, 1–9. [Google Scholar]
- Jiang, X.; Yang, F.C.; Guo, Z.G. Superwetting surfaces for filtration separation of high-viscosity raw petroleum/water mixtures. J. Mater. Chem. A 2022, 10, 14273–14292. [Google Scholar] [CrossRef]
- Saien, J.; Kharazi, M.; Yarie, M.; Zolfigol, M.A. A Systematic investigation of a surfactant type nano gemini ionic liquid and simultaneous abnormal salts effects on the crude oil/water interfacial tension. Ind. Eng. Chem. Res. 2019, 58, 3583–3594. [Google Scholar] [CrossRef]
- Nazhipkyzy, M.; Assylkhanova, D.; Araylim, N.; Seitkazinova, A.; Özsin, G.; Varol, E.A. Effective separation of petroleum oil-water mixtures via flexible and re-usable hydrophobic soot-coated melamine sponge. J. Water Process. Eng. 2022, 49, 103032. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Bae, T.-H. Recent advances in mixed-matrix membranes for light hydrocarbon (c(1)–c(3)) separation. Membranes 2022, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Cao, J.F.; Liu, W.; Liao, J.F.; Xie, W.J.; Huang, W. Prediction of Product Properties of Catalytic Diesel Hydrocracking with Different Aromatic Mass Fractions. Contemp. Chem. Ind. 2021, 50, 1897–1899+1904. [Google Scholar]
- Sun, H.; Shen, B.X. Effect of Binder on Adsorption of N-alkanes on 5A Molecular Sieve. Petrochem. Ind. 2011, 40, 279–284. [Google Scholar]
- Broughton, D.B.; Gerhold, C.G. Continuous Sorption Process Employing Fixed Bed of Sorbent and Moving Inlets and Outlets. U.S. Patent 2,985,589, 23 May 1961. [Google Scholar]
- Huo, Q.; Dou, T.; Zhao, Z.; Pan, H. Synthesis and application of a novel mesoporous zeolite L in the catalyst for the HDS of FCC gasoline. Appl. Catal. A Gen. 2010, 381, 101–108. [Google Scholar] [CrossRef]
- Ban, S.; Van Laak, A.; De Jongh, P.E.; Van der Eerden, J.P.; Vlugt, T.J. Adsorption selectivity of benzene/propene mixtures for various zeolites. J. Phys. Chem. C 2007, 111, 17241–17248. [Google Scholar] [CrossRef]
- Costa, A.A.; Wilson, W.B.; Wang, H.; Campiglia, A.D.; Dias, J.A.; Dias, S.C. Comparison of BEA, USY and ZSM-5 for the quantitative extraction of polycyclic aromatic hydrocarbons from water samples. Microporous Mesoporous Mater. 2012, 149, 186–192. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Wang, S. Selective adsorption of CO on CuCl/Y adsorbent prepared using CuCl2 as precursor: Equilibrium and thermodynamics. Chem. Eng. J. 2016, 290, 418–427. [Google Scholar] [CrossRef]
- Song, R.T.; Yao, J.; Yang, M.; Ye, Z.B. Insights into High-Performance and Selective Elimination of Cationic Dye from Multicomponent Systems by Using Fe-Based Metal–Organic Frameworks. Langmuir 2022, 38, 9400–9409. [Google Scholar] [CrossRef]
- Gu, J.J.; Gao, Y.M.; Zhang, J.L.; Li, W.; Dong, Y.Z.; Han, Y. Hydrochlorination of Acetylene Catalyzed by an Activated Carbon-Supported Ammonium Hexachlororuthenate Complex. Catalysts 2017, 7, 17. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.H.; Gao, H.Y. Recent progress in porous intermetallics: Synthesis mechanism, pore structure, and material properties. J. Mater. Sci. Technol. 2021, 74, 89–104. [Google Scholar] [CrossRef]
- Qu, D.; Feng, X.; Li, N.; Ma, X.; Shang, C.; Chen, X.D. Adsorption of heterocyclic sulfur and nitrogen compounds in liquid hydrocarbons on activated carbons modified by oxidation: Capacity, selectivity and mechanism. RSC Adv. 2016, 6, 41982–41990. [Google Scholar] [CrossRef]
- Pang, Y.; Huang, Y.; Li, W.; Feng, L.; Shen, X. Conjugated polyelectrolyte/graphene multilayer films for simultaneous electrochemical sensing of three monohydroxylated polycyclic aromatic hydrocarbons. ACS Appl. Nano Mater. 2019, 2, 7785–7794. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, L.; Wu, F.; Huang, B.; Liao, Z.; Yu, Z.; Hu, L.; Zhou, Y.; Chen, Y. Regulation of the polar groups in n-Type conjugated polyelectrolytes as electron transfer layer for inverted polymer solar cells. Macromolecules 2018, 51, 8197–8204. [Google Scholar] [CrossRef]
- Costa, A.S.; Romão, L.P.; Araújo, B.R.; Lucas, S.C.; Maciel, S.T.; Wisniewski, A., Jr.; Alexandre, M.D. Environmental strategies to remove volatile aromatic fractions (BTEX) from petroleum industry wastewater using biomass. Bioresour. Technol. 2012, 105, 31–39. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Core flood study for enhanced oil recovery through ex-situ bioaugmentation with thermo- and halo-tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Bioresour. Technol. 2016, 220, 175–182. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Yue, B.; Nie, Y.; Chai, Y.; Wu, G.; Li, J.; Han, X.; Day, S.J.; Thompson, S.P.; et al. Cascade adsorptive separation of light hydrocarbons by commercial zeolites. J. Energy Chem. 2022, 72, 299–305. [Google Scholar] [CrossRef]
- Yao, Z.L.; Zhao, Y.Z. Advances in Adsorption and Separation Technology of N-alkanes. Prog. Chem. Ind. 2003, 22, 589–592. [Google Scholar]
- Cao, D.; Li, Z.; Wang, Z.; Wang, H.; Gao, S.; Wang, Y.; Shi, Y.; Qiu, J.; Zhao, Y.; Wang, J. Highly Dispersed Ionic Liquids in Mesoporous Molecular Sieves Enable a Record NH3 Absorption. ACS Sustain. Chem. Eng. 2021, 9, 16363–16372. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Z.; Xu, S.; Zheng, A.; Wu, P.; Li, B.; Yuan, X.; Wei, Y.; Liu, Z. Cavity-controlled diffusion in 8-membered ring molecular sieve catalysts for shape selective strategy. J. Catal. 2019, 37, 51–62. [Google Scholar] [CrossRef]
- Kang, D.W.; Ju, S.E.; Kim, D.W.; Kang, M.; Kim, H.; Hong, C.S. Emerging porous materials and their composites for NH3 gas removal. Adv. Sci. 2020, 7, 2002142. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Shen, B.X. Optimized utilization of naphtha: Liquid adsorption separation of linear paraffins in naphtha with n-pentane as the desorbent. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 235–245. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wang, H.C.; Qiao, X.F.; Yang, Y.Q.; Wang, D.H.; Wang, H.G. Study on adsorption and separation of n-alkanes with 5A molecular sieve adsorbent. Petrochemical 2021, 50, 534. [Google Scholar]
- Santi, K. Zeolites in Industrial Separation and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 219. [Google Scholar]
- He, J.; Yan, A.Z. Properties of 5A Dewaxed Molecular Sieve IV. Effect of Ca2+ Exchange Degree on Desorption and Diffusion in n-alkane Molecular Sieve. Chin. J. Pet. 1995, 11, 13–20. [Google Scholar]
- Yang, C.T.; Janda, A.; Bell, A.T.; Lin, L.C. Atomistic investigations of the effects of Si/Al ratio and Al distribution on the adsorption selectivity of n-Alkanes in Brønsted Acid zeolites. J. Phys. Chem. C 2018, 122, 9397–9410. [Google Scholar] [CrossRef]
- Robert, F.D.; Bahman, E.; Matheus, D.M. Understanding the unique sorption of alkane-α, ω-diols in silicalite-1. J. Chem. Phys. 2018, 149, 072331. [Google Scholar]
- Wang, L.Y.; Yang, G.M.; Xin, J. Research progress on the removal of aromatic hydrocarbons from diesel fuel by adsorption process. Petrochem. Technol. Appl. 2021, 39, 66–69. [Google Scholar]
- Xu, Y.Q.; Wang, Z.Y.; Pang, X.Y.; Xiao, L.G. Analysis on the scheme of diesel hydrocracking to reduce diesel-gasoline ratio. Zhongwai Energy 2016, 21, 85–89. [Google Scholar]
- Zhang, C.; Wang, B.; Xiang, J.; Su, C.; Mu, C.; Wen, F.; Liu, Z. Microwave absorption properties of CoS2 nanocrystals embedded into reduced graphene oxide. ACS Appl. Mater. Interfaces 2017, 9, 28868–28875. [Google Scholar] [CrossRef]
- Al-Jammal, N.; Abdullah, T.A.; Juzsakova, T.; Zsirka, B.; Cretescu, I.; Vágvölgyi, V.; Sebestyén, V.; Le Phuoc, C.; Rasheed, R.T.; Domokos, E. Functionalized carbon nanotubes for hydrocarbon removal from water. J. Environ. Chem. Eng. 2020, 8, 103570. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, L.; Luo, Y.; Wang, C.R. The Effect of the Polyaromatic Hydrocarbon in the Formation of Fullerenes. Angew. Chem. Int. Ed. 2020, 59, 3942–3947. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, T.; Kose, H.S.; Karanfil, T. Adsorption of aromatic compounds by carbonaceous adsorbents: A comparative study on granular activated carbon, activated carbon fiber, and carbon nanotubes. Environ. Sci. Technol. 2010, 44, 6377–6383. [Google Scholar] [CrossRef]
- Daley, M.A.; Tandon, D.; Economy, J.; Hippo, E.J. Elucidating the porous structure of activated carbon fibers using direct and indirect methods. Carbon 1996, 34, 1191–1200. [Google Scholar] [CrossRef]
- Bandosz, T.J. Activated Carbon Surfaces in Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Khaled, M. Adsorption performance of multiwall carbon nanotubes and graphene oxide for removal of thiophene and dibenzothiophene from model diesel fuel. Res. Chem. Intermed. 2015, 41, 9817–9833. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Sridhar, K.; Chen, B.H. Removal of polycyclic aromatic hydrocarbons from water by magnetic activated carbon nanocomposite from green tea waste. J. Hazard Mater. 2021, 415, 125701. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B.X. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef]
- Zhang, J.; Li, R.; Ding, G.; Wang, Y.; Wang, C. Sorptive removal of phenanthrene from water by magnetic carbon nanomaterials. J. Mol. Liq. 2019, 293, 111540. [Google Scholar] [CrossRef]
- Bayazit, Ş.S.; Yildiz, M.; Aşçi, Y.S.; Şahin, M.; Bener, M.; Eğlence, S.; Salam, M.A. Rapid adsorptive removal of naphthalene from water using graphene nanoplatelet/MIL-101 (Cr) nanocomposite. J. Alloys Compd. 2017, 701, 740–749. [Google Scholar] [CrossRef]
- Aksu, Z.; Yener, J. A comparative adsorption/biosorption study of mono-chlorinated phenols onto various sorbents. Waste Manag. 2001, 21, 695–702. [Google Scholar] [CrossRef]
- Elangovan, S.P.; Ogura, M.; Zhang, Y.; Chino, N.; Okubo, T. Silicoaluminophosphate molecular sieves as a hydrocarbon trap. Appl. Catal. B 2005, 57, 31–36. [Google Scholar] [CrossRef]
- Maretto, M.; Vignola, R.; Williams, C.D.; Bagatin, R.; Latini, A.; Papini, M.P. Adsorption of hydrocarbons from industrial wastewater onto a silica mesoporous material: Structural and thermal study. Micropor. Mesopor. Mater. 2015, 203, 139–150. [Google Scholar] [CrossRef]
- Zhan, C.; Jana, S.C. Shrinkage reduced polyimide-graphene oxide composite aerogel for oil absorption. Microporous Mesoporous Mater. 2020, 307, 110501. [Google Scholar] [CrossRef]
- Cesar Filho, M.C.; Matias, T.; Duraes, L.; Valente, A.J. Efficient simultaneous removal of petroleum hydrocarbon pollutants by a hydrophobic silica aerogel-like material. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 550–560. [Google Scholar] [CrossRef]
- Štandeker, S.; Novak, Z.; Knez, Ž. Removal of BTEX vapours from waste gas streams using silica aerogels of different hydrophobicity. J. Hazard. Mater. 2009, 165, 1114–1118. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, X.; Zhang, X.; Li, H. The synergistic adsorption of pyrene and copper onto Fe (III) functionalized mesoporous silica from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 39–45. [Google Scholar] [CrossRef]
- Palantavida, S.; Peng, B.; Sokolov, I. Absorption of organic compounds by mesoporous silica discoids. Micropor Mesopor Mat. 2020, 306, 110379. [Google Scholar] [CrossRef]
- Hernández Maldonado, A.J.; Yang, R.T. Denitrogenation of transportation fuels by zeolites at ambient temperature and pressure. Angew. Chem. 2004, 116, 1022–1024. [Google Scholar] [CrossRef]
- Ono, T.; Sugimoto, T.; Shinkai, S.; Sada, K. Lipophilic polyelectrolyte Gels as super-absorbent polymers for nonpolar organic solvents. Nat. Mater. 2007, 6, 429–433. [Google Scholar] [CrossRef]
- Chung, T.C.M.; Jeong, Y.; Chen, Q.; Kleinhammes, A.; Wu, Y. Synthesis of microporous boron-substituted Carbon (B/C) materials using polymeric precursors for hydrogen physisorption. J. Am. Chem. Soc. 2008, 130, 6668–6669. [Google Scholar] [CrossRef]
- Xue, W.; Hamley, I.W.; Huglin, M.B. Rapid swelling and deswelling of thermoreversible hydrophobically modified poly(N-Isopropylacrylamide) hydrogels prepared by freezing polymerisation. Polymer 2002, 43, 5181–5186. [Google Scholar] [CrossRef]
- Nam, C.; Li, H.X.; Chung, T.C.M. Petrogel: New hydrocarbon (petroleum) absrbent based on polyolefin polymers. Macromolecules 2016, 49, 5427–5437. [Google Scholar] [CrossRef]
- Nam, C.; Li, H.X.; Chung, T.C.M. Practical petroleum spill recovery by a combination of polyolefin absorbent and mechanical skimmer. ACS Sustain. Chem. Eng. 2018, 6, 12036–12045. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, M.; Wang, H.; Zeng, G.; Huang, D.; Cheng, M.; Liu, Y.; Xue, W.; Wang, Z. The application of different typological and structural MOFs-based materials for the dyes adsorption. Coord. Chem. Rev. 2019, 380, 471–483. [Google Scholar] [CrossRef]
- Zou, D.; Liu, D. Understanding the modifications and applications of highly stable porous frameworks via UiO-66. Mater. Today Chem. 2019, 12, 139–165. [Google Scholar] [CrossRef]
- Maes, M.; Trekels, M.; Boulhout, M.; Schouteden, S.; Vermoortele, F.; Alaerts, L.; Heurtaux, D.; Seo, Y.K.; Hwang, Y.K.; Chang, J.S.; et al. Selective removal of N-heterocyclic aromatic contaminants from fuels by Lewis acidic metal–organic frameworks. Angew. Chem. Int. Ed. 2011, 50, 4210–4214. [Google Scholar] [CrossRef]
- Makowski, W.; Mańko, M.; Zabierowski, P.; Mlekodaj, K.; Majda, D.; Szklarzewicz, J.; Łasocha, W. Unusual adsorption behavior of volatile hydrocarbons on MOF-5 studied using thermodesorption methods. Thermochim. Acta 2014, 587, 1–10. [Google Scholar] [CrossRef]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Ramli, A.; Abu Bakar, N.H.; Saad, B.; Rozaini, M.N.; Isiyaka, H.A.; Osman, A.M.; Sulieman, A. An overview and evaluation of highly porous adsorbent materials for polycyclic aromatic hydrocarbons and phenols removal from wastewater. Water 2020, 12, 2921. [Google Scholar] [CrossRef]
- Bibi, S.; Yasin, T.; Hassan, S.; Riaz, M.; Nawaz, M. Chitosan/CNTs green nanocomposite membrane: Synthesis, swelling and polyaromatic hydrocarbons removal. Mater. Sci. Eng. C 2015, 46, 359–365. [Google Scholar] [CrossRef]
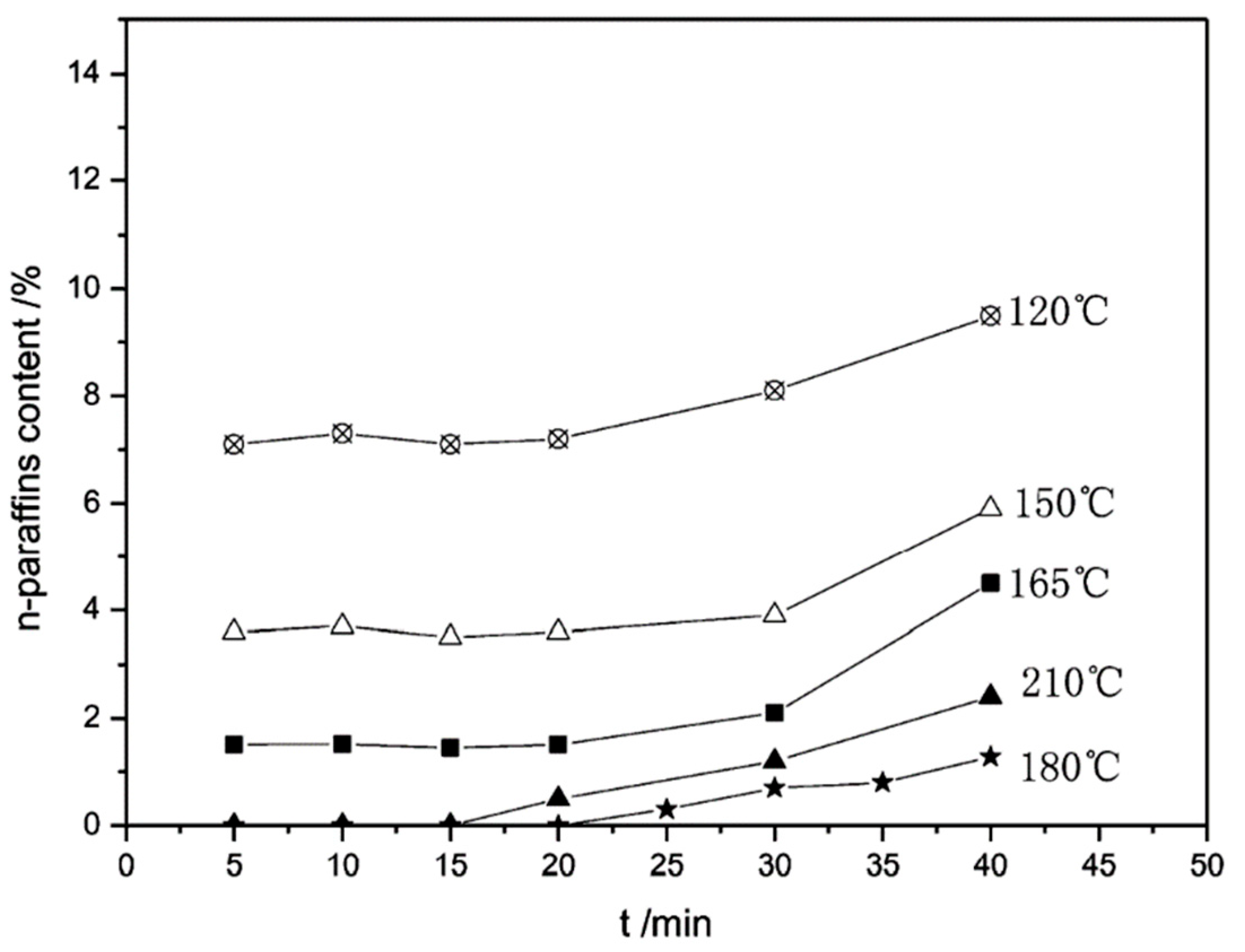
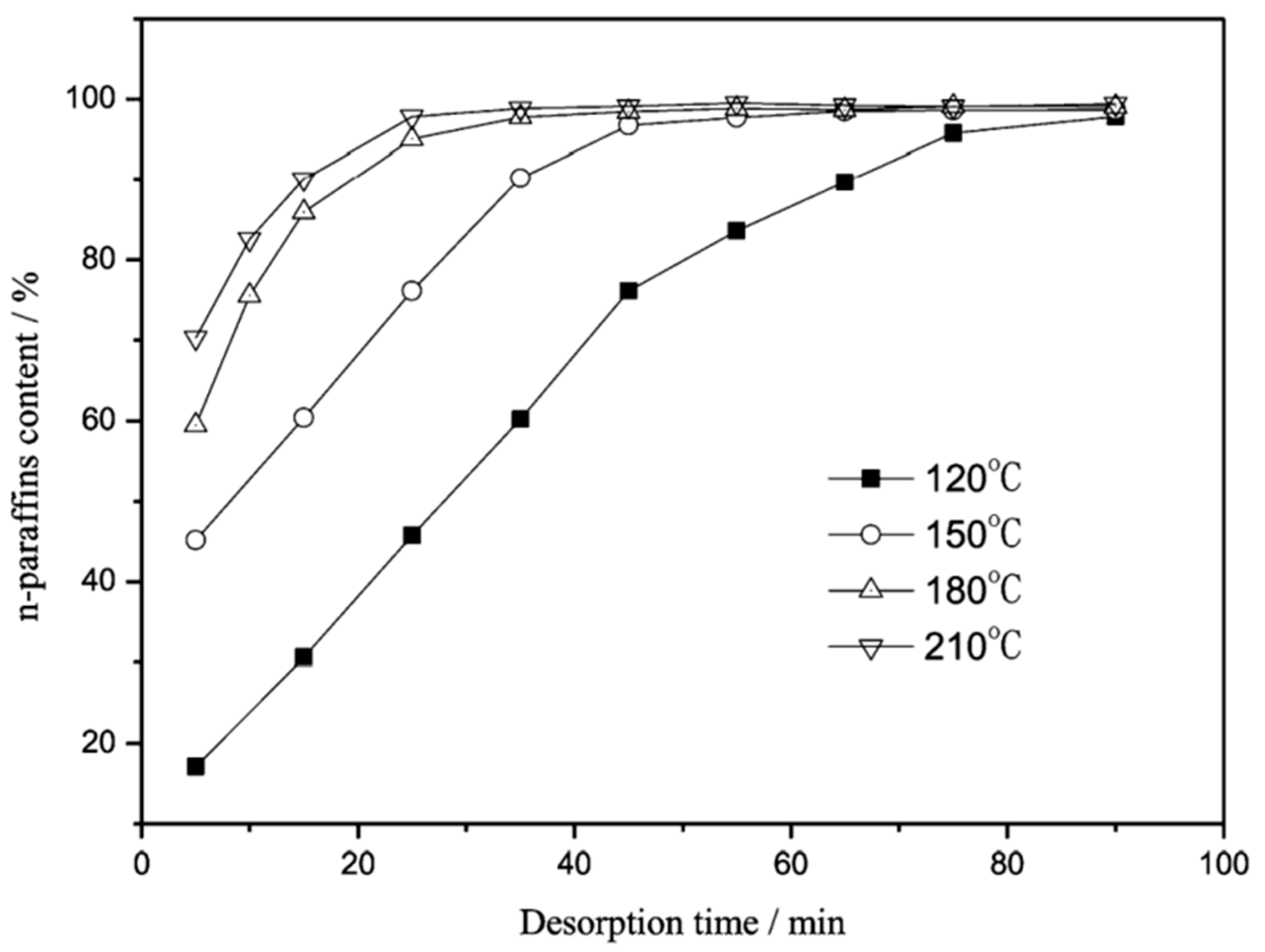

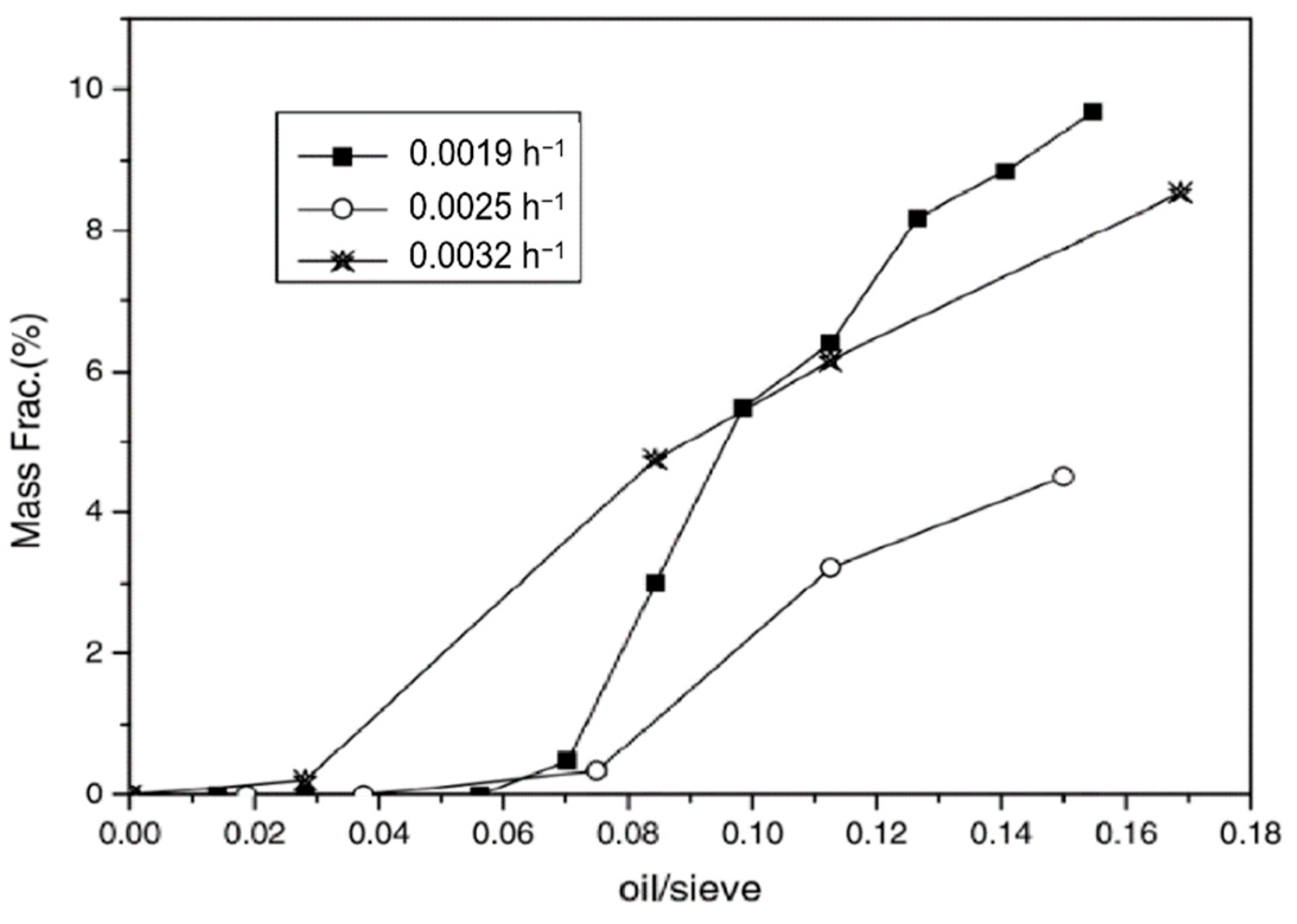
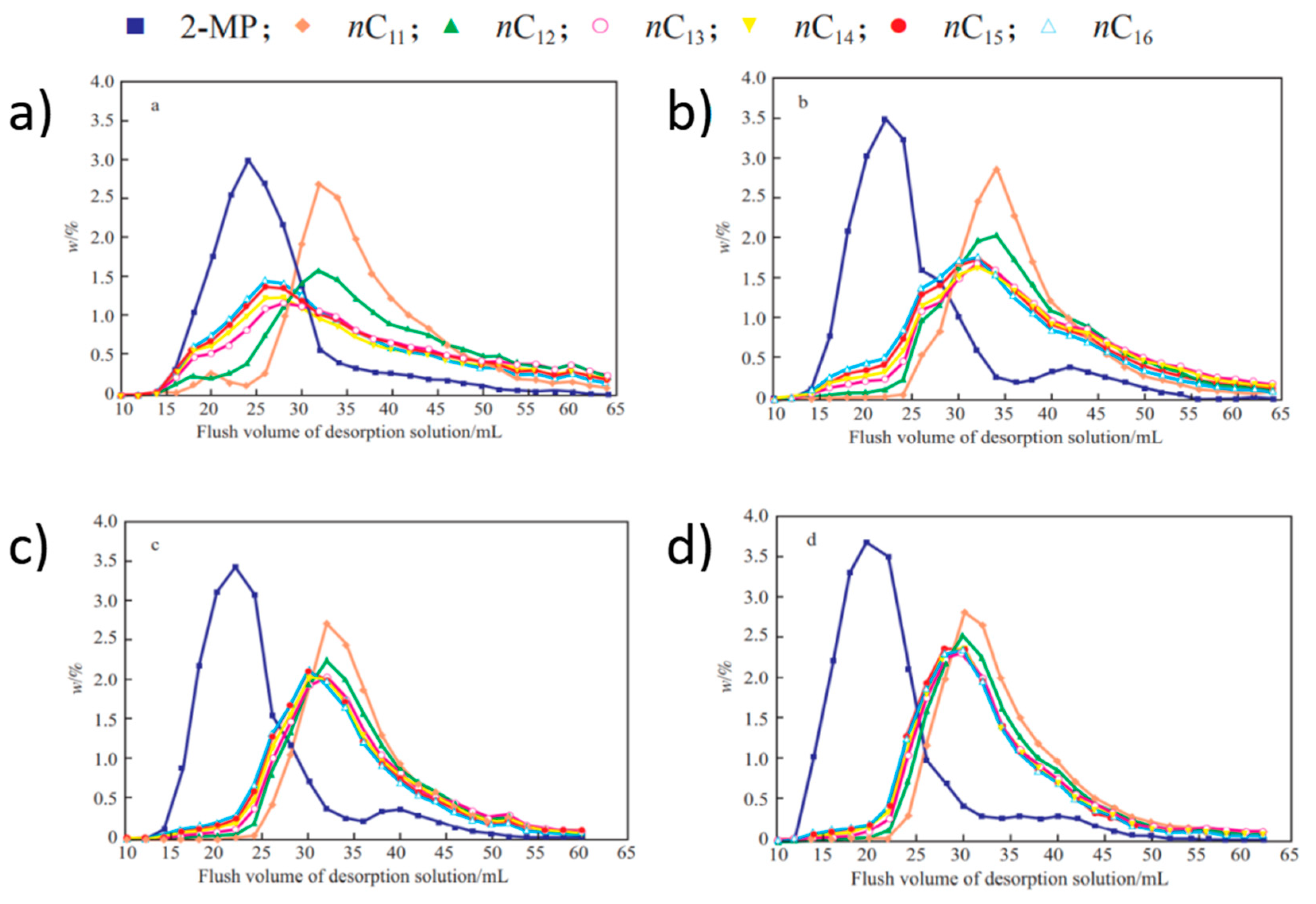


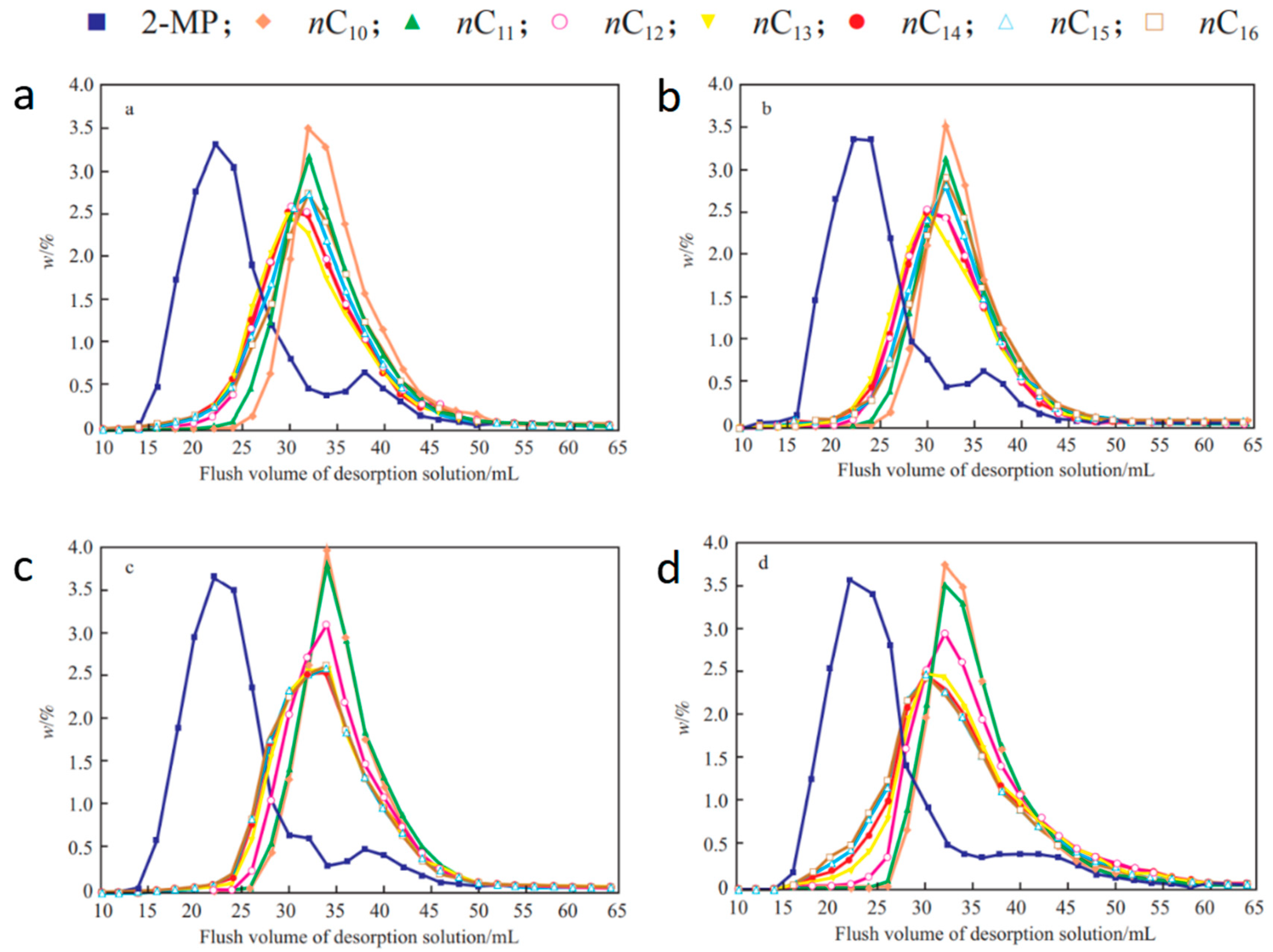
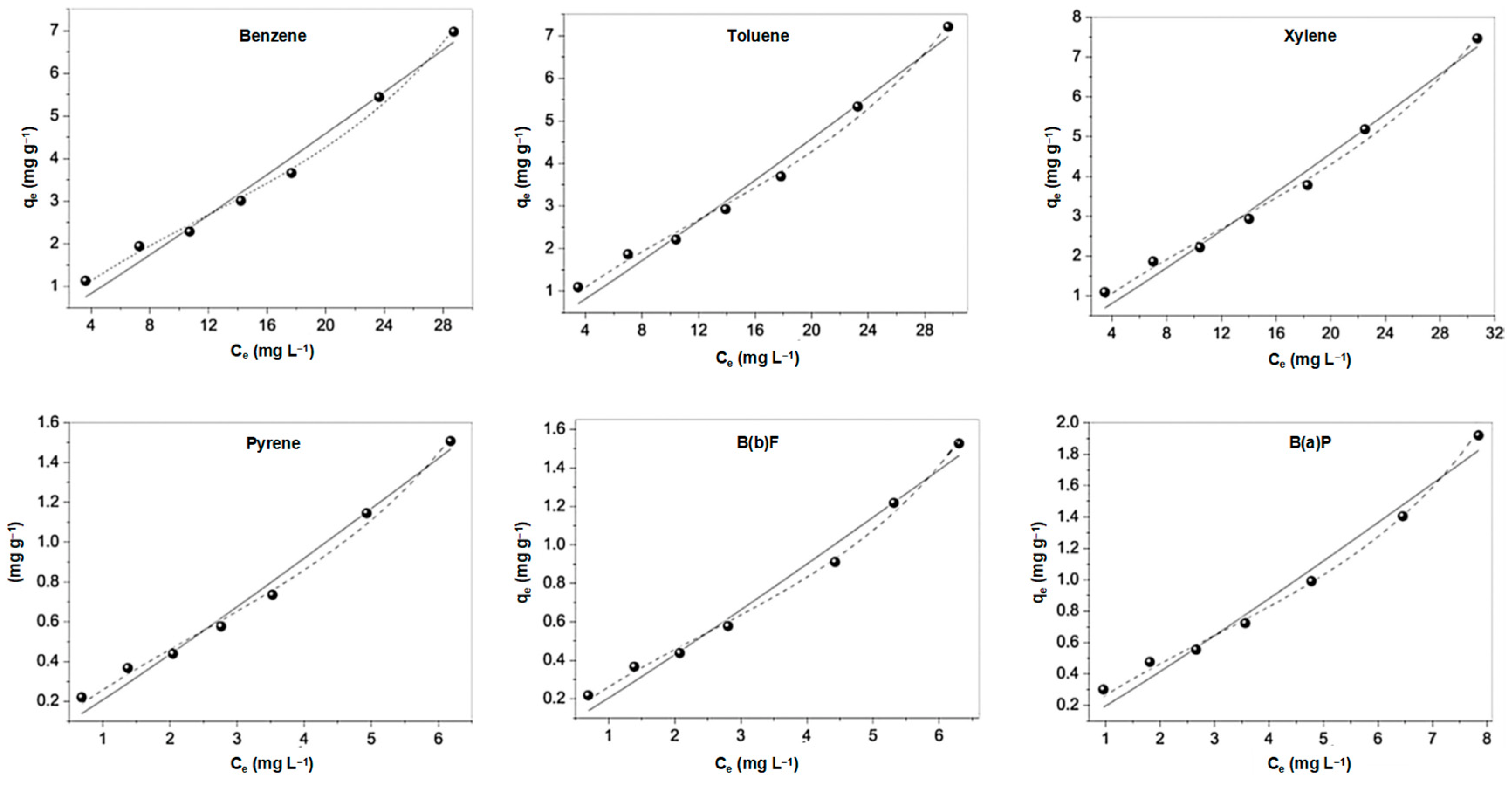
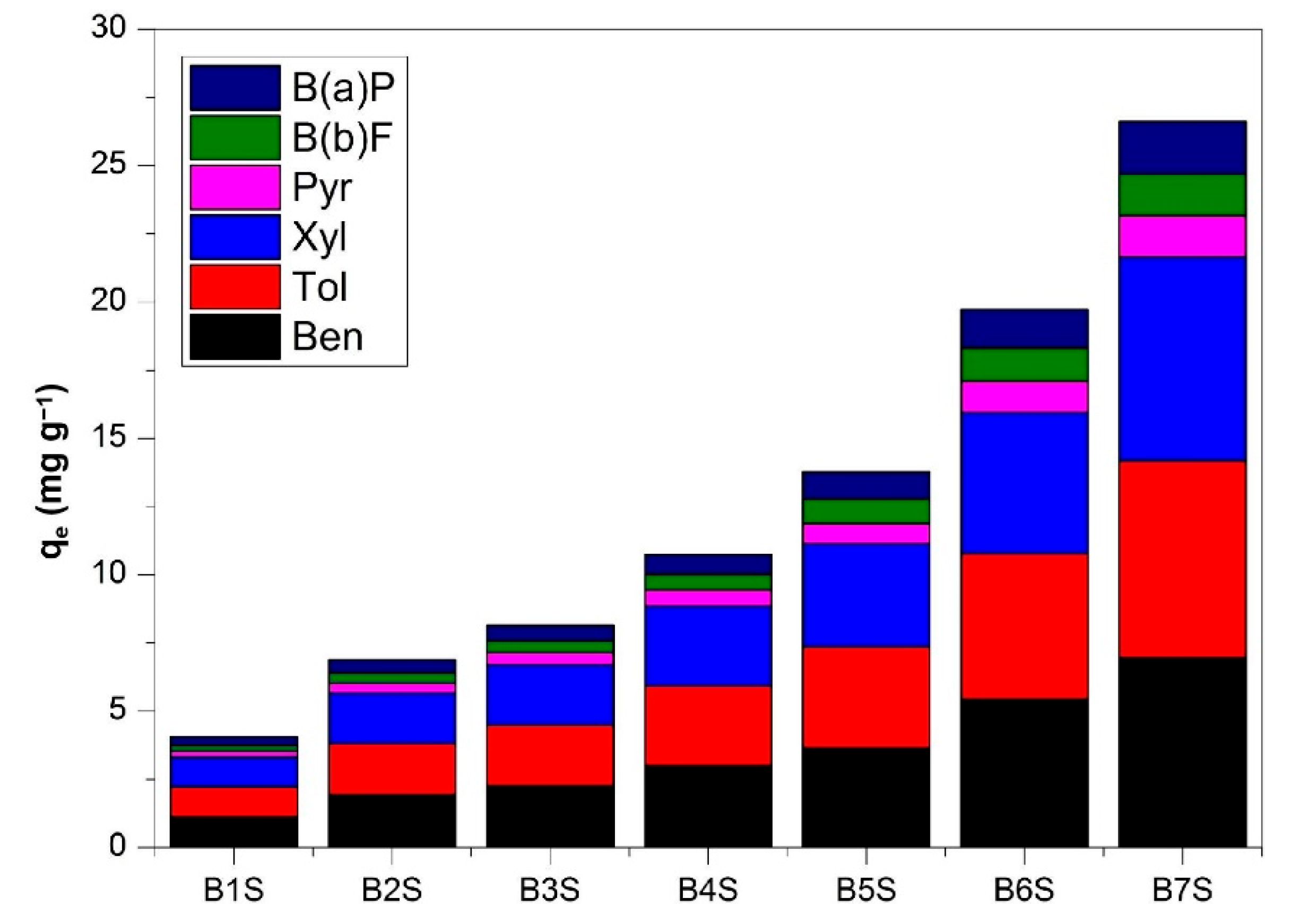
| Item | Temperature/°C | nC11 | nC12 | nC13 | nC14 | nC15 | nC16 |
|---|---|---|---|---|---|---|---|
| ΔVR/mL | 120 | 9.56 | 10.28 | 7.84 | 4.83 | 4.45 | 3.88 |
| 150 | 12.20 | 12.33 | 12.84 | 12.48 | 10.89 | 9.91 | |
| 180 | 11.74 | 11.19 | 10.65 | 10.25 | 9.77 | 9.62 | |
| 200 | 11.75 | 10.77 | 10.12 | 9.79 | 9.61 | 9.78 | |
| W1/2/mL | 120 | 10.64 | 17.10 | 22.38 | 19.47 | 18.45 | 17.44 |
| 150 | 9.67 | 14.41 | 18.50 | 18.98 | 16.67 | 15.65 | |
| 180 | 9.25 | 11.01 | 12.43 | 12.44 | 11.99 | 11.91 | |
| 200 | 10.22 | 10.91 | 11.48 | 11.67 | 11.61 | 11.73 | |
| R | 120 | 0.90 | 0.74 | 0.48 | 0.32 | 0.31 | 0.28 |
| 150 | 1.29 | 1.04 | 0.93 | 0.88 | 0.84 | 0.80 | |
| 180 | 1.31 | 1.14 | 1.01 | 0.98 | 0.95 | 0.94 | |
| 200 | 1.21 | 1.07 | 0.98 | 0.94 | 0.92 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Zang, J.; Guo, C.; Li, B.; Li, B.; Zhang, X.; Chen, T. Research Progress on Adsorption and Separation of Petroleum Hydrocarbon Molecules by Porous Materials. Separations 2023, 10, 17. https://doi.org/10.3390/separations10010017
Yu H, Zang J, Guo C, Li B, Li B, Zhang X, Chen T. Research Progress on Adsorption and Separation of Petroleum Hydrocarbon Molecules by Porous Materials. Separations. 2023; 10(1):17. https://doi.org/10.3390/separations10010017
Chicago/Turabian StyleYu, Haibin, Jiazhong Zang, Chunlei Guo, Bin Li, Ben Li, Xueyin Zhang, and Tiehong Chen. 2023. "Research Progress on Adsorption and Separation of Petroleum Hydrocarbon Molecules by Porous Materials" Separations 10, no. 1: 17. https://doi.org/10.3390/separations10010017
APA StyleYu, H., Zang, J., Guo, C., Li, B., Li, B., Zhang, X., & Chen, T. (2023). Research Progress on Adsorption and Separation of Petroleum Hydrocarbon Molecules by Porous Materials. Separations, 10(1), 17. https://doi.org/10.3390/separations10010017





