Abstract
The enantiomeric separation of antifungal compounds is an arduous task in pharmaceutical and biomedical fields due to the different properties that each diastereoisomer presents. The enantioseparation of a group of fungicides (sulconazole, bifonazole, triadimefon and triadimenol) using supercritical fluid chromatography was achieved in this work. For this goal, four different chiral columns based on polysaccharide derivatives, as well as the effect of different chromatographic parameters such as temperature, type and percentage of organic modifier (methanol, ethanol and isopropanol), were thoroughly investigated. The inversion of the elution order of enantiomers as a result of a change in the stationary phase or organic modifier was also evaluated by employing a circular dichroism detector. The best separation conditions, in terms of the enantioresolution and analysis time, were obtained with the Lux® Cellulose-2 column using isopropanol as the organic modifier.
1. Introduction
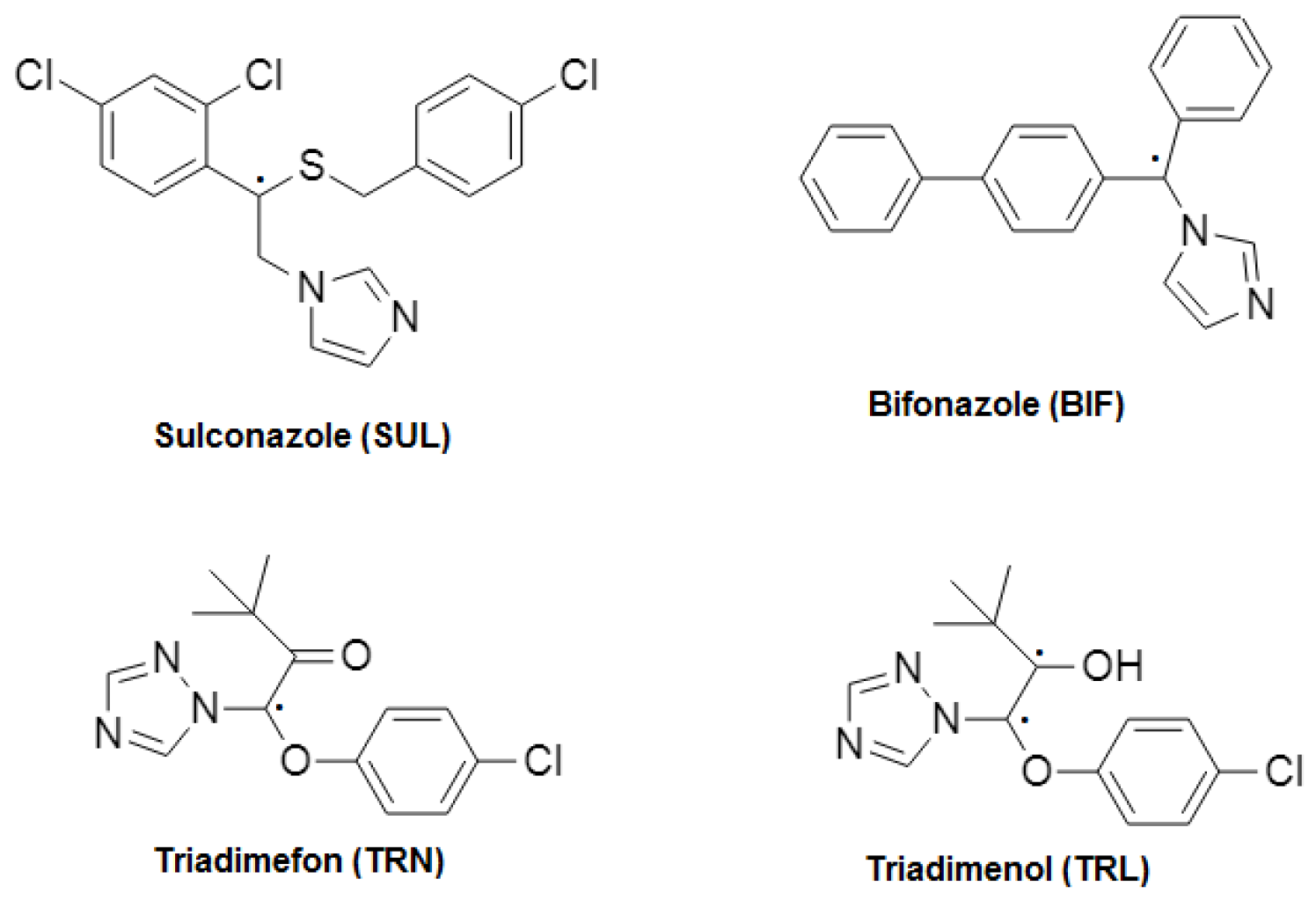
Azoles are used extensively in pharmacology leads and for agricultural crop protection [1,2]. Azole fungicides such as sulconazole and bifonazole have well-documented activities against superficial skin infections, whereas triadimefon and triadimenol have most widely been employed to control plant diseases. All of them have one or two asymmetric carbon atoms in their structure, but whereas the first two are imidazole derivatives, the others include a triazole ring (see Figure 1). The antifungals selected share the same mechanism of action inhibiting the ergosterol synthesis via cytochrome P450 [3].
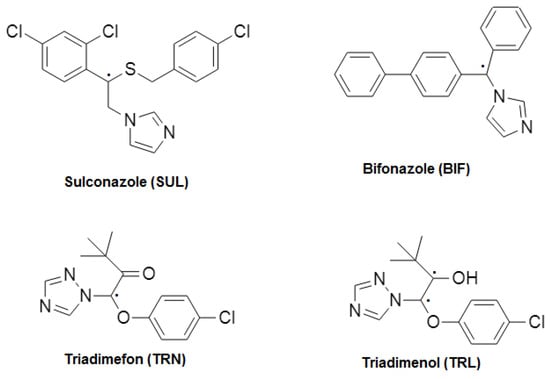
Figure 1.
Structure of the azole fungicides studied.
It is well known that the bioactivity of stereoisomers depends on their specific stereochemistry, affecting their pharmacological, toxicological and pharmacokinetic properties, as well as their accumulation or degradation in the environment. Considering these differences between pairs of enantiomers, the development of enantioselective analytical methods is an important task. Several analytical techniques have been applied for the enantiomeric separation of antifungal compounds, such as gas chromatography (GC) [4], capillary electrophoresis (CE) [5,6,7,8,9,10,11], high-performance liquid chromatography (HPLC) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] and supercritical fluid chromatography (SFC) [14,32,33,34,35,36,37,38,39]. Commercially or hand-made chiral stationary phases (CSPs), based on different cellulose [12,14,15,16,19,20,21,22,23,25,26,27,28,29,30,31] or amylose [13,15,17,22] derivatives and mobile phases typically used for normal-phase mode (mixtures of n-hexane/alcohol with additives), polar organic mode (alcohol or acetonitrile) or reversed-phase mode (aqueous solutions of acids or salts mixed with alcohol or acetonitrile), have been extensively employed in HPLC. Other kind of CSP, such as amino acids derivatives [18] or emamectin [24], have also been studied in liquid chromatography. Fortunately, applications with environmental-friendly techniques, such as SFC, have increased in the last few years. Chiral separations are one of the fields in which SFC has been employed with great success. High efficiencies along with a reduction in the analysis time and waste generation are the main advantages of SFC compared to HPLC due to the physico-chemical properties of the supercritical fluids. The advantages of chiral SFC have been discussed in numerous papers and reviews [40,41,42,43]. Chiral columns based on polysaccharide derivatives [14,15,32,33,34,35,36,37,39] have been widely used in SFC due to their powerful enantioseparation ability, but other chiral selectors, such as 4-chlorophenylcarbamoylated β-cyclodextrin or 3,5-di-methylphenylcarbamoylated β-cyclodextrin, have also been employed, with different degree of success, for the enantiomeric separation of azole fungicides [37].
Although the enantiomeric separation of azole fungicides is a widely studied topic, in most works, the enantioseparation was studied individually. To our knowledge, there are just two papers that describe the simultaneous enantioseparation of several chiral triazole fungicides. M. Lou et al. [31] performed the simultaneous enantioseparation of four chiral triazoles using HPLC, obtaining the baseline separation in 50 min. On the other hand, Yang et al. [39] used SFC-MS/MS and determined five chiral triazole fungicides. Nevertheless, the baseline enantiomeric separation of all of the enantiomers was not achieved, and the determination was performed using MS in tandem. Taking into account that chiral azole fungicides are broadly employed by humans, the study of their enantiomeric environmental behaviors and risks are of great interest. Moreover, in environmental samples, these compounds come up in the presence of each other. Thus, methods that enable the simultaneous chiral separation of several azole fungicides are mandatory.
The purpose of this work was to achieve the simultaneous enantiomeric separation of four azole fungicides (sulconazole, bizonazole, triadimefon and triadimenol), widely employed not only in agricultural but also in medical activities, using SFC. For this purpose, five different chiral columns (Chiralcel® OD, Lux® Cellulose-2, Reflect™ I-cellulose C and Lux® Amylose-2) were evaluated and the effect of different chromatographic conditions was examined in order to obtain the best enantioseparation in the shortest analysis time.
2. Materials and Methods
2.1. Chemical and Materials
HPLC grade quality organic solvents employed (methanol, ethanol, isopropanol) were obtained from LAB-SCAN (Dublin, Ireland). Solid standards of sulconazole (SUL) and bifonazole (BIF) were acquired from Sigma-Aldrich (Madrid, Spain), and triadimefon (TRN) and triadimenol (TRL) were purchased from Dr. Ehrenstofer (Augsburg, Germany). All standards are racemic mixtures, except for TRL standard, for which the threo diastereoisomer predominates. The standard stock solutions at 1000 µg/mL level prepared in methanol were stored at 4 °C. Methanol was also employed as solvent to prepare the working solutions by dilution. Carbon dioxide was of SFC grade and purchased from Carburos Metálicos (Barcelona, Spain).
2.2. Chiral Columns
Four commercially available chiral columns were tested. They were purchased from Phenomenex (Torrance, CA, USA), Daicel Chiral Technologies (Illkirch, France) and Regis Technologies (Chicago, IL, USA). Their characteristics are shown in Table 1.

Table 1.
Different chiral columns employed in SFC method development.
2.3. Supercritical Fluid Chromatography
2.3.1. Instrumentation
An SFC system manufactured by Jasco (Tokyo, Japan) was employed for performing all of the experiments. Carbon dioxide and the modifier were supplied, making use of two pumps, PU-2080-CO2 and PU-2080, respectively. The injection volume was set at 10 µL in the autosampler AS-2059-SF model. The columns were thermostated in a CO-2065 oven, while a BP-2080 pressure regulator controlled the pressure. The instrument was provided with two detectors: a MD-2015 photodiode-array detector (PDA) and a CD-4095 circular dichroism detector (CD), which were employed. System control and data acquisition were performed by ChromNav 1.009.02 software from Jasco (Tokyo, Japan).
2.3.2. Chromatographic Conditions
Lux® Cellulose 2 (250 × 4.6 mm; 5 μm) column was selected due to its better chromatographic performance. Analysis conditions were set as follows: the supercritical fluid used as mobile phase was a mixture of carbon dioxide and isopropanol with a constant flow rate of 2 mL/min, a pressure of 150 bar and a column temperature of 35 °C. An elution gradient was set as follows: initial composition of organic modifier was 5% for 5 min, it was increased to 22% at 10 min and it was finally increased to 45% at 11 min, which was held to the end of the analysis. CD detection was performed at 220 nm. Under optimal SFC conditions, all compounds were eluted in 25 min with enantiomeric resolutions and separations higher than 1.5.
3. Results and Discussion
3.1. Selection of the Stationary Phase and Composition of the Mobile Phase
Different columns were evaluated in detail to select the most suitable one. The chiral selectors investigated included cellulose and amylose carbamate derivatives (see Table 1). According to previous experiments, the initial conditions were 35 °C, 150 bar and 2 mL/min. The compounds studied possess diverse functional groups that can interact with the stationary phases. Thus, the use of an organic modifier was necessary to decrease the retention. In this way, the effect of three organic modifiers (methanol, ethanol and isopropanol) was studied.
The Chiralcel® OD column did not provide any kind of enantioresolution for any of the compounds analyzed, regardless of the modifier employed. Therefore, it was discarded for the rest of the work.
The most relevant results obtained with the columns Lux® Cellulose-2, Lux® Amylose-2 and Reflect™ I-Cellulose C, using different organic modifiers, are shown in Table 2 and Table 3. As can be seen, SUL and BIF presented the highest retentions on these kinds of columns, which require the use of high percentages of the organic modifier to obtain reasonable analysis times. For both compounds, Lux® Cellulose-2 and Lux® Amylose-2 columns provided the highest enantioresolutions with any of the organic modifier used. The retention of BIF was similar on both columns, but SUL clearly showed a lower retention on the Lux® Amylose-2. It should be noted that the Reflect™ I-cellulose C column had a shorter length, which could justify the lower enantioresolutions and retention times. Several assays were performed decreasing the percentage of the modifier. As expected, the retention increased and the enantioresolution increased slightly, but the baseline resolution was not achieved with this last column. TRN and TRL presented the lowest retentions and the enantiomeric resolution was achieved with a lower percentage of the organic modifier. For TRN, the best results were obtained with the Lux® Cellulose-2 and Reflect™ I-cellulose C columns, with Lux® Cellulose-2 being the one that provided the highest enantioresolutions. In the case of TRL, the best results were obtained using the columns Lux® Cellulose-2 and Lux® Amylose-2, with the Lux® Cellulose-2 column being the one that provided the highest retentions.

Table 2.
Effect of the organic modifier on the enantiomeric separation of sulconazole and bifonazole. Chromatographic conditions for three different chiral columns at the same chromatographic conditions of temperature (35 °C), pressure (150 bar) and flow rate (2 mL/min).

Table 3.
Effect of the organic modifier on the enantiomeric separation of triadimefon and triadimenol. Chromatographic conditions for three different chiral columns at the same chromatographic conditions of temperature (35 °C), pressure (150 bar) and flow rate (2 mL/min).
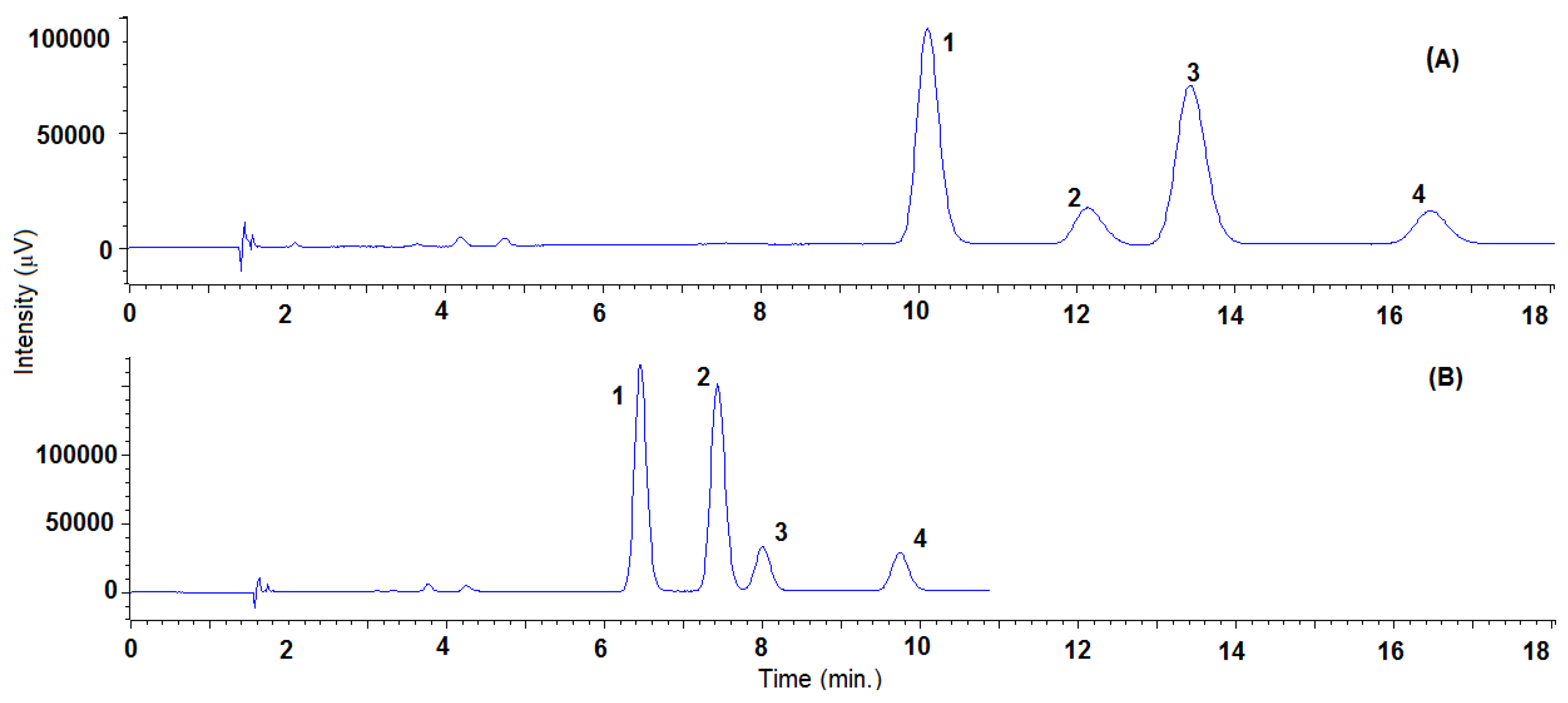
Concerning the effect of the organic modifier, a decrease in the polarity of the organic modifier generally causes an increase in the retention, consequently in the order of methanol < ethanol < isopropanol solvent. Nevertheless, in the case of TRL, when using the Lux® Cellulose-2 column, isopropanol provided the lower retentions and satisfactory resolutions between the four stereoisomers. This behavior has also been noticed for other compounds using polysaccharide-based columns [44,45], and could be due to the higher hydrogen-bond-accepting ability of isopropanol. As a general rule, methanol provided the highest enantioresolutions with all of the CSP checked, except for SUL. In this case, and for any of the columns studied, no sign of enantioseparation was observed using methanol, even at low percentages. The highest enantioresolution for SUL was obtained using isopropanol and the Lux® Cellulose-2 column. In addition, in the cases of TRN, using the Lux® Amylose-2 and Reflect™ I-cellulose C columns, and TRL, using the Lux® Amylose-2 column, isopropanol provided the highest enantioresolutions. It is important to note that, when employing the Lux® Cellulose-2 column, the four stereoisomers changed the elution order when methanol was replaced by isopropanol (see Figure 2 and Table S1 to check system suitability parameters). Using methanol, the two first eluted enantiomers were those of the threo form, which was predominant in the standard and thus provided the biggest peak.
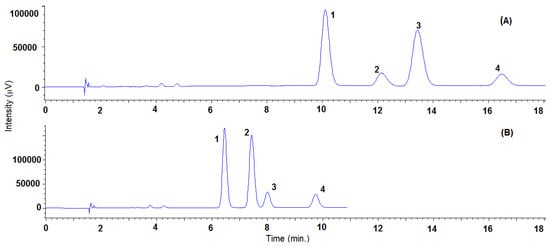
Figure 2.
Representative SFC-UV (220 nm) chromatogram obtained with Lux® Cellulose-2 column of triadimenol standard using isopropanol (A) or methanol (B) as a modifier under the same conditions in isocratic elution (8%), temperature (35 °C), pressure (150 bar) and flow rate (2 mL/min). Peaks 1: TRL-Threo-1, 2: TRL-Eritro-1; 3: TRL-Threo-2; 4: TRL-Erytro-2. System suitability parameters are summarized in Table S1.
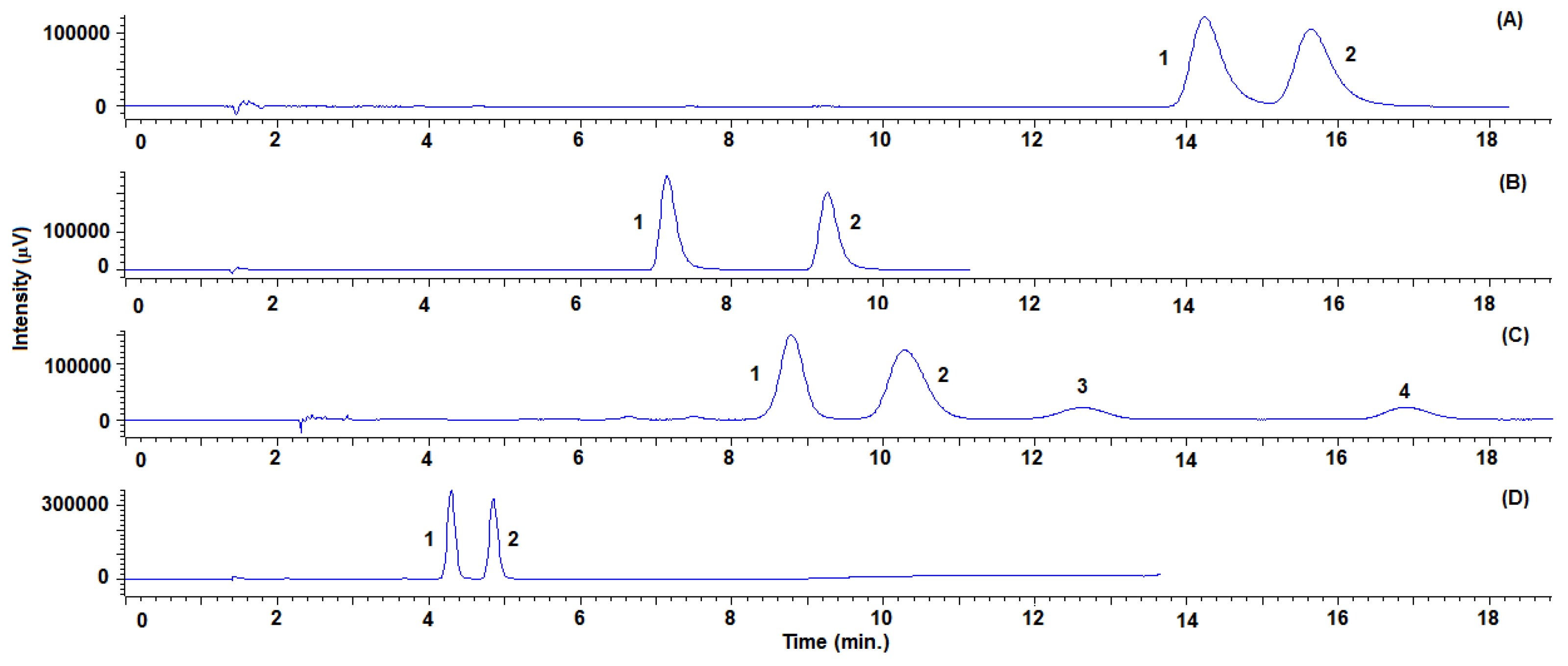
The chromatographic conditions in terms of columns and organic modifiers were different in order to obtain the best separation of the enantiomers for each azole compound (see Table 2 and Table 3). Figure 3 shows the chromatograms with the conditions selected, taking into account the better enantioresolutions, shortest analysis time and best peak shapes (see Tables S1 and S2 to check system suitability parameters). The Lux® Cellulose-2 column was chosen for sulconazole, bifonazole and triadimefon, whereas Lux® Amylose-2 was the selected column for triadimenol. Nevertheless, considering all of the compounds as a whole, and with a view to reach the simultaneous enantiomeric separation of the five pairs of enantiomers, the Lux® Cellulose-2 column and isopropanol as the organic modifier should be chosen. In this way, different gradients of the modifier were assayed and the best results in reference to the enantioresolution and analysis time were acquired with the following conditions: it was initially maintained at 5% for 5.0 min, increased to 22% at 10 min and finally increased to 45% at 11 min, which was held for 14 min. As can be seen in Figure 4, all of the stereoisomers were baseline resolved within an analysis time of 25 min (see Table S3 to check system suitability parameters).
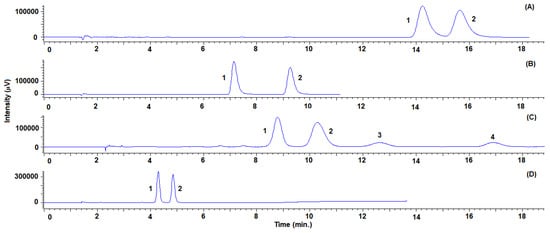
Figure 3.
Representative SFC-UV (220 nm) chromatograms for sulconazole (A), bifonazole (B), triadimefon standard solutions (D) with the Lux® Cellulose-2 column and triadimenol standard solution (C) with the Lux® Amylose-2 column. System suitability parameters are summarized in Table S2.
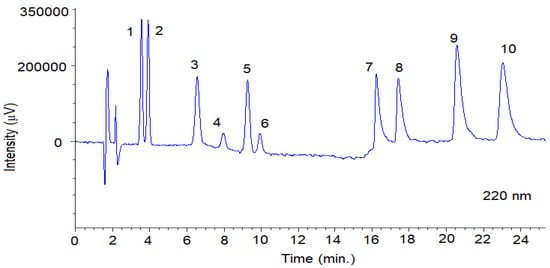
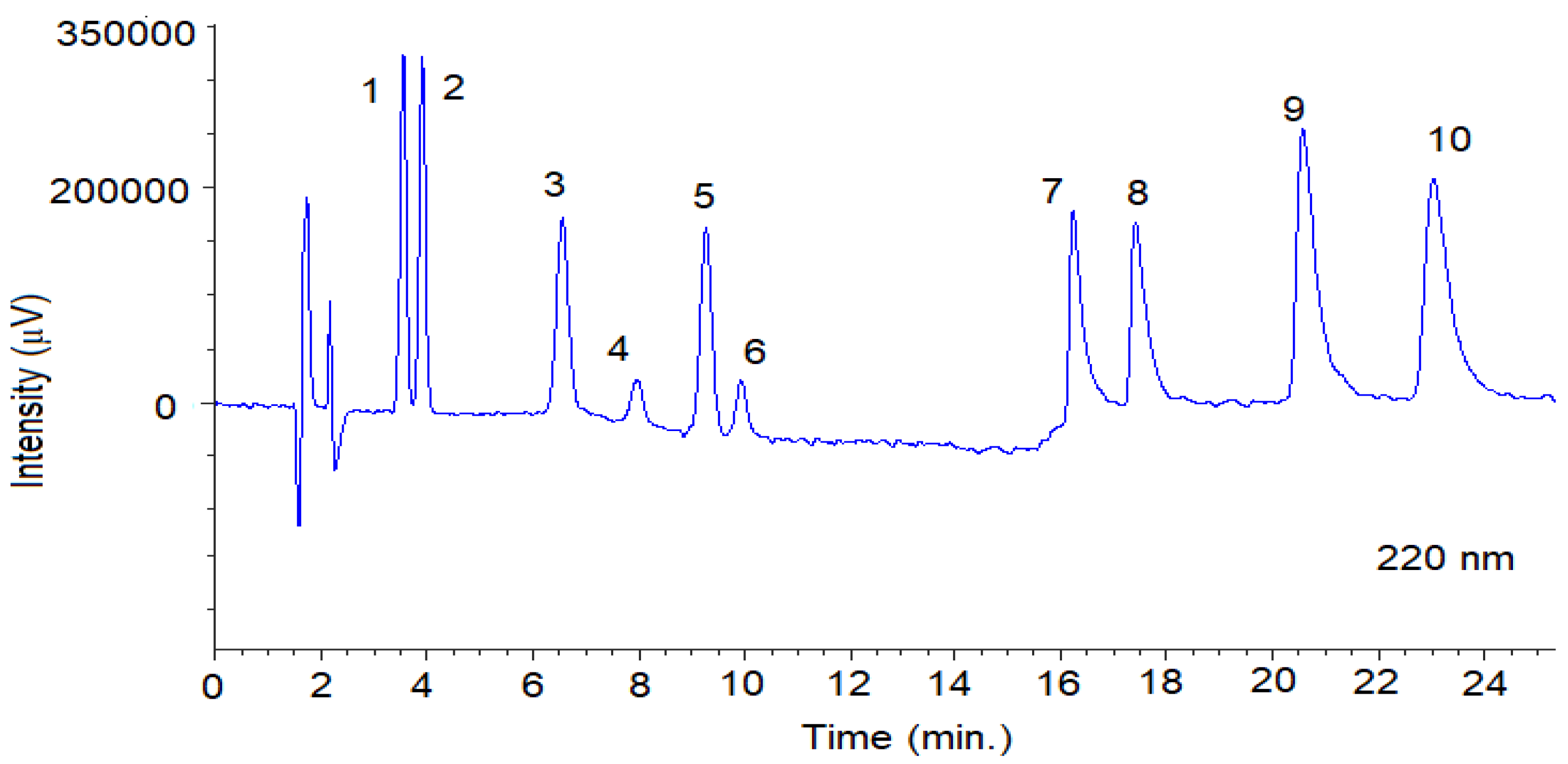
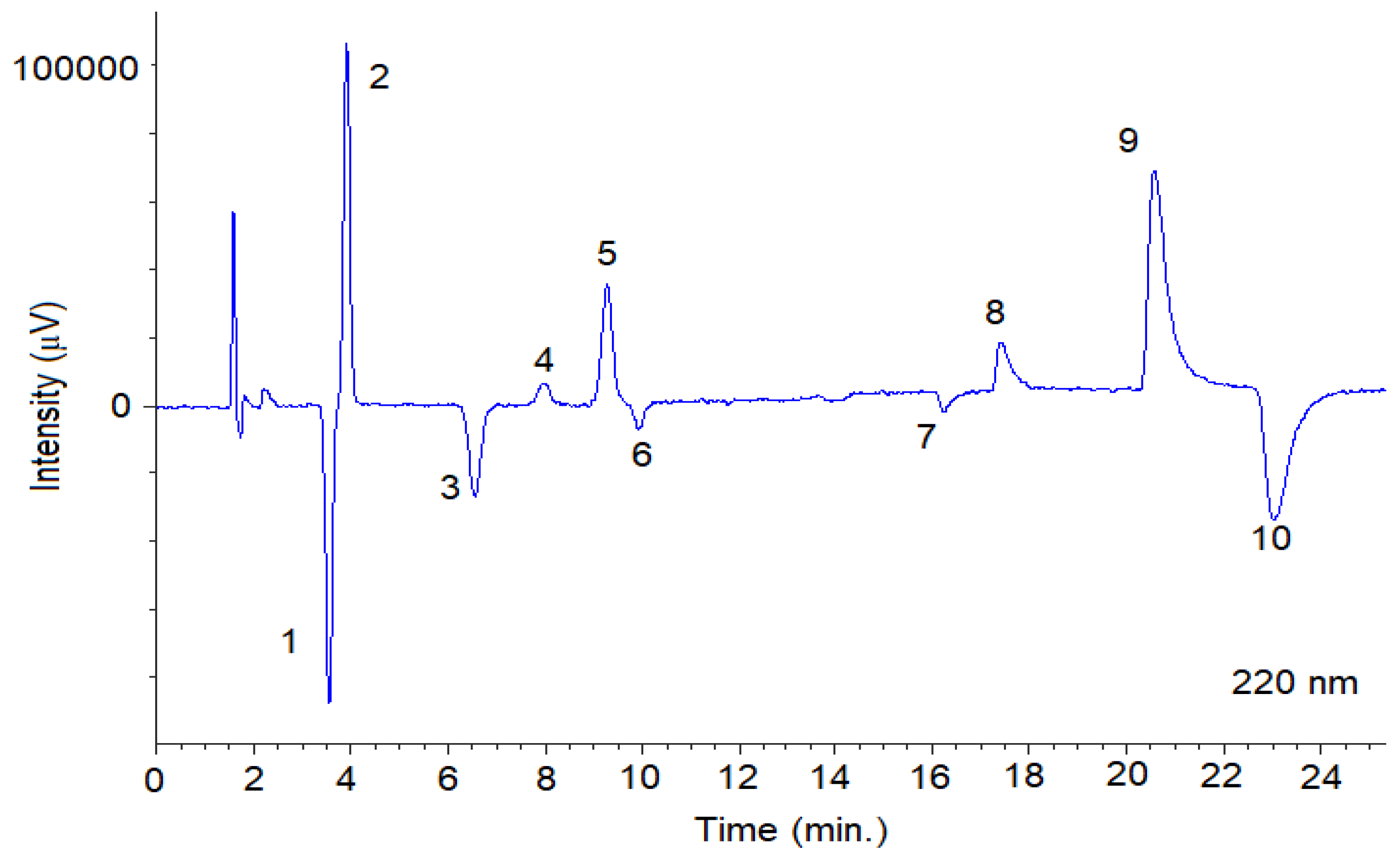
Figure 4.
Representative SFC-UV (220 nm) chromatogram obtained from a mixture of the fungicides with optimal chromatographic conditions using Lux® Cellulose-2 column. The SFC conditions are summarized in Section 2.3.1. Peaks 1: TRN-1; 2: TRN-2; 3: TRL-Threo-1, 4: TRL-Erytro-1; 5: TRL-Threo-2; 6: TRL-Erytro-2; 7: BIF-1; 8: BIF-2; 9: SUL-1; 10: SUL-2. System suitability parameters are summarized in Table S3.
3.2. Determination of the Elution Order
In order to check if there was an inversion of the enantiomer elution order caused by the change in the CSP or the type of modifier, the elution order of the enantiomers was evaluated for each compound and with the combinations of the CSP/modifier that provided baseline enantioresolutions. For this purpose, a CD detector was used and a wavelength of 220 nm was selected for the measurements. The other chromatographic conditions were 35 °C, 2 mL/min and 150 bar.
In Table 4, the results obtained are shown. Positive signs (+) refer to CD positive signals, whereas the (−) signs correspond to CD negative signals, as shown in Figure 5.

Table 4.
Variations in enantiomers elution with CD detector using different best chromatographic conditions in terms of columns and modifiers.
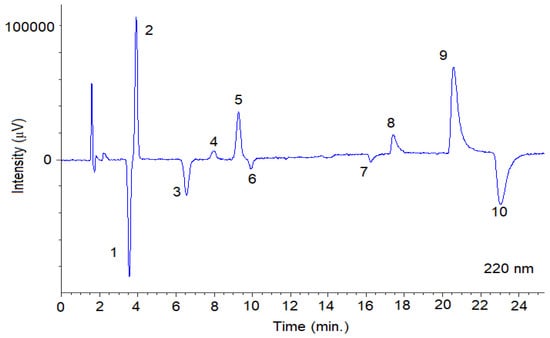
Figure 5.
Representative SFC-CD (220 nm) chromatogram obtained from a mixture of the fungicides with optimal chromatographic conditions using Lux® Cellulose-2 column. The SFC conditions are summarized in Section 2.3.1. Peaks 1: TRN-1; 2: TRN-2; 3: TRL-Threo-1; 4: TRL-Erytro-1; 5: TRL-Threo-2; 6: TRL-Erytro-2; 7: BIF-1; 8: BIF-2; 9: SUL-1; 10: SUL-2.
The elution order of SUL and TRN enantiomers did not vary when changing the CSP or the organic modifier. On the contrary, BIF enantiomers inverted the elution order when the CSP was changed. Using methanol and the Lux® Cellulose-2 column, the first eluted enantiomers had a positive CD signal, whereas, when using the Lux® Amylose-2 column, they had a negative one. This reveals a change in retention mechanisms when changing the organic modifier. The order of elution could not be studied with the Reflect I Cellulose C column since it did not provide enough of an enantioresolution.
Regarding TRL, only three combinations of column/modifier allowed for the baseline separation of the four stereoisomers. Each of them gave different results: with the Lux® Cellulose-2 column, thanks to the difference in the peak size, a change in the elution order of the stereoisomers when changing from methanol (the enantiomers of the threo form eluted the first) to isopropanol had already been observed. The CD detector allowed us to determine that only the central enantiomers, which turned out to be the positive ones, exchanged their elution order, whereas the extreme ones, the negative enantiomers, maintained their relative position. With the Lux® Amylose-2 column, using isopropanol, the first two eluted enantiomers were those of the threo form (the biggest peaks), and the last ones were those of the erythro form (the smallest peaks). This behavior was also observed using the Lux® Cellulose-2 column and methanol as the modifier. If only the information provided by the PDA detector were available, we could think that there was no inversion in the elution order of the four stereoisomers. However, the use of the CD detector allowed us to conclude that there was a reversal of the elution order within each pair of enantiomers, as can be observed in Table 4.
3.3. Effect of Temperature
Temperature is a very relevant parameter in the control of chiral separations, since it can affect both retention and selectivity, which, in turn, influence the enantioresolution [32,46]. When working with binary fluids at a constant pressure, an increase in temperature produces a decrease in density. In SFC, its effect is a controversial point: in some cases, an increase in temperature produces a decrease in retention, but in others, it leads to an increase, or even has no very significant effect on retention [32,47].
The selectivity (α) of a chromatographic separation is determined as:
where tr1 and tr2 are the retention times of two consecutive peaks, and t0 the dead time (which is defined as the time elapsed between the injection and the elution of the peak corresponding to a non-retained compound).
The selectivity can be related to the absolute temperature (T) through the Van’t Hoff equation:
where R is the ideal gas constant, and ΔΔH° and ΔΔS° are the differences between the changes in the enthalpy and entropy of the interaction of the enantiomers with the stationary phase and the mobile phase.
When ΔΔH° and ΔΔS° are independent of temperature, the plotting of the natural logarithm of selectivity (ln α) versus the inverse of temperature (1/T) provides a straight line. The Van’t Hoff equation can also be expressed as a function of the natural logarithm of the retention factor k, which is calculated as:
where tr is the retention time and t0 is the dead time.
The Van’t Hoff equation as a function of the natural logarithm of the retention factor is expressed as:
where Φ is the phase relationship, which, in this case, is equal to 1.
Thus, the retention and selectivity of the chromatographic separation are affected by two different terms: the enthalpic term, which decreases as the temperature increases, and the entropic term, which is independent of temperature.
The Van’t Hoff equation also makes it possible to determine the isoelution temperature, which is the temperature for which the selectivity (α) is equal to 1, and it is defined as the temperature at which the pair of enantiomers coelute. Once this temperature is exceeded, there would again be separation between the enantiomers, with a reversal in the elution order; that is, in selectivity. This temperature coincides with the inverse of the intersection point of the straight line with the x-axis in the representation of the natural logarithm of the selectivity versus 1/T.
In this work, the effect of temperature was evaluated using the Lux® Cellulose-2 column because it provided the best results for all of the compounds studied. The flow rate and pressure were maintained as constant at 2 mL/min and 150 bar, respectively, and the temperature was varied between 20 and 35 °C.
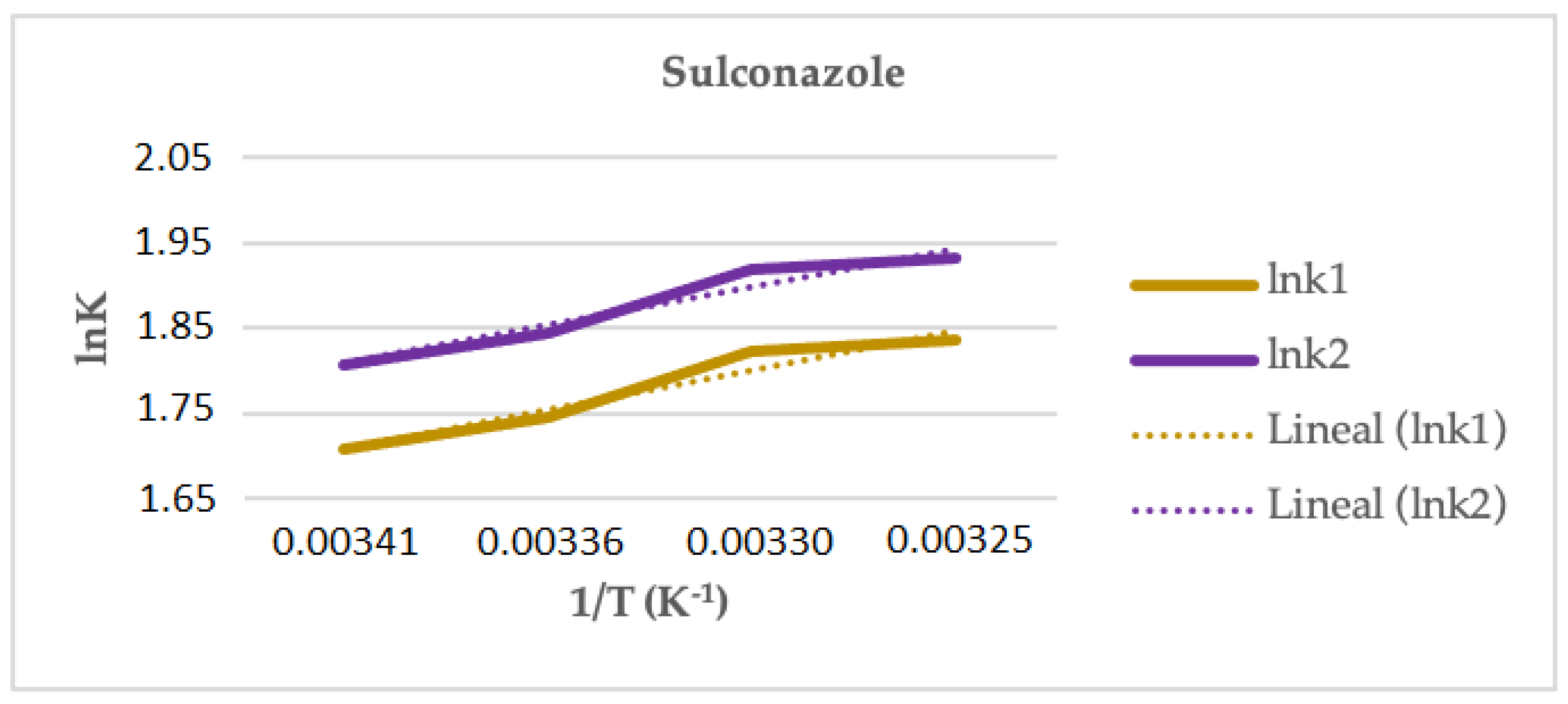
In the case of SUL, the study was performed using the two modifiers that provided enantiomeric separations (30% methanol and 30% ethanol). Linear graphs for the representation of the Van’t Hoff equations were obtained only in the case of using ethanol and plotting the ln k (see Figure 6). Using isopropanol, an increase in the retention and the resolution was observed when the temperature increased, but the Van’t Hoff graphs were nonlinear, which could be attributed to a change in the mechanism of the enantioseparation with the temperature. In Table 5, the thermodynamic parameters estimated for the enantiomeric separation of SUL, using ethanol as modifier, are shown. Since the isoelution temperature (−149.2 °C) is below the working temperature range, the enantiomeric separation is entropically driven and the selectivity increases with an increasing temperature.
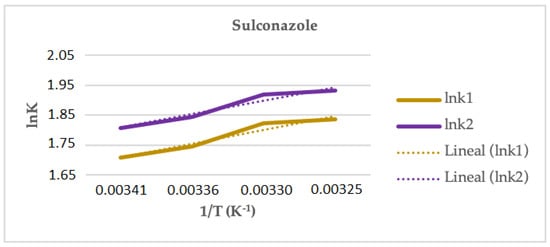
Figure 6.
Representation of ln k versus 1/T (Equation (4)) for sulconazole in Lux® Cellulose-2 column and 30% of ethanol.

Table 5.
Thermodynamic parameters and isoelution temperatures for sulconazole using a Lux® Cellulose-2 column.
The effect of temperature on the enantiomeric separation of BIF was studied using methanol, ethanol and isopropanol. The results obtained are shown in Table 6. For all of the modifiers studied, the retention decreased with an increasing temperature, but neither the selectivity factor nor the resolution showed a clear trend. The representations of ln α and ln k against the inverse of the temperature were nonlinear in any case, which can be attributed to the fact that variations in the retention mechanism are produced when the working temperature is modified.

Table 6.
Effect of temperature on the enantiomeric separation of bifonazole. Chromatographic conditions: Lux® Celulose-2 column, 150 bar and flow rate of 2 mL/min.
In Table 7, the results obtained for TRN are shown. For all of the modifiers assayed, the retention increased when the temperature increased, whereas the selectivity decreased, especially using methanol and ethanol. When the representations of ln α were obtained, there was only a linear behavior in the case of using methanol and ethanol. With isopropanol, it was the plot of ln k that provided a good linear fit. In all cases, it was possible to calculate the isoelution temperature (see Table 8), obtaining values above the working temperature range when using methanol and ethanol, which indicates that, in these cases, the enantioseparation is enthalpically driven and the selectivity decreases with an increasing temperature. The isoelution temperature obtained in the case of using isopropanol was lower than the working range, so, in this case, the separation is entropically handled, and the selectivity increases with an increasing temperature.

Table 7.
Effect of temperature on the enantiomeric separation of triadimefon. Chromatographic conditions: Lux® Celulose-2 column, 150 bar and flow rate of 2 mL/min.

Table 8.
Thermodynamic parameters and isoelution temperatures for triadimefon using a Lux® Cellulose-2 column.
In the case of TRL, the effect of temperature was investigated using methanol because it provided the highest resolutions, and the elution order of each pair of enantiomers was not mixed. The values of selectivity were calculated for each pair of enantiomers. As is shown in Table 9, the retention increased and selectivity decreased when the temperature increased. For the erytro enantiomers, the plots of ln α were linear. On the contrary, for the threo enantiomers, a linear fit was obtained for ln k. In all cases, the isoelution temperatures (see Table 10) were above the working temperatures range; thus, the separation is enthalpically driven, and the selectivity decreases with an increasing temperature, as was observed experimentally.

Table 9.
Effect of temperature on the enantiomeric separation of triadimenol. Chromatographic conditions: Lux® Celulose-2 column, 150 bar and flow rate of 2 mL/min.

Table 10.
Thermodynamic parameters and isoelution temperatures for triadimenol using a Lux® Cellulose-2 column.
4. Conclusions
Taking into account the tests carried out with the different columns and organic modifiers, the best column for the optimal separation of the enantiomers of all of the compounds studied in a single run was Lux® Cellulose-2. However, the Lux® Amylose-2 column also allowed for the enantiomeric resolution of all fungicides, but only individually. In contrast, the Reflect™ I-Cellulose C column only showed efficacy in the separation of triadimefon, while the Chiralcel® OD column did not allow any enantiomeric separation. Among the different modifiers employed, and from a general point of view, methanol provided the highest enantioresolutions, except in the case of sulconazole, for which the best results were obtained with isopropanol. Thus, considering the simultaneous enantiomeric separation of the five pairs of enantiomers, isopropanol was selected as the organic modifier and the separation was achieved within 25 min by employing an elution gradient. An inversion of the enantiomer elution order was observed for bifonazole and tradinemol, and this was associated with a change in the chiral stationary phase. In the case of BIF, a reversal of elution was caused when methanol was used as the modifier, and the CSP changed from the Lux® Cellulose-2 column to the Lux® Amylose-2 one. The same effect was observed for triadimenol employing isopropanol when these two CSPs were exchanged. It is significant that, if only the information provided by the PDA detector were available, it would have been thought that there was no inversion in the elution order of the enantiomers when the Lux® Cellulose-2 column was replaced by the Lux® Amylose-2 one. Considering the effect of the temperature on the enantiomeric separation and using the Lux® Cellulose-2 column, the results obtained showed that, in all cases, the retention increased with an increasing temperature, except for sulconazole, for which the opposite effect was observed. The isoelution temperature could be estimated for all of the compounds, except for bifonazole and sulconazole using isopropanol. The values revealed that the enantiomeric separation of the compounds was enthalpically driven, except for sulconazole using ethanol and triadimenon using isopropanol, for which an entropically driven behaviour was noticed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10010009/s1, Table S1: System suitability parameters referring to chromatograms of Figure 2; Table S2: System suitability parameters referring to chromatograms of Figure 3; Table S3: System suitability parameters referring to chromatogram of Figure 4.
Author Contributions
Conceptualization, L.T. and A.M.A.; methodology, L.T., I.M., B.M.-G. and M.T.M.; formal analysis, L.T. and A.M.A.; investigation, L.T. and I.M.; resources, L.T.; data collection and curation, L.T., B.M.-G., M.T.M., S.V. and A.M.A.; writing—original draft preparation, L.T., S.V., B.M.-G. and A.M.A.; writing—review and editing, L.T., S.V., M.T.M. and A.M.A.; visualization, L.T. and A.M.A.; supervision, L.T. and A.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets generated during the current study are contained within this article and the Supplementary Materials, or are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jørgensen, L.N.; Heick, T.M. Azole use in agriculture, horticulture, and wood preservation—Is it indispensable? Front. Cell. Infect. Microbiol. 2021, 11, 730297. [Google Scholar] [CrossRef] [PubMed]
- Safiei, M.; Peyton, L.; Hashemzadeh, M.; Foroumadi, A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorg. Chem. 2020, 104, 104240. [Google Scholar] [CrossRef] [PubMed]
- Draskau, M.K.; Svingen, T. Azole fungicide and their endocrine disrupting properties: Perspectives on sex hormone-dependent reproductive development. Front. Toxicol. 2022, 4, 883254. [Google Scholar] [CrossRef] [PubMed]
- Bicchi, C.; Cravotto, G.; D’amato, A.; Rubiolo, P.; Galli, A.; Galli, M. Cyclodextrin derivatives in gas chromatography separation of racemates with different volatility. Part XV: 6-o-t-Butyldimethylsilyl-versus 6-o-t-Heayldimethylsilyl- β and -γ Derivates. J. Microcolumn Sep. 1999, 11, 487–500. [Google Scholar] [CrossRef]
- Wu, Y.S.; Lee, H.K.; Li, S.F. Simultaneous chiral separation of triadimefon and triadimenol by sulfated beta-cyclodextrin-mediated capillary electrophoresis. Electrophoresis 2000, 21, 1611–1619. [Google Scholar] [CrossRef]
- Li, W.; Zhao, L.; Zhang, H.; Chen, X.; Chen, S.; Zhu, Z.; Hong, Z.; Chai, Y. Enantioseparation of new triadimenol antifungal active compounds by electrokinetic chromatography and molecular modeling study of chiral recognition mechanisms. Electrophoresis 2014, 35, 2855–2862. [Google Scholar] [CrossRef]
- Wei-Li, W.; Bao-Yuan, G.; Jin-Ming, L. Ultra-high concentration of amylose for chiral separations in capillary electrophoresis. J. Chromatogr. A 2009, 1216, 1484–1489. [Google Scholar] [CrossRef]
- Salido-Fortuna, S.; Marina, M.L.; Castro-Puyana, M. Enantiomeric determination of econazole and sulconazole by electrokinetic chromatography using hydroxypropyl-β-cyclodextrin combined with ionic liquids based on L-lysine and L-glutamic acid. J. Chromatogr. A 2020, 1621, 461085. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Zhu, F.; Chen, B.; Zhang, Q.; Du, S.; Li, P. Study of the enantioseparation capability of chiral dual system based on chondroitin sulfate C in CE. Electrophoresis 2015, 36, 607–614. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Crego, A.L.; Marina, M.L.; García-Ruiz, C. Enantioselective separation of azole compounds by EKC. Reversal of migration order of enantiomers with CD concentration. Electrophoresis 2007, 28, 2667–2674. [Google Scholar] [CrossRef]
- Salido-Fortuna, S.; Greño, M.; Castro-Puyana, M.; Marina, M.L. Amino acid chiral ionic liquids combined with hydroxypropyl-β-cyclodextrin for drug enantioseparation by capillary electrophoresis. J. Chromatogr. A 2019, 1607, 460375. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, H.Y.; Ali, I. Comparative study of the enantiomeric resolution of chiral antifungal drugs econazole, miconazole and sulconazole by HPLC on various cellulose chiral columns in normal phase mode. J. Pharm. Biom. Anal. 2002, 27, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, H.Y.; Ali, I. Comparison of the chiral resolution of econazole, miconazole, and sulconazole by HPLC using normal-phase amylose CSPs. Anal. Bioanal. Chem. 2001, 370, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Nelander, H.; Andersson, S.; Öhlén, K. Evaluation of the chiral recognition properties as well as the column performance of four chiral stationary phases based on cellulose (3,5-dimethylphenylcarbamate) by parallel HPLC and SFC. J. Chromatogr. A 2011, 1218, 9397–9405. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, M.; Wang, X.; Wang, X.; Xu, H.; Wang, Q.; Wang, M. HPLC-MS/MS enantioseparation of triazole fungicides using polysaccharide-based stationary phases. J. Sep. Sci. 2012, 35, 773–777. [Google Scholar] [CrossRef]
- Ma, S.; Wang, L.; Guo, G.; Tang, J.; Yu, J. The enantioseparation and determination of sulconazole enantiomers in rat plasma and tissues by a chiral HPLC-ESI-MS/MS method and its application to studies on stereoselective pharmacokinetics and tissue distribution. New J. Chem. 2022, 46, 4262–4271. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y.; Gaitonde, V.D.; Singh, P.; Rawat, M.S.M.; Sharma, B. Chiral separations of imidazole antifungal drugs on AmyCoat RP column in HPLC. Chromatographia 2009, 70, 223–227. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Ali, I. Enantiomeric resolution of some imidazole antifungal agents on chiralpak WH chiral stationary phase using HPLC. Chromatographia 2001, 54, 200–202. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, L.; Lun, J.; Zhao, M.; Guo, X. Enantiomeric separation and molecular docking study of seven imidazole antifungal drugs on a cellulose tris-(3,5-dimethylphenylcarbamate) chiral stationary phase. New J. Chem. 2020, 44, 18337–18346. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Liu, Y.; Yu, J.; Guo, X. Immobilized Cellulose-Based Chiralpak IC Chiral Stationary Phase for Enantioseparation of Eight Imidazole Antifungal Drugs in Normal-Phase, Polar Organic Phase and Reversed-Phase Conditions Using High-Performance Liquid Chromatography. Chromatographia 2019, 82, 649–660. [Google Scholar] [CrossRef]
- Bielejewska, A.; Duszczyk, K.; Zukowski, J. Effect of (+) or (−) camphorsulfonic acid additives to the mobile phase on enantioseparations of some basic drugs on a Chiralcel OD column. J. Chromatogr. A 2005, 1083, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mskhiladze, A.; Karchkhadze, M.; Dadianidze, A.; Fanali, S.; Farkas, T.; Chankvetadze, B. Enantioseparation of chiral antimycotic drugs by HPLC with polysaccharide-based chiral columns and polar organic mobile phases with emphasis on enantiomer elution order. Chromatographia 2013, 76, 1449–1458. [Google Scholar] [CrossRef]
- Podolska, M.; Bialecka, W.; Kulik, A.; Kwiatkowska-Puchniarz, B.; Mazurek, A. HPLC method for separating enantiomers of imidazole derivatives-antifungal compounds. Acta Pol. Pharm. 2017, 74, 777–784. [Google Scholar] [PubMed]
- Bi, C.; Zhao, E.; Liu, Y.; Qiu, J.; Zhou, Z. Direct optical resolution of chiral pesticides by HPLC on emamectin CSP under normal phase conditions. J. Chromatogr. Rel. 2006, 29, 1601–1607. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, S.; Liu, D.; Wang, P.; Zhou, Z. Direct enantiomeric resolutions of chiral triazole pesticides by high-performance liquid chromatography. J. Biochem. Biophys. Methods 2005, 62, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Qiu, J.; Li, L.; Li, W.; Zhou, Z.; Liu, F.; Qiu, L. Stereoselective separation and determination of triadimefon and triadimenol in wheat, straw, and soil by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2012, 35, 166–173. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Shuang, Y.; Li, L. The preparation of a new 3,5-dichlorophenylcarbamated cellulose-bonded stationary phase and its application for the enantioseparation and determination of chiral fungicides by LC-MS/MS. Talanta 2019, 202, 494–506. [Google Scholar] [CrossRef]
- Qiu, J.; Dai, S.; Zheng, C.; Yang, S.; Chai, T.; Bie, M. Enantiomeric separation of triazole fungicides with 3-μm and 5-μml particle chiral columns by reverse-phase high-performance liquid chromatography. Chirality 2011, 23, 479–486. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Lin, K.; Zhu, X.; Liu, W. Enantiomer separation of triazole fungicides by high-performance liquid chromatography. Chirality 2019, 21, 421–427. [Google Scholar]
- Li, Y.; Dong, F.; Liu, X.; Xu, J.; Li, J.; Kong, Z.; Chen, X.; Liang, X.; Zheng, Y. Simultaneous enantioselective determination of triazole fungicides in soil and water by chiral liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2012, 1224, 51–60. [Google Scholar] [CrossRef]
- Luo, M.; Liu, D.; Zhou, Z.; Wang, P. A new chiral residue analysis method for triazole fungicides in water using dispersive liquid-liquid microextraction (DLLME). Chirality 2013, 25, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Toribio, L.; del Nozal, M.J.; Bernal, J.L.; Alonso, C.; Jiménez, J.J. Enantiomeric separation of several antimycotic azole drugs using supercritical fluid chromatography. J. Chromatogr A 2007, 4, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, S.; Zeng, L. Effects of Hexane in Supercritical Fluid Chromatography for the Separation of Enantiomers. Chirality 2016, 28, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Del Nozal, M.J.; Toribio, L.; Bernal, J.L.; Castaño, N. Separation of triadimefon and triadimenol enantiomers and diastereoisomers by supercritical fluid chromatography. J. Chromatogr. A 2003, 986, 135–141. [Google Scholar] [CrossRef]
- Toribio, L.; Bernal, J.L.; Martín, M.T.; Bernal, J.; del Nozal, M.J. Effects of organic modifier and temperature on the enantiomeric separation of several azole drugs using supercritical fluid chromatography and the Chiralpak AD column. Biomed. Chromatogr. 2013, 28, 152–158. [Google Scholar] [CrossRef]
- Toribio, L.; del Nozal, M.J.; Bernal, J.L.; Alonso, C.; Jiménez, J.J. Enantiomeric resolution of bifonazole by supercritical fluid chromatography. J. Sep. Sci. 2006, 29, 1373–1378. [Google Scholar] [CrossRef]
- Mai, B.; Fan, J.; Jiang, Y.; He, R.; Lai, Y.; Zhang, W. Fast enantioselective determination of triadimefon in different matrices by supercritical fluid chromatography. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2019, 4, 1126–1127. [Google Scholar]
- Yao, Z.; Li, X.; Miao, Y.; Lin, M.; Xu, M.; Wang, Q.; Zhang, H. Simultaneous enantioselective determination of triadimefon and its metabolite triadimenol in edible vegetable oil by gel permeation chromatography and ultraperformance convergence chromatography/tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 8849–8859. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, X.; Shao, J.; Xiong, W.; Ji, Y.; Liu, S.; Tang, G.; Deng, H.; Wang, Y. A rapid method for the simultaneous stereoselective determination of the triazole fungicides in tobacco by supercritical fluid chromatography-tandem mass spectrometry combined with pass-through cleanup. J. Chromatogr. A 2021, 1642, 462040. [Google Scholar] [CrossRef]
- West, C. Recent trends in chiral supercritical fluid chromatography. TrAC-Trends Anal. Chem. 2019, 120, 115648. [Google Scholar]
- Kalikova, K.; Slechtova, T.; Vozka, J.; Tesarova, E. Supercritical fluid chromatography as a tool for enantioselective separation: A review. Anal. Chim. Acta 2014, 821, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Ji, Y.; Tang, S.; Yang, F.; Tang, G.; Shi, H.; Lee, H.K. Application of Chiral and Achiral Supercritical Fluid Chromatography in Pesticide Analysis: A Review. J. Chromatogr. A 2020, 1634, 461684. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Raja, R.; Alam, S.D.; Shirsath, V.; Jain, A.K.; Locatelli, M.; David, V. A comparison of chiral separations by supercritical fluid chromatography and high-performance liquid chromatography. J. Liq. Chromatogr. Relat. 2021, 44, 550–563. [Google Scholar] [CrossRef]
- Toribio, L.; Alonso, C.; del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J. Enantiomeric separation of chiral sulfoxides by supercritical fluid chromatography. J. Sep. Sci. 2006, 29, 1363–1372. [Google Scholar] [CrossRef]
- Ares, A.M.; Bernal, J.; Janvier, A.; Toribio, L.J. Chiral and achiral separation of ten flavanones using supercritical fluid chromatography. Application to bee pollen analysis. J. Chromatogr. A 2022, 1685, 463633. [Google Scholar]
- Phinney, K.W.; Stringham, R.W. Chiral Separations Using Supercritical Fluid Chromatography. In Chiral Separation Techniques: A Practical Approach, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 135–154. [Google Scholar]
- Dai, J.; Zhang, Y.; Wang-Iverson, D.B.; Tymiak, A. Supercritical Fluid Chromatography. In ADME-Enabling Technologies in Drug Design and Development; Zhang, D., Surapaneni, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 363–380. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).