Biscuits Polyphenol Content Fortification through Herbs and Grape Seed Flour Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biscuits
2.2. Reagents Used
2.3. Assessment of Total Polyphenols Content
2.4. Assessment of Antioxidant Capacity
2.5. Texture Assessment
2.6. Sensory Analysis
2.7. Colour Measurement
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of Antioxidant Capacity and Total Amount of Polyphenols
3.2. Texture
3.3. Sensory Analysis
3.4. Colour
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krystyjan, M.; Gumul, D.; Ziobro, R.; Korus, A. The fortification of biscuits with bee pollen and its effect on physicochemical and antioxidant properties in biscuits. LWT Food Sci. Tech. 2015, 63, 640–646. [Google Scholar] [CrossRef]
- Ramis-Ramos, G. Antioxidants Synthetic antioxidants. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, UK, 2003; pp. 265–275. [Google Scholar]
- Sati, Y.A.; Al-Dalain, A.H.; Al-Fraihat, E.T. Effect of Aromatic Plant Essential Oils on Oxidative Stability of Sunflower Oil during Heating and Storage. Pakistan. J. Nutr. 2011, 10, 864–870. [Google Scholar]
- Ibrahium, M.I.; Abd El-Ghany, M.E.; Ammar, M.S. Effect of Clove Essential Oil as Antioxidant and Antimicrobial Agent on Cake Shelf Life. World J. Dairy Food Sci. 2013, 8, 140–146. [Google Scholar]
- Sitzmann, J.; Habegger, R.; Schnitzler, W.H.; Grassmann, J. Comparative analysis of antioxidant activities of fourteen Mentha essential oils and their components. Chem. Biodiverse 2014, 11, 1978–1989. [Google Scholar] [CrossRef]
- Dhartiben, B.K.; Bhumika, K.D.; Kishor Kumar, D.A. Spices and Herbs as a Source of Natural Antioxidants for Food. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 280–288. [Google Scholar]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef]
- Abdullahi, M. Biopotency role of culinary spices and herbs and their chemical constituents in health and commonly used spices in Nigerian dishes and snacks. Afr. J. Food Sci. 2011, 5, 111–124. [Google Scholar]
- Reddy, V.; Urooj, A.; Kumar, A. Evaluation of antioxidant activity of some plant extracts and their application in biscuits. Food Chem. 2005, 90, 317–321. [Google Scholar] [CrossRef]
- Paakki, M.; Sandell, M.; Hopia, A. Visual attractiveness depends on colourfulness and colour contrasts in mixed salads. Food Qual. Prefer. 2019, 76, 81–90. [Google Scholar] [CrossRef]
- Calvo, C.; Salvador, A.; Fiszman, S.M. Influence of colour intensity on the perception of colour and sweetness in various fruit-flavoured yoghurts. Eur. Food Res. Tech. 2001, 213, 99–103. [Google Scholar] [CrossRef]
- Kadlec, K.; Kmínek, M.; Kadlec, P. Měření Barvy Potravin. Technologie Potravin-Měření a Řízení v Potravinářských a Biotechnologických Výrobách; Key Publishing: Ostrava, Czech Republic, 2015; pp. 455–461. [Google Scholar]
- Brosnan, T.; Sun, D.W. Inspection and grading of agricultural and food products by computer vision systems—A review. Comput. Electron. Agric. 2002, 36, 193–213. [Google Scholar] [CrossRef]
- Du, C.J.; Sun, D.W. Recent developments in the applications of image processing techniques for food quality evaluation. Trends Food Sci. Technol. 2004, 15, 230–249. [Google Scholar] [CrossRef]
- Pudil, F.; Schwarzová, M.; Pipek, P. Kontrola kvality analýzou obrazu. Náš Chov. 1996, 56, 16–17. [Google Scholar]
- Hrušková, M.; Švec, I.; Hofmanova, T.; Dvořáková, J. Image analysis–comparison of recipe composition effect. Proc. Eng. 2012, 42, 955–963. [Google Scholar] [CrossRef][Green Version]
- Pauly, A.B.; Pareyt, M.A.; Lambrecht, E.F.; Delcour, J.A. Flours from wheat cultivars of varying hardness produces semi-sweet biscuits with varying textural and structural properties. LWT Food Sci. Technol. 2013, 53, 452–457. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity stress enhances colour parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food. Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Use of Plantago major seed mucilage as a novel edible coating incorporated with Anethum graveolens essential oil on shelf life extension of beef in refrigerated storage. Int. J. Biol. Macromol. 2017, 94, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Pack. Shelf Life. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). General Guidance for Design of Test. Rooms; 8589:2007; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Bajaj, S.; Urooj, A.; Prabhasankar, P. Effect of incorporation of mint on texture, colour and sensory parameters of biscuits. Int. J. Food Prop. 2006, 9, 691–700. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Auty, M.; Arendt, E.K.; Gallagher, E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur. Food Res. Tech. 2010, 230, 437. [Google Scholar] [CrossRef]
- Dvorackova, E.; Snoblova, M.; Chromcova, L.; Hrdlicka, P. Effects of extraction methods on the phenolic compounds contents and antioxidant capacities of cinnamon extracts. Food Sci. Biotechnol. 2015, 24, 1201–1207. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Shahid, M.Z.; Saima, H.; Yasmin, A.; Nadeem, M.T.; Imran, M.; Afzaal, M. Antioxidant capacity of cinnamon extract for palm oil stability. Lipids Health Dis. 2018, 17, 116. [Google Scholar] [CrossRef]
- Joly, N.; Souidi, K.; Depraetere, D.; Wils, D.; Martin, P. Potato By-Products as a Source of Natural Chlorogenic Acids and Phenolic Compounds: Extraction, Characterization, and Antioxidant Capacity. Molecules 2021, 26, 177. [Google Scholar] [CrossRef]
- Antonic, B.; Dordevic, D.; Jancikova, S.; Holeckova, D.; Tremlova, B.; Kulawik, P. Effect of Grape Seed Flour on the Antioxidant Profile, Textural and Sensory Properties of Waffles. Processes 2021, 9, 131. [Google Scholar] [CrossRef]
- Rosales Soto, M.U.; Brown, K.; Ross, C.F. Antioxidant activity and consumer acceptance of grape seed flour-containing food products. Inter. J. Food Sci. Technol. 2012, 47, 592–602. [Google Scholar] [CrossRef]
- Benabdallah, A.; Rahmoune, C.; Boumendjel, M.; Aissi, O.; Messaoud, C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria (Open Access). Asian Pac. J. Trop. Biol. 2016, 6, 760–766. [Google Scholar] [CrossRef]
- Singh, R.; Shushni, M.A.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef]
- Aksoylu, Z.; Çağindi, Ö.; Köse, E. Effects of blueberry, grape seed powder and poppy seed incorporation on physicochemical and sensory properties of biscuit. J. Food Qual. 2015, 38, 164–174. [Google Scholar] [CrossRef]
- Dev, M.; Ghosh, M.; Bhattacharyya, D.K. Physico-chemical, Antimicrobial, and Organoleptic Properties of Roasted Aromatic Spice (Clove Bud) in Baked Product. Appl. Biochem. Biotechnol. 2021, 193, 1813–1835. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Lim, J.A.; Lee, J.H. Quality and Antioxidant Properties of Cookies Supplemented with Cinnamon Powder. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1457–1461. [Google Scholar] [CrossRef]
- Starowicz, M.; Lelujka, E.; Ciska, E.; Lamparski, G.; Sawicki, T.; Wronkowska, M. The Application of Lamiaceae Lindl. Promotes Aroma Compounds Formation, Sensory Properties, and Antioxidant Activity of Oat and Buckwheat-Based Cookies. Molecules 2020, 25, 5626. [Google Scholar] [CrossRef]
- Bajaj, S.; Urooj, A.; Prabhasankar, P. Antioxidative properties of mint (Mentha spicata L.) and its application in biscuits. Curr. Res. Nutr. Food Sci. J. 2016, 4, 209–216. [Google Scholar] [CrossRef]
- Ng, S.H.; Wan Rosli, W.I. Effect of Cinnamon Powder Addition on Nutritional Composition, Physical Properties and Sensory Acceptability of Butter Biscuits. Malays. J. Nutr. 2014, 20, 245–253. [Google Scholar]
- Özvural, E.B.; Vural, H. Grape seed flour is a viable ingredient to improve the nutritional profile and reduce lipid oxidation of frankfurters. Meat Sci. 2011, 88, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, S.; Ning, J.; Chen, Q.; Zhang, Z. Evaluating green tea quality based on multisensor data fusion combining hyperspectral imaging and olfactory visualization systems. J. Sci. Food Agric. 2019, 99, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Markovic, I.; Ilic, J.; Markovic, D.; Simonovic, V.; Kosanic, N. Colour measurement of food products using CIE L* a* b* and RGB colour space. J. Hyg. Eng. Des. 2013, 4, 50–53. [Google Scholar]
- Lu, H.; Zheng, H. Fractal colour: A new approach for evaluation of acrylamide contents in biscuits. Food Chem. 2012, 134, 2521–2525. [Google Scholar] [CrossRef]
- Bade, A.A.; Dale, M.P.; Jemshid, K.K. Quality assessment of biscuits using computer vision. ICTACT J. Image Video Process. 2016, 7, 1285–1289. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Ahmed, Z.S.; Hussein, A.M.S. Exploring the suitability of incorporating tiger nut flour as novel ingredient in gluten-free biscuit. Polish J. Food Nutr. Sci. 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Camire, M.E.; Chaovanalikit, A.; Dougherty, M.P.; Briggs, J. Blueberry and grape anthocyanins as breakfast cereal colourants. J. Food Sci. 2002, 67, 438–441. [Google Scholar] [CrossRef]
- Acun, S.; Gül, H. Effects of grape pomace and grape seed flours on cookie quality. Qual. Assur. Saf. Crop. 2014, 6, 81–88. [Google Scholar] [CrossRef]

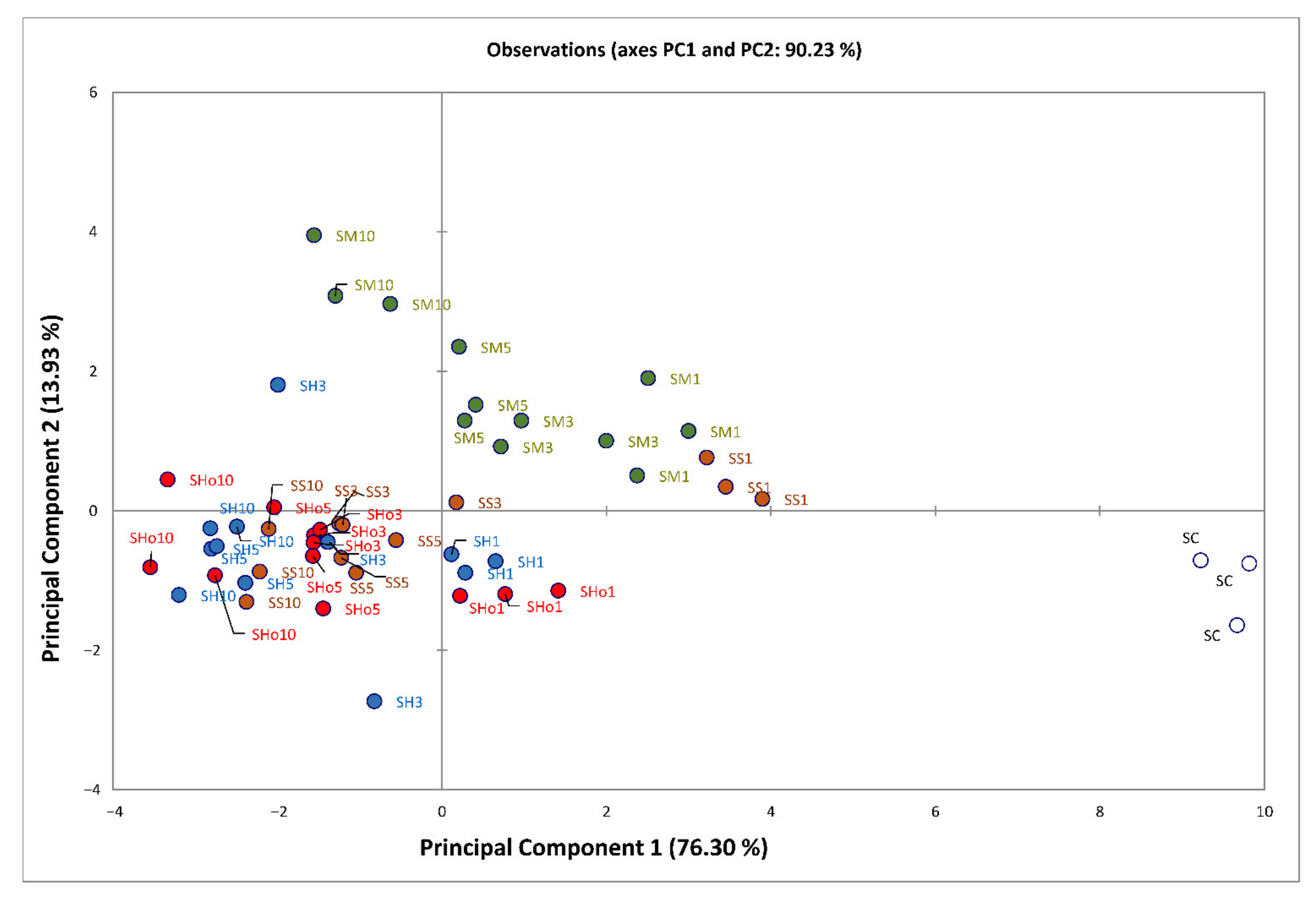
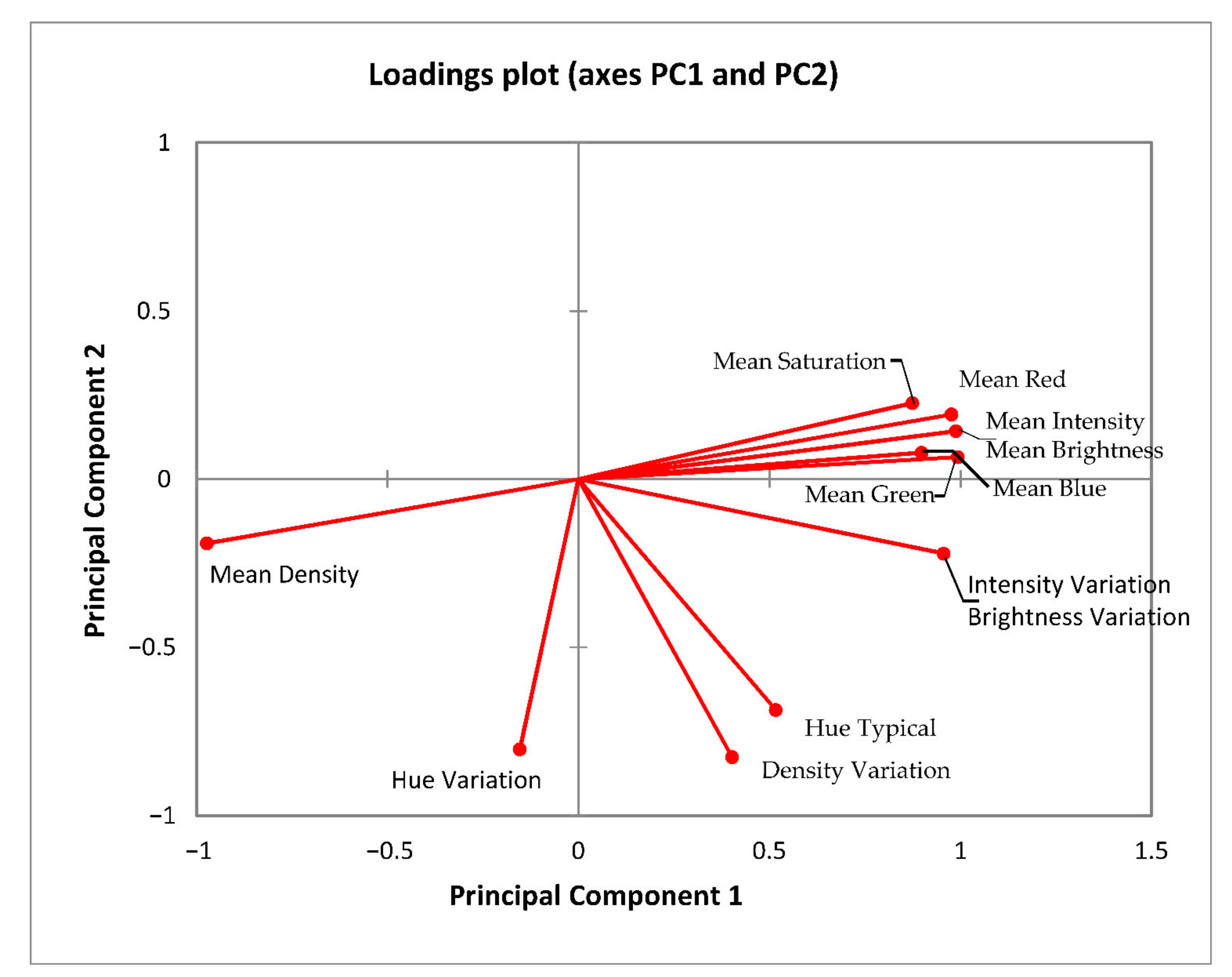
| Samples | Polyphenols (mg GAE/g) | DPPH (%) | FRAP (μmol Trolox/g) |
|---|---|---|---|
| Biscuits with the addition of grape seed flour (Vitis vinifera) | |||
| SC | 0.20 ± 0.00 a | 15.41 ± 1.16 a | 1.02 ± 0.01 a |
| SHo1 | 0.43 ± 0.00 b | 34.97 ± 1.02 b | 5.24 ± 0.05 b |
| SHo3 | 0.59 ± 0.00 c | 42.39 ± 2.35 c | 8.94 ± 0.08 c |
| SHo5 | 0.70 ± 0.00 d | 55.05 ± 5.96 d | 11.02 ± 0.11 d |
| SHo10 | 0.97 ± 0.00 e | 64.11 ± 3.60 e | 17.49 ± 0.13 e |
| Biscuits with the addition of peppermint (Mentha piperita L.) | |||
| SC | 0.20 ± 0.00 a | 15.4 ± 0.001 a | 1.02 ± 0.01 a |
| SM1 | 0.62 ± 0.00 b | 44.8 ± 0.001 b | 6.70 ± 0.05 b |
| SM3 | 1.08 ± 0.00 c | 61.5 ± 0.000 c | 10.73 ± 0.08 c |
| SM5 | 1.49 ± 0.00 d | 67.0 ± 0.004 c | 9.11 ± 0.08 d |
| SM10 | 2.34 ± 0.00 e | 84.2 ± 0.000 d | 9.59 ± 0.07 e |
| Biscuits with the addition of cloves (Eugenia caryophyllata) | |||
| SC | 0.20 ± 0.00 a | 15.4 ± 0.001 a | 1.02 ± 0.01 a |
| SH1 | 0.88 ± 0.00 b | 77.4 ± 0.003 b | 24.45 ± 0.08 b |
| SH3 | 1.60 ± 0.01 c | 90.5 ± 0.000 c | 25.08 ± 0.05 c |
| SH5 | 2.41 ± 0.05 d | 90.7 ± 0.001 c | 102.23 ± 0.65 d |
| SH10 | 4.51 ± 0.02 e | 92.6 ± 0.000 c | 208.42 ± 1.11 e |
| Biscuits with the addition of cinnamon (Cinnamomum verum) | |||
| SC | 0.20 ± 0.00 a | 15.4 ± 0.001 a | 1.02 ± 0.01 a |
| SS1 | 0.41 ± 0.00 b | 40.9 ± 0.003 b | 2.54 ± 0.01 b |
| SS3 | 0.35 ± 0.00 c | 79.7 ± 0.002 c | 3.89 ± 0.04 c |
| SS5 | 0.42 ± 0.00 d | 81.5 ± 0.005 c | 10.77 ± 0.01 d |
| SS10 | 1.10 ± 0.00 e | 90.1 ± 0.000 d | 36.61 ± 0.30 e |
| Pure herbs and spices | |||
| Peppermint (Mentha piperita L.) | 25.02 ± 0.01 | 20.57 ± 0.000 | 240.00 ± 0.38 |
| Cinnamon (Cinnamomum verum) | 101.59 ± 0.04 | 71.48 ± 0.001 | 1322.57 ± 18.08 |
| Cloves (Eugenia caryophyllata) caryophyllata) | 219.09 ± 0.01 | 85.18 ± 0.002 | 3316.52 ± 14.22 |
| Grape seed flour | 5.75 ± 0.02 | 65.24 ± 0.61 | 225.23 ± 1.89 |
| Sample | Hardness (g) | Fracturability (g) |
|---|---|---|
| Biscuits with the addition of grape seed flour (Vitis vinifera) | ||
| SC | 10,068.8 ± 1098.25 a | 7737.46 ± 809.73 a |
| SHo1 | 2085.52 ± 737.36 b | 3924.94 ± 220.71 b |
| SHo3 | 1806.74 ± 446.09 b | 3798.7 ± 425.94 b |
| SHo5 | 2934.78 ± 584.75 b | 2689.79 ± 399.6 b |
| SHo10 | 2142.81 ± 197.44 b | 3458.87 ± 980.54 b |
| Biscuits with the addition of peppermint (Mentha piperita L.) | ||
| SC | 10,068.8 ± 1098.25 a | 7737.46 ± 809.73 a |
| SM1 | 3112.33 ± 1295.61 b | 4524.03 ± 288.96 b |
| SM3 | 4637.05 ± 880.37 b | 4468.19 ± 1382.39 b |
| SM5 | 3322.03 ± 1506.43 b | 4160.94 ± 882.68 b |
| SM10 | 9504.44 ± 1460.19 a | 7803.87 ± 828.18 a |
| Biscuits with the addition of cloves (Eugenia caryophyllata) | ||
| SC | 10,068.8 ± 1098.25 a | 7737.46 ± 809.73 a |
| SH1 | 3401.11 ± 1454.35 b | 3941.56 ± 667.56 b |
| SH3 | 1195.27 ± 550.54 b | 1794.38 ± 691.11 b |
| SH5 | 2153.17 ± 462.01 b | 2555.33 ± 516.5 b |
| SH10 | 2279.71 ± 1339.79 b | 2569.36 ± 1158.18 b |
| Biscuits with the addition of cinnamon (Cinnamomum verum) | ||
| SC | 10,068.8 ± 1098.25 a | 7737.46 ± 809.73 a |
| SS1 | 4917.92 ± 129.69 b | 4880.96 ± 992.69 b |
| SS3 | 2014 ± 412.89 b | 4312.43 ± 872.71 b |
| SS5 | 3004.65 ± 1139.35 b | 4179.81 ± 406.29 b |
| SS10 | 4045.75 ± 1738.87 b | 5747.69 ± 352.3 b |
| Sample | Colour | Consistency | Smell | Taste | Sweetness | Overall Impression | Price (Kč) |
|---|---|---|---|---|---|---|---|
| Biscuits with the addition of grape seed flour (Vitis vinifera) | |||||||
| SC | 17.57 ± 12.66 a | 72.14 ± 16.89 a | 29.14 ± 13.86 a | 37.00 ± 17.57 | 35.43 ± 14.52 | 39.57 ± 22.26 | 7.86 ± 11.07 |
| SHo1 | 57.29 ± 9.72 c | 47.71 ± 7.93 c | 40.00 ± 11.37 | 29.14 ± 15.03 | 40.43 ± 13.83 | 34.57 ± 17.06 | 9.14 ± 10.21 |
| SHo3 | 68.14 ± 9.42 b,c | 45.29 ± 2.93 b,c | 42.14 ± 9.92 | 39.86 ± 13.75 | 43.29 ± 13.76 | 55.43 ± 14.12 | 18.71 ± 10.00 |
| SHo5 | 76.00 ± 11.17 b | 44.86 ± 7.43 b,c | 44.14 ± 16.36 b | 38.00 ± 19.58 | 42.43 ± 14.00 | 39.29 ± 22.48 | 11.00 ± 10.66 |
| SHo10 | 73.57 ± 13.81 b | 48.43 ± 6.88 b | 47.57 ± 10.77 b | 24.00 ± 6.98 | 36.14 ± 14.40 | 32.71 ± 19.66 | 6.43 ± 7.48 |
| Biscuits with the addition of peppermint (Mentha piperita L.) | |||||||
| SC | 17.57 ± 12.66 a | 72.14 ± 16.89 a | 29.14 ± 13.86 a | 37 ± 17.57 | 35.43 ± 14.52 | 39.57 ± 22.26 a | 7.86 ± 11.07 a |
| SM1 | 49.5 ± 13.37 c | 45.67 ± 4.33 b | 54.92 ± 13.87 b | 44.17 ± 20.1 | 45.92 ± 11.59 | 48.25 ± 14.58 a | 21.42 ± 13.45 b,c |
| SM3 | 56.08 ± 11.58 b,c | 50.67 ± 6.8 b | 55.67 ± 13.87 b | 40.25 ± 21.96 | 40.33 ± 18.72 | 37.75 ± 19.48 a | 12.58 ± 9.95 b |
| SM5 | 62.75 ± 9.5 b | 49.92 ± 8.5 b | 55.08 ± 18.99 b | 37.33 ± 23.37 | 32 ± 16.28 | 34.33 ± 18.46 a | 11.17 ± 9.34 b |
| SM10 | 73.17 ± 7.28 b | 57.25 ± 13.34 b | 69.5 ± 21.6 b | 29.92 ± 29.96 | 26.58 ± 13.27 | 18.58 ± 12.06 b | 3.13 ± 5.33 c |
| Biscuits with the addition of cloves (Eugenia caryophyllata) | |||||||
| SC | 17.57 ± 12.66 a | 72.14 ± 16.89 a | 29.14 ± 13.86 a | 37 ± 17.57 | 35.43 ± 14.52 a | 39.57 ± 22.26 a | 7.86 ± 11.07 a,b |
| SH1 | 56.42 ± 6.6 b | 43.08 ± 9.89 b | 55.75 ± 16.11 b | 49.33 ± 16.52 | 42.25 ± 16.71 a | 49.25 ± 14.7 a | 20.42 ± 17.08 a |
| SH3 | 65.92 ± 10.89 b,c | 44.5 ± 11.07 b | 54.58 ± 15.16 b | 38.75 ± 22.62 | 35.25 ± 17.39 a | 38.17 ± 13.89 a,b | 10.42 ± 11.2 a,b |
| SH5 | 70.83 ± 9.67 c,d | 50.9 ± 12.75 b | 56.75 ± 19.92 b | 36 ± 25.73 | 28.42 ± 12.62 a,b | 28.17 ± 9.93 b,c | 4.96 ± 6.72 a,b |
| SH10 | 77.83 ± 12.93 d | 55.75 ± 12.84 a,b | 55.75 ± 12.84 b | 31.17 ± 35.91 | 15.75 ± 12.56 b | 15.50 ± 10.97 c | 4.04 ± 6.99 b |
| Biscuits with the addition of cinnamon (Cinnamomum verum) | |||||||
| SC | 17.57 ± 12.66 a | 72.14 ± 16.89 a | 29.14 ± 13.86 a | 37 ± 17.57 a | 35.43 ± 14.52 a | 39.57 ± 22.26 a | 7.86 ± 11.07 a |
| SS1 | 40.14 ± 18.84 c | 55.57 ± 15.53 b | 48.71 ± 8.22 b | 60.14 ± 19.76 b | 56.14 ± 15.2 c | 58.86 ± 15.98 b | 26.57 ± 19.27 c |
| SS3 | 68.86 ± 10.96 b,c | 47.00 ± 9.85 b | 48 ± 9.54 b | 65.57 ± 20.65 b | 52.43 ± 8.02 b,c | 63.43 ± 15.98 b | 30.29 ± 19.29 c |
| SS5 | 62.00 ± 14.28 b,c | 58.00 ± 15.31 b | 50.14 ± 13.95 b | 61.29 ± 24.03 b | 48.57 ± 11.77 b,c | 51.86 ± 23.13 b | 27 ± 18.65 c |
| SS10 | 76.71 ± 10.58 b,c | 47.57 ± 14.68 b | 54.43 ± 16.35 b | 38.86 ± 16.53 b | 34.86 ± 11.23 b | 39.43 ± 15.97 a | 12.29 ± 12.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Král, O.; Javůrková, Z.; Dordevic, D.; Pospiech, M.; Jančíková, S.; Fursova, K.; Tremlová, B. Biscuits Polyphenol Content Fortification through Herbs and Grape Seed Flour Addition. Processes 2021, 9, 1455. https://doi.org/10.3390/pr9081455
Král O, Javůrková Z, Dordevic D, Pospiech M, Jančíková S, Fursova K, Tremlová B. Biscuits Polyphenol Content Fortification through Herbs and Grape Seed Flour Addition. Processes. 2021; 9(8):1455. https://doi.org/10.3390/pr9081455
Chicago/Turabian StyleKrál, Ondřej, Zdeňka Javůrková, Dani Dordevic, Matej Pospiech, Simona Jančíková, Kseniia Fursova, and Bohuslava Tremlová. 2021. "Biscuits Polyphenol Content Fortification through Herbs and Grape Seed Flour Addition" Processes 9, no. 8: 1455. https://doi.org/10.3390/pr9081455
APA StyleKrál, O., Javůrková, Z., Dordevic, D., Pospiech, M., Jančíková, S., Fursova, K., & Tremlová, B. (2021). Biscuits Polyphenol Content Fortification through Herbs and Grape Seed Flour Addition. Processes, 9(8), 1455. https://doi.org/10.3390/pr9081455








