Impact of Particle Size on the Rheological Properties and Amylolysis Kinetics of Ungelatinized Cassava Flour Suspensions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Devices
2.2. Substrate and Enzyme
2.3. Total Starch, Reducing Sugar and Hydrolysis Yield
2.4. Solvent Retention Capacity of Cassava Flour (SRC)
2.5. Ex-Situ Rheometry
2.6. In-Situ Rheometry
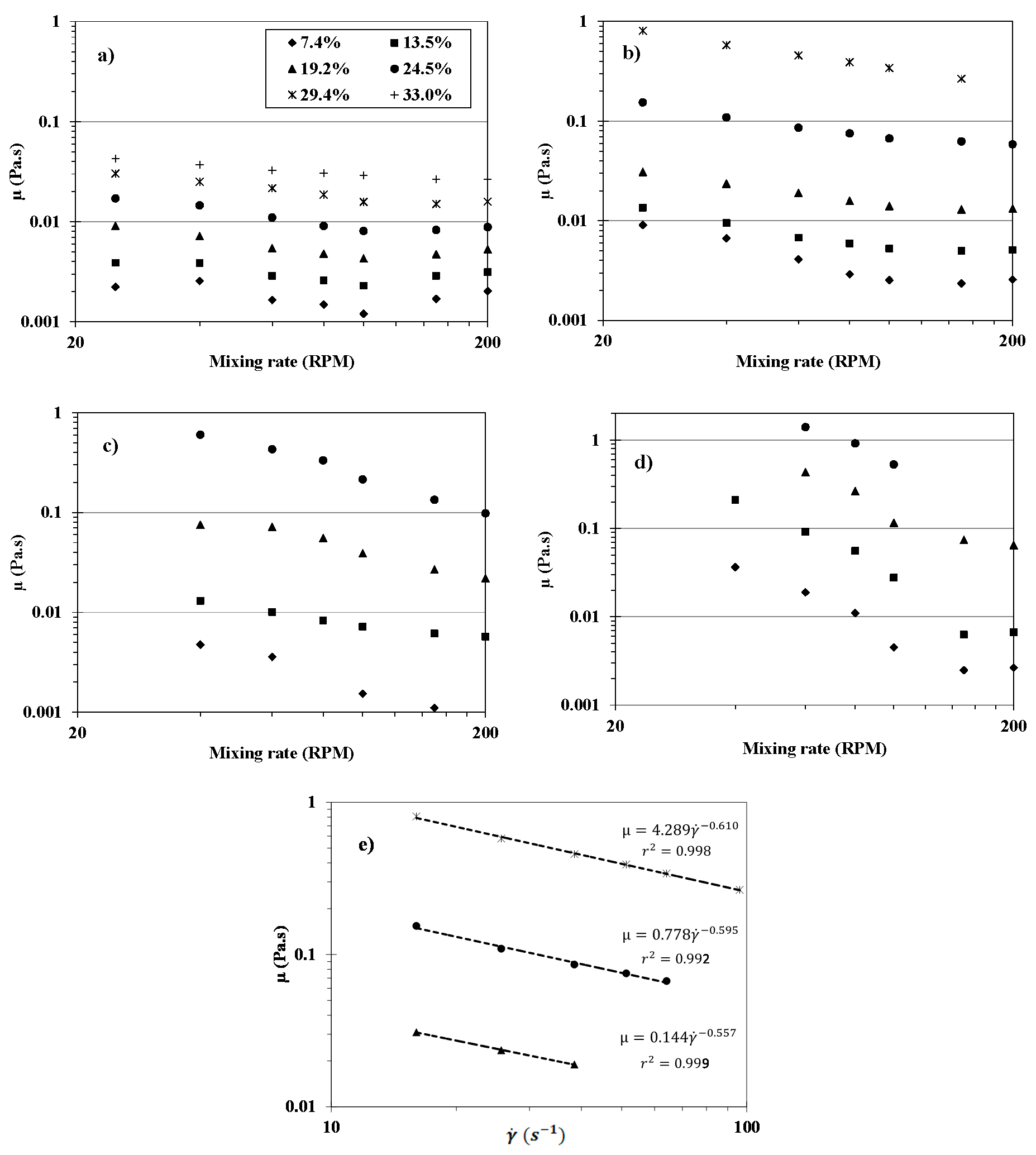
2.7. Viscosity Measurement of Initial Suspensions
2.8. Enzymatic Hydrolysis
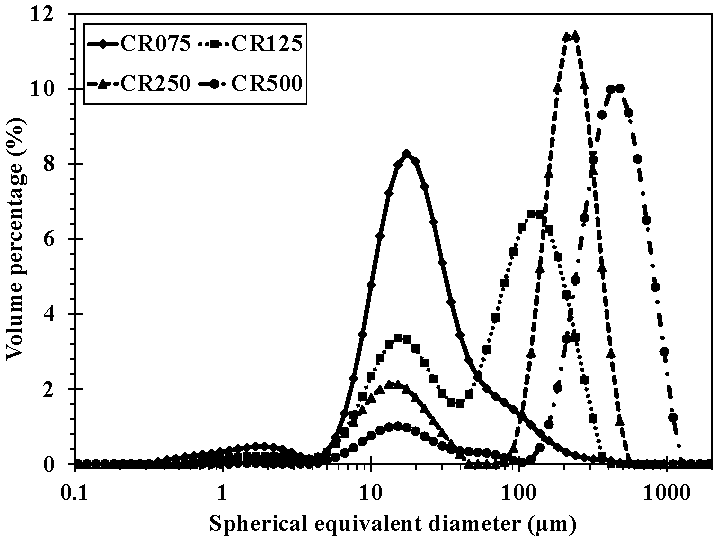
2.9. Particle Size Distribution
3. Results and Discussions
3.1. Cassava Flour Characteristics
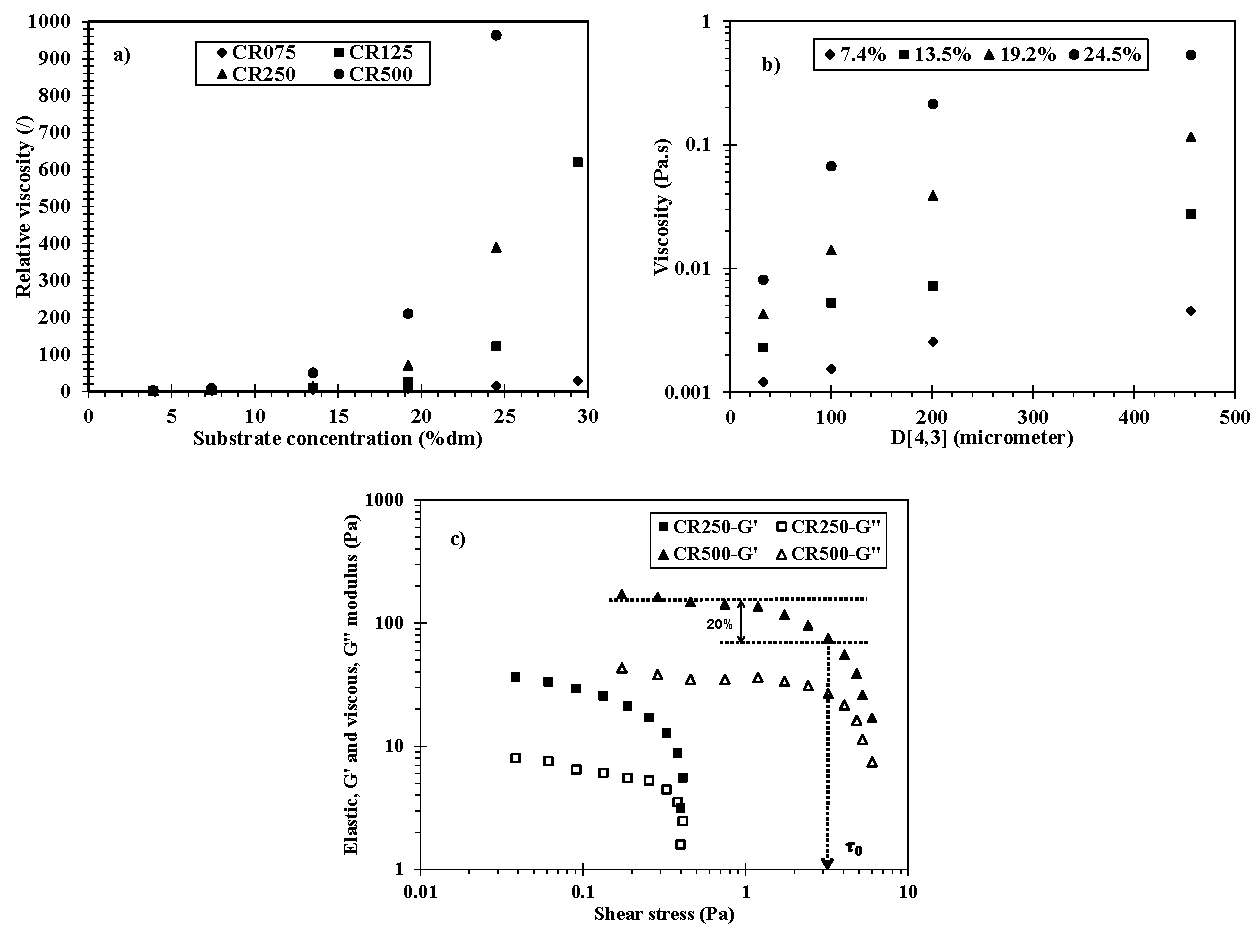
3.2. Rheology of Initial Flour Suspensions
3.3. Bioconversion Rate
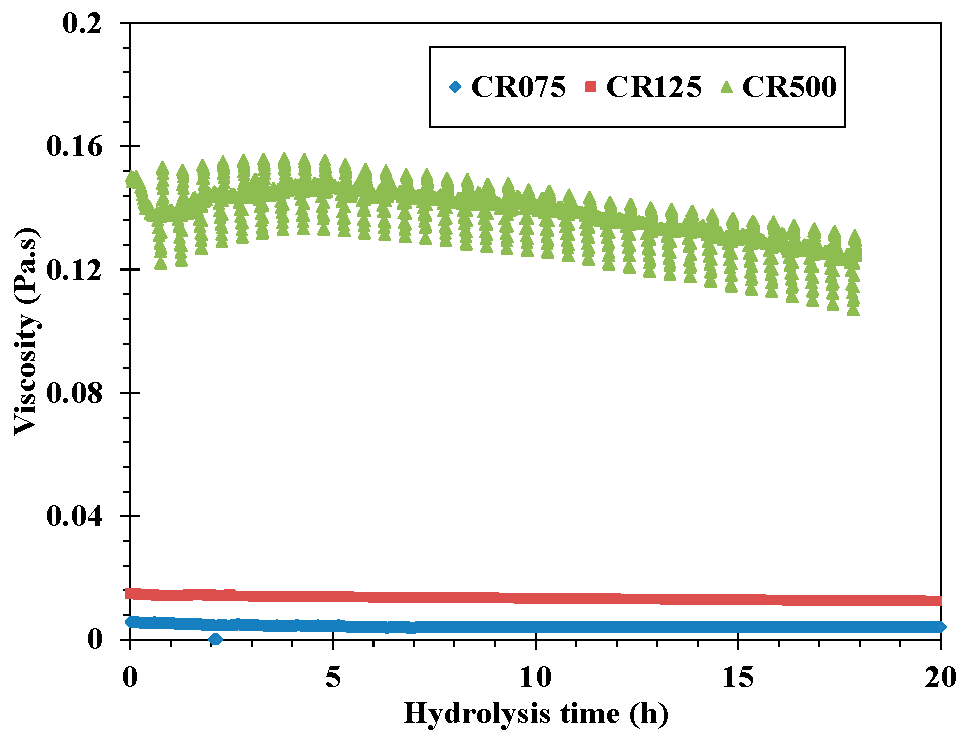
3.4. Rheological Behavior during Hydrolysis
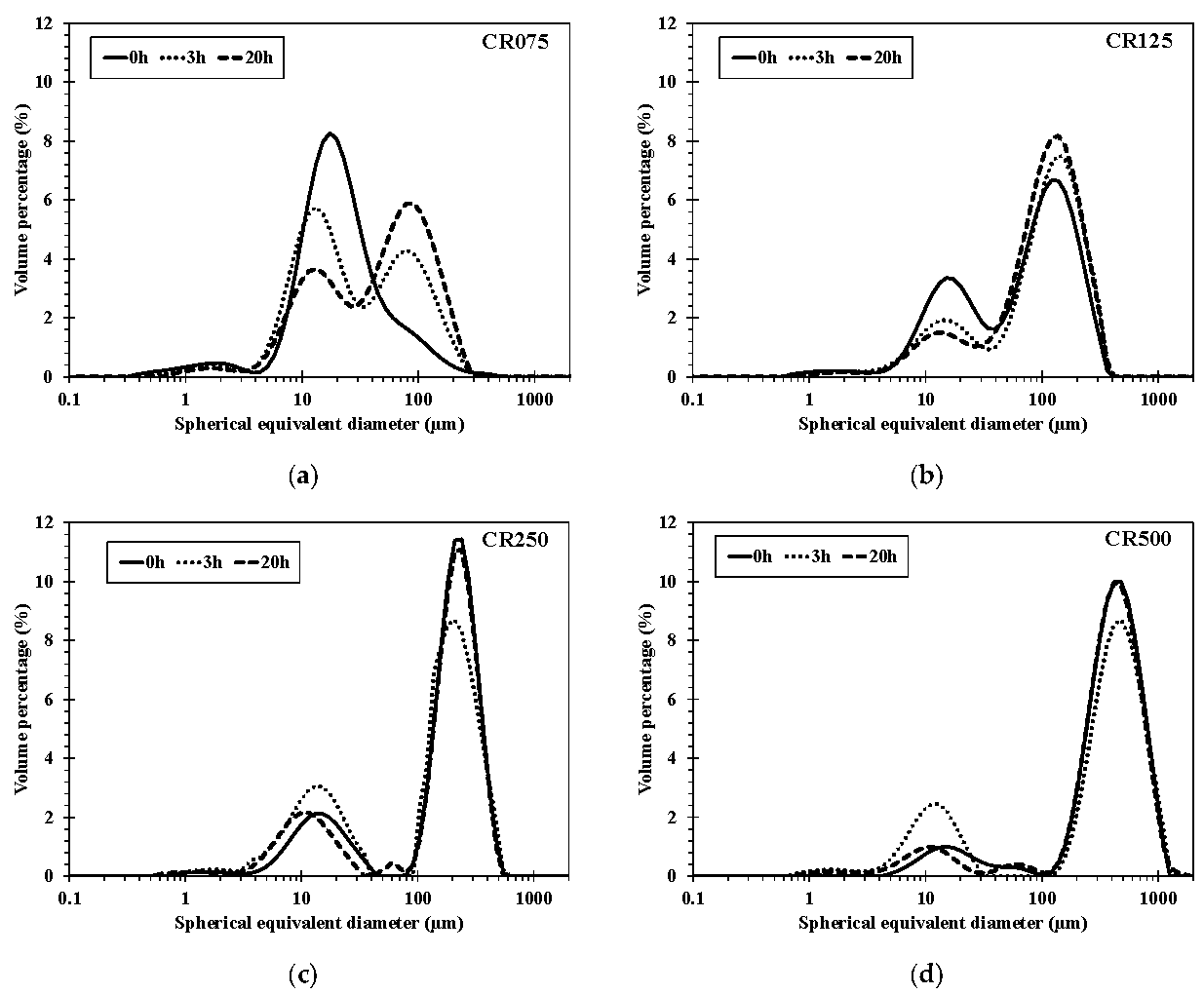
3.5. Particle Size Evolution during Hydrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| C | Torque (mixing system) | (N.m) |
| dm | Dry matter content | (%) |
| G′ | Elastic modulus | (Pa) |
| G″ | Viscous modulus | (Pa) |
| K | Consistency index | (Pa·sn) |
| Kp | Power constant | (/) |
| Ks | Metzner–Otto constant | (/) |
| ms | Quantity of substrate | (g humid matter) |
| n | Power-law index | (/) |
| N | Rotation speed | (round per second) |
| Np | Power number | (/) |
| P | Power consumption | (W) |
| Re | Reynolds number | (/) |
| Shear rate | (s−1) | |
| ρ | Density | (kg·m−3) |
| µ | Viscosity | (Pa·s) |
| τ | Shear stress | (Pa) |
| τ0 | Yield stress | (Pa) |
References
- Robertson, G.H.; Wong, D.W.S.; Lee, C.C.; Wagschal, K.; Smith, M.R.; Orts, W.J. Native or Raw Starch Digestion: A Key Step in Energy Efficient Biorefining of Grain. J. Agric. Food Chem. 2006, 54, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Galvez, A. Analyzing cold enzyme starch hydrolysis technology in new ethanol plant design. Ethanol Prod. Mag. 2005, 11, 58–60. [Google Scholar]
- Blazek, J.; Gilbert, E.P. Effect of Enzymatic Hydrolysis on Native Starch Granule Structure. Biomacromolecules 2010, 11, 3275–3289. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, B. Hydrolysis of granular corn starch with controlled pore size. J. Cereal Sci. 2012, 56, 316–320. [Google Scholar] [CrossRef]
- Li, J.; Vasanthan, T.; Bressler, D.C. Improved cold starch hydrolysis with urea addition and heat treatment at subgelatinization temperature. Carbohydr. Polym. 2012, 87, 1649–1656. [Google Scholar] [CrossRef]
- Li, Z.; Cai, L.; Gu, Z.; Shi, Y.-C. Effects of Granule Swelling on Starch Saccharification by Granular Starch Hydrolyzing Enzyme. J. Agric. Food Chem. 2014, 62, 8114–8119. [Google Scholar] [CrossRef]
- Shariffa, Y.N.; Karim, A.A.; Fazilah, A.; Zaidul, I.S.M. Enzymatic hydrolysis of granular native and mildly heat-treated tapioca and sweet potato starches at sub-gelatinization temperature. Food Hydrocoll. 2009, 23, 434–440. [Google Scholar] [CrossRef]
- Textor, S.D.; Hill, G.A.; Macdonald, D.G.; Denis, E.S. Cold enzyme hydrolysis of wheat starch granules. Can. J. Chem. Eng. 1998, 76, 87–93. [Google Scholar] [CrossRef]
- Uthumporn, U.; Zaidul, I.S.M.; Karim, A.A. Hydrolysis of granular starch at sub-gelatinization temperature using a mixture of amylolytic enzymes. Food Bioprod. Process. 2010, 88, 47–54. [Google Scholar] [CrossRef]
- Cinelli, B.A.; Castilho, L.R.; Freire, D.M.G.; Castro, A.M. A brief review on the emerging technology of ethanol production by cold hydrolysis of raw starch. Fuel 2015, 150, 721–729. [Google Scholar] [CrossRef]
- Srichuwong, S.; Fujiwara, M.; Wang, X.H.; Seyama, T.; Shiroma, R.; Arakane, M.; Mukojima, N.; Tokuyasu, K. Simultaneous saccharification and fermentation (SSF) of very high gravity (VHG) potato mash for the production of ethanol. Biomass Bioenerg. 2009, 33, 890–898. [Google Scholar] [CrossRef]
- Poonsrisawat, A.; Wanlapatit, S.; Paemanee, A.; Eurwilaichitr, L.; Piyachomkwan, K.; Champreda, V. Viscosity reduction of cassava for very high gravity ethanol fermentation using cell wall degrading enzymes from Aspergillus aculeatus. Process Biochem. 2014, 49, 1950–1957. [Google Scholar] [CrossRef]
- Poonsrisawat, A.; Wanlapatit, S.; Wansuksri, R.; Piyachomkwan, K.; Paemanee, A.; Gamonpilas, C.; Eurwilaichitr, L.; Champreda, V. Synergistic effects of cell wall degrading enzymes on rheology of cassava root mash. Process Biochem. 2016, 51, 2104–2111. [Google Scholar] [CrossRef]
- Zhang, L.A.; Chen, Q.A.; Jin, Y.L.; Xue, H.L.; Guan, J.F.; Wang, Z.Y.; Zhao, H. Energy-saving direct ethanol production from viscosity reduction mash of sweet potato at very high gravity (VHG). Fuel Process. Technol. 2010, 91, 1845–1850. [Google Scholar] [CrossRef]
- Wiman, M.; Palmqvist, B.; Tornberg, E.; Liden, G. Rheological characterization of dilute acid pretreated softwood. Biotechnol. Bioeng. 2011, 108, 1031–1041. [Google Scholar] [CrossRef]
- Charoenkul, N.; Uttapap, D.; Pathipanawat, W.; Takeda, Y. Physicochemical characteristics of starches and flours from cassava varieties having different cooked root textures. LWT Food Sci. Technol. 2011, 44, 1774–1781. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Le, T.M.; Chu-Ky, S. Pilot scale simultaneous saccharification and fermentation at very high gravity of cassava flour for ethanol production. Ind. Crop. Prod. 2014, 56, 160–165. [Google Scholar] [CrossRef]
- Le, T.; Anne-Archard, D.; Coma, V.; Cameleyre, X.; Lombard, E.; To, K.A.; Pham, T.A.; Nguyen, T.C.; Fillaudeau, L. Using in-situ viscosimetry and morphogranulometry to explore hydrolysis mechanisms of filter paper and pretreated sugarcane bagasse under semi-dilute suspensions. Biochem. Eng. J. 2017, 127, 9–20. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Anne-Archard, D.; Cameleyre, X.; Lombard, E.; To, K.A.; Fillaudeau, L. Bio-catalytic hydrolysis of paper pulp using in- and ex-situ multi-physical approaches: Focus on semidilute conditions to progress towards concentrated suspensions. Biomass Bioenergy 2019, 122, 28–36. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Anne-Archard, D.; Coma, V.; Cameleyre, X.; Lombard, E.; Binet, C.; Nouhen, A.; To, K.A.; Fillaudeau, L. In situ rheometry of concentrated cellulose fibre suspensions and relationships with enzymatic hydrolysis. Bioresour. Technol. 2013, 133, 563–572. [Google Scholar] [CrossRef]
- Deshors, M.; Guais, O.; Neugnot-Roux, V.; Cameleyre, X.; Fillaudeau, L.; Francois, J.M. Combined in situ Physical and ex-situ Biochemical Approaches to Investigate in vitro Deconstruction of Destarched Wheat Bran by Enzymes Cocktail Used in Animal Nutrition. Front. Bioeng. Biotechnol. 2019, 7, 158. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Eduardo, M.; Svanberg, U.; Oliveira, J.; Ahrne, L. Effect of Cassava Flour Characteristics on Properties of Cassava-Wheat-Maize Composite Bread Types. Int. J. Food Sci. 2013, 2013, 305407. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Boger, D.V. Measuring the flow properties of yield stress fluids. Annu. Rev. Fluid Mech. 1992, 24, 47–88. [Google Scholar] [CrossRef]
- Dinkgreve, M.; Paredes, J.; Denn, M.M.; Bonn, D. On different ways of measuring “the” yield stress. J. Non-Newton. Fluid Mech. 2016, 238, 233–241. [Google Scholar] [CrossRef]
- Sun, A.; Gunasekaran, S. Yield Stress in Foods: Measurements and Applications. Int. J. Food Prop. 2009, 12, 70–101. [Google Scholar] [CrossRef]
- Metzner, A.B.; Otto, R.E. Agitation of non-Newtonian fluids. Aiche J. 1957, 3, 3–10. [Google Scholar] [CrossRef]
- Becker, A.; Hill, S.E.; Mitchell, J.R. Milling—A Further Parameter Affecting the Rapid Visco Analyser (RVA) Profile. Cereal Chem. 2001, 78, 166–172. [Google Scholar] [CrossRef]
- White, G.A.; Doucet, F.J.; Hill, S.E.; Wiseman, J. Physicochemical properties and nutritional quality of raw cereals for newly weaned piglets. Anim. Int. J. Anim. Biosci. 2008, 2, 867–878. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Arfat, Y.A. Functional, rheological, microstructural and antioxidant properties of quinoa flour in dispersions as influenced by particle size. Food Res. Int. 2018, 116, 302–311. [Google Scholar] [CrossRef]
- Berski, W.; Krystyjan, M.; Zięć, G.; Litwinek, D.; Gambuś, H. The influence of starch hydrolyzate on physicochemical properties of pastes and gels prepared from oat flour and starch. J. Cereal Sci. 2016, 70, 16–22. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bhat, K.K.; Raghuveer, K.G. Rheology of Bengal gram (Cicer arietinum) flour suspensions. J. Food Eng. 1992, 17, 83–96. [Google Scholar] [CrossRef]
- Quemada, D. Modélisation Rhéologique Structurelle. Dispersions Concentrées et Fluides Complexes; Tec & Doc Lavoisier: Paris, France, 2006. [Google Scholar]
- Román, L.; Reguilón, M.P.; Gómez, M. Physicochemical characteristics of sauce model systems: Influence of particle size and extruded flour source. J. Food Eng. 2018, 219, 93–100. [Google Scholar] [CrossRef]
- Prakash, M.; Haridasa Rao, P.; Susheelamma, N.S.; Prabhakar, J.V. Rheological characteristics of native and steamed wheat flour suspensions. J. Cereal Sci. 1998, 28, 281–289. [Google Scholar] [CrossRef]
- Dasari, R.; Dunaway, K.; Berson, R. A scraped surface bioreactor for enzymatic saccharification of pretreated corn stover slurries. Energy Fuel 2009, 23, 492–497. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Foudari, M.; Al-Salman, F.; Almusallam, A.S. Effect of particle size and temperature on rheological, thermal, and structural properties of pumpkin flour dispersion. J. Food Eng. 2014, 124, 43–53. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Jassar, S.; Thomas, L. A comparison in rheological, thermal, and structural properties between Indian Basmati and Egyptian Giza rice flour dispersions as influenced by particle size. Food Hydrocoll. 2015, 48, 72–83. [Google Scholar] [CrossRef]
- Chung, C.; Degner, B.; McClements, D.J. Physicochemical characteristics of mixed colloidal dispersions: Models for foods containing fat and starch. Food Hydrocoll. 2013, 30, 281–291. [Google Scholar] [CrossRef]
- Naguleswaran, S.; Li, J.; Vasanthan, T.; Bressler, D.; Hoover, R. Amylolysis of large and small granules of native triticale, wheat and corn starches using a mixture of α-amylase and glucoamylase. Carbohydr. Polym. 2012, 88, 864–874. [Google Scholar] [CrossRef]
- Mahasukhonthachat, K.; Sopade, P.A.; Gidley, M.J. Kinetics of starch digestion in sorghum as affected by particle size. J. Food Eng. 2010, 96, 18–28. [Google Scholar] [CrossRef]
- De la Hera, E.; Gomez, M.; Rosell, C.M. Particle size distribution of rice flour affecting the starch enzymatic hydrolysis and hydration properties. Carbohydr. Polym. 2013, 98, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Oates, C.G. Towards an understanding of starch granule structure and hydrolysis. Trends Food Sci. Technol. 1997, 8, 375–382. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Gidley, M.J. Relationship between granule size and in vitro digestibility of maize and potato starches. Carbohydr. Polym. 2010, 82, 480–488. [Google Scholar] [CrossRef]
- Colonna, P.; Leloup, V.; Buleon, A. Limiting factors of starch hydrolysis. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S17–S32. [Google Scholar] [PubMed]
- Al-Rabadi, G.J.S.; Gilbert, R.G.; Gidley, M.J. Effect of particle size on kinetics of starch digestion in milled barley and sorghum grains by porcine alpha-amylase. J. Cereal Sci. 2009, 50, 198–204. [Google Scholar] [CrossRef]
- Warren, F.J.; Royall, P.G.; Gaisford, S.; Butterworth, P.J.; Ellis, P.R. Binding interactions of α-amylase with starch granules: The influence of supramolecular structure and surface area. Carbohydr. Polym. 2011, 86, 1038–1047. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Chu-Ky, S.; Luong, H.N.; Nguyen, H.V. Effect of Pretreatment Methods on Enzymatic Kinetics of Ungelatinized Cassava Flour Hydrolysis. Catalysts 2020, 10, 760. [Google Scholar] [CrossRef]

| Parameters | CR075 | CR125 | CR250 | CR500 |
|---|---|---|---|---|
| Starch content (% dm) | 82.0 ± 1.0 a | 81.4 ± 0.7 a | 78.4 ± 1.3 b | 77.3 ± 0.8 b |
| SRC (%) | 103.3 ± 0.5 a | 166.7 ± 1.8 b | 237.6 ± 1.2 c | 259.6 ± 0.9 d |
| D[4,3] (µm) | 33.2 ± 0.4 a | 100.4 ± 1.2 b | 201.1 ± 2.1 c | 456.2 ± 8.9 d |
| Concentration (% dm) | Coefficient | CR075 | CR125 | CR250 | CR500 |
|---|---|---|---|---|---|
| 19.2 | n (/) | 0.70 | 0.44 | 0.48 | 0.37 |
| K (Pa·sn) | 0.04 | 0.14 | 0.83 | 4.05 | |
| r2 (/) | 0.99 | 0.99 | 0.96 | 0.94 | |
| 24.5 | n (/) | 0.66 | 0.41 | 0.36 | 0.23 |
| K (Pa·sn) | 0.09 | 0.78 | 2.08 | 7.40 | |
| r2 (/) | 0.99 | 0.99 | 0.99 | 0.99 | |
| 29.4 | n (/) | 0.61 | 0.39 | n.r | n.r |
| K (Pa·sn) | 0.11 | 4.3 | |||
| r2 (/) | 0.99 | 0.99 |
| Hydrolysis Time (h) | CR075 | CR125 | CR250 | CR500 |
|---|---|---|---|---|
| 6 | 30.2 ± 0.4 a | 28.5 ± 0.2 b | 28.2 ± 0.7 b | 26.0 ± 1.1 c |
| 12 | 46.3 ± 0.3 a | 38.5 ± 0.5 b | 38.4 ± 1.4 b | 37.5 ± 1.2 b |
| 24 | 61.6 ± 1.0 a | 58.4 ± 0.7 b | 54.8 ± 0.5 c | 52.9 ± 0.3 d |
| 48 | 77.2 ± 0.9 a | 76.6 ± 1.2 a | 69.2 ± 0.7 b | 66.9 ± 0.6 c |
| 72 | 90.7 ± 0.6 a | 89.5 ± 0.3 a | 79.3 ± 0.7 b | 78.0 ± 0.4 b |
| Hydrolysis Time (h) | 0 | 0.75 | 2.25 | 4.25 | 10.25 | 17.75 |
|---|---|---|---|---|---|---|
| n (/) | 0.37 | 0.56 | 0.66 | 0.71 | 0.72 | 0.72 |
| K (Pa·sn) | 4.05 | 0.85 | 0.59 | 0.47 | 0.44 | 0.45 |
| r2 (/) | 0.94 | 0.99 | 0.97 | 0.96 | 0.92 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.C.; Fillaudeau, L.; Anne-Archard, D.; Chu-Ky, S.; Luong, H.N.; Vu, T.T.; Nguyen, T.H.D.; Nguyen, V.H. Impact of Particle Size on the Rheological Properties and Amylolysis Kinetics of Ungelatinized Cassava Flour Suspensions. Processes 2021, 9, 989. https://doi.org/10.3390/pr9060989
Nguyen TC, Fillaudeau L, Anne-Archard D, Chu-Ky S, Luong HN, Vu TT, Nguyen THD, Nguyen VH. Impact of Particle Size on the Rheological Properties and Amylolysis Kinetics of Ungelatinized Cassava Flour Suspensions. Processes. 2021; 9(6):989. https://doi.org/10.3390/pr9060989
Chicago/Turabian StyleNguyen, Tien Cuong, Luc Fillaudeau, Dominique Anne-Archard, Son Chu-Ky, Hong Nga Luong, Thu Trang Vu, Thi Hoai Duc Nguyen, and Van Hung Nguyen. 2021. "Impact of Particle Size on the Rheological Properties and Amylolysis Kinetics of Ungelatinized Cassava Flour Suspensions" Processes 9, no. 6: 989. https://doi.org/10.3390/pr9060989
APA StyleNguyen, T. C., Fillaudeau, L., Anne-Archard, D., Chu-Ky, S., Luong, H. N., Vu, T. T., Nguyen, T. H. D., & Nguyen, V. H. (2021). Impact of Particle Size on the Rheological Properties and Amylolysis Kinetics of Ungelatinized Cassava Flour Suspensions. Processes, 9(6), 989. https://doi.org/10.3390/pr9060989






