Brassica oleracea var. capitata L. Alleviates Indomethacin-Induced Acute Gastric Injury by Enhancing Anti-Inflammatory and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement of Vitamin U in BOE
2.3. Animals and Treatment
2.4. Histopathology
2.5. Alcian Blue Binding Assay
2.6. Measurement of Total Hexose, Sialic Acid, and Collagen
2.7. ELISA
2.8. Quantitative Polymerase Chain Reaction (qPCR)
2.9. Measurement of Myeloperoxidase (MPO) Activity
2.10. Lipid Peroxidation Assay
2.11. Measurement of Glutathione Concentration
2.12. Measurement of Catalase and Superoxide Dismutase Activities
2.13. Statistical Analyses
3. Results
3.1. HPLC Analysis for Quantifying Vitamin U in BOE
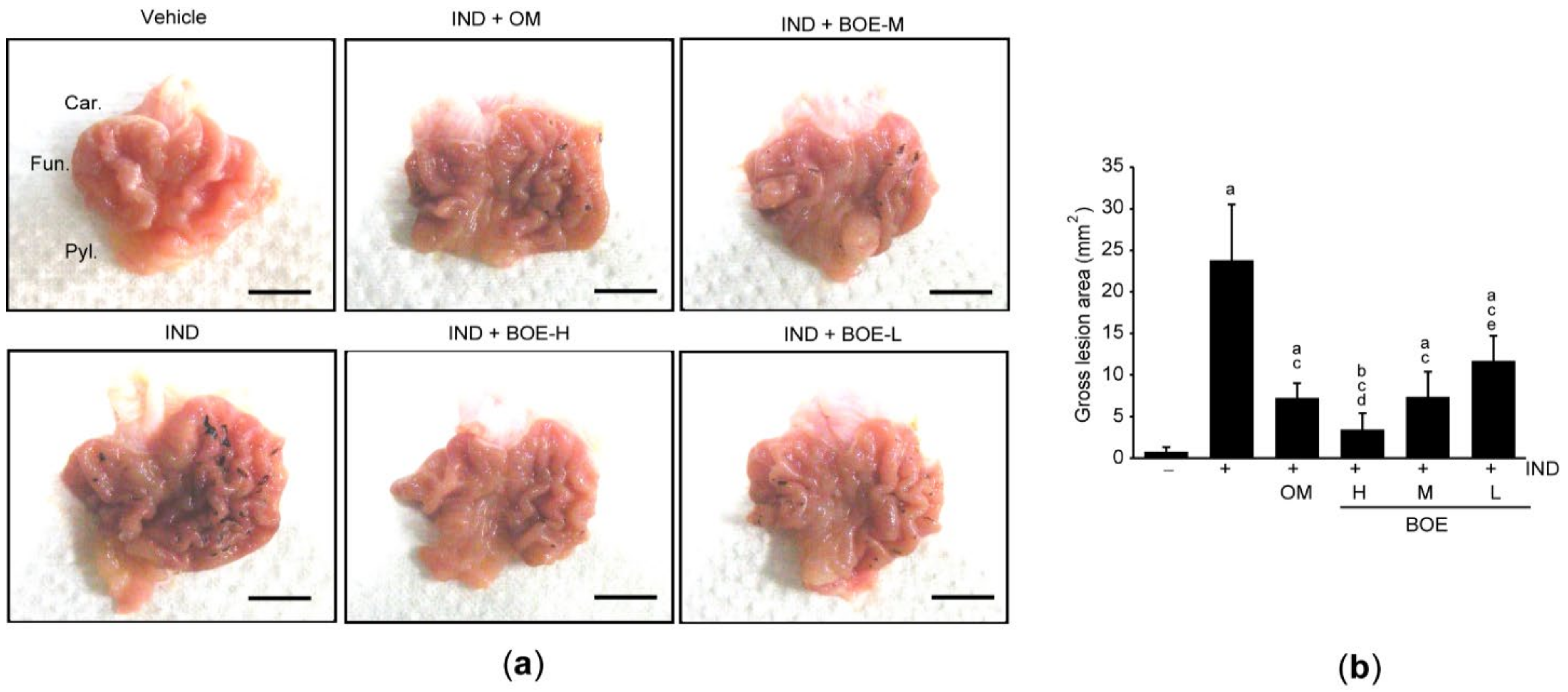
3.2. Pre-Administration of BOE Alleviates IND-Mediated Gastric Injury in Rats
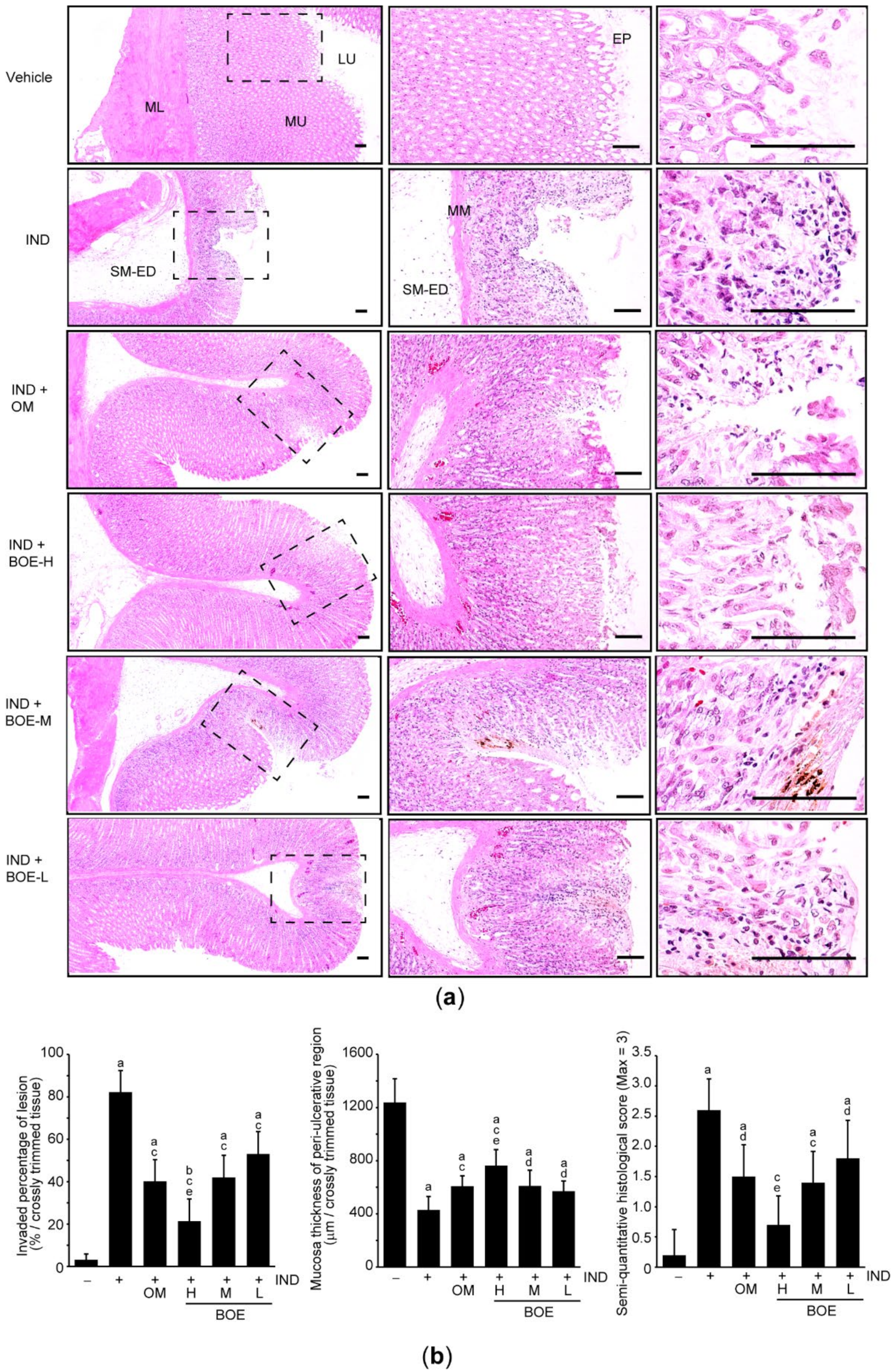
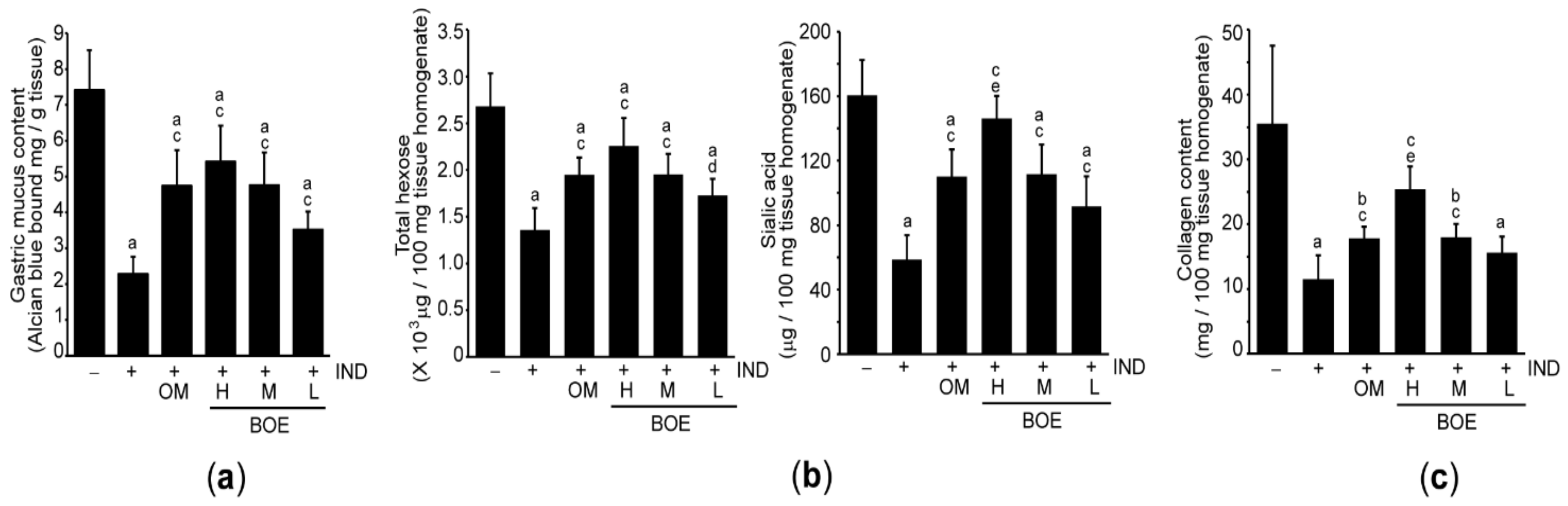
3.3. Pre-Administration of BOE Restores Integrity of the Gastric Mucus Impaired by IND
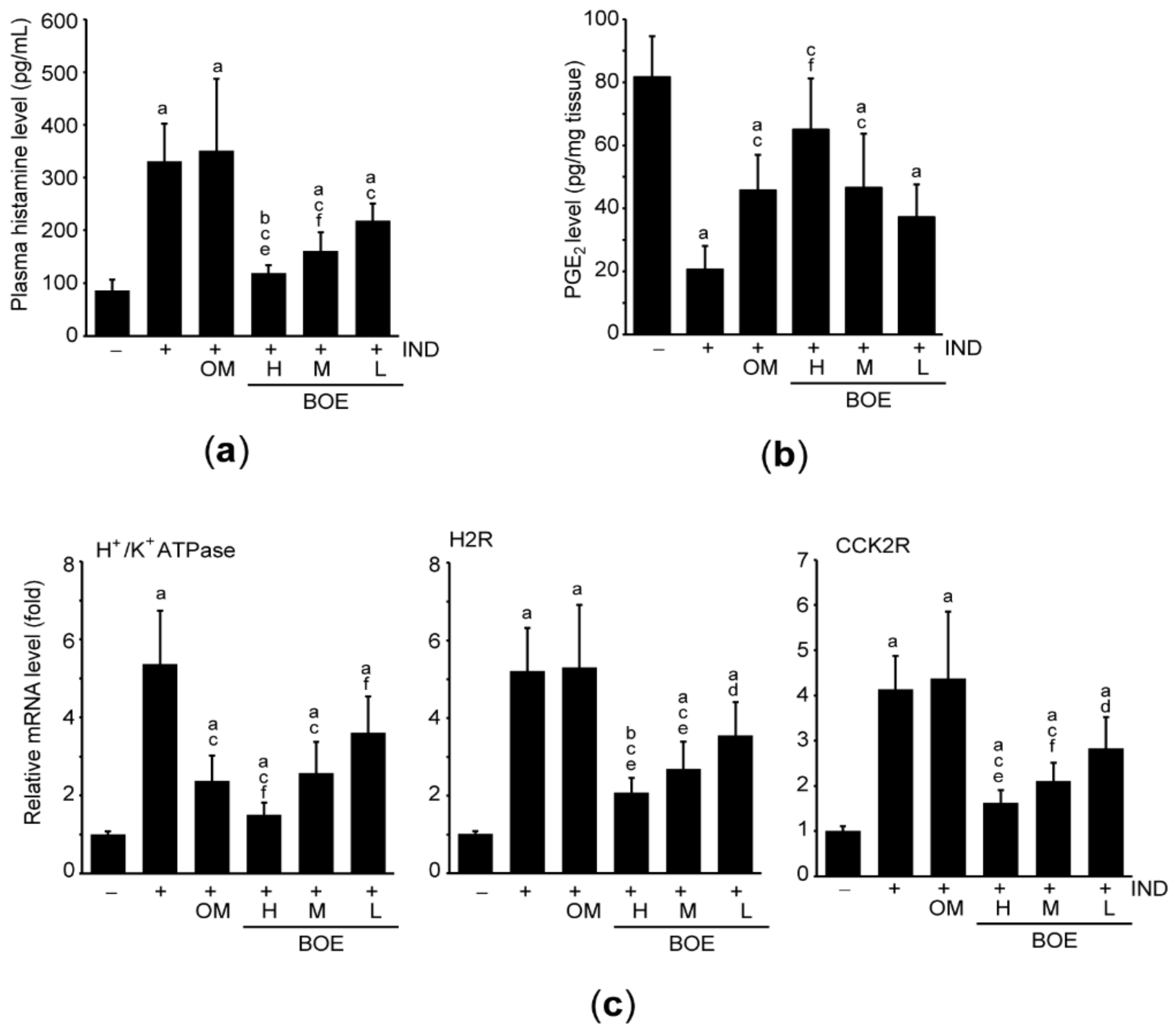
3.4. Pre-Administration of BOE Downregulates Essential Factors for Secreting Gastric Acid
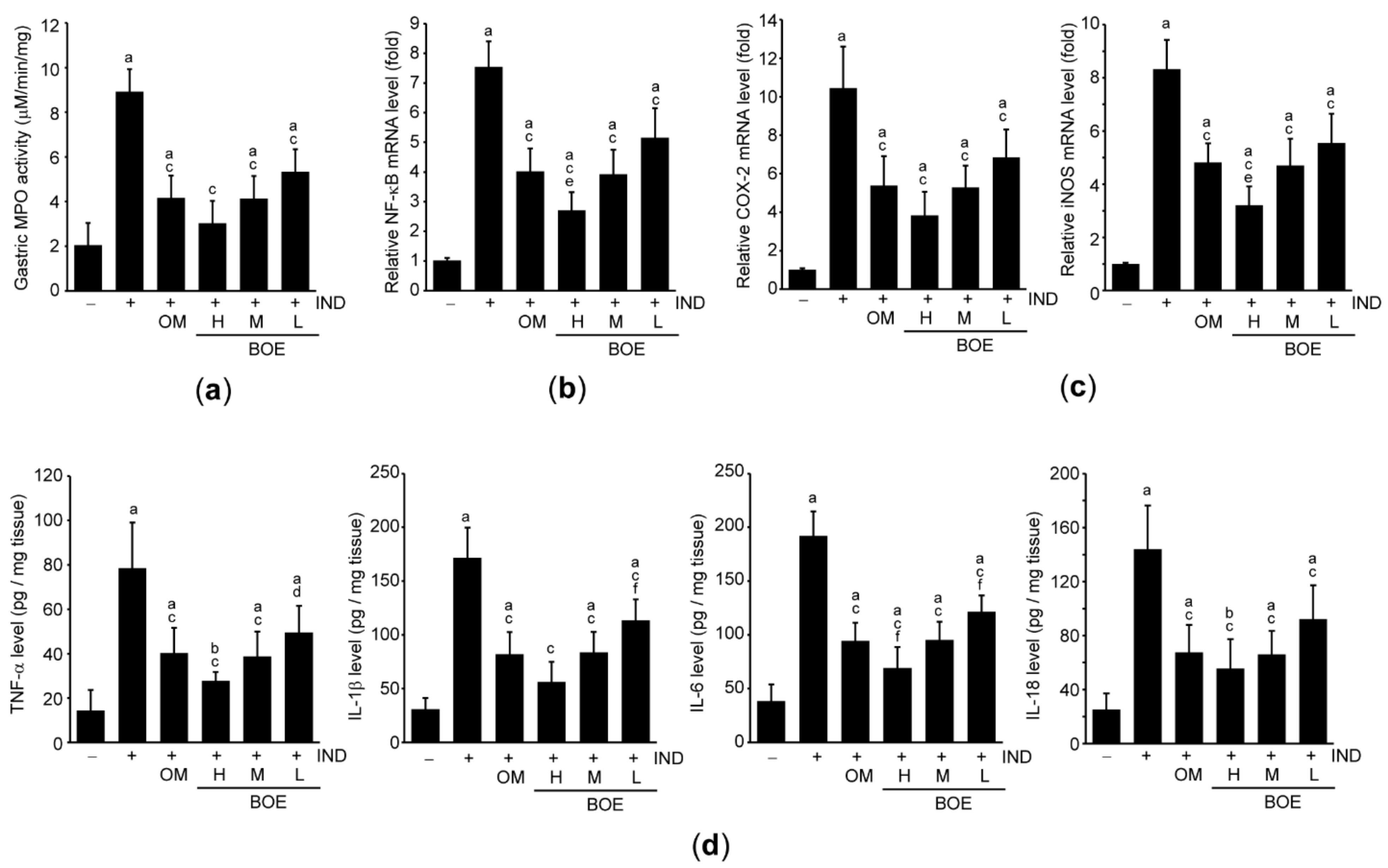
3.5. Pre-Administration of BOE Attenuates IND-Mediated Gastric Inflammation
3.6. Pre-Administration of BOE Suppresses Lipid Peroxidation by Enhancing Antioxidant Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Melcarne, L.; García-Iglesias, P.; Calvet, X.; Luigi, M.; Pilar, G.-I.; Xavier, C. Management of NSAID-associated peptic ulcer disease. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 723–733. [Google Scholar] [CrossRef]
- Coxib and traditional NSAID Trialists’ (CNT) Collaboration; Bhala, N.; Emberson, J.; Merhi, A.; Abramson, S.; Arber, N.; Baron , J.A.; Bombardier, C.; Cannon, C.; Farkouh, M.E.; et al. Vascular and upper gastrointestinal effects of non-steroidal an-ti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet 2013, 382, 769–779. [Google Scholar] [CrossRef]
- Beck, P.L.; Xavier, R.; Lu, N.; Nanda, N.N.; Dinauer, M.; Podolsky, D.K.; Seed, B. Mechanisms of NSAID-induced gastroin-testinal injury defined using mutant mice. Gastroenterology 2000, 119, 699–705. [Google Scholar] [CrossRef]
- Villegas, I.; Martin, M.J.; La Casa, C.; Motilva, V.; De La Lastra, C.A. Effects of meloxicam on oxygen radical generation in rat gastric mucosa. Inflamm. Res. 2000, 49, 361–366. [Google Scholar] [CrossRef]
- Lim, J.-M.; Song, C.-H.; Park, S.-J.; Park, D.-C.; Jung, G.-W.; Cho, H.-R.; Bashir, K.M.I.; Ku, S.K.; Choi, J.-S. Protective effects of triple fermented barley extract (FBe) on indomethacin-induced gastric mucosal damage in rats. BMC Complement. Altern. Med. 2019, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Katary, M.A.; Salahuddin, A. Gastroprotective effect of vanillin on indomethacin-induced gastric ulcer in rats: Protective pathways and anti-secretory mechanism. Clin. Exp. Pharmacol. Physiol. 2017, 7, 100232. [Google Scholar] [CrossRef]
- Sharma, A.V.; Ganguly, K.; Paul, S.; Maulik, N.; Swarnakar, S. Curcumin Heals Indomethacin-Induced Gastric Ulceration by Stimulation of Angiogenesis and Restitution of Collagen Fibers via VEGF and MMP-2 Mediated Signaling. Antioxidants Redox Signal. 2012, 16, 351–362. [Google Scholar] [CrossRef]
- De Carvalho, C.A.; Fernandes, K.M.; Matta Pinto, S.L.; da Silva, M.B.; de Oliveira, L.L.; Fonseca, C.C. Evaluation of antiulcerogenic activity of aqueous extract of Brassica oleracea var. capitata (cabbage) on wistar rat gastric ulceration. Arq. Gastroenterol. 2011, 48, 276–282. [Google Scholar] [CrossRef]
- Šamec, D.; Pavlović, I.; Salopek-Sondi, B. White cabbage (Brassica oleracea var. capitata f. alba): Botanical, phytochemical and pharmacological review. Phytochem. Rev. 2017, 16, 117–135. [Google Scholar] [CrossRef]
- Thangam, R.; Suresh, V.; Rajkumar, M.; Vincent, J.D.; Gunasekaran, P.; Anbazhagan, C.; Kaveri, K.; Kannan, S. Antioxidant and In Vitro Anticancer Effect of 2-Pyrrolidinone Rich Fraction of Brassica oleracea var. capitata Through Induction of Apoptosis in Human Cancer Cells. Phytotherapy Res. 2013, 27, 1664–1670. [Google Scholar] [CrossRef]
- Yang, D.K. Cabbage (Brassica oleracea var. capitata) Protects against H2O2-Induced Oxidative Stress by Preventing Mitochondrial Dysfunction in H9c2 Cardiomyoblasts. Evid. Based Complement. Altern. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morales-López, J.; Centeno-Álvarez, M.; Nieto-Camacho, A.; López, M.G.; Pérez-Hernández, E.; Pérez-Hernández, N.; Fernández-Martínez, E. Evaluation of antioxidant and hepatoprotective effects of white cabbage essential oil. Pharm. Biol. 2016, 55, 233–241. [Google Scholar] [CrossRef]
- Assad, T.; Khan, R.A.; Feroz, Z. Evaluation of hypoglycemic and hypolipidemic activity of methanol extract of Brassica oleracea. Chin. J. Nat. Med. 2014, 12, 648–653. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Ogunsuyi, O.B.; Oyeleye, S.I.; Dada, A.F.; Boligon, A.A. Cabbage and cucumber extracts exhibited anticholinesterase, antimonoamine oxidase and antioxidant properties. J. Food Biochem. 2017, 41, e12358. [Google Scholar] [CrossRef]
- Cheney, G. Rapid Healing of Peptic Ulcers in Patients Receiving Fresh Cabbage Juice. Calif. Med. 1949, 70, 10–15. [Google Scholar]
- Cheney, G.; Waxler, S.H.; Miller, I.J. VITAMIN U THERAPY OF PEPTIC ULCER—Experience at San Quentin Prison. Calif. Med. 1956, 84, 39–42. [Google Scholar]
- Cheney, G. Anti-peptic ulcer dietary factor (vitamin “U”) in the treatment of peptic ulcer. J. Am. Diet. Assoc. 1950, 26, 668–672. [Google Scholar]
- Ben Hadda, T.; ElSawy, N.A.; Header, E.A.M.; Mabkhot, Y.N.; Mubarak, M.S. Effect of garlic and cabbage on healing of gastric ulcer in experimental rats. Med. Chem. Res. 2014, 23, 5110–5119. [Google Scholar] [CrossRef]
- Kim, M.-R.; Kim, T.-I.; Choi, B.-R.; Kim, M.B.; Cho, I.J.; Lee, K.-W.; Ku, S.K. Brassica oleracea Prevents HCl/Ethanol-Induced Gastric Damages in Mice. Appl. Sci. 2020, 11, 16. [Google Scholar] [CrossRef]
- Russell, R.I. Non-steroidal anti-inflammatory drugs and gastrointestinal damage-problems and solutions. Postgrad. Med. J. 2001, 77, 82–88. [Google Scholar] [CrossRef]
- Ribeiro, A.R.S.; Valença, J.D.D.N.; Santos, J.D.S.; Boeing, T.; Da Silva, L.M.; De Andrade, S.F.; Albuquerque-Júnior, R.L.; Thomazzi, S.M. The effects of baicalein on gastric mucosal ulcerations in mice: Protective pathways and anti-secretory mechanisms. Chem. Interact. 2016, 260, 33–41. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Song, C.-H.; Lee, J.-E.; Choi, S.H.; Ku, S.-K.; Park, S.-J. Effects of platycodin D on reflux esophagitis due to modulation of antioxidant defense systems. Evid. Based Complement. Alternat. Med. 2018, 2018, 7918034. [Google Scholar] [CrossRef]
- Matsui, H.; Shimokawa, O.; Kaneko, T.; Nagano, Y.; Rai, K.; Hyodo, I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J. Clin. Biochem. Nutr. 2011, 48, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.; Naumann, M. NF-κB signaling in gastric cancer. Toxins 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Ito, Y.; Saegusa, Y.; Iwai, T.; Goso, Y.; Ikezawa, T.; Ishihara, K. Effects of combination treatment with famotidine and methylmethionine sulfonium chloride on the mucus barrier of rat gastric mucosa. J. Gastroenterol. Hepatol. 2009, 24, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ohara, S.; Ichikawa, T.; Saigenji, K.; Hotta, K. Mechanisms for cytoprotection by vitamin U from ethanol-induced gastric mucosal damage in rats. Dig. Dis. Sci. 1996, 41, 49–54. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Jiang, N.; Choi, S.-H.; Lim, Y.P.; Park, J.-T.; Al-Dhabi, N.A.; Kim, S.-J. Metabolite profiling of phenolics, anthocyanins and favonols in cabbage (Brassica oleracea var. capitata). Ind. Crops Prod. 2014, 60, 8–14. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Lee, M.-K.; Chun, J.-H.; Seo, J.M.; Lee, S.-W.; Al-Dhabi, N.A.; Kim, S.-J. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.). Food Chem. 2014, 145, 77–85. [Google Scholar] [CrossRef]
- Kolgazi, M.; Cilingir, S.; Yilmaz, O.; Gemici, M.; Yazar, H.; Ozer, S.; Acikel-Elmas, M.; Arbak, S.; Suyen, G.G. Caffeic acid attenuates gastric mucosal damage induced by ethanol in rats via nitric oxide modulation. Chem. Interact. 2020, 334, 109351. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Boeing, T.; Somensi, L.B.; Cury, B.J.; Espíndola, V.L.; França, T.C.S.; De Almeida, M.O.; Arruda, C.; Bastos, J.K.; Da Silva, L.M.; et al. Hydroalcoholic extract from Baccharis dracunculifolia recovers the gastric ulcerated tissue, and p -coumaric acid is a pivotal bioactive compound to this action. BioFactors 2019, 45, 479–489. [Google Scholar] [CrossRef]
- Li, Q.; Hu, X.; Xuan, Y.; Ying, J.; Fei, Y.; Rong, J.; Zhang, Y.; Zhang, J.; Liu, C.; Liu, Z. Kaempferol protects ethanol-induced gastric ulcers in mice via pro-inflammatory cytokines and NO. Acta Biochim. Biophys. Sin. 2018, 50, 246–253. [Google Scholar] [CrossRef] [PubMed]
- AlKushi, A.G.R.; ElSawy, N.A.M. Quercetin attenuates, indomethacin-induced acute gastric ulcer in rats. Folia Morphol. 2017, 76, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Masuda, H.; Shimamura, Y.; Sugiyama, C.; Takabayashi, F. Improvement Effects of Wasabi (Wasabia japonica) Leaves and Allyl Isothiocyanate on Stomach Lesions of Mongolian Gerbils Infected with Helicobacter pylori. Nat. Prod. Commun. 2017, 12, 595–598. [Google Scholar] [CrossRef]
- Zeren, S.; Bayhan, Z.; Kocak, F.E.; Kocak, C.; Akcılar, R.; Bayat, Z.; Simsek, H.; Duzgun, S.A. Gastroprotective effects of sul-foraphane and thymoquinone against acetylsalicylic acid-induced gastric ulcer in rats. J. Surg. Res. 2016, 203, 348–359. [Google Scholar] [CrossRef] [PubMed]
- A El-Shinnawy, N.; A Abd-Elmageid, S.; A Alshailabi, E.M. Evaluation of antiulcer activity of indole-3-carbinol and/or omeprazole on aspirin-induced gastric ulcer in rats. Toxicol. Ind. Heal. 2012, 30, 357–375. [Google Scholar] [CrossRef]
- Huilgol, S.V.; Kumar, V.H. Evaluation of antiulcerogenic potential of antioxidant α-tocopherol in pylorus-ligated albino rats. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 81–85. [Google Scholar] [CrossRef]
- Abdel-Raheem, I.T. Gastroprotective Effect of Rutin against Indomethacin-Induced Ulcers in Rats. Basic Clin. Pharmacol. Toxicol. 2010, 107, 742–750. [Google Scholar] [CrossRef]
- De Barros, M.P.; Lemos, M.; Maistro, E.L.; Leite, M.F.; Sousa, J.P.B.; Bastos, J.K.; De Andrade, S.F. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J. Ethnopharmacol. 2008, 120, 372–377. [Google Scholar] [CrossRef]
- Min, Y.S.; Yim, S.H.; Bai, K.L.; Choi, H.J.; Jeong, J.H.; Song, H.J.; Park, S.Y.; Ham, I.; Whang, W.K.; Sohn, U.D. The effects of apigenin-7-O-beta-d-glucuronopyranoside on reflux oesophagitis and gastritis in rats. Auton. Autacoid Pharmacol. 2005, 25, 85–91. [Google Scholar] [CrossRef]
- Singh, P.; Bhargava, V.K.; Garg, S.K. Effect of melatonin and beta-carotene on indomethacin induced gastric mucosal injury. Indian J. Physiol. Pharmacol. 2002, 46, 229–234. [Google Scholar]
- Wallace, J.L. Prostaglandins, NSAIDs, and Gastric Mucosal Protection: Why Doesn’t the Stomach Digest Itself? Physiol. Rev. 2008, 88, 1547–1565. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Engevik, A.C.; Kaji, I.; Goldenring, J.R. The Physiology of the Gastric Parietal Cell. Physiol. Rev. 2020, 100, 573–602. [Google Scholar] [CrossRef]
- Kunikata, T.; Tanaka, A.; Miyazawa, T.; Kato, S.; Takeuchi, K. 16,16-Dimethyl prostaglandin E2 inhibits indomethacin-induced small intestinal lesions through EP3 and EP4 receptors. Dig. Dis. Sci. 2002, 47, 894–904. [Google Scholar] [CrossRef]
- Suzuki, K.; Araki, H.; Mizoguchi, H.; Furukawa, O.; Takeuchi, K. Prostaglandin E inhibits indomethacin-induced gastric lesions through EP-1 receptors. Digestion 2001, 63, 92–101. [Google Scholar] [CrossRef]
- Takeuchi, K.; Amagase, K. Roles of cyclooxygenase, prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr. Pharm. Des. 2018, 24, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Arfors, K.-E.; McKnight, G. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology 1991, 100, 878–883. [Google Scholar] [CrossRef]
- Żebrowska-Nawrocka, M.; Agnieszka, W.; Jacek, P.; Jeleń, A.; Adrian, K.; Dagmara, S.-K.; Sałagacka-Kubiak, A.; Balcerczak, E. NFKB2 gene expression in patients with peptic ulcer diseases and gastric cancer. Mol. Biol. Rep. 2020, 47, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Zhou, P.-Y.; Jia, M.; Cheng, X.-S.; Jia, Y.-T.; Xu, S.-G. Absence of NF-κB subunit p50 ameliorates cold immobilization stress-induced gastric ulcers. Biochem. Biophys. Res. Commun. 2013, 434, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, J.E.; Jung, E.H.; Jung, J.Y.; Jung, D.H.; Ku, S.K.; Cho, I.J.; Kim, S.C. Hemistepsin A ameliorates acute inflam-mation in macrophages via inhibition of nuclear factor-κB and activation of nuclear factor erythroid 2-related factor 2. Food Chem. Toxicol. 2018, 111, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Grandjean-Laquerriere, A.; Antonicelli, F.; Gangloff, S.C.; Guenounou, M.; Le Naour, R. UVB-induced IL-18 production in human keratinocyte cell line NCTC 2544 through NF-kappaB activation. Cytokine 2007, 37, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and other nutrigenomic Nrf2 activators: Can the clinician’s ex-pectation be matched by the reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2–Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Oztay, F.; Tunali, S.; Kayalar, O.; Yanardag, R. The protective effect of vitamin U on valproic acid-induced lung toxicity in rats via amelioration of oxidative stress. J. Biochem. Mol. Toxicol. 2020, 34, 22602. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]

| Gene Name | Nucleotide Sequence (5′→3′) | GenBank Accession No. | Amplicon Size (bp) | |
|---|---|---|---|---|
| H+/K+ ATPase | Sense | ATCATTGGACGCATCGCCTCTCTGG | NM_012509.1 | 420 |
| Antisense | GTCTTCTGTGGTGTCCGCCGTGTGG | |||
| H2R | Sense | ATGGAGCCCAATGGCACAG | NM_012965.3 | 105 |
| Antisense | GCCAGCAATGGTGATGAGGA | |||
| CCK2R | Sense | CAGCAGGCCGGTGATAAGA | D50608.1 | 245 |
| Antisense | GGTGGACATGAGAAGGTGT | |||
| NF-κB | Sense | GCGCATCCAGACCAACAATAA | LC369719.1 | 425 |
| Antisense | GCCGAAGCTGCATGGACACT | |||
| COX-2 | Sense | TGCGATGCTCTTCCGAGCTGTGCT | NM_017232 | 472 |
| Antisense | TCAGGAAGTTCCTTATTTCCTTTC | |||
| iNOS | Sense | CACCACCCTCCTTGTTCAAC | U26686.1 | 132 |
| Antisense | CAATCCACAACTCGCTCCAA | |||
| GAPDH | Sense | TGGTGAAGGTCGGTGTGAAC | NM_017008.4 | 190 |
| Antisense | TTCCCATTCTCAGCCTTGAC | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryou, S.H.; Cho, I.J.; Choi, B.-R.; Kim, M.B.; Kwon, Y.S.; Ku, S.K. Brassica oleracea var. capitata L. Alleviates Indomethacin-Induced Acute Gastric Injury by Enhancing Anti-Inflammatory and Antioxidant Activity. Processes 2021, 9, 372. https://doi.org/10.3390/pr9020372
Ryou SH, Cho IJ, Choi B-R, Kim MB, Kwon YS, Ku SK. Brassica oleracea var. capitata L. Alleviates Indomethacin-Induced Acute Gastric Injury by Enhancing Anti-Inflammatory and Antioxidant Activity. Processes. 2021; 9(2):372. https://doi.org/10.3390/pr9020372
Chicago/Turabian StyleRyou, Seong Hwan, Il Je Cho, Beom-Rak Choi, Moon Bong Kim, Young Sam Kwon, and Sae Kwang Ku. 2021. "Brassica oleracea var. capitata L. Alleviates Indomethacin-Induced Acute Gastric Injury by Enhancing Anti-Inflammatory and Antioxidant Activity" Processes 9, no. 2: 372. https://doi.org/10.3390/pr9020372
APA StyleRyou, S. H., Cho, I. J., Choi, B.-R., Kim, M. B., Kwon, Y. S., & Ku, S. K. (2021). Brassica oleracea var. capitata L. Alleviates Indomethacin-Induced Acute Gastric Injury by Enhancing Anti-Inflammatory and Antioxidant Activity. Processes, 9(2), 372. https://doi.org/10.3390/pr9020372







