Abstract
The utilization of cosmetic raw materials for medicinal purposes in a microneedle patch containing hyaluronic acid (HA) and Lonicerae flos ethanol extract (LEE) has increased based on their eco-friendly characteristics. The moisturizing effects of this microneedle patch were assessed on the forearms of 20 female participants, and our results showed an increase between 29.60% and 88.54% in skin moisture content at the same time points relative to the untreated group. Apart from confirming the safety of HA and Lonicerae flos, our findings may also promote the use of HA and medicinal herbs in relevant industries, indicating their potential as raw ingredients for the development of cosmetic products and topical dermatological drugs. The results suggested that HA, Lonicerae flos, and other combinations of chemical and natural products based on traditional oriental medicine could beneficially affect skin health.
1. Introduction
Recently, there has been an increase in the development of cosmetics containing natural ingredients [1]. Moreover, the number of beauty-related products containing clinically approved ingredients is gradually increasing [2]. Hyaluronic acid (HA), a type of glycosaminoglycan consisting of D-glucuronic acid and N-acetyl-D-glucosamine, is found in the skin, cartilage, and various body parts, and is known to have moisturizing effects [3]. Each cell in the human body contains a gene that synthesizes HA, and the known HA synthesis metabolic pathway includes hyaluronic acid synthase (HAS)-1, -2, -3, TGF-β (trans forming growth factor β), PDGF BB (platelet derived growth factor BB), FGF (fibroblast growth factor), and EGF (epidermal growth factor) [4].
Even though it is widely known that hyaluronic acid is closely related to the moisture condition of the skin, there are various medical limitations in injection of hyaluronic acid from outside the body, so if we consider solutions in promoting the synthesis of hyaluronic acid in human cells together, we can achieve a more effective moisturizing effect [5].
Thus, various methods have been proposed to promote natural HA synthesis, and among all such methods, the use of natural ingredients has recently drawn the most attention owing to its safety [6]. In addition, the increased demand for beauty products containing oriental medicine ingredients comes from social demands regarding eco-friendly concepts and the interest in K-beauty, based on Korean cultural content in the global market [7].
Lonicerae flos is the name of herbal medicine, used as Lonicera japonica Thunberg’s unflowered flower buds [8]. It has been traditionally used as a medical raw material in East Asia, and is applied to various inflammatory diseases including infectious diseases [9]. The major components of Lonicerae flos include Chlorogenic acid, Neochlorogenic acid, Caffeic acid, Cynarin, and Lonicerin, among which Chlorogenic acid is known as a representative indicator component [10]. Today, in modern medicine, its antiviral, antibacterial and anti-inflammatory activities have been confirmed, and it is used as a raw material for various medicines [11].
Currently, many countries, including Korea, prohibit animal experiments to check safety and effectiveness of cosmetics and beauty-care items for beauty purposes [12]. Therefore, it can be said that the human testing being controlled under the manual is almost the only way to obtain the most important scientific basis for the product [13].
In this study, clinical testing was conducted with the approval of the Clinical Test Ethics Committee (Semyung University Oriental Medicine Hospital) in accordance with the clinical trial guidelines of the Ministry of Food and Drug Safety to confirm the effectiveness of the HA microneedle patch, which is important for maintaining skin moisture content, and Lonicerae flos ethanol extract (LEE), which is known to exert a marked effect on HA levels in human cells. The results of our study confirmed effective skin moisturization.
2. Materials and Methods
2.1. Experimental Materials
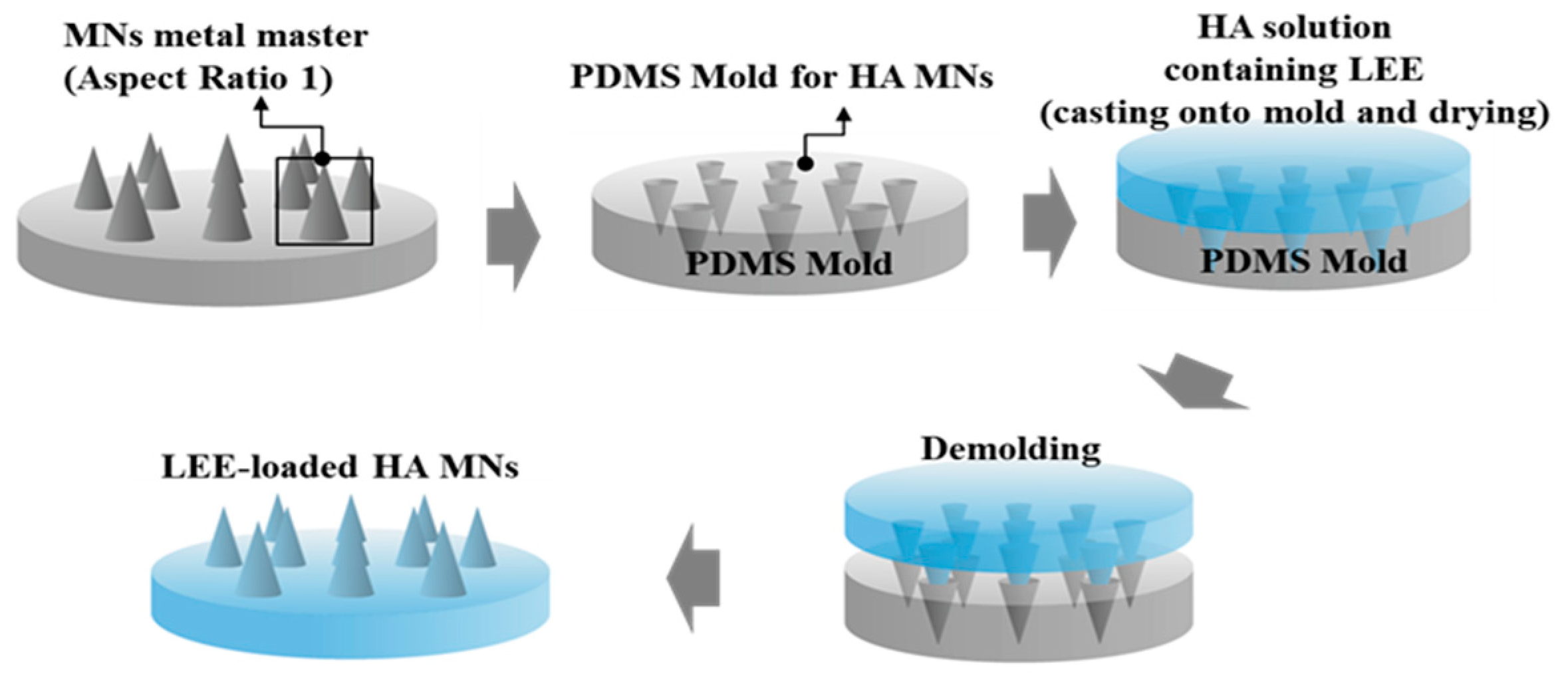
The microneedle patch containing HA and LEE was prepared by Baybiotech (Cheng-ju, Korea), and Lonicerae flos was purchased from Puremind Co. (Yong-choen, Korea). The samples were stored in laboratory at the BIT College of Convergence at Seowon University (Cheng-ju, Korea). Lonicerae flos (100 g) was macerated in 600 mL of 70% (v/v) ethanol solvent three times for 24 h. The solution was filtered using filter paper and collected in a bottle. It was then run in a rotary evaporator and freeze-dried for 3 days. The extraction yield was 21.2%. Using LEE, we created a HA microneedle patch and soluble essence. The soluble essence was diluted in glycerin and used in a clinical test at a concentration of 1%. In addition, LEE-loaded dissolving HA microneedle arrays (MNs) were prepared by a simple molding process. Details of the preparation procedure have been presented in our previous studies [14,15,16]. In brief, the MN metal master structure consisting of square pyramids with an aspect ratio of 1 (base width 250 μm, height 250 μm) was manufactured using a micromilling technique. By inversely replicating the shape of the master structure, the MN molds were prepared using Polydimethylsiloxane (PDMS). Briefly, a PDMS monomer and curing agent (9:1 w/w) were mixed and poured onto the master structure. After 4 h at 60 °C, the PDMS molds were detached from the master structure. The LEE-loaded HA MNs were fabricated by a simple molding process and casting an HA solution containing 3% (w/w) HA (Bio Sodium Hyaluronate, SK Bioland, Cheng-ju, Korea) and 0.9% (w/w) LEE in distilled water. HA and LEE powders were completely dissolved in distilled water by vortexing for 20 min. The HA solution was poured onto the PDMS mold. The mold was dried in an oven at 35 °C for 8 h. Eventually, the LEE-loaded HA MNs were detached from the mold and stored in a desiccator containing silica gel for further use. The fabrication process of a typical MN is shown in Figure 1 [14,15,16].
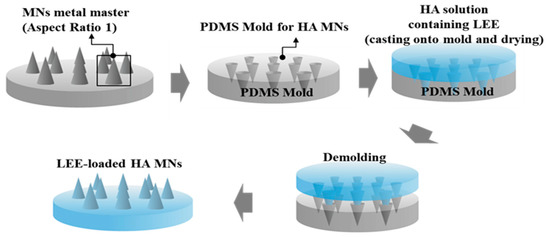
Figure 1.
Schematic diagram for the fabrication process of hyaluronic acid (HA) microneedle arrays (MNs) containing Lonicerae flos ethanol extract (LEE).
2.2. Specification of Natural Products
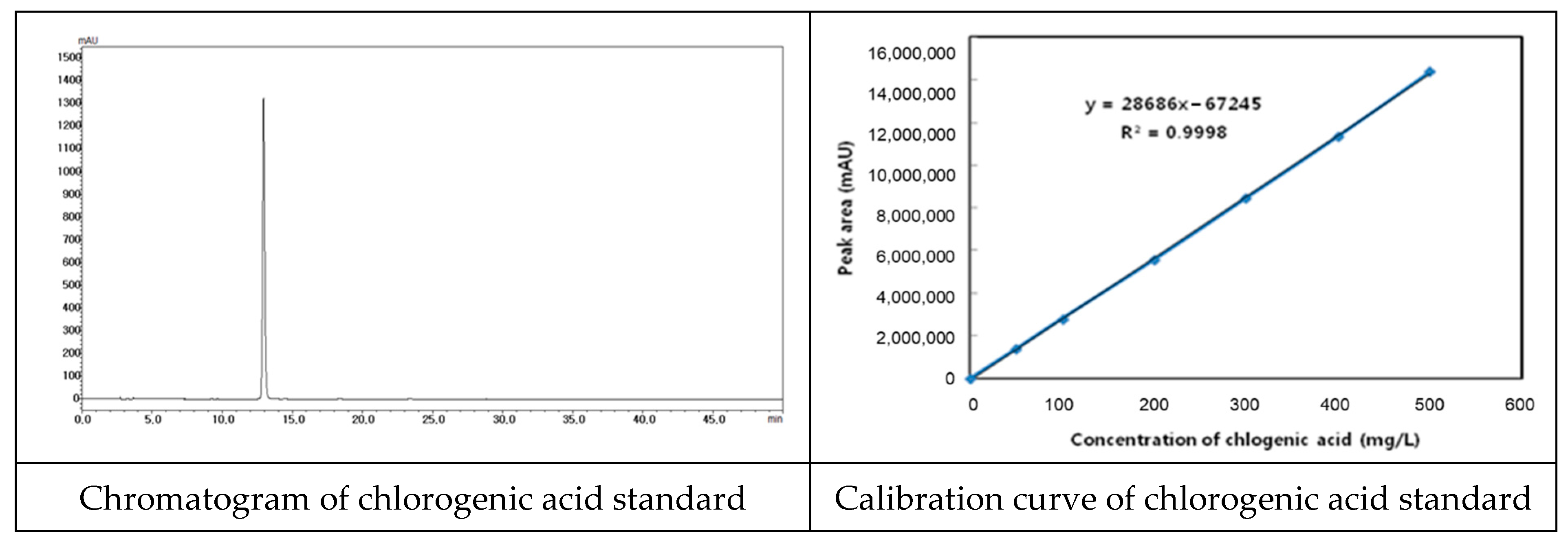
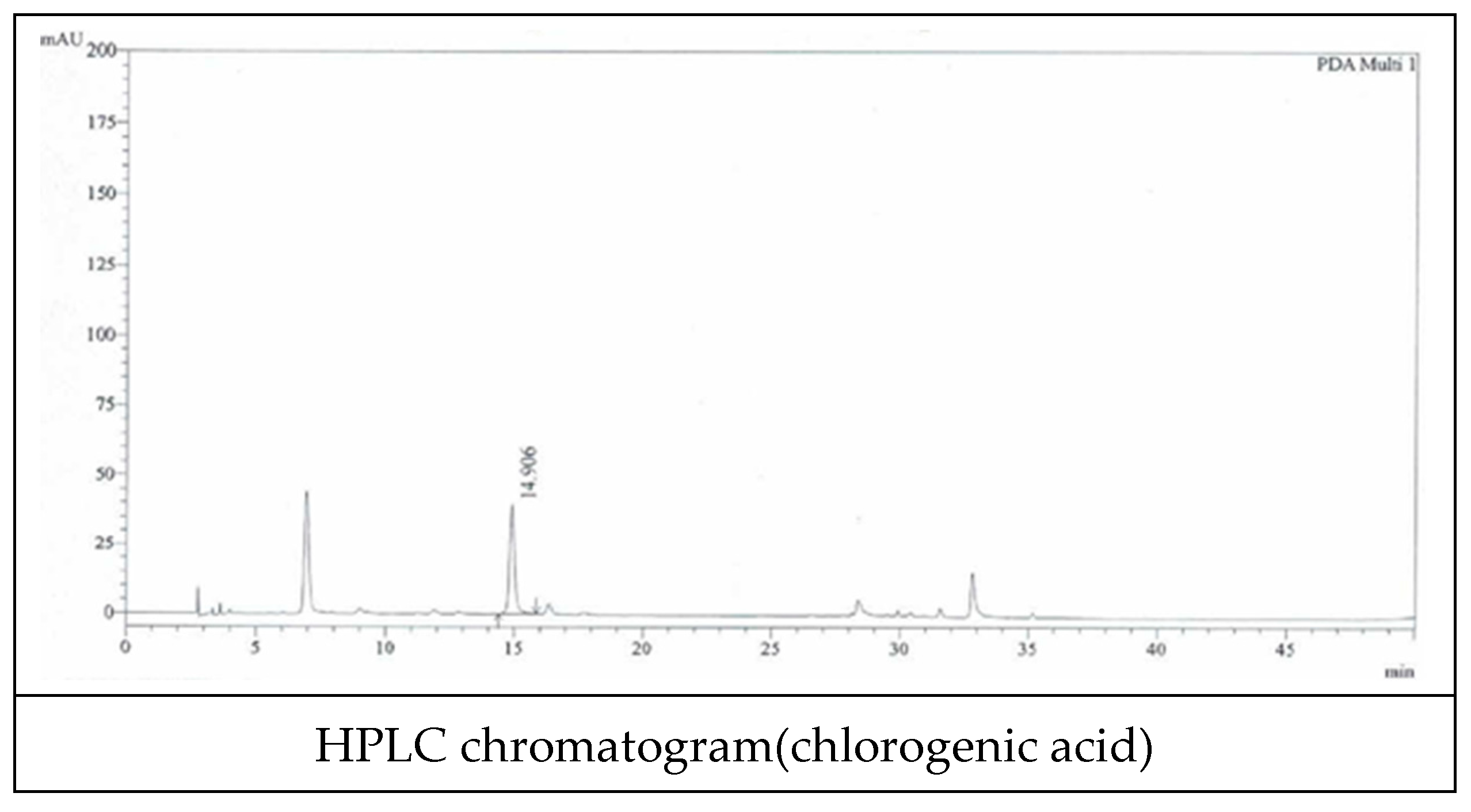
We used Lonicerae flos that was inspected by the Ministry of Korea Food and Drug Safety (KFDA). We also specified quality using known indicator components (Figure 2). Chlorogenic acid was used as an indicator component, and quantitative analysis was performed using HPLC (Figure 3 and Table 1).
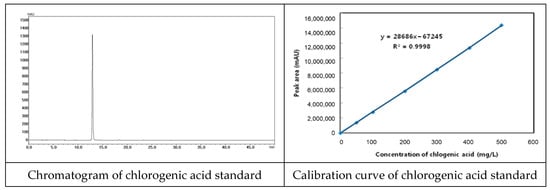
Figure 2.
Chromatogram of chlorogenic acid standard and calibration curve.
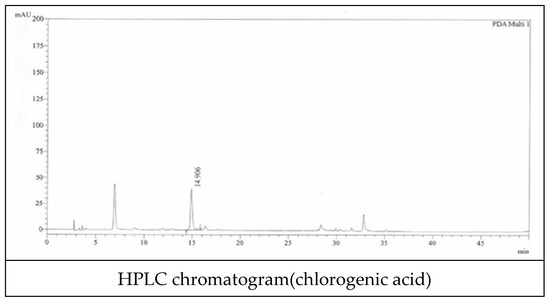
Figure 3.
HPLC chromatogram of extracts from Lonicerae Flos.

Table 1.
HPLC peak table.
2.3. Participant Selection
The present study was approved by the Institutional Review Board (IRB) of Semyung University Oriental Medicine Hospital (IRB No. SMCTC-053-18-004) and was conducted between 30 May 2018, and 29 May 2019. Subjects aged 30–55 years who satisfied the inclusion criteria and did not meet the exclusion criteria were enrolled in this study. The average age was 45.4 years. For the measurement of various parameters of the test sample, the participants were informed about the research procedure by our researchers through verbal and written communication. Participants began their participation after submitting their signed consent forms. A total of 20 women who satisfied the inclusion criteria were finally selected. The effectiveness of the HA micro needle patch with LEE soluble essence in improving skin moisturization was assessed, and a skin irritation test was performed in accordance with the guidelines for clinical assessment of cosmetic products released by the Ministry of Food and Drug Safety.
2.4. Skin Irritation Test
Skin irritation symptoms, such as erythema, swelling, itchiness, and burning sensation, were assessed and graded.
2.5. Test Method
The moisturizing effects of the extracts were assessed on the forearms. The extract was applied under the same experimental conditions in a clean and dry state after the patients rested in an air-conditioned room for at least 30 min (22 ± 2 °C, RH 40–60%). A corneometer (CM 825, Courage and Khazaka Electronic Co., Cologne, Germany) was used to measure moisture content. This optical measuring system measures the amount of skin drawn into the probe using electronic capacitance. The moisture content and electrostatic load capacity are proportional to each other; thus, the higher the moisture, the higher is the measured value. An arbitrary unit (AU) was used as the measurement unit. Clinical efficacy evaluation was performed by setting three groups: HA micro needle patch with LEE soluble essence (EG), HA micro needle patch (CG), and untreated (NG). In the first group, 2 µL/cm2 of LEE soluble essence was applied with a micropipette to each of the four forearm test sites (1.5 × 1.5 cm each). After 30 min of absorption, the HA microneedle patch was removed, and moisture content was measured. The clinical assessment was approved by the IRB.
2.6. Test Schedule and Procedure
Skin irritation test and moisture contents assessments using a corneometer were performed after the participants used the test sample in the order of 0.5, 2, 4, 6, 8 h according to the instructions. Adverse events of the test sample were also assessed through a standard questionnaire survey and inspection.
2.7. Statistical Analysis
Changes in skin moisture content before extract application and at 0.5, 2, 4, 6, and 8 h after application were statistically analyzed with a student, paired t-test, and ANOVA test using IBM SPSS ver. 18.0 (IBM Co, NY, USA). The level of statistical significance was set at p < 0.05.
3. Results and Discussion
3.1. Age Range of the Participants
The participants were women aged 30–55 years (mean 45.4 years). The proportion of women in their 30s, 40s, and 50s was 15%, 60%, and 25%, respectively. A total of 20 subjects participated in the test, and none dropped out or quit during the research period (Table 2).

Table 2.
Moisture content values of the experiment according to duration of test sample use (Unit: AU).
3.2. Skin Irritation Test
No abnormal skin reactions against the test sample were observed by the examiners or reported by the participants. While some natural products have been shown to cause skin irritation, the microneedle patch containing HA and LEE was shown to be safe when applied topically.
3.3. Moisture Content Analysis Results
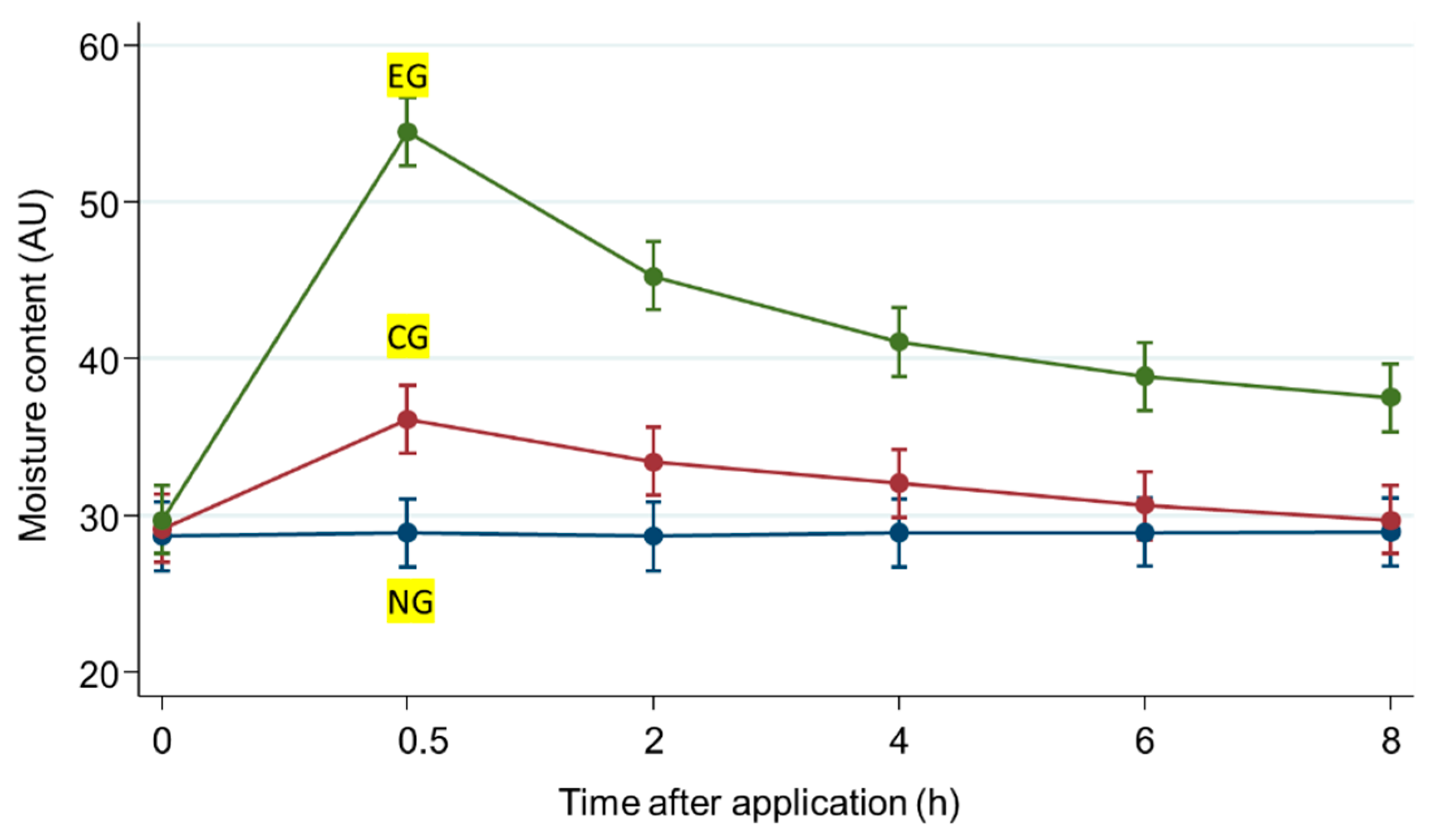
The effectiveness of the test sample for moisturizing the skin was subjectively and clinically assessed in accordance with the guidelines for the clinical assessment of cosmetic products released by the Ministry of Food and Drugs Safety. The skin moisture content values were measured at 0.5, 2, 4, 6, and 8 h after application, and the results are shown in Table 2 and Figure 4. The mean values of moisture content after 8 h were 28.95 AU in the untreated group, 29.72 AU in the microneedle patch group, and 37.52 AU in the microneedle patch with LEE soluble essence group. The treatment samples significantly improved skin moisture content (p < 0.05). Based on this result, EG increased by 88.54% after 0.5 h and 29.60% after 8 h compared to NG.
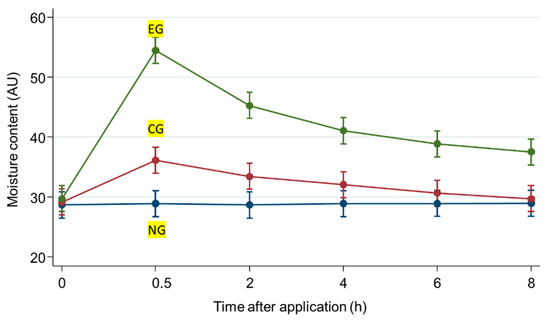
Figure 4.
Moisture content values of the experiment. Values are expressed as the mean ± SD (n = 20). Time, hours; EG, experimental group; CG, control group; NG, untreated group.
3.4. Changes in Skin Moisture Content
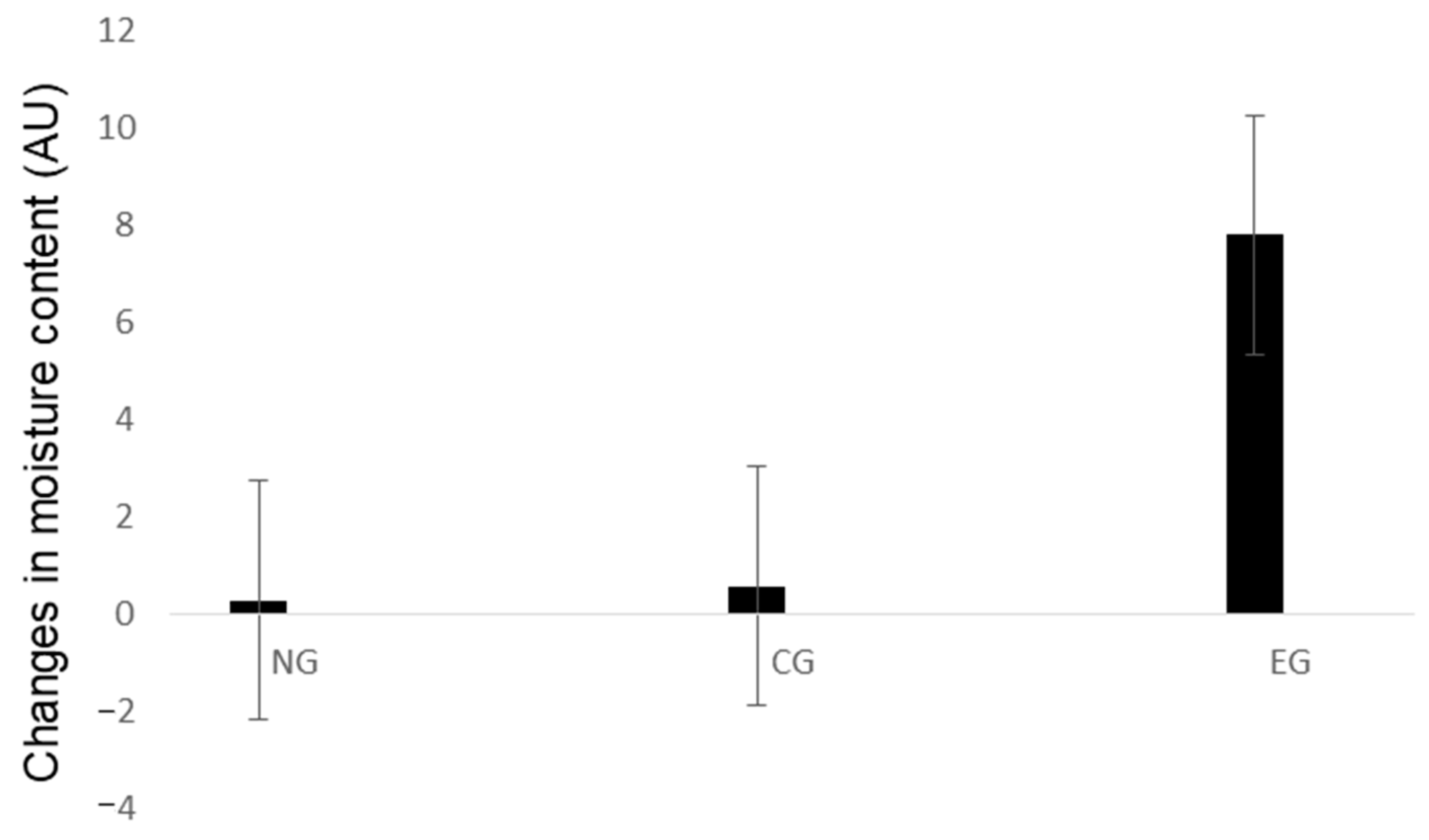
Changes in skin moisture content were measured by subtracting the values before and after sample application (Table 3 and Figure 5). The skin moisture content value significantly increased at 8 h after application (p < 0.05), indicating that HA microneedle patch with LEE soluble essence significantly improved skin moisturizing content.

Table 3.
Changes in moisture content values in each group before and after the experiment.
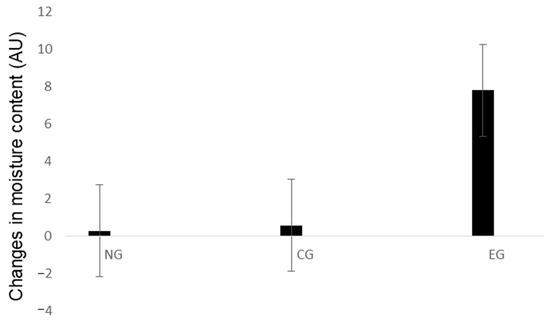
Figure 5.
Changes in moisture content values. Time, hours; EG, experimental group; CG, control group; NG, untreated group.
The moisture content in the experimental group was significantly higher than that in the untreated group at 8 h (Table 4).

Table 4.
Comparison of moisture content values between the groups after the experiment.
The skin moisture content value significantly increased at 8 h after application (p < 0.05), indicating that HA microneedle patch with LEE soluble essence significantly improved skin moisturizing content.
The results of the clinical assessments showed that the HA microneedle patch with LEE soluble essence contributed toward improving skin moisture content. Lonicerae flos, a natural raw ingredient used in oriental medicine, has been used to treat febrile diseases. Recently, various studies related to compounds useful for skin moisturization have been conducted, and these compounds are actively used in skin moisturizing products. In addition, needle patches containing HA have recently been developed. Their usefulness and marketability are continuously increasing; therefore, it is necessary to provide a scientific basis for their clinical use. In this study, we produced a HA needle patch with LEE soluble essence, and we conducted a clinical study to investigate its effect on human skin moisturization.
The results suggest that using the HA microneedle patch with LEE soluble essence as well as a combination of skin moisturizing effects, based on the previously known moisturizing effect, have certain effects on skin health.
4. Conclusions
In the present study, the effectiveness of HA and Lonicerae flos for improving skin moisturization was clinically assessed following approval from the IRB. Improvement in skin moisturizing was significantly higher with HA microneedle patch with LEE soluble essence (EG) than HA microneedle patch without LEE soluble essence (CG) after the application. These results confirm that Lonicerae flos can improve skin moisture content and maintain skin health. Lonicerae flos is known to contain saponins, flavonoids, and iridoids along with chlorogenic acid. Saponin and iridoid glycosides have been known to have toxic reactions to some animal cells. Therefore, it is necessary to identify the relationship between individual components contained in Lonicerae flos in advanced studies. In addition, these results suggest a scientific basis for adding the combination of HA and Lonicerae flos to existing moisturizing formulas. Furthermore, our results can be used as basic data to increase the effectiveness of skin moisturizing natural raw materials.
Author Contributions
Conceptualization, H.-Y.H. and S.-J.L.; methodology, H.-Y.H.; software, S.-K.H.; formal analysis, H.-Y.H. and S.-K.H.; investigation, H.-Y.H. and S.-K.H.; resources, H.-Y.H. and S.-J.L.; data curation, H.-Y.H. and S.-K.H.; writing—original draft preparation, H.-Y.H., S.-J.L. and S.-K.H.; writing—review and editing, H.-Y.H.; visualization, S.-K.H.; supervision, H.-Y.H.; project administration, H.-Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Semyung University Oriental Medicine Hospital (protocol code SMCTC-053-18-004 and date of approval 10 May 2018).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yoo, I.D.; Kim, J.P.; Kim, W.G.; Yun, B.S.; Ryoo, I.J. Development of new natural antioxidants for cosmeceuticals. J. Soc. Cosmet. Sci. Korea 2005, 31, 349–357. [Google Scholar]
- Seo, Y.K.; Koh, J.S.; Lee, W.C. Investigation of the study plan and statistical method of functional cosmetics on human skin. J. Soc. Cosmet. Sci. Korea 2013, 39, 105–115. [Google Scholar]
- Lee, P.J.; Ha, H.Y. Effect of Lonicera japonica ethanol extract on hyaluronic acid production in HaCaT cells. J. Investig. Cosmetol. 2018, 14, 441–446. [Google Scholar]
- Song, H.J.; Jin, M.H.; Lee, S.H. Effect of ferulic acid isolated from Cnidium officinale on the synthesis of hyaluronic acid. J. Soc. Cosmet. Sci. Korea 2013, 39, 281–288. [Google Scholar]
- Han, M.S.; Park, Y.J.; Kim, H.S. Evaluation of automated assays for measuring serum hyaluronic acid: For the diagnosis of Rheumatoid Arthritis. Lab Med. Online 2014, 4, 98–104. [Google Scholar] [CrossRef][Green Version]
- Han, S.K.; Ha, H.Y. The moisturizing effect of the co-administration of hyaluronic acid micro fill patch and Scutellariae Radix ethanol extract on human skin. J. Investig. Cosmetol. 2017, 13, 21–25. [Google Scholar]
- Park, H.J.; Hahn, J.P.; Park, J.H. A study on korean cosmeceutical firm’s market penetration strategy for southeast asia -focused on indonesia and malaysia. J. Korea Trade Res. Assoc. 2015, 40, 75–101. [Google Scholar]
- Kim, S.M.; Shin, D.I.; Yoon, S.T.; Song, H.S. Distribution and habitat chracteristics of Lonicera japonica Thunb. in the inland and the seashore areas of Korea. Korean J. Med. Crop Sci. 2007, 15, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, Y.J.; Park, S.N. Synergistic antimicrobial effect of Lonicera japonica and Magnolia obovata Extracts and Potential as a Plant-Derived Natural Preservative. J. Microbiol. Biotechnol. 2018, 28, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Li, W.; Fu, C.; Song, Y.; Fu, Q. Lonicerae japonicae flos and Lonicerae flos: A systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochem. Rev. 2020, 19, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Kwon, O.S.; Jin, J.H.; Son, K.H.; Kim, H.P. Inhibition of experimental systemic inflammation (septic inflammation) and chronic bronchitis by new phytoformula BL containing Broussonetia papyrifera and Lonicera japonica. Biomol. Ther. 2013, 21, 66–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.K.; Sin, J.S.; Kim, J.H.; Eom, J.H.; Kim, H.S.; Park, K.L. Evaluation of phototoxicity for cosmetics and alternative method. J. Soc. Cosmet. Sci. Korea 2005, 31, 245–251. [Google Scholar]
- Jo, G.W.; Hwang, C.Y.; Hong, S.H.; Kim, N.K. A systematic review for the development of cosmetic clinical trial protocol. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2013, 26, 104–117. [Google Scholar] [CrossRef][Green Version]
- Lee, S.J. Fabrication and characterization of micropatterned elastomeric PDMS mold. J. Adv. Eng. Technol. 2017, 10, 335–339. [Google Scholar]
- Min, H.K.; Kim, J.A.; Kim, J.C.; Lee, S.J. Preparation and characterization of superparamagnetic microneedles (SMMNs) containing magnetic cubosome nanoparticles (MCNs). J. Adv. Eng. Technol. 2019, 12, 103–108. [Google Scholar]
- Han, S.K.; Ha, H.Y.; Lee, S.J. Fabrication of microneedles array combining biodegradable hyaluronic acid and herbal medicine Lonicera flos. Polymer (Korea) 2019, 43, 540–546. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).