Effects and Risk Assessment of the Polycyclic Musk Compounds Galaxolide® and Tonalide® on Marine Microalgae, Invertebrates, and Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Selections
2.2. Acute Toxicity Test
2.2.1. Microalgae Growth Inhibition Test
2.2.2. Artemia Toxicity Test
2.2.3. Sea Urchin Toxicity Test
2.2.4. Mussels Larvae Development Test
2.2.5. Fish Larva Mortality Test
2.3. Statistical Analysis
3. Results
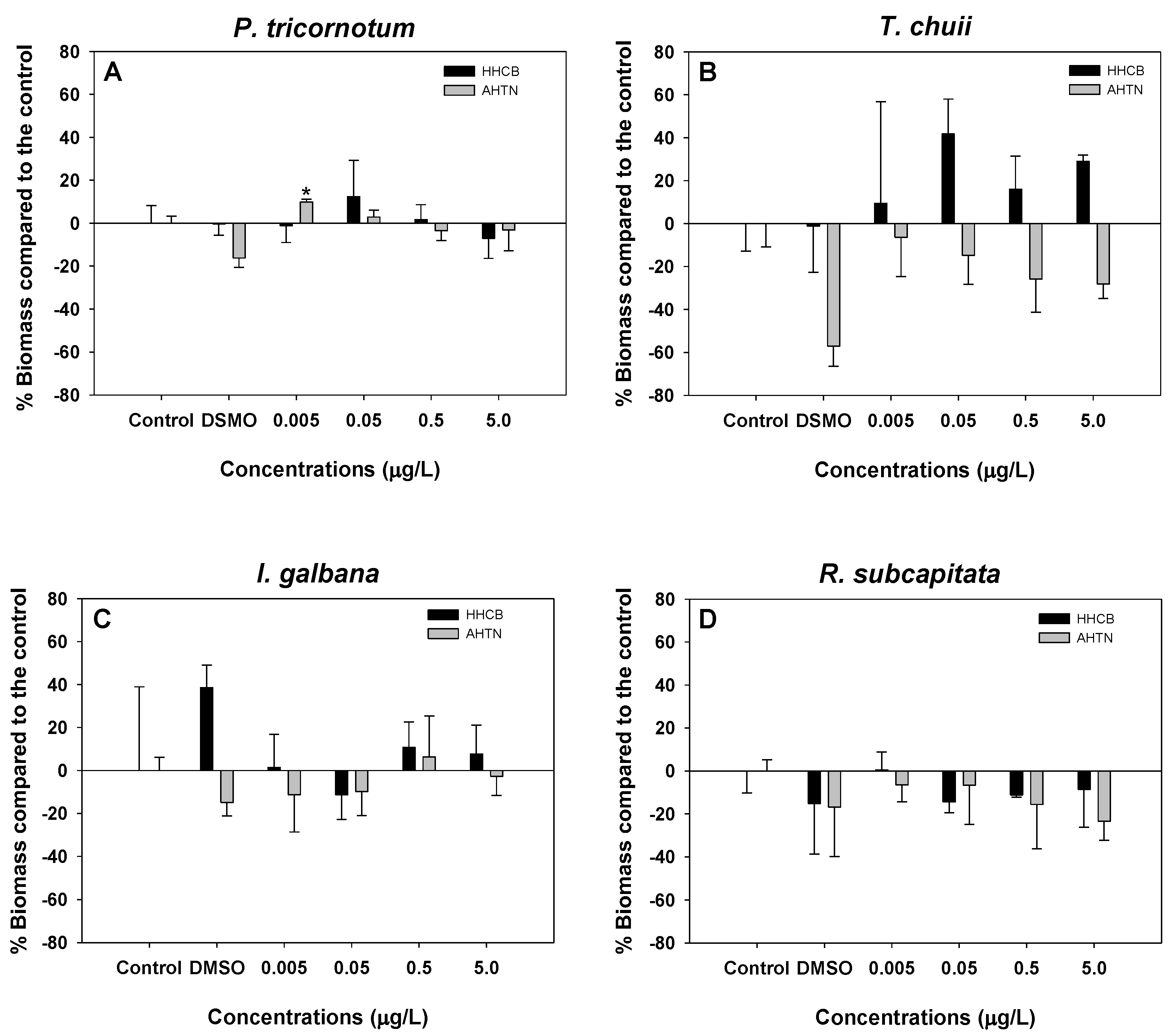
3.1. Microalgae Growth Inhibition Tests
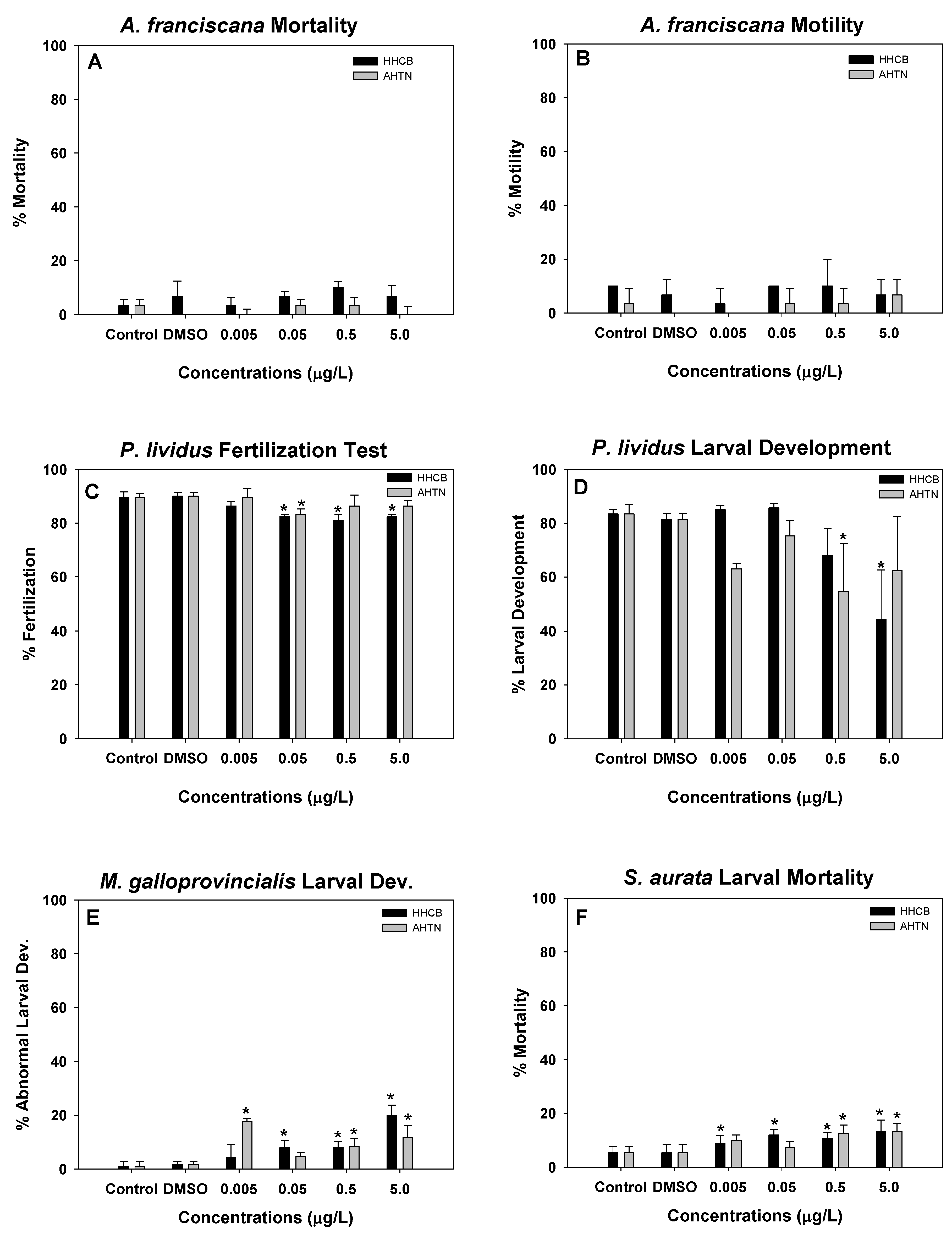
3.2. Artemia, Sea Urch, Mussels, and Fish Early Life Stage Toxicity Tests
3.3. Risk Quotient (RQ)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OSPAR. Musk Xylene and Other Musks. Hazard. Subst. Ser. 2004, 1–160. Available online: https://www.ospar.org/documents?v=6978 (accessed on 15 February 2021).
- Gao, Y.; Ji, Y.; Li, G.; Mai, B.; An, T. Bioaccumulation and ecotoxicity increase during indirect photochemical transformation of polycyclic musk tonalide: A modeling study. Water Res. 2016, 105, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Dietrich, D.R.; Hitzfeld, B.C. Bioaccumulation and ecotoxicity of synthetic musks in the aquatic environment. Handb. Environ. Chem. 2004, 3, 233–244. [Google Scholar] [CrossRef]
- Balk, F.; Ford, R.A. Environmental risk assessment for the polycyclic musks, AHTN and HHCB II. Effect assessment and risk characterisation. Toxicol. Lett. 1999, 111, 81–94. [Google Scholar] [CrossRef]
- Bester, K.; Hühnerfuss, H.; Lange, W.; Rimkus, G.G.; Theobald, N. Results of non target screening of lipophilic organic pollutants in the German Bight II: Polycyclic musk fragrances. Water Res. 1998, 32, 1857–1863. [Google Scholar] [CrossRef]
- Díaz-Garduño, B.; Pintado-Herrera, M.; Biel-Maeso, M.; Rueda-Márquez, J.; Lara-Martín, P.; Perales, J.; Manzano, M.; Garrido-Pérez, C.; Martín-Díaz, M. Environmental risk assessment of effluents as a whole emerging contaminant: Efficiency of alternative tertiary treatments for wastewater depuration. Water Res. 2017, 119, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Otto, T.; Pilz, K. Polycyclic musk fragrances in different environmental compartments in Berlin (Germany). Water Res. 2001, 35, 121–128. [Google Scholar] [CrossRef]
- Kannan, K.; Reiner, J.L.; Yun, S.H.; Perrotta, E.E.; Tao, L.; Johnson-Restrepo, B.; Rodan, B.D. Polycyclic musk compounds in higher trophic level aquatic organisms and humans from the United States. Chemosphere 2005, 61, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H. Occurrence of synthetic musk fragrances in marine mammals and sharks from Japanese coastal waters. Environ. Sci. Technol. 2007, 41, 2216–2222. [Google Scholar] [CrossRef]
- Sumner, N.R.; Guitart, C.; Fuentes, G.; Readman, J.W. Inputs and distributions of synthetic musk fragrances in an estuarine and coastal environment; a case study. Environ. Pollut. 2010, 158, 215–222. [Google Scholar] [CrossRef]
- Villa, S.; Assi, L.; Ippolito, A.; Bonfanti, P.; Finizio, A. First evidences of the occurrence of polycyclic synthetic musk fragrances in surface water systems in Italy: Spatial and temporal trends in the Molgora River (Lombardia Region, Northern Italy). Sci. Total. Environ. 2012, 416, 137–141. [Google Scholar] [CrossRef]
- Dsikowitzky, L.; Schwarzbauer, J.; Littke, R. Distribution of polycyclic musks in water and particulate matter of the Lippe River (Germany). Org. Geochem. 2002, 33, 1747–1758. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. Environmentally friendly analysis of emerging contaminants by pressurized hot water extraction–stir bar sorptive extraction–derivatization and gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-B.; Lee, D.-H.; Lee, Y.S.; Kannan, K. Occurrence and accumulation patterns of polycyclic aromatic hydrocarbons and synthetic musk compounds in adipose tissues of Korean females. Chemosphere 2012, 86, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Sasaki, H.; Takemura, A.; Yoshioka, M.; Tanabe, S.; Kannan, K. Bioaccumulation, Temporal Trend, and Geographical Distribution of Synthetic Musks in the Marine Environment. Environ. Sci. Technol. 2005, 39, 3430–3434. [Google Scholar] [CrossRef]
- Rüdel, H.; Böhmer, W.; Schröter-Kermani, C. Retrospective monitoring of synthetic musk compounds in aquatic biota from German rivers and coastal areas. J. Environ. Monit. 2006, 8, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Vandermeersch, G.; Lourenço, H.M.; Alvarez-Muñoz, D.; Cunha, S.; Diogène, J.; Cano-Sancho, G.; Sloth, J.J.; Kwadijk, C.; Barcelo, D.; Allegaert, W.; et al. Environmental contaminants of emerging concern in seafood—European database on contaminant levels. Environ. Res. 2015, 143, 29–45. [Google Scholar] [CrossRef]
- Gatermann, R.; Biselli, S.; Hühnerfuss, H.; Rimkus, G.G.; Hecker, M.; Karbe, L. Synthetic Musks in the Environment. Part 1: Species-Dependent Bioaccumulation of Polycyclic and Nitro Musk Fragrances in Freshwater Fish and Mussels. Arch. Environ. Contam. Toxicol. 2002, 42, 437–446. [Google Scholar] [CrossRef]
- Cunha, S.; Trabalón, L.; Jacobs, S.; Castro, M.; Fernandez-Tejedor, M.; Granby, K.; Verbeke, W.; Kwadijk, C.; Ferrari, F.; Robbens, J.; et al. UV-filters and musk fragrances in seafood commercialized in Europe Union: Occurrence, risk and exposure assessment. Environ. Res. 2018, 161, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Balk, F.; Ford, R.A. Environmental risk assessment for the polycyclic musks AHTN and HHCB in the EU. I. Fate and exposure assessment. Toxicol. Lett. 1999, 111, 57–79. [Google Scholar] [CrossRef]
- Breitholtz, M.; Wollenberger, L.; Dinan, L. Effects of four synthetic musks on the life cycle of the harpacticoid copepod Nitocra spinipes. Aquat. Toxicol. 2003, 63, 103–118. [Google Scholar] [CrossRef]
- Carlsson, G.; Orn, S.; Andersson, P.L.; Söderström, H.; Norrgren, L. The impact of musk ketone on reproduction in zebrafish (Danio rerio). Mar. Environ. Res. 2000, 50, 237–241. [Google Scholar] [CrossRef]
- Carlsson, G.; Norrgren, L. Synthetic musk toxicity to early life stages of zebrafish (Danio rerio). Arch. Environ. Contam. Toxicol. 2004, 46, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Gooding, M.P.; Newton, T.J.; Bartsch, M.R.; Hornbuckle, K.C. Toxicity of Synthetic Musks to Early Life Stages of the Freshwater Mussel Lampsilis cardium. Arch. Environ. Contam. Toxicol. 2006, 51, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, Q.; Liu, S.; Xiu, Z. Acute toxicity, biochemical and gene expression responses of the earthworm Eisenia fetida exposed to polycyclic musks. Chemosphere 2011, 83, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Luckenbach, T.; Epel, D. Nitromusk and Polycyclic Musk Compounds as Long-Term Inhibitors of Cellular Xenobiotic Defense Systems Mediated by Multidrug Transporters. Environ. Heal. Perspect. 2005, 113, 17–24. [Google Scholar] [CrossRef]
- Parolini, M.; Magni, S.; Traversi, I.; Villa, S.; Finizio, A.; Binelli, A. Environmentally relevant concentrations of galaxolide (HHCB) and tonalide (AHTN) induced oxidative and genetic damage in Dreissena polymorpha. J. Hazard. Mater. 2015, 285, 1–10. [Google Scholar] [CrossRef]
- Pedersen, S.; Selck, H.; Salvito, D.; Forbes, V. Effects of the polycyclic musk HHCB on individual- and population-level endpoints in Potamopyrgus antipodarum. Ecotoxicol. Environ. Saf. 2009, 72, 1190–1199. [Google Scholar] [CrossRef]
- Wollenberger, L.; Breitholtz, M.; Kusk, K.O.; Bengtsson, B.-E. Inhibition of larval development of the marine copepod Acartia tonsa by four synthetic musk substances. Sci. Total. Environ. 2003, 305, 53–64. [Google Scholar] [CrossRef]
- Yamauchi, R.; Ishibashi, H.; Hirano, M.; Mori, T.; Kim, J.-W.; Arizono, K. Effects of synthetic polycyclic musks on estrogen receptor, vitellogenin, pregnane X receptor, and cytochrome P450 3A gene expression in the livers of male medaka (Oryzias latipes). Aquat. Toxicol. 2008, 90, 261–268. [Google Scholar] [CrossRef]
- European Commission. European Union Risk Assessment Report on AHTN. Rep. AHTN 2008. [Google Scholar] [CrossRef]
- European Commission. European Union Risk Assessment Report on HHCB. Rep. HHCB 2008. [Google Scholar] [CrossRef]
- Human & Environmental Risk Assessment on ingredients of Household Cleaning Products (HERA). Polycyclic musks AHTN (CAS 1506-02-1) and HHCB (CAS 1222-05-05). Scenario 2004. [Google Scholar]
- ECHA. Chapter R.10: Characterisation of dose [concentration]-response for environment. In Guidance on Information Requirements and Chemical Safety Assessment; European Chemicals Agency: Helsinki, Finland, 2008; pp. 1–65. [Google Scholar]
- De Lange, H.; Sala, S.; Vighi, M.; Faber, J. Ecological vulnerability in risk assessment—A review and perspectives. Sci. Total. Environ. 2010, 408, 3871–3879. [Google Scholar] [CrossRef] [PubMed]
- Fleeger, J.W.; Carman, K.R.; Nisbet, R.M. Indirect effects of contaminants in aquatic ecosystems. Sci. Total. Environ. 2003, 317, 207–233. [Google Scholar] [CrossRef]
- Johnston, E.L.; Roberts, D.A. Contaminants reduce the richness and evenness of marine communities: A review and meta-analysis. Environ. Pollut. 2009, 157, 1745–1752. [Google Scholar] [CrossRef]
- Brink, P.J.V.D. Ecological Risk Assessment: From Book-Keeping to Chemical Stress Ecology. Environ. Sci. Technol. 2008, 42, 8999–9004. [Google Scholar] [CrossRef][Green Version]
- Díaz-Garduño, B.; Rueda-Márquez, J.; Manzano, M.; Garrido-Pérez, C.; Martín-Díaz, M. Are combined AOPs effective for toxicity reduction in receiving marine environment? Suitability of battery of bioassays for wastewater treatment plant (WWTP) effluent as an ecotoxicological assessment. Mar. Environ. Res. 2016, 114, 1–11. [Google Scholar] [CrossRef]
- Meriç, S.; De Nicola, E.; Iaccarino, M.; Gallo, M.; Di Gennaro, A.; Morrone, G.; Warnau, M.; Belgiorno, V.; Pagano, G. Toxicity of leather tanning wastewater effluents in sea urchin early development and in marine microalgae. Chemosphere 2005, 61, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I.; Campaña, O.; Lubián, L.; Blasco, J. Calcium alginate immobilized marine microalgae: Experiments on growth and short-term heavy metal accumulation. Mar. Pollut. Bull. 2005, 51, 823–829. [Google Scholar] [CrossRef]
- Yu, Y.; Kong, F.; Wang, M.; Qian, L.; Shi, X. Determination of short-term copper toxicity in a multispecies microalgal population using flow cytometry. Ecotoxicol. Environ. Saf. 2007, 66, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Okumura, J.K.Y.; Koyama, J.; Takaku, H.; Satoh, H.; Okumura, Y. Influence of Organic Solvents on the Growth of Marine Microalgae. Arch. Environ. Contam. Toxicol. 2001, 41, 123–128. [Google Scholar] [CrossRef]
- Latała, A.; Nędzi, M.; Stepnowski, P. Acute toxicity assessment of perfluorinated carboxylic acids towards the Baltic microalgae. Environ. Toxicol. Pharmacol. 2009, 28, 167–171. [Google Scholar] [CrossRef]
- Mariani, L.; De Pascale, D.; Faraponova, O.; Tornambè, A.; Sarni, A.; Giuliani, S.; Ruggiero, G.; Onorati, F.; Magaletti, E. The use of a test battery in marine ecotoxicology: The acute toxicity of sodium dodecyl sulfate. Environ. Toxicol. 2006, 21, 373–379. [Google Scholar] [CrossRef]
- OECD. No. 210: Fish, Early-Life Stage Toxicity Test. OECD Test 1992, 2, 1–24. [Google Scholar] [CrossRef]
- Environment Canada. Biological test method: Fertilization assay using echinoids (sea urchins and sand dollars). In Method Dev. Appl. Sect.; 2011; p. 111. Available online: http://publications.gc.ca/collections/collection_2011/ec/En49-7-1-27-eng.pdf (accessed on 15 February 2021).
- US EPA. Guidelines for Exposure Assessment. Risk Assess. Forum 1992, 57, 22888–22938. [Google Scholar]
- US EPA. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 5th ed.; U.S. Environmental Protection AgencyOffice of Water: Washington, DC, USA, 2002. [Google Scholar]
- Ehiguese, F.O.; Fernandez, M.D.C.C.; Lara-Martín, P.A.; Martín-Díaz, M.L.; Araújo, C.V. Avoidance behaviour of the shrimp Palaemon varians regarding a contaminant gradient of galaxolide and tonalide in seawater. Chemosphere 2019, 232, 113–120. [Google Scholar] [CrossRef]
- Garrido-Perez, M.C.; Perales-VargasMachuca, J.A.; Nebot-Sanz, E.; Sales-Márquez, D.; Garrido-Pérez, M.D.C. Effect of the test media and toxicity of LAS on the growth of Isochrysis galbana. Ecotoxicol. 2008, 17, 738–746. [Google Scholar] [CrossRef]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana hustedt, and Detonula confervacea (CLEVE) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Kilham, S.S.; Kreeger, D.A.; Lynn, S.G.; Goulden, C.E.; Herrera, L. COMBO: A defined freshwater culture medium for algae and zooplankton. Hydrobiol. 1998, 377, 147–159. [Google Scholar] [CrossRef]
- Beiras, R.; Vázquez, E.; Bellas, J.; Lorenzo, J.; Fernández, N.; Macho, G.; Mariño, J.; Casas, L. Sea-urchin Embryo Bioassay for in situ Evaluation of the Biological Quality of Coastal Seawater. Estuarine, Coast. Shelf Sci. 2001, 52, 29–32. [Google Scholar] [CrossRef]
- ASTM International. Standard guide for conducting acute toxicity test starting with embryos of four species of saltwater bivalve molluscs. 2012, 21, E724–E798. [Google Scholar] [CrossRef]
- Yoshioka, K.; Saito, M.; Oh, K.-B.; Nemoto, Y.; Matsuoka, H.; Natsume, M.; Abe, H. Intracellular Fate of 2-NBDG, a Fluorescent Probe for Glucose Uptake Activity, inEscherichia coliCells. Biosci. Biotechnol. Biochem. 1996, 60, 1899–1901. [Google Scholar] [CrossRef]
- Zhang, H.; Bayen, S.; Kelly, B.C. Multi-residue analysis of legacy POPs and emerging organic contaminants in Singapore’s coastal waters using gas chromatography–triple quadrupole tandem mass spectrometry. Sci. Total. Environ. 2015, 523, 219–232. [Google Scholar] [CrossRef]
- Schreurs, R.H.; Quaedackers, M.E.; Seinen, W.; Van Der Burg, B. Transcriptional Activation of Estrogen Receptor ERα and ERβ by Polycyclic Musks Is Cell Type Dependent. Toxicol. Appl. Pharmacol. 2002, 183, 1–9. [Google Scholar] [CrossRef]
- Składanowski, A.; Stepnowski, P.; Kleszczyński, K.; Dmochowska, B. AMP deaminase in vitro inhibition by xenobiotics. Environ. Toxicol. Pharmacol. 2005, 19, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tumová, J.; Šauer, P.; Golovko, O.; Ucun, O.K.; Grabic, R.; Máchová, J.; Kroupová, H.K. Effect of polycyclic musk compounds on aquatic organisms: A critical literature review supplemented by own data. Sci. Total. Environ. 2019, 651, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Sibila, M.; Garrido, M.; Perales, J.; Quiroga, J. Ecotoxicity and biodegradability of an alkyl ethoxysulphate surfactant in coastal waters. Sci. Total. Environ. 2008, 394, 265–274. [Google Scholar] [CrossRef] [PubMed]
- OECD. Proposal for updating guideline 201 freshwater alga and cyanobacteria, growth inhibition test. In OECD Guidelines for Testing of Chemicals; OECD: Paris, France, 2002; pp. 1–21. [Google Scholar]
- Ding, T.; Li, W.; Cai, M.; Jia, X.; Yang, M.; Yang, B.; Li, J. Algal toxicity, accumulation and metabolic pathways of galaxolide. J. Hazard. Mater. 2020, 384, 121360. [Google Scholar] [CrossRef]
- Ferreira, C.S.G.; Nunes, B.A.; Henriques-Almeida, J.M.D.M.; Guilhermino, L. Acute toxicity of oxytetracycline and florfenicol to the microalgae Tetraselmis chuii and to the crustacean Artemia parthenogenetica. Ecotoxicol. Environ. Saf. 2007, 67, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Hadjispyrou, S.; Kungolos, A.; Anagnostopoulos, A. Toxicity, Bioaccumulation, and Interactive Effects of Organotin, Cadmium, and Chromium on Artemia franciscana. Ecotoxicol. Environ. Saf. 2001, 49, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.H.; Guasto, J.S.; Zimmer, R.K.; Stocker, R.; Riffell, J.A. Sperm chemotaxis promotes individual fertilization success in sea urchins. J. Exp. Biol. 2016, 219, 1458–1466. [Google Scholar] [CrossRef]
- Capolupo, M.; Díaz-Garduño, B.; Martín-Díaz, M.L. The impact of propranolol, 17α-ethinylestradiol, and gemfibrozil on early life stages of marine organisms: Effects and risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 32196–32209. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Martínez, G.V.; DelValls, A.T.; Martín-Díaz, M.L. Yes, caffeine, ibuprofen, carbamazepine, novobiocin and tamoxifen have an effect on Corbicula fluminea (Müller, 1774). Ecotoxicol. Environ. Saf. 2015, 120, 142–154. [Google Scholar] [CrossRef]
- Paredes, E.; Perez, S.; Rodil, R.; Quintana, J.; Beiras, R. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere 2014, 104, 44–50. [Google Scholar] [CrossRef]
- Beiras, R.; Fernandez, N.; Bellas, J.; Besada, V.; González-Quijano, A.; Nunes, T. Integrative assessment of marine pollution in Galician estuaries using sediment chemistry, mussel bioaccumulation, and embryo-larval toxicity bioassays. Chemosphere 2003, 52, 1209–1224. [Google Scholar] [CrossRef]
- Bellas, J.; Granmo, Å.; Beiras, R. Embryotoxicity of the antifouling biocide zinc pyrithione to sea urchin (Paracentrotus lividus) and mussel (Mytilus edulis). Mar. Pollut. Bull. 2005, 50, 1382–1385. [Google Scholar] [CrossRef]
- Losso, C.; Picone, M.; Novelli, A.A.; Delaney, E.; Ghetti, P.F.; Ghirardini, A.V. Developing Toxicity Scores for Embryotoxicity Tests on Elutriates with the Sea Urchin Paracentrotus lividus, the Oyster Crassostrea gigas, and the Mussel Mytilus galloprovincialis. Arch. Environ. Contam. Toxicol. 2007, 53, 220–226. [Google Scholar] [CrossRef]
- Aguirre-Martínez, G.; Owuor, M.; Garrido-Pérez, C.; Salamanca, M.; Del Valls, T.; Martín-Díaz, M. Are standard tests sensitive enough to evaluate effects of human pharmaceuticals in aquatic biota? Facing changes in research approaches when performing risk assessment of drugs. Chemosphere 2015, 120, 75–85. [Google Scholar] [CrossRef] [PubMed]
| Country/Location | HHCB | AHTN | Reference |
|---|---|---|---|
| Germany (North Sea) | 0.09–4.8 | 0.08–2.6 | [6] |
| Germany (Elbe Estuary) | 95–136 | 65–200 | [6] |
| United Kingdom (Tamar Estuarine—Plym Sound) | 6.00–30 | 3.00–15 | [11] |
| Spain (Bay of Cadiz) | 230 ± 0.1 | NA | [14] |
| Singapore (Coastal water) | 1.66–21.8 | 0.244–1.85 | [58] |
| Microalgae Growth | Embryo-Larval Development | ||||
| P. tricornutum | I. galbana | P. lividus | M. galloprovincialis | ||
| HHCB | EC50 | NC | NC | 4.063 (0.963–120.731) | NC |
| EC10 | 0.127(NC) | 5.22(NC) | 0.004 (0.000–0.025) | 0.188(0.074–0.390) | |
| MEC/PNEC | 0.378–18.110 | 0.009–0.440 | 1200–57500 | 25.532–1223.404 | |
| Risk | Yes | No | Yes | Yes | |
| ANTH | EC50 | NC | NC | NC | NC |
| EC10 | 0.002(0.000 – 0.014) | 0.328(NC) | 0.006 (NC) | ||
| MEC/PNEC | 24–1150 | 0.146–7.0122 | NC | 800–38333.330 | |
| Risk | Yes | Yes | NC | Yes | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehiguese, F.O.; González-Delgado, M.J.; Garrido-Perez, C.; Araújo, C.V.M.; Martin-Diaz, M.L. Effects and Risk Assessment of the Polycyclic Musk Compounds Galaxolide® and Tonalide® on Marine Microalgae, Invertebrates, and Fish. Processes 2021, 9, 371. https://doi.org/10.3390/pr9020371
Ehiguese FO, González-Delgado MJ, Garrido-Perez C, Araújo CVM, Martin-Diaz ML. Effects and Risk Assessment of the Polycyclic Musk Compounds Galaxolide® and Tonalide® on Marine Microalgae, Invertebrates, and Fish. Processes. 2021; 9(2):371. https://doi.org/10.3390/pr9020371
Chicago/Turabian StyleEhiguese, Friday Ojie, M. Judit González-Delgado, Carmen Garrido-Perez, Cristiano V. M. Araújo, and M. Laura Martin-Diaz. 2021. "Effects and Risk Assessment of the Polycyclic Musk Compounds Galaxolide® and Tonalide® on Marine Microalgae, Invertebrates, and Fish" Processes 9, no. 2: 371. https://doi.org/10.3390/pr9020371
APA StyleEhiguese, F. O., González-Delgado, M. J., Garrido-Perez, C., Araújo, C. V. M., & Martin-Diaz, M. L. (2021). Effects and Risk Assessment of the Polycyclic Musk Compounds Galaxolide® and Tonalide® on Marine Microalgae, Invertebrates, and Fish. Processes, 9(2), 371. https://doi.org/10.3390/pr9020371








