Redox Effects of Molecular Hydrogen and Its Therapeutic Efficacy in the Treatment of Neurodegenerative Diseases
Abstract
1. Introduction
2. Characteristics of Molecular Hydrogen
3. Administration Routes of Hydrogen
4. H2 Acts as an Antioxidant Agent
5. Anti-Inflammatory Effects of H2 in Different Neurodegenerative Disease Models
6. Effects of Molecular Hydrogen on Animal and Human Models of Neurodegenerative Diseases
7. Hydrogen Therapy in Neonatal Brain Disorders
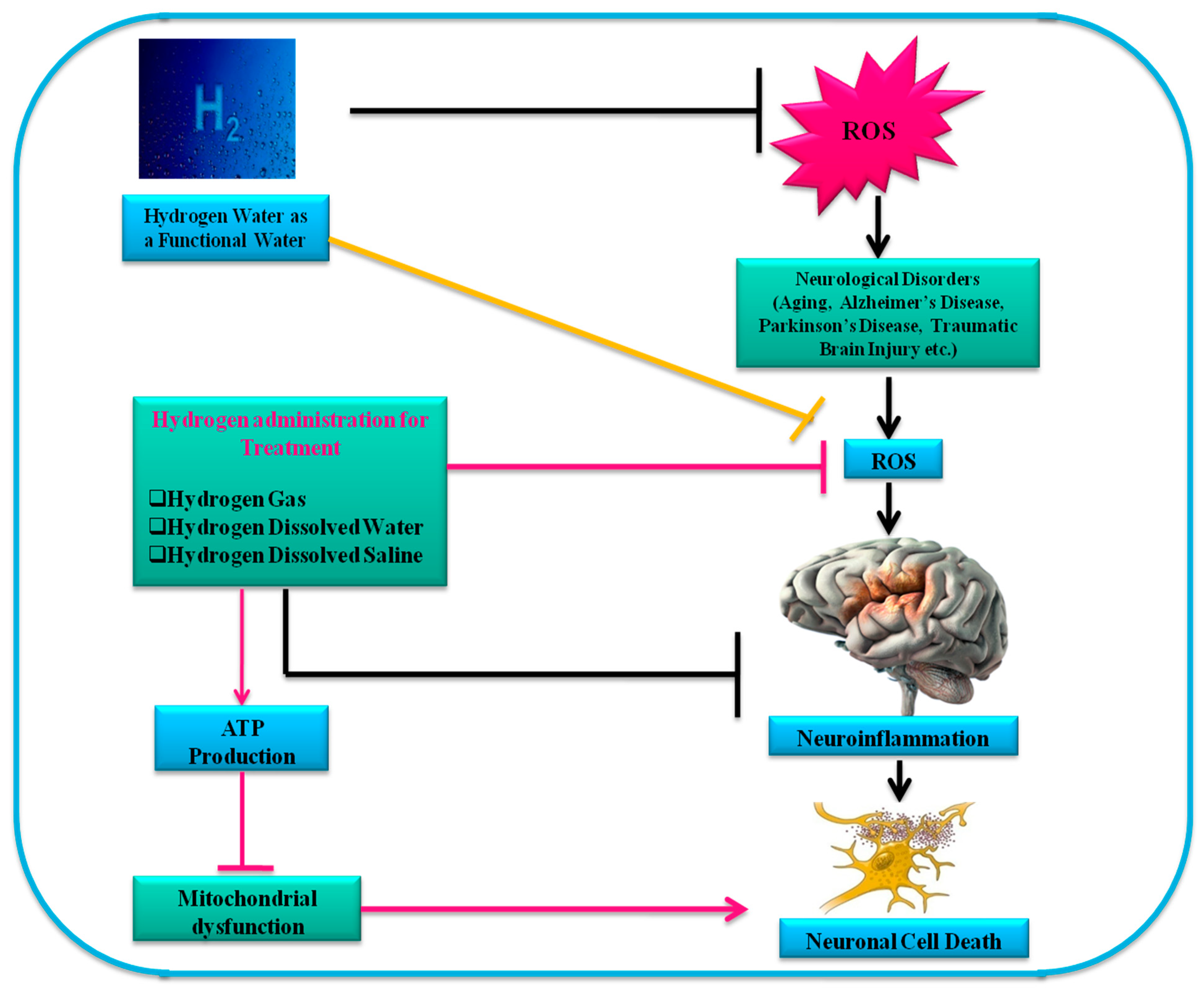
8. Mechanisms of Hydrogen Treatment in Neurodegenerative Diseases
9. Studies Related to Hydrogen Therapy in Neurodegenerative Diseases
10. Other Neurological Disorders
11. Therapeutic Efficacy of H2 Molecule
12. Novel Advantages of H2 Molecule
13. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OH | Hydroxyl radical |
| AD | Alzheimer’s disease |
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine triphosphate |
| Aβ | Amyloid beta |
| BDNF | Brain-derived neurotrophic factor |
| CAT | Catalase |
| CCL-2 | C-C motif chemokine ligand 2 |
| CNS | Central nervous system |
| FGF21 | Fibroblast growth factor 21 |
| FIRS | Inflammatory fetal response syndrome |
| GHSR | Growth hormone secretagogue receptor |
| GPx | Glutathione peroxidase |
| HD | Hemodialysis |
| HO-1 | Heme oxygenase-1 |
| HRW | Hydrogen-rich water |
| HS | Hydrogen dissolved saline |
| HW | H2-dissolved water (or H2-water) |
| IL | Interleukin |
| IR | Ischemia-reperfusion |
| IRI | Ischemia-reperfusion injury |
| JNK | c-Jun N-terminal Kinase |
| LPS | Lipopolysaccharides |
| LTP | Long-term potentiation |
| MAPK | Mitogen-activated protein kinase |
| MTTP | 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine |
| ND | Neurodegenerative disease |
| NF-κB | Nuclear factor κB |
| NLRP3 | NLR Family Pyrin Domain Containing 3 |
| Nrf2 | Nuclear factor-E2-related factor 2 |
| ONOO- | Peroxynitrite |
| OS | Oxidative stress |
| PD | Parkinson’s disease |
| ROS | Reactive oxygen species |
| SNpc | Substantia nigra pars compacta |
| SOD | Superoxide dismutase |
| TBI | Traumatic brain injury |
| TNF-α | Tumor necrosis factor-α |
References
- Tarozzi, A. Oxidative stress in neurodegenerative diseases: From preclinical studies to clinical applications. J. Clin. Med. 2020, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.; Moon, I.S. Neuroprotection against oxidative stress: Phytochemicals targeting TrkB signaling and the Nrf2-ARE antioxidant system. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Singh, E.; Devasahayam, G. Neurodegeneration by oxidative stress: A review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020, 1–8. [Google Scholar] [CrossRef]
- Yeung, A.W.; Tzvetkov, N.T.; Georgieva, M.G.; Ognyanov, I.V.; Kordos, K.; Jóźwik, A.; Kühl, T.; Perry, G.; Petralia, M.C.; Mazzon, E.; et al. Reactive oxygen species and their impact in neurodegenerative diseases: Literature landscape analysis. Antioxid. Redox Signal. 2021, 34, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hickey, M.J.; Borgstahl, G.E.; Hallewell, R.A.; Lepock, J.R.; O’Connor, D.; Hsieh, Y.; Nick, H.S.; Silverman, D.N.; Tainer, J.A. Crystal structure of Y34F mutant human mitochondrial manganese superoxide dismutase and the functional role of tyrosine 34. Biochemistry 1998, 37, 4722–4730. [Google Scholar] [CrossRef]
- Bošković, M.; Grabnar, I.; Terzič, T.; Plesničar, B.K.; Vovk, T. Oxidative stress in schizophrenia patients treated with long-acting haloperidol decanoate. Psychiatry.Res. 2013, 210, 761–768. [Google Scholar] [CrossRef]
- Ambani, L.M.; Van Woert, M.H.; Murphy, S. Brain peroxidase and catalase in Parkinson Disease. Arch. Neurol. 1975, 32, 114–118. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef]
- Terlecky, S.R.; Koepke, J.I.; Walton, P.A. Peroxisomes and aging. Biochim. Biophy. Acta 2006, 1763, 1749–1754. [Google Scholar] [CrossRef]
- Begum, R.; Kim, C.S.; Fadriquela, A.; Bajgai, J.; Jing, X.; Kim, D.H.; Kim, S.K.; Lee, K.J. Molecular hydrogen protects against oxidative stress-induced RAW 264.7 macrophage cells through the activation of Nrf2 and inhibition of MAPK signaling pathway. Mol. Cell Toxicol. 2020, 16, 103–118. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta 2012, 1820, 586–594. [Google Scholar] [CrossRef]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef]
- Noda, M.; Fujita, K.; Hamner, M.A.; Yamafuji, M.; Akimoto, N.; Kido, M.A.; Tanaka, Y.; Nakabeppu, Y.; Ransom, B.R. Molecular hydrogen protects against central nervous system white matter ischemic injury. In Proceedings of the SfN 42nd Annual Meeting, New Orleans, LA, USA, 13–17 October 2012; Volume 660, p. 14. [Google Scholar]
- Fujita, K.; Nakabeppu, Y.; Noda, M. Therapeutic effects of hydrogen in animal models of Parkinson’s disease. Parkinson Dis. 2011, 2011, 307875. [Google Scholar] [CrossRef]
- Ito, M.; Hirayama, M.; Yamai, K.; Goto, S.; Ichihara, M.; Ohno, K.; Ito, M. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med. Gas. Res. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Nagatani, K.; Nawashiro, H.; Takeuchi, S.; Tomura, S.; Otani, N.; Osada, H.; Wada, K.; Katoh, H.; Tsuzuki, N.; Mori, K. Safety of intravenous administration of hydrogen-enriched fluid in patients with acute cerebral ischemia: Initial clinical studies. Med. Gas. Res. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishijima, Y.; Ohta, S.; Sakamoto, M.; Kinone, K.; Horikosi, T.; Tamaki, M.; Takeshita, H.; Futatuki, T.; Ohishi, W.; et al. Hydrogen gas inhalation treatment in acute cerebral infarction: A randomized controlled clinical study on safety and neuroprotection. J. Stroke. Cerebrovasc. Dis. 2017, 26, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Laher, I.; Kura, B.; Slezak, J. Hydrogen gas: From clinical medicine to an emerging ergogenic molecule for sports athletes. Can. J. Physiol. Pharmacol. 2019, 97, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; de Mattos, G.F.; Settineri, R.; Costa, C.; Ellithorpe, R.; Rosenblatt, S.; Ohta, S. Clinical effects of hydrogen administration: From animal and human diseases to exercise medicine. Int. J. Clin. Med. 2016, 7, 32–76. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Y.; Wang, T.; Wei, S.J.; Block, M.L.; Wilson, B.; Liu, B.; Hong, J.S. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem. 2004, 279, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Spulber, S.; Edoff, K.; Hong, L.; Morisawa, S.; Shirahata, S.; Ceccatelli, S. Molecular hydrogen reduces LPS-induced neuroinflammation and promotes recovery from sickness behaviour in mice. PLoS ONE 2012, 7, e42078. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Kotani, T.; Ito, M.; Nagai, T.; Ichinohashi, Y.; Yamada, K.; Ohno, K.; Kikkawa, F.; Toyokuni, S. Maternal molecular hydrogen administration ameliorates rat fetal hippocampal damage caused by in utero ischemia–reperfusion. Free Radic. Biol. Med. 2014, 69, 324–330. [Google Scholar] [CrossRef]
- Yang, L.; Li, D.; Chen, S. Hydrogen water reduces NSE, IL-6, and TNF-α levels in hypoxic-ischemic encephalopathy. Open Med. 2016, 11, 399–406. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxid. Med. Cell Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef]
- Huang, C.S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic. Res. 2010, 44, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Abraini, J.H.; Gardette-Chauffour, M.C.; Martinez, E.; Rostain, J.C.; Lemaire, C. Psychophysiological reactions in humans during an open sea dive to 500 m with a hydrogen-helium-oxygen mixture. J. Appl. Physiol. 1994, 76, 1113–1118. [Google Scholar] [CrossRef]
- Fontanari, P.; Badier, M.; Guillot, C.; Tomei, C.; Burnet, H.; Gardette, B.; Jammes, Y. Changes in maximal performance of inspiratory and skeletal muscles during and after the 7.1-MPa Hydra 10 record human dive. Eur. J. Appl. Physiol. 2000, 81, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishijima, Y.; Adachi, N.; Sakamoto, M.; Kudo, Y.; Kaneko, K.; Nakao, A.; Imaoka, T. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 level. Med. Gas Res. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Ohsawa, I.; Nishimaki, K.; Yamagata, K.; Ishikawa, M.; Ohta, S. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem. Biophys. Res. Commun. 2008, 377, 1195–1198. [Google Scholar] [CrossRef]
- Nagata, K.; Nakashima-Kamimura, N.; Mikami, T.; Ohsawa, I.; Ohta, S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology 2009, 34, 501–508. [Google Scholar] [CrossRef]
- Sobue, S.; Yamai, K.; Ito, M.; Ohno, K.; Ito, M.; Iwamoto, T.; Ichihara, M. Simultaneous oral and inhalational intake of molecular hydrogen additively suppresses signaling pathways in rodents. Mol. Cell. Biochem. 2015, 403, 231–241. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Sun, X.; Li, R.; Sun, Q.; Cai, J.; Zhang, W. Hydrogen saline offers neuroprotection by reducing oxidative stress in a focal cerebral ischemia-reperfusion rat model. Med. Gas. Res. 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Grochowska, M.; Laskus, T.; Radkowski, M. Gut microbiota in neurological disorders. Arch. Immunol. Ther. Exp. 2019, 67, 375–383. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Han, C.; Ma, K.; Xia, Y.; Wan, F.; Yin, S.; Kou, L.; Sun, Y.; Wu, J.; Hu, J.; et al. Hydralazine protects nigrostriatal dopaminergic neurons from MPP+ and MPTP induced neurotoxicity: Roles of Nrf2-ARE signaling pathway. Front. Neurol. 2019, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006, 443, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.W. Triple endobutton technique in acromioclavicular joint reduction and reconstruction. Ann. Acad. Med. Singap. 2008, 37, 294. [Google Scholar]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R.J. Therapeutics, Nrf2–ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Clin. Pharm. Therap. 2016, 157, 84–104. [Google Scholar]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L.J. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef]
- Jakel, R.J.; Townsend, J.A.; Kraft, A.D.; Johnson, J.A. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007, 1144, 192–201. [Google Scholar] [CrossRef]
- Innamorato, N.G.; Jazwa, A.; Rojo, A.I.; García, C.; Fernández-Ruiz, J.; Grochot–Przeczek, A.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Cuadrado, A. Different susceptibility to the Parkinson’s toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS ONE 2010, 5, e11838. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, G.; Liu, S.Y.; Li, J.B.; Wang, J.F.; Bo, L.L.; Qian, L.R.; Sun, X.J.; Deng, X.M. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J. Surg. Res. 2011, 167, e339–e344. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lenahan, C.; Boling, W.; Tang, J.; Zhang, J.H. Molecular hydrogen application in stroke: Bench to bedside. Curr. Pharm. Des. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Manaenko, A.; Lekic, T.; Ma, Q.; Zhang, J.; Tang, J. Hydrogen inhalation ameliorated mast cell mediated brain injury after ICH in mice. Crit. Care Med. 2013, 41, 1266. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J. Hydrogen, a potential safeguard for graft-versus-host disease and graft ischemia-reperfusion injury? Clinics 2016, 71, 544–549. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, W.F.; Sun, H.Y.; Fan, D.F.; Nakao, A.; Cai, J.M.; Yan, G.; Zhou, W.P.; Shen, R.X.; Yang, J.M.; et al. Hydrogen-rich saline protects against liver injury in rats with obstructive jaundice. Liver Int. 2010, 30, 958–968. [Google Scholar] [CrossRef]
- Choi, J.; An, E.S.; Ban, Y.H.; Seo, D.W.; Kim, T.S.; Lee, S.P.; Kim, Y.B. Hydrogen-enriched water eliminates fine particles from the lungs and blood by enhancing phagocytic activity. J. Biomed. Res. 2017, 31, 503–511. [Google Scholar]
- Nishida, T.; Hayashi, T.; Inamoto, T.; Kato, R.; Ibuki, N.; Takahara, K.; Tanda, N. Dual gas treatment with hydrogen and carbon monoxide attenuates oxidative stress and protects from renal ischemia-reperfusion injury. Transplant. Proc. 2018, 1, 250–258. [Google Scholar] [CrossRef]
- Nishimaki, K.; Asada, T.; Ohsawa, I.; Nakajima, E.; Ikejima, C.; Yokota, T.; Ohta, S. Effects of molecular hydrogen assessed by an animal model and a randomized clinical study on mild cognitive impairment. Curr. Alzheimer Res. 2018, 15, 482–492. [Google Scholar] [CrossRef]
- Hayashi, T.; Yoshioka, T.; Hasegawa, K.; Miyamura, M.; Mori, T.; Ukimura, A.; Ishizaka, N. Inhalation of hydrogen gas attenuates left ventricular remodeling induced by intermittent hypoxia in mice. Am. J. Physiol. 2011, 301, H1062–H1069. [Google Scholar] [CrossRef]
- Tamaki, N.; Orihuela-Campos, R.C.; Fukui, M.; Ito, H.O. Hydrogen-rich water intake accelerates oral palatal wound healing via activation of the Nrf2/antioxidant defense pathways in a rat model. Oxid. Med. Cell Longev. 2016, 2016, 5679040. [Google Scholar] [CrossRef]
- Zhou, P.; Lin, B.; Wang, P.; Pan, T.; Wang, S.; Chen, W.; Liu, S. The healing effect of hydrogen-rich water on acute radiation-induced skin injury in rats. J. Radiat. Res. 2019, 60, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Does H2 alter mitochondrial bioenergetics via GHS-R1α activation? Theranostics 2017, 7, 1330–1332. [Google Scholar] [CrossRef]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Ohtsuka, C.; Maeda, T.; Hirayama, M.; Abe, T.; Watanabe, H.; Hatano, T. Randomized, double-blind, multicenter trial of hydrogen water for Parkinson’s disease. Mov. Disord. Clin. Pract. 2018, 33, 1505–1507. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Abe, T.; Ohtsuka, C.; Maeda, T.; Hirayama, M.; Watanabe, H.; Hatano, T. A randomized double-blind multi-center trial of hydrogen water for Parkinson’s disease: Protocol and baseline characteristics. BMC Neurol. 2016, 16, 66. [Google Scholar] [CrossRef]
- Lin, C.P.; Chuang, W.C.; Lu, F.J.; Chen, C.Y. Anti-oxidant and anti-inflammatory effects of hydrogen-rich water alleviate ethanol-induced fatty liver in mice. World J. Gastroenterol. 2017, 23, 4920–4934. [Google Scholar] [CrossRef]
- Kura, B.; Bagchi, A.K.; Singal, P.K.; Barancik, M.; LeBaron, T.W.; Valachova, K.; Šoltés, L.; Slezák, J. Molecular hydrogen: Potential in mitigating oxidative-stress-induced radiation injury. Can. J. Physiol. Pharm. 2018, 97, 287–292. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, D.; Liu, Y.; Chen, X.; Zhang, H.; Shen, F.; Liu, X.; Fu, J. Hydrogen-rich water attenuates oxidative stress in rats with traumatic brain injury via Nrf2 pathway. J. Surg. Res. 2018, 228, 238–246. [Google Scholar] [CrossRef]
- Chen, H.G.; Xie, K.L.; Han, H.Z.; Wang, W.N.; Liu, D.Q.; Wang, G.L.; Yu, Y.H. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. Int. J. Surg. 2013, 11, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—An update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, H.; Fan, Y.; Li, L.; Fang, J.; Yang, W. Hydrogen-rich saline attenuates cardiac and hepatic injury in doxorubicin rat model by inhibiting inflammation and apoptosis. Mediat. Inflamm. 2016, 2016, 1320365. [Google Scholar] [CrossRef]
- Tamura, T.; Hayashida, K.; Sano, M.; Onuki, S.; Suzuki, M. Efficacy of inhaled hydrogen on neurological outcome following brain ischemia during post-cardiac arrest care (HYBRID II trial): Study protocol for a randomized controlled trial. Trials 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haam, S.; Lee, J.G.; Paik, H.C.; Park, M.S.; Lim, B.J. Hydrogen gas inhalation during ex vivo lung perfusion of donor lungs recovered after cardiac death. J. Heart. Lung Transplant. 2018, 37, 1271–1278. [Google Scholar] [CrossRef]
- Abisso, T.G.; Adzavon, Y.M.; Zhao, P.; Zhang, X.; Liu, M.; Ma, X. Current progress in molecular hydrogen medication: Protective and therapeutic uses of hydrogen against different disease scenarios. Intern. Med. 2020, 10, 314. [Google Scholar]
- Imai, K.; Kotani, T.; Tsuda, H.; Mano, Y.; Nakano, T.; Ushida, T.; Hirakawa, A. Neuroprotective potential of molecular hydrogen against perinatal brain injury via suppression of activated microglia. Free Radic. Biol. Med. 2016, 91, 154–163. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xiong, S.; Zhang, J.; Wang, J.; Sun, A.; Mei, X.; Wang, Q. Protective effects of hydrogen-rich saline on ulcerative colitis rat model. J. Surg. Res. 2013, 185, 174–181. [Google Scholar] [CrossRef]
- Chen, C.H.; Manaenko, A.; Zhan, Y.; Liu, W.W.; Ostrowki, R.P.; Tang, J.; Zhang, J.H. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience 2010, 169, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kajiyama, S.; Amano, A.; Kondo, Y.; Sasaki, T.; Handa, S.; Fujinawa, H. Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem. Biophys. Res. Commun. 2008, 375, 346–350. [Google Scholar] [CrossRef]
- Nakayama, M.; Nakano, H.; Hamada, H.; Itami, N.; Nakazawa, R.; Ito, S.A. Novel bioactive haemodialysis system using dissolved dihydrogen (H2) produced by water electrolysis: A clinical trial. Nephrol. Dial. Transplant. 2010, 25, 3026–3033. [Google Scholar] [CrossRef]
- Domoki, F.; Oláh, O.; Zimmermann, A.; Németh, I.; Tóth-Szűki, V.; Hugyecz, M.; Bari, F. Hydrogen is neuroprotective and preserves cerebrovascular reactivity in asphyxiated newborn pigs. Pediatr. Res. 2010, 68, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Seike, T.; Yutsudo, N.; Ohno, M.; Yamada, H.; Yamaguchi, H.; Katafuchi, T. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS ONE 2009, 4, e7247. [Google Scholar] [CrossRef]
- Fu, Y.; Ito, M.; Fujita, Y.; Ito, M.; Ichihara, M.; Masuda, A.; Ohsawa, I. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett. 2009, 453, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011, 70, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Zhang, J.H.; Cai, J.M.; Cao, Y.P.; Sun, X.J. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010, 1328, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Liu, Q.; Yang, R.; Zhang, J.H.; Cao, Y.P.; Sun, X.J. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-κB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neurosci. Lett. 2011, 491, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Noe, E.; Ferri, J.; Colomer, C.; Moliner, B.; Chirivella, J. APOE genotype and verbal memory recovery during and after emergence from post-traumatic amnesia. Brain Inj. 2010, 24, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Shimmura, S.; Kubota, S.; Miyashita, H.; Kato, N.; Noda, K.; Ozawa, Y.; Usui, T.; Ishida, S.; Umezawa, K.; et al. Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Investig. Ophthal. Vis. Sci. 2011, 52, 427–433. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Q.; Mao, Y.; Xu, S.; Xia, C.; Shi, X.; Sun, X. Hydrogen-rich saline protects against spinal cord injury in rats. Neurochem. Res. 2010, 35, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Eckermann, J.M.; Chen, W.; Jadhav, V.; Hsu, F.P.; Colohan, A.R.; Tang, J.; Zhang, J.H. Hydrogen is neuroprotective against surgically induced brain injury. Med. Gas. Res. 2011, 1, 7. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, K.; Li, J.; Xu, N.; Gong, G.; Wang, G.; Xiong, L. Beneficial effects of hydrogen gas against spinal cord ischemia–reperfusion injury in rabbits. Brain Res. 2011, 1378, 125–136. [Google Scholar] [CrossRef]
- Matchett, G.A.; Fathali, N.; Hasegawa, Y.; Jadhav, V.; Ostrowski, R.P.; Martin, R.D.; Dorotta, I.R.; Sun, X.; Zhang, J.H. Hydrogen gas is ineffective in moderate and severe neonatal hypoxia–ischemia rat models. Brain Res. 2009, 1259, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishijima, Y.; Adachi, N.; Tachibana, S.; Chitoku, S.; Mukaihara, S.; Nawashiro, H. Improved brain MRI indices in the acute brain stem infarct sites treated with hydroxyl radical scavengers, edaravone and hydrogen, as compared to edaravone alone. A non-controlled study. Med. Gas. Res. 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Oharazawa, H.; Igarashi, T.; Yokota, T.; Fujii, H.; Suzuki, H.; Machide, M.; Ohsawa, I. Protection of the retina by rapid diffusion of hydrogen: Administration of hydrogen-loaded eye drops in retinal ischemia–reperfusion injury. Investig. Ophthalmol. Vis. Sci. 2010, 51, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Taura, A.; Kikkawa, Y.S.; Nakagawa, T.; Ito, J. Hydrogen protects vestibular hair cells from free radicals. Acta. Otolaryngol. 2010, 130, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kashio, A.; Sakamoto, T.; Suzukawa, K.; Kakigi, A.; Yamasoba, T. Hydrogen in drinking water attenuates noise-induced hearing loss in guinea pigs. Neurosci. Lett. 2011, 487, 12–16. [Google Scholar] [CrossRef]
- Terasaki, Y.; Ohsawa, I.; Terasaki, M.; Takahashi, M.; Kunugi, S.; Dedong, K.; Ishikawa, A. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L415–L426. [Google Scholar] [CrossRef]
- Qian, L.; Cao, F.; Cui, J.; Wang, Y.; Huang, Y.; Chuai, Y.; Cai, J. The potential cardio protective effects of hydrogen in irradiated mice. J. Radiat. Res. 2010, 51, 741–747. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y.; Okumura, M. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Asthma. Res. Pract. 2010, 90, 1344–1351. [Google Scholar]
- Fang, Y.; Fu, X.J.; Gu, C.; Xu, P.; Wang, Y.; Yu, W.R.; Yao, M. Hydrogen-rich saline protects against acute lung injury induced by extensive burn in rat model. J. Burn. Care Res. 2011, 32, e82–e91. [Google Scholar] [CrossRef]
- Fukuda, K.I.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2007, 361, 670–674. [Google Scholar] [CrossRef]
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010, 77, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hamasaki, T.; Nakamichi, N.; Kashiwagi, T.; Komatsu, T.; Ye, J.; Teruya, K.; Abe, M.; Yan, H.; Kinjo, T.; et al. Suppressive effects of electrolyzed reduced water on alloxan-induced apoptosis and type 1 diabetes mellitus. Cytotechnology 2011, 63, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Adachi, T. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, C.E.; Boylan, G.B.; Murray, D.M. Short and long term prognosis in perinatal asphyxia: An update. World. J. Clin. Pediatr. 2016, 5, 67–74. [Google Scholar] [CrossRef]
- Kriz, J. Inflammation in ischemic brain injury: Timing is important. Crit. Rev. Neurobiol. 2006, 18, 145–157. [Google Scholar] [CrossRef]
- Cai, J.; Kang, Z.; Liu, W.W.; Luo, X.; Qiang, S.; Zhang, J.H.; Li, R. Hydrogen therapy reduces apoptosis in neonatal hypoxia–ischemia rat model. Neurosci. Lett. 2008, 441, 167–172. [Google Scholar] [CrossRef]
- Cai, J.; Kang, Z.; Liu, K.; Liu, W.; Li, R.; Zhang, J.H.; Sun, X. Neuroprotective effects of hydrogen saline in neonatal hypoxia–ischemia rat model. Brain Res. 2009, 1256, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Oláh, O.; Tóth-Szűki, V.; Temesvári, P.; Bari, F.; Domoki, F. Delayed neurovascular dysfunction is alleviated by hydrogen in asphyxiated newborn pigs. Neonatology 2013, 104, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Kalkman, C.J.; Peelen, L.; Moons, K.G.; Veenhuizen, M.; Bruens, M.; Sinnema, G.; de Jong, T.P. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology 2009, 110, 805–812. [Google Scholar] [CrossRef]
- Yonamine, R.; Satoh, Y.; Kodama, M.; Araki, Y.; Kazama, T. Coadministration of hydrogen gas as part of the carrier gas mixture suppresses neuronal apoptosis and subsequent behavioral deficits caused by neonatal exposure to sevoflurane in mice. Anesthesiology 2013, 118, 105–113. [Google Scholar] [CrossRef]
- Takaenoki, Y.; Satoh, Y.; Araki, Y.; Kodama, M.; Yonamine, R.; Yufune, S.; Kazama, T. Neonatal exposure to sevoflurane in mice causes deficits in maternal behavior later in adulthood. Anesthesiology 2014, 120, 403–415. [Google Scholar] [CrossRef]
- JazvinšćakJembrek, M.; Hof, P.R.; Šimić, G. Ceramides in Alzheimer’s disease: Key mediators of neuronal apoptosis induced by oxidative stress and Aβ accumulation. Oxid. Med. CellLongev. 2015, 2015, 346783. [Google Scholar]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Clos, A.L.; Jackson, G.R.; Kayed, R. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 2011, 6, 39. [Google Scholar] [CrossRef]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Tan, L. The NLRP3 inflammasome in Alzheimer’s disease. Mol. Neurobiol. 2013, 48, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.D.; Wu, X.B.; Jiang, R.; Hao, D.P.; and Liu, Y. Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 50–55. [Google Scholar]
- Lin, C.L.; Huang, W.N.; Li, H.H.; Huang, C.N.; Hsieh, S.; Lai, C.; Lu, F.J. Hydrogen-rich water attenuates amyloid β-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem. Biol. Interact. 2015, 240, 12–21. [Google Scholar] [CrossRef]
- Yao, H.; Zhao, D.; Khan, S.H.; Yang, L. Role of autophagy in prion protein-induced neurodegenerative diseases. ActaBiochim. Biophy. Sin. 2013, 45, 494–502. [Google Scholar] [CrossRef]
- Henderson, L.E.; Abdelmegeed, M.A.; Yoo, S.H.; Rhee, S.G.; Zhu, X.; Smith, M.A.; Song, B.J. Enhanced phosphorylation of Bax and its translocation into mitochondria in the brains of individuals affiliated with Alzheimer’s disease. Open Neurol. J. 2017, 11, 48–58. [Google Scholar] [CrossRef]
- Han, B.; Zhou, H.; Jia, G.; Wang, Y.; Song, Z.; Wang, G.; Pan, S.; Bai, X.; Lv, J.; Sun, B. MAPK s and Hsc70 are critical to the protective effect of molecular hydrogen during the early phase of acute pancreatitis. FEBS J. 2016, 283, 738–756. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Peng, Y.; Qin, C.; Fan, F.; Liu, J.; Long, J. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Aβ clearance. Free Radic. Res. 2018, 52, 1311–1322. [Google Scholar] [CrossRef]
- Fitzpatrick, J.L.; Mize, A.L.; Wade, C.B.; Harris, J.A.; Shapiro, R.A.; Dorsa, D.M. Estrogen-mediated neuroprotection against β-amyloid toxicity requires expression of estrogen receptor α or β and activation of the MAPK pathway. J. Neurochem. 2002, 82, 674–682. [Google Scholar] [CrossRef]
- Hollands, C.; Tobin, M.K.; Hsu, M.; Musaraca, K.; Yu, T.S.; Mishra, R.; Lazarov, O. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer’s disease by compromising hippocampal inhibition. Mol. Neurodegener. 2017, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Huang, C.S.; Inoue, T.; Yamashita, T.; Ishida, T.; Kang, K.M.; Nakao, A. Drinking hydrogen water ameliorated cognitive impairment in senescence-accelerated mice. J. Clin. Biochem. Nutr. 2010, 46, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Kerr, M.E.; Kim, Y.; Kamboh, M.I.; Beers, S.R.; Conley, Y.P. Apolipoprotein E4 allele presence and functional outcome after severe traumatic brain injury. J. Neurotrauma 2007, 24, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef]
- Kikkawa, Y.S.; Nakagawa, T.; Horie, R.T.; Ito, J. Hydrogen protects auditory hair cells from free radicals. Neuroreport 2009, 20, 689–694. [Google Scholar] [CrossRef]
- Kikkawa, Y.S.; Nakagawa, T.; Taniguchi, M.; Ito, J. Hydrogen protects auditory hair cells from cisplatin-induced free radicals. Neurosci. Lett. 2014, 579, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, H.; Ruan, F.; Chen, X.; Zheng, G.; Kang, M.; Sun, X. Hydrogen-rich saline alleviates experimental noise-induced hearing loss in guinea pigs. Neuroscience 2012, 209, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, S193–S197. [Google Scholar] [CrossRef]
- Ji, X.; Liu, W.; Xie, K.; Liu, W.; Qu, Y.; Chao, X.; Fei, Z. Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res. 2010, 1354, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Dohi, K.; Kraemer, B.C.; Erickson, M.A.; McMillan, P.J.; Kovac, A.; Flachbartova, Z.; Banks, W.A. Molecular hydrogen in drinking water protects against neurodegenerative changes induced by traumatic brain injury. PLoS ONE 2014, 9, e108034. [Google Scholar] [CrossRef]
- Liu, F.T.; Xu, S.M.; Xiang, Z.H.; Li, X.N.; Li, J.; Yuan, H.B.; Sun, X.J. Molecular hydrogen suppresses reactive astrogliosis related to oxidative injury during spinal cord injury in rats. CNS. Neurosci. Ther. 2014, 20, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Yamafuji, M.; Tachibana, T.; Nakabeppu, Y.; Noda, M.; Nakaya, H. Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci. Rep. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Andrews, Z.B. The extra-hypothalamic actions of ghrelin on neuronal function. Trends Neurosci. 2011, 34, 31–40. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y.; Okumura, M. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L646–L656. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Imoto, A.; Kamimura, N.; Nishimaki, K.; Ichimiya, H.; Yokota, T.; Ohta, S. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Landucci, G.; Tugnoli, A.; Cozzani, V. Inherent safety key performance indicators for hydrogen storage systems. J. Hazard Mater. 2008, 159, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Molecular hydrogen: An inert gas turns clinically effective. Ann. Med. 2015, 47, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Shen, L.; Ge, J.W.; Zhang, R.F. The transfer of hydrogen from inert gas to therapeutic gas. Med. Gas. Res. 2017, 7, 265. [Google Scholar] [PubMed]
| Diseases Category. | Species | Route of Administration | References |
|---|---|---|---|
| Alzheimer’s disease | Animal | Saline | [79,80] |
| Parkinson’s disease | Animal | Water | [76] |
| Corneal alkali-burn | Animal | Instillation | [82] |
| Spinal cord ischemia/reperfusion | Animal | Saline | [83] |
| Surgically induced brain injury | Animal | Gas | [84] |
| Spinal cord | Animal | Saline | [83,85] |
| Spinal cord injury | Animal | Saline | [85] |
| Senile dementia in senescence-accelerated mice | Animal | Water | [33,53] |
| Moderate to severe neonatal brain hypoxia | Animal | Gas | [86] |
| Cerebral infarction | Animal, Human | Gas, saline | [53,87] |
| Glaucoma | Animal | Instillation | [88] |
| Ear, hearing loss | Tissue, Animal | Medium, water | [89,90] |
| Radiation-induced lung injury | Animal | Saline | [91,92] |
| Lung transplantation | Animal | Gas | [93] |
| Burn-induced lung injury | Animal | Saline | [94] |
| Liver ischemia/reperfusion | Animal | Gas | [95] |
| Kidney transplantation | Animal | Water | [96] |
| Diabetes mellitus type I | Animal | Water | [97] |
| Diabetes mellitus type II | Human | Water | [98] |
| Author | Animals/Cells | Model | Results | References |
|---|---|---|---|---|
| Nagata et al. | Mice | Dementia induced by chronic physical restraint stress | Molecular hydrogen inhibited memory and learning from stress | [33] |
| Lin et al. | Human neuroblastoma SK-N-MC cells | AD | AMPK-Sirt1-FoxO3a pathway and excessive ROS neutralization to protect the neuron is not regulated by hydrogen-rich water | [113] |
| Nishimaki et al. | Mice | Dementia | In apolipoprotein genotype carriers, molecular hydrogen enhances cognition | [53] |
| Hou et al. | Mice | AD | Water-rich in hydrogen inhibits NLRP3 and diminishes the signal pathway of estrogen-ERβ-BDNF | [117] |
| Li et al. | Rats | AD | The saline-rich hydrogen enhances the memory by inhibiting OS and reducing interleukin-6 and TNF-α and activating astrocytes | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh Thi, T.; Akter, R.; Goh, S.H.; Kim, C.-S.; Lee, K.-J. Redox Effects of Molecular Hydrogen and Its Therapeutic Efficacy in the Treatment of Neurodegenerative Diseases. Processes 2021, 9, 308. https://doi.org/10.3390/pr9020308
Rahman MH, Bajgai J, Fadriquela A, Sharma S, Trinh Thi T, Akter R, Goh SH, Kim C-S, Lee K-J. Redox Effects of Molecular Hydrogen and Its Therapeutic Efficacy in the Treatment of Neurodegenerative Diseases. Processes. 2021; 9(2):308. https://doi.org/10.3390/pr9020308
Chicago/Turabian StyleRahman, Md. Habibur, Johny Bajgai, Ailyn Fadriquela, Subham Sharma, Thuy Trinh Thi, Rokeya Akter, Seong Hoon Goh, Cheol-Su Kim, and Kyu-Jae Lee. 2021. "Redox Effects of Molecular Hydrogen and Its Therapeutic Efficacy in the Treatment of Neurodegenerative Diseases" Processes 9, no. 2: 308. https://doi.org/10.3390/pr9020308
APA StyleRahman, M. H., Bajgai, J., Fadriquela, A., Sharma, S., Trinh Thi, T., Akter, R., Goh, S. H., Kim, C.-S., & Lee, K.-J. (2021). Redox Effects of Molecular Hydrogen and Its Therapeutic Efficacy in the Treatment of Neurodegenerative Diseases. Processes, 9(2), 308. https://doi.org/10.3390/pr9020308








