Fluid Characteristics of Biodiesel Produced from Palm Oil with Various Initial Water Contents
Abstract
1. Introduction
2. Experimental Details
2.1. Production of Palm-Oil Biodiesel Added with Water Content
2.2. Measurement of Fluid Characteristics of Biodiesel from Feedstock Oil with Water
3. Results and Discussion
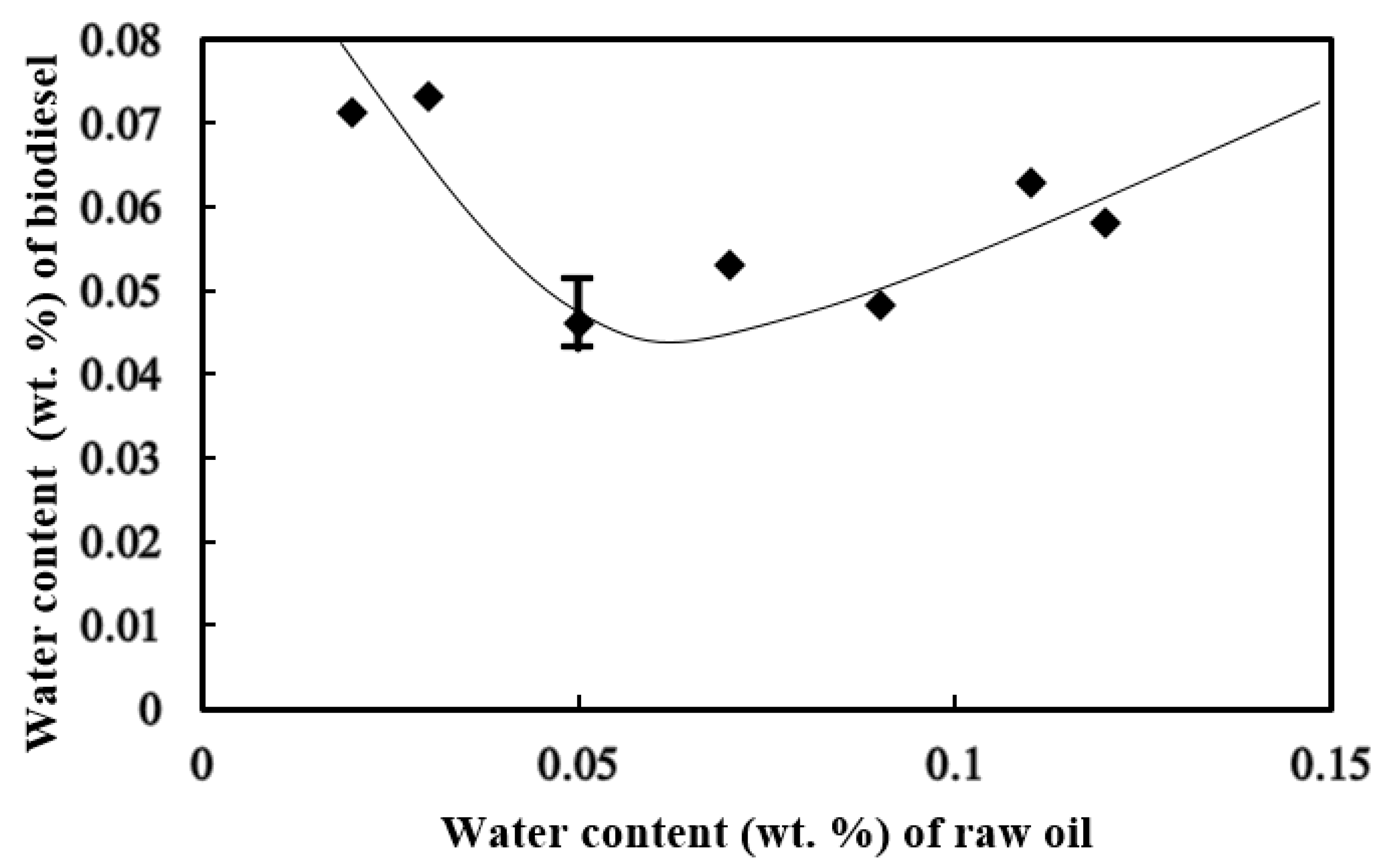
3.1. Water Content in the Biodiesel
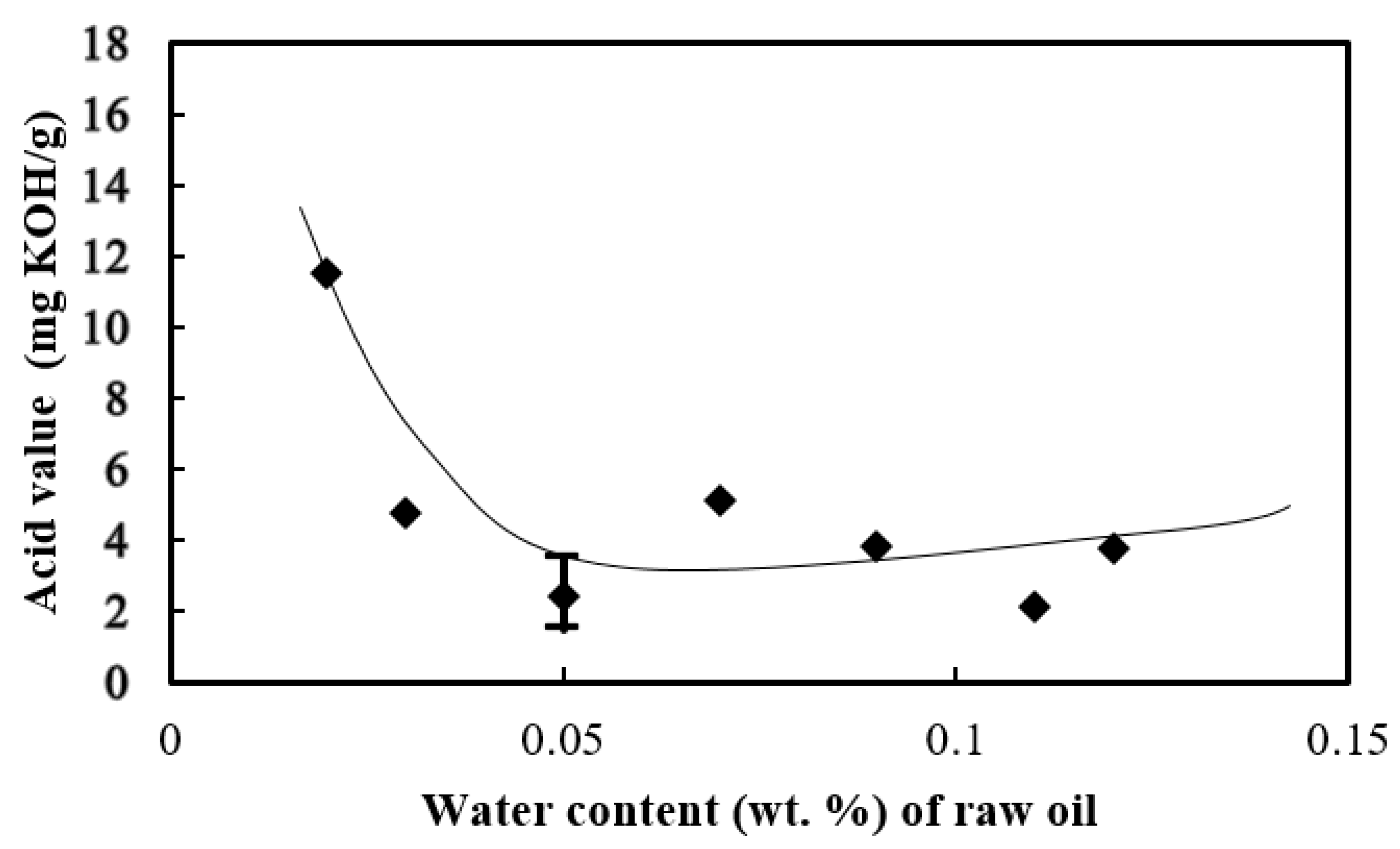
3.2. Acid Value of the Biodiesel
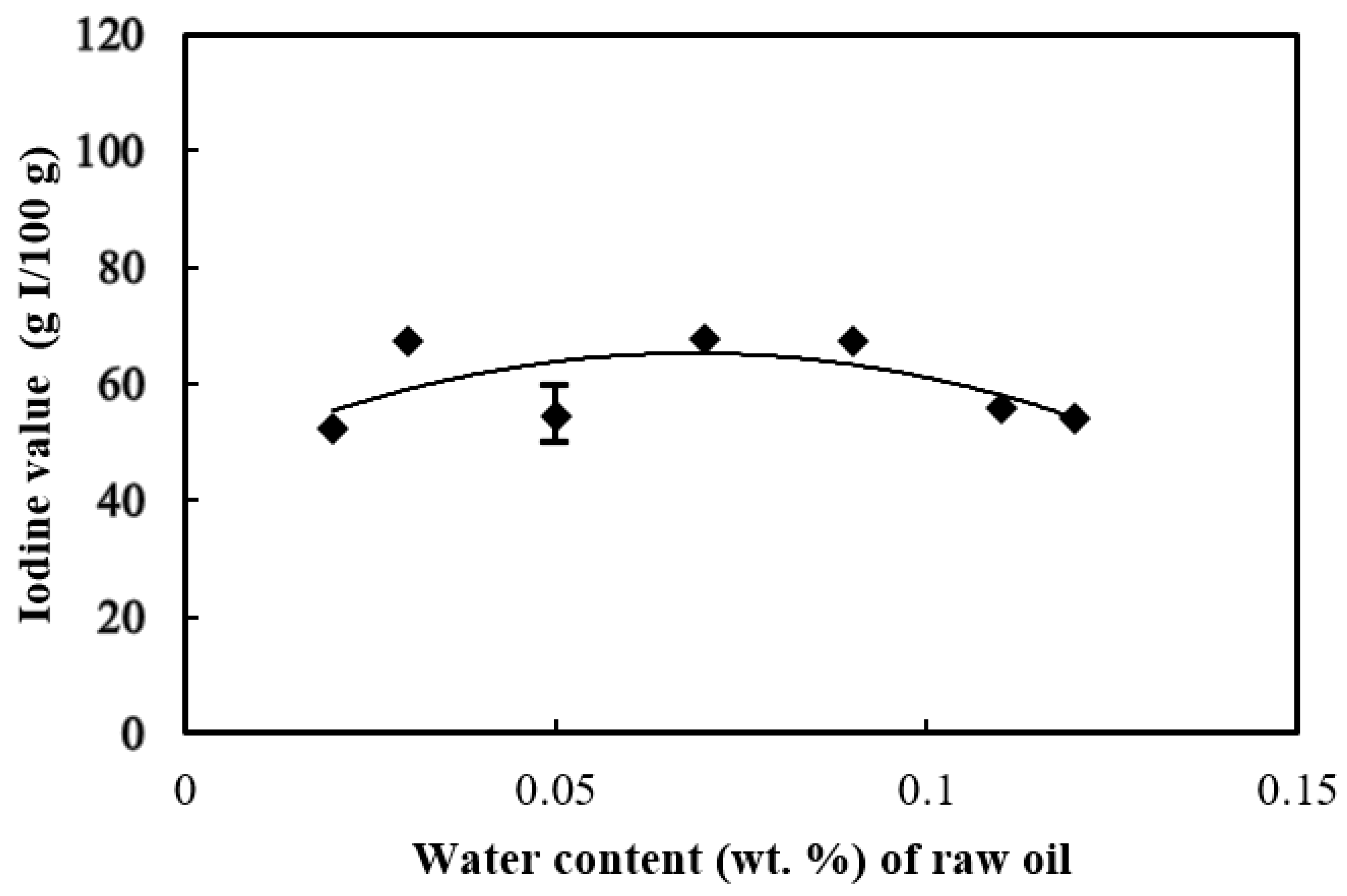
3.3. Iodine Value of the Biodiesel
3.4. Kinematic Viscosity of the Biodiesel
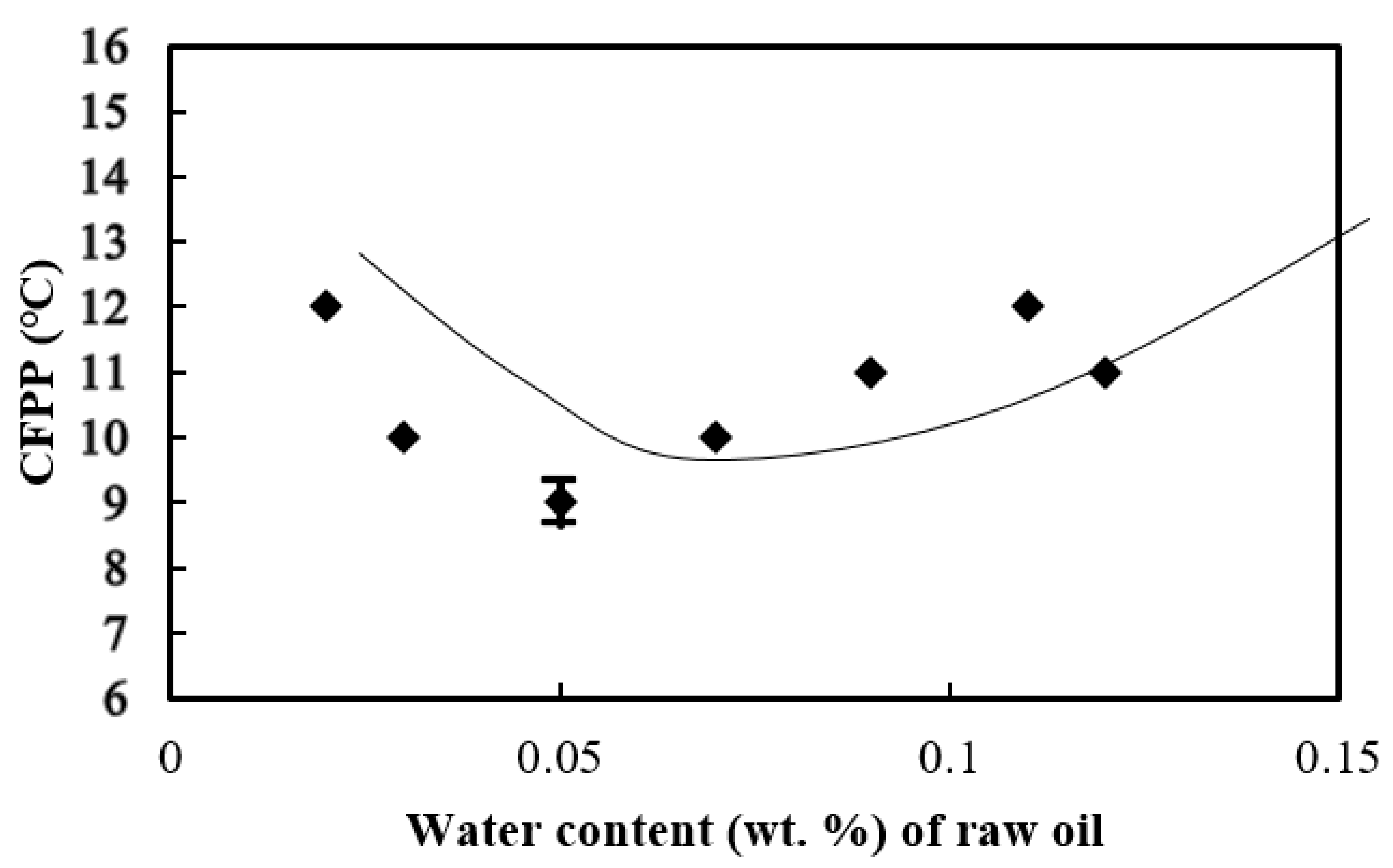
3.5. Cold Filter Plugging Point of the Biodiesel
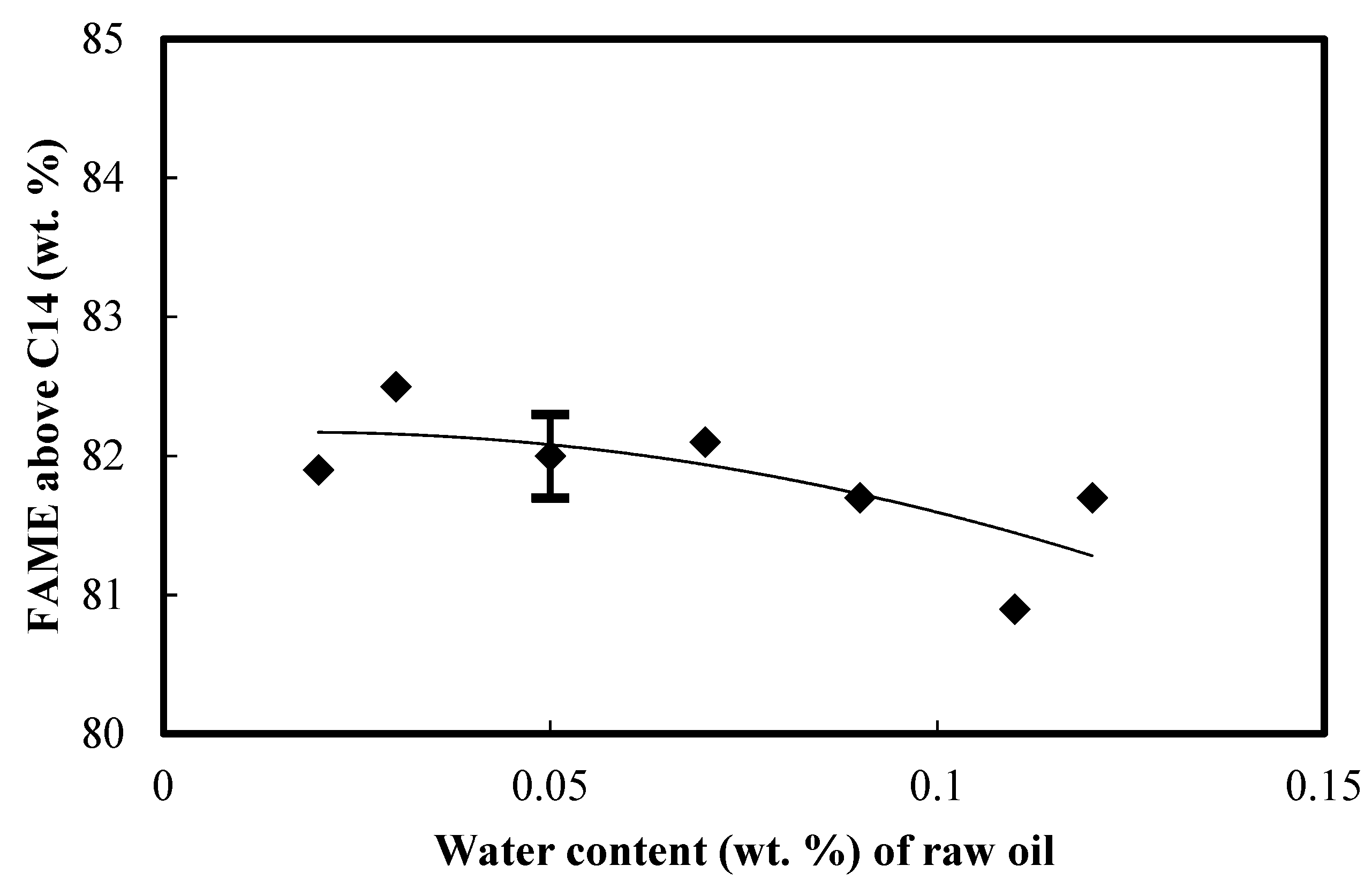
3.6. Fatty Acid Methyl Esters (FAME) above C14 of the Biodiesel
4. Conclusions
- (1)
- Initial water content of 0.05 wt. % added to feedstock oil facilitated the formation of the lowest water content in the biodiesel product. The transesterification reaction was enhanced with adequate water content in the reactant mixture.
- (2)
- Water in raw palm oil enhanced the hydrolysis of fatty acids, causing the production of free fatty acids and increasing the acid value. The lowest acid value of the biodiesel was found when the feedstock oil had 0.05 wt. % initial water added.
- (3)
- The biodiesels produced in this study came from the non-drying oil group, based on their iodine values ranging between 52 and 67 g I/100 g biodiesel. The lowest iodine value of biodiesel was produced from the feedstock oil with 0.02 wt. % initial water added, due to the formation of the lowest amount of unsaturated fatty acids.
- (4)
- Biodiesel composed of more long or saturated fatty acids is prone to greater kinematic viscosity. The highest kinematic viscosity corresponding to the lowest iodine value occurred in the FAME made from the feedstock oil with 0.02 wt. % water added.
- (5)
- Biodiesel consisting of more unsaturated fatty acids tends to have a lower cold filter plugging point (CFPP). Biodiesel made from palm oil with 0.05 wt. % water added had the lowest CFPP of 9 °C and a corresponding lower iodine value due to the formation of more unsaturated fatty acids, of which oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3) amounted to 56.85 wt. %. Biodiesel produced from palm oil with 0.03 wt. % initial water added appeared to have the highest content of FAME above C14.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, P.-Q.; Zhong, Y.-M.; Hu, Z.; Lou, D.-M. Size distributions, PAHs and inorganic ions of exhaust particles from a heavy duty diesel engine using B20 biodiesel with different exhaust aftertreatments. Energy 2017, 141, 898–906. [Google Scholar] [CrossRef]
- Peng, D.-X. Biodiesel Improves Lubricity of Low-Sulfur Petro-Diesels. Chem. Technol. Fuels Oils 2017, 52, 699–703. [Google Scholar] [CrossRef]
- Gharehghani, A.; Mirsalim, M.; Hosseini, R. Effects of waste fish oil biodiesel on diesel engine combustion characteristics and emission. Renew. Energy 2017, 101, 930–936. [Google Scholar] [CrossRef]
- Yadav, A.K.; Khan, M.E.; Pal, A. Biodiesel production from oleander (Thevetia Peruviana) oil and its performance testing on a diesel engine. Korean J. Chem. Eng. 2016, 34, 340–345. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Pastore, C. Metal hydrated-salts as efficient and reusable catalysts for pre-treating waste cooking oils and animal fats for an effective production of biodiesel. Renew. Energy 2019, 143, 1193–1200. [Google Scholar] [CrossRef]
- Shi, W.J.; Liu, H.Q.; Feng, S.B.; Zheng, E.L. Study on preparation of biodiesel with low acid value rapeseed oil. Renew. Energy Resour. 2009, 27, 37–39. [Google Scholar]
- Chanakaewsomboon, I.; Tongurai, C.; Photaworn, S.; Kungsanant, S.; Nikhom, R. Investigation of saponification mechanisms in biodiesel production: Microscopic visualization of the effects of FFA, water and the amount of alkaline catalyst. J. Environ. Chem. Eng. 2020, 8, 103538. [Google Scholar] [CrossRef]
- Fan, P.; Xing, S.; Wang, J.; Fu, J.; Yang, L.; Yang, G.; Miao, C.; Lv, P. Sulfonated imidazolium ionic liquid-catalyzed transesterification for biodiesel synthesis. Fuel 2017, 188, 483–488. [Google Scholar] [CrossRef]
- Widziewicz-Rzońca, K.; Tytła, M.; Majewski, G.; Rogula-Kopiec, P.; Loska, K.; Rogula-Kozłowska, W. Strongly and loosely bound water in ambient particulate matter—qualitative and quantitative determination by Karl Fischer Coulometric meth-od. Sustainability 2020, 12, 6196. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, C.; Igou, T.; Liu, P.; Hu, Z.; Van Ginkel, S.W.; Chen, Y. Effect of water content on [Bmim][HSO4] assisted in-situ transesterification of wet Nannochloropsis oceanica. Appl. Energy 2018, 226, 461–468. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Son, J.; Lee, J.W. Solvo-thermal in situ transesterification of wet spent coffee grounds for the production of biodiesel. Bioresour. Technol. 2018, 249, 494–500. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Kaneki, Y.; Yamakoshi, Y. H2SO4/CaO-Catalyzed Process for Biodiesel Production from High Acid Value Jatropha curcas Crude Oil. J. Chem. Eng. Jpn. 2011, 44, 529–533. [Google Scholar] [CrossRef]
- Amoah, J.; Quayson, E.; Hama, S.; Yoshida, A.; Hasunuma, T.; Ogino, C.; Kondo, A. Simultaneous conversion of free fatty acids and triglycerides to biodiesel by immobilized Aspergillus oryzae expressing Fusarium heterosporum lipase. Biotechnol. J. 2017, 12, 1600400. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Sarkar, A.; Chakraborty, J.P. Influence of Alternate Fuels on the Performance and Emission from Internal Combustion Engines and Soot Particle Collection Using Thermophoretic Sampler: A Comprehensive Review. Waste Biomass Valorization 2018, 10, 2801–2823. [Google Scholar] [CrossRef]
- Eze, V.C.; Phan, A.N.; Harvey, A.P. Intensified one-step biodiesel production from high water and free fatty acid waste cooking oils. Fuel 2018, 220, 567–574. [Google Scholar] [CrossRef]
- Alptekin, E.; Canalci, M. Optimization of transesterification for methyl ester production from chicken fat. Fuel 2011, 90, 2630–2638. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Toste, F.D.; Bell, A.T. Mechanism and kinetics of isobutene formation from ethanol and acetone over ZnxZryOz. ACS Catal. 2019, 9, 10588–10604. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, K.; Zheng, Y.; Wang, F.; Deng, L.; Tan, T. Lipase Candida sp. 99-125Coupled with β-cyclodextrin as additive synthesized the human milk fat substitutes. J. Mol. Catal. B Enzym. 2016, 125, 1–5. [Google Scholar] [CrossRef]
- Kara, K.; Ouanji, F.; Lotfi, E.M.; El Mahi, M.; Kacimi, M.; Ziyad, M. Biodiesel production from waste fish oil with high free fatty acid content from Moroccan fish-processing industries. Egypt. J. Pet. 2018, 27, 249–255. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, X.; Yu, X.; Huyan, Z.; Wang, X. Rapid and Simultaneous Determination of the Iodine Value and Saponification Number of Edible Oils by FTIR Spectroscopy. Eur. J. Lipid Sci. Technol. 2018, 120, 1700396. [Google Scholar] [CrossRef]
- Serra, J.L.; de Cruz Rodrigues, A.M.; de Freitas, R.A.; de Almeida Meirelles, A.J.; Darnet, S.H.; Meller da Silva, L.H. Alternative sources of oils and fats from Amazonian plants: Fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef]
- Aslam, M.M.; Khan, A.A.; Cheema, H.M.N.; Hanif, M.A.; Azeem, M.W.; Azmat, M.A. Novel mutant camelina and jatropha as valuable feedstocks for biodiesel production. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tomak, E.D.; Hughes, M.; Yildiz, U.C.; Viitanen, H. The combined effects of boron and oil heat treatment on beech and Scots pone wood properties. Part 1: Boron leaching, thermogravimetric analysis, and chemical composition. J. Mater. Sci. 2011, 46, 598–607. [Google Scholar] [CrossRef]
- Vescovi, V.; Rojas, M.J.; Baraldo, A., Jr.; Botta, D.C.; Montes Santana, F.A.; Costa, J.P.; Machado, M.S.; Honda, V.K.; de Lima Camargo Giordano, R.; Tardioli, P.W. Lipase-catalyzed production of biodiesel by hydrolysis of waste cooking oil followed by esterification of free fatty acids. J. Am. Oil Chem. Soc. 2016, 93, 1615–1624. [Google Scholar] [CrossRef]
- Plötz, T.; von Hanstein, A.S.; Krümmel, B.; Laporte, A.; Mehmeti, I.; Lenzen, S. Structure-toxicity relationships of saturated and unsaturated free fatty acids for elucidating the lipotoxic effects in human EndoC-βH1 beta-cells. Biochim. Biophys. Acta. Mol. Basis. Dis. 2019, 1865, 165525. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Lu, C. Development perspectives of promising lignocellulose feedstocks for production of advanced generation biofuels: A review. Renew. Sustain. Energy Rev. 2021, 136, 110445. [Google Scholar] [CrossRef]
- Lepri, F.G.; Chaves, E.S.; Vieira, M.A.; Ribeiro, A.S.; Curtius, A.J.; DeOliveira, L.C.C.; Decampos, R.C. Determination of Trace Elements in Vegetable Oils and Biodiesel by Atomic Spectrometric Techniques—A Review. Appl. Spectrosc. Rev. 2011, 46, 175–206. [Google Scholar] [CrossRef]
- Nezihe, A.; Elif, D.; Özlem, Y.; Tunçer, E.A. Microwave Heating Application to Produce Dehydrated Castor Oil. Ind. Eng. Chem. Res. 2011, 50, 398–403. [Google Scholar] [CrossRef]
- Giakoumis, E.G. Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew. Energy 2018, 126, 403–419. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition. Fuel 2018, 222, 574–585. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Devarajan, Y.; Mahalingam, A.; Nagappan, B. Emissions analysis on diesel engine fueled with palm oil biodiesel and pentanol blends. J. Oil Palm Res. 2017, 29, 380–386. [Google Scholar] [CrossRef]
- Sørensen, G.; Sørensen, K.B.; Hansen, H.O.; Nygaard, S.D. Fuel for the Future: Development of New Fuels, e.g., Biofuels. Appl. Microbiol. Mol. Biol. Oilfield Syst. 2010, 5, 219–228. [Google Scholar] [CrossRef]
- Li, B.; Liu, G.; Ren, S.; Chen, L.; Teng, H.; Lu, X.; Gao, J. Non-isothermal crystallization kinetics of waxy crude oil. Pet. Sci. Technol. 2018, 37, 282–289. [Google Scholar] [CrossRef]
- Yuan, M.-H.; Chen, Y.-H.; Chen, J.-H.; Luo, Y.-M. Dependence of cold filter plugging point on saturated fatty acid profile of biodiesel blends derived from different feedstocks. Fuel 2017, 195, 59–68. [Google Scholar] [CrossRef]
- Lv, P.; Cheng, Y.; Yang, L.; Yuan, Z.; Li, H.; Luo, W. Improving the low temperature flow properties of palm oil biodiesel: Addition of cold flow improver. Fuel Process. Technol. 2013, 110, 61–64. [Google Scholar] [CrossRef]
- Wahyudi; Wardana, I.N.G.; Widodo, A.; Wijayanti, W. Improving Vegetable Oil Properties by Transforming Fatty Acid Chain Length in Jatropha Oil and Coconut Oil Blends. Energies 2018, 11, 394. [Google Scholar] [CrossRef]
- Wu, L.; Wei, T.-Y.; Tong, Z.-F.; Zou, Y.; Lin, Z.-J.; Sun, J.-H. Bentonite-enhanced biodiesel production by NaOH-catalyzed transesterification of soybean oil with methanol. Fuel Proc. Technol. 2016, 144, 334–340. [Google Scholar] [CrossRef]
- Idowu, I.; Pedrola, M.O.; Wylie, S.; Teng, K.; Kot, P.; Phipps, D.; Shaw, A. Improving biodiesel yield of animal waste fats by combination of a pre-treatment technique and microwave technology. Renew. Energy 2019, 142, 535–542. [Google Scholar] [CrossRef]

| Fatty Acids Chemical Structure | Composition (wt. %) |
|---|---|
| Myristic acid C14:0 | 1.14 |
| Palmitic acid C16:0 | 36.93 |
| Palmitoleic acid C16:1 | 0.22 |
| Stearic acid C18:0 | 3.95 |
| Oleic acid C18:1 | 45.07 |
| Linoleic acid C18:2 | 11.68 |
| Linolenic acid C18:3 | 0.10 |
| Arachidic acid C20:0 | 0.34 |
| Eicosenoic acid C20:1 | 0.17 |
| Behenic acid C22:0 | 0.17 |
| Erucic acid C22:1 | 0.05 |
| Lignoceric acid C24:0 | 0.10 |
| Docosahexaenoic acid C22:6 | 0.06 |
| Total saturated fatty acids | 42.63 |
| Monounsaturated fatty acids | 45.51 |
| Polyunsaturated fatty acids | 11.84 |
| Total FAME | 97.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Ma, L. Fluid Characteristics of Biodiesel Produced from Palm Oil with Various Initial Water Contents. Processes 2021, 9, 309. https://doi.org/10.3390/pr9020309
Lin C-Y, Ma L. Fluid Characteristics of Biodiesel Produced from Palm Oil with Various Initial Water Contents. Processes. 2021; 9(2):309. https://doi.org/10.3390/pr9020309
Chicago/Turabian StyleLin, Cherng-Yuan, and Lei Ma. 2021. "Fluid Characteristics of Biodiesel Produced from Palm Oil with Various Initial Water Contents" Processes 9, no. 2: 309. https://doi.org/10.3390/pr9020309
APA StyleLin, C.-Y., & Ma, L. (2021). Fluid Characteristics of Biodiesel Produced from Palm Oil with Various Initial Water Contents. Processes, 9(2), 309. https://doi.org/10.3390/pr9020309








