Microencapsulation of Flaxseed Oil—State of Art
Abstract
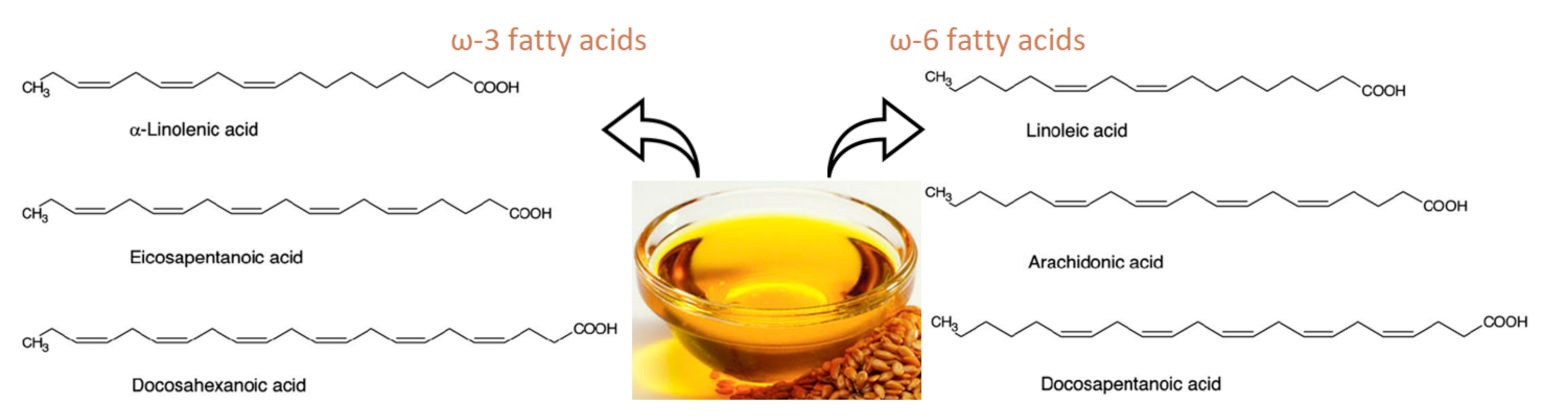
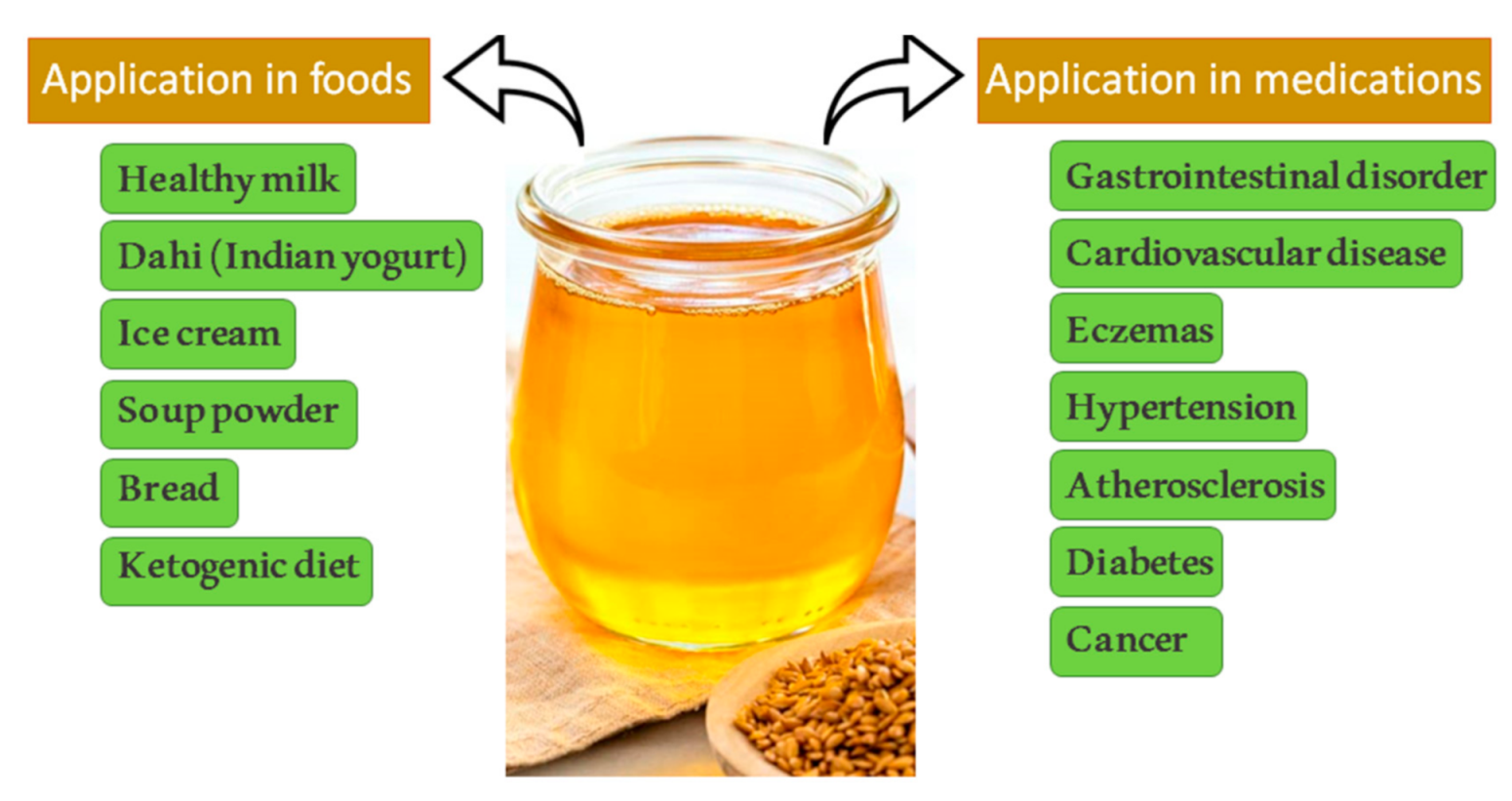
1. Introduction
2. Microencapsulation
2.1. Emulsification
2.2. Spray-Drying
2.3. Freeze-Drying
2.4. Matrix (Wall Material)
2.5. Emulsifier
3. Characterization of Microencapsulated Flaxseed Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A Potential Source of Food, Feed and Fiber. Crit. Rev. Food Sci. Nutr. 2011, 51, 210–222. [Google Scholar] [CrossRef]
- Toure, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Tulbek, M.C.; Xu, Y. Flaxseed. Adv. Food Nutr. Res. 2006, 51, 1–97. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M.K. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef]
- Gibson, R.A.; Muhlhausler, B.; Makrides, M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsatu-rated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child Nutr. 2011, 7, 17–26. [Google Scholar] [CrossRef]
- Ludwig, D.S. The Ketogenic Diet: Evidence for Optimism but High-Quality Research Needed. J. Nutr. 2019, 150, 1354–1359. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Sihag, M.K.; Singh, A.K.; Arora, S.; Sabikhi, L. Fortification of dahi (Indian yoghurt) with omega-3 fatty acids using microencapsulated flaxseed oil microcapsules. J. Food Sci. Technol. 2016, 53, 2422–2433. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Sihag, M.K.; Singh, A.K.; Arora, S.; Sabikhi, L. Oxidative stability of alpha-linolenic acid (ω-3) in flaxseed oil microcapsules fortified market milk. Int. J. Dairy Technol. 2017, 70, 188–196. [Google Scholar] [CrossRef]
- Gowda, A.; Sharma, V.; Goyal, A.; Singh, A.K.; Arora, S. Process optimization and oxidative stability of omega-3 ice cream fortified with flaxseed oil microcapsules. J. Food Sci. Technol. 2018, 55, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Morales, E.; Contreras, K.; Ceballos, C.; Acevedo, F.; Villarroel, M.; Shene, C. Development of a soup powder en-riched with microencapsulated linseed oil as a source of omega-3 fatty acids. Eur. J. Lipid Sci. Technol. 2012, 114, 423–433. [Google Scholar] [CrossRef]
- Gallardo, G.; Guida, L.; Martinez, V.; Lopez, M.C.; Bernhardt, D.; Blasco, R.; Pedroza-Islas, R.; Hermida, L.G. Microencapsulation of linseed oil by spray drying for functional food application. Food Res. Int. 2013, 52, 473–482. [Google Scholar] [CrossRef]
- Harvey, K.L.; Holcomb, L.E.; Kolwicz, J.S.C. Ketogenic Diets and Exercise Performance. Nutrients 2019, 11, 2296. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary flaxseed as a strategy for improv-ing human health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef]
- Dell, C.A.; Likhodii, S.S.; Musa, K.; Ryan, M.A.; Burnham, W.M.I.; Cunnane, S.C. Lipid and fatty acid profiles in rats con-suming different high-fat ketogenic diets. Lipids 2001, 36, 373–378. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Mokos, Z.B. Omega-3 versus Omega-6 polyunsaturated fatty acids in the pre-vention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Zou, X.G.; Chen, X.L.; Hu, J.N.; Wang, Y.F.; Gong, D.M.; Zhu, X.M.; Deng, Z.Y. Comparisons of proximate compositions, fatty ac-ids profile and micronutrients between fiber and oil flaxseeds (Linum usitatissimum L.). J. Food Compos. Anal. 2017, 62, 168–176. [Google Scholar] [CrossRef]
- Shahidi, F. Bailey’s Industrial Oil and Fat Products, Volumes 1–6, 6th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Gunstone, F.D. Vegetable Oils in Food Technology: Composition, Properties and Uses, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Holstun, J.; Zetocha, D. An Analysis of Flaxseed Utilization in the Health Food Industry; North Dakota State University: Fargo, ND, USA, 1994. [Google Scholar]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Liu, T.T.; Yang, T.S. Optimization of emulsification and microencapsulation of evening primrose oil and its oxidative stabil-ity during storage by response surface methodology. J. Food Qual. 2011, 34, 64–73. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nano-materials. 1st Nano Update. Arabian J. Chem. 2012, 5, 379–417. [Google Scholar] [CrossRef]
- Aronson, M.P. The role of free surfactant in destabilizing oil-in-water emulsions. Langmuir 1989, 5, 494–501. [Google Scholar] [CrossRef]
- Nazari, M.; Mehrnia, M.A.; Jooyandeh, H.; Barzegar, H. Preparation and characterization of water in sesame oil microemul-sion by spontaneous method. J. Food Process Eng. 2019, 42, e13032. [Google Scholar] [CrossRef]
- Charcosset, C. Preparation of emulsions and particles by membrane emulsification for the food processing industry. J. Food Eng. 2009, 92, 241–249. [Google Scholar] [CrossRef]
- Shima, M.; Kobayashi, Y.; Fujii, T.; Tanaka, M.; Kimura, Y.; Adachi, S.; Matsuno, R. Preparation of fine W/O/W emulsion through membrane filtration of coarse W/O/W emulsion and disappearance of the inclusion of outer phase solution. Food Hydrocoll. 2004, 18, 61–70. [Google Scholar] [CrossRef]
- Gaikwad, S.G.; Pandit, A.B. Ultrasound emulsification: Effect of ultrasonic and physicochemical properties on dispersed phase volume and droplet size. Ultrason. Sonochem. 2008, 15, 554–563. [Google Scholar] [CrossRef]
- Stang, M.; Schuchmann, H.; Schubert, H. Emulsification in High-Pressure Homogenizers. Eng. Life Sci. 2001, 1, 151–157. [Google Scholar] [CrossRef]
- Friberg, S.E.; Corkery, R.W.; Blute, I.A. Phase Inversion Temperature (PIT) Emulsification Process. J. Chem. Eng. Data 2011, 56, 4282–4290. [Google Scholar] [CrossRef]
- Charcosset, C.; Limayem, I.; Fessi, H. The membrane emulsification process—A review. J. Chem. Technol. Biotechnol. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Lapez-Montilla, J.C.; Herrera-Morales, P.E.; Pandey, S.; Shah, D.O. Spontaneous emulsification: Mechanisms, physicochemi-cal aspects, modeling, and applications. J. Dispers. Sci. Technol. 2002, 23, 219–268. [Google Scholar] [CrossRef]
- Solans, C.; Morales, D.; Homs, M. Spontaneous emulsification. Curr. Opin. Colloid Interface Sci. 2016, 22, 88–93. [Google Scholar] [CrossRef]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Mishra, M. Overview of Encapsulation and Controlled Release. In Handbook of Encapsulation and Controlled Release; CRC Press: Boca Raton, FL, USA, 2015; pp. 3–19. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehen-sive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Spray drying, freeze drying and related processes for food ingredient and nutraceutical encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2012; pp. 73–109. [Google Scholar]
- Mori, C.; Kadota, K.; Tozuka, Y.; Shimosaka, A.; Yoshida, M.; Shirakawa, Y. Application of nozzleless electrostatic atomiza-tion to encapsulate soybean oil with solid substances. J. Food Eng. 2019, 246, 25–32. [Google Scholar] [CrossRef]
- Keogh, M. Spray-Dried Microencapsulated Fat Powders. In Food Plant Economics; CRC Press: Boca Raton, FL, USA, 2005; pp. 477–483. [Google Scholar]
- Anandharamakrishnan, C.; Ishwarya, S.P. Spray Drying Techniques for Food Ingredient Encapsulation; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry. Technol. 2019, 38, 235–258. [Google Scholar] [CrossRef]
- Haseley, P.; Oetjen, G.-W. Foundations and Process Engineering. In Freeze-Drying, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Garcia-Amezquita, L.E.; Welti-Chanes, J.; Vergara-Balderas, F.T.; Bermúdez-Aguirre, D. Freeze-drying: The Basic Process. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Comparison of spray drying, drum drying and freeze drying for β-carotene encapsulation and preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Bora, A.F.M.; Li, X.; Zhu, Y.; Du, L. Improved Viability of Microencapsulated Probiotics in a Freeze-Dried Banana Powder during Storage and Under Simulated Gastrointestinal Tract. Probiotics Antimicrob. Proteins 2018, 11, 1330–1339. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X. Encapsulation of food ingredients. Crit. Rev. Food Sci. Nutr. 1993, 33, 501–547. [Google Scholar] [CrossRef] [PubMed]
- Can Karaca, A.; Low, N.; Nickerson, M. Encapsulation of flaxseed oil using a benchtop spray-dryer for legume pro-tein-maltodextrin microcapsule preparation. J. Agric. Food Chem. 2013, 61, 5148–5155. [Google Scholar] [CrossRef]
- Kinyanjui, T.; Artz, W.; Mahungu, S. EMULSIFIERS|Organic Emulsifiers. In Encyclopedia of Food Sciences and Nutrition; Elsevier BV: Amsterdam, The Netherlands, 2003; pp. 2070–2077. [Google Scholar]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & SonsJohn Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Nabilah, A.; Handayani, S.; Setiasih, S.; Rahayu, D.U.C.; Hudiyono, S. Emulsifier and antimicrobial activity against Propi-onibacterium acnes and Staphylococcus epidermidis of oxidized fatty acid esters from hydrolyzed castor oil. In IOP Con-ference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Yang, X.; Li, S.; Yan, J.; Xia, J.; Huang, L.; Li, M.; Ding, H.; Xu, L. Effect of different combinations of emulsifier and wall materials on physical properties of spray-dried microencapsulated swida wilsoniana oil. J. Bioresour. Bioprod. 2020, 5, 44–50. [Google Scholar] [CrossRef]
- Thirundas, R.; Gadhe, K.S.; Syed, I.H. Optimization of Wall Material Concentration in Preparation of Flaxseed Oil Powder Using Response Surface Methodology. J. Food Process. Preserv. 2012, 38, 889–895. [Google Scholar] [CrossRef]
- Barroso, A.K.M.; Pierucci, A.P.T.R.; Freitas, S.P.; Torres, A.G.; Da Rocha-Leão, M.H.M. Oxidative stability and sensory eval-uation of microencapsulated flaxseed oil. J. Microencapsul. 2014, 31, 193–201. [Google Scholar] [CrossRef]
- Onsaard, E.; Onsaard, W. Microencapsulated Vegetable Oil Powder. In Microencapsulation–Processes, Technologies and Industrial Applications; IntechOpen: London, UK, 2019. [Google Scholar]
- Mourtzinos, I.; Biliaderis, C.G. Principles and applications of encapsulation technologies to food materials. In Thermal and Nonthermal Encapsulation Methods; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Mohseni, F.; Goli, S.A.H. Encapsulation of flaxseed oil in the tertiary conjugate of oxidized tannic acid-gelatin and flaxseed (Linum usitatissimum) mucilage. Int. J. Biol. Macromol. 2019, 140, 959–964. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Adhikari, B. Microencapsulation of flaxseed oil in flaxseed protein and flaxseed gum complex coacervates. Food Res. Int. 2016, 86, 1–8. [Google Scholar] [CrossRef]
- Quispe-Condori, S.; Saldaña, M.D.; Temelli, F. Microencapsulation of flax oil with zein using spray and freeze drying. LWT 2011, 44, 1880–1887. [Google Scholar] [CrossRef]
- Tonon, R.V.; Pedro, R.B.; Grosso, C.; Hubinger, M.D. Microencapsulation of Flaxseed Oil by Spray Drying: Effect of Oil Load and Type of Wall Material. Dry. Technol. 2012, 30, 1491–1501. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Chang, C.; Low, N.H.; Nickerson, M.T. Encapsulation of flaxseed oil within native and modified lentil protein-based microcapsules. Food Res. Int. 2016, 81, 17–24. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Sihag, M.K.; Tomar, S.K.; Arora, S.; Sabikhi, L.; Singh, A.K. Development and physico-chemical characteri-zation of microencapsulated flaxseed oil powder: A functional ingredient for omega-3 fortification. Powder Technol. 2015, 286, 527–537. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microen-capsulation of flaxseed oil by spray-drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Fioramonti, S.A.; Stepanic, E.M.; Tibaldo, A.M.; Pavón, Y.L.; Santiago, L.G. Spray dried flaxseed oil powdered microcapsules obtained using milk whey proteins-alginate double layer emulsions. Food Res. Int. 2019, 119, 931–940. [Google Scholar] [CrossRef]
- Fioramonti, S.A.; Rubiolo, A.C.; Santiago, L.G. Characterisation of freeze-dried flaxseed oil microcapsules obtained by mul-tilayer emulsions. Powder Technol. 2017, 319, 238–244. [Google Scholar] [CrossRef]
- Bajaj, P.R.; Bhunia, K.; Kleiner, L.; Melito, H.S.J.; Smith, D.; Ganjyal, G.; Sablani, S. Improving functional properties of pea protein isolate for microencapsulation of flaxseed oil. J. Microencapsul. 2017, 34, 218–230. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Influence of emulsion composition and ultrasonication time on flaxseed oil powder properties. Powder Technol. 2014, 264, 54–60. [Google Scholar] [CrossRef]
- Domian, E.; Brynda-Kopytowska, A.; Marzec, A. Functional Properties and Oxidative Stability of Flaxseed Oil Microencap-sulated by Spray-drying Using Legume Proteins in Combination with Soluble Fiber or Trehalose. Food Bioprocess Technol. 2017, 10, 1374–1386. [Google Scholar] [CrossRef]
- Pedro, R.B.; Tonon, R.V.; Hubinger, M.D. Effect of Oil Concentration on the Microencapsulation of Flaxseed Oil By Spray-drying. In Jornadas Internacionais Sobre Avanços na Tecnologia de Filmes e Coberturas Funcionais em Alimentos, 3, 2011; Unicamp: Campinas, Brazil, 2011; pp. 1–5. [Google Scholar]


| Emulsification Techniques | Description | Reference | |
|---|---|---|---|
| High-Energy Consuming Methods | Ultrasound generator | Due to the ultrasound (physical shear force), fine droplets are created. At a certain range of sound, a source pressure amplitude cavitation takes place and emulsification of the immiscible liquids occurs. | [30] |
| High pressure homogenizer | In a homogenizer, with the help of a pump the liquid is pressed with high pressure to a narrow channel, which offers shear force on immersible liquids. It creates cavitation and leads to emulsion with a small droplet size. | [31] | |
| Low-Energy Techniques | Phase inversion temperature | Due to the change in factors, such as temperature or pH, the activity of the emulsifier in terms of its hydrophilic—lipophilic balance is affected. It helps create the emulsion. | [32] |
| Membrane emulsification | Membrane emulsification is performed with the porous membrane. Hydrophilic liquid (oil) in a dispersed phase is pressed through the membrane pores to a continuous phase, generally hydrophilic liquid and the emulsion is formed in a continuous phase. | [33] | |
| Spontaneous emulsification | In spontaneous emulsification, the immiscible liquids, such as oil and water along with the emulsifier create the emulsion without an external energy source. | [34,35] | |
| Matrix | Source | Characteristics | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Gum Arabic | Extracted from Acacia senegal (L.) or Acacia seyal (L.). | 1. It is a mixture of polysaccharides, oligosaccharides, and glycoproteins. 2. Hydrolysis of polysaccharides produce arabinose, galactose, rhamnose, and glucuronic acid. 3. It is soluble in water. | 1. Well accepted film-forming ability. 2. It has an emulsifying property due to the presence of protein. 3. Low viscosity in aqueous solution. 4. Stable in aqueous emulsion. 5. High solubility in aqueous solution. 6. Good retention of flavour. | 1. Expensive. 2. Variable availability and quality. 3. Limited potentiality to prevent oxidation of the encapsulated item. | [40,43] |
| Maltodextrin | Enzymatically derived from corn (Zea mays), potato (Solanum tuberosum L.), rice (Oryza sativa), and wheat (Triticum aestivum L.) starches. | Maltodextrin consists of D-glucose, linked with α(1→4) glycosidic bond. Maltodextrin can be of variable length according to the degree of polymerization. Typically, it varies from 3 to 17 glucose units. Maltodextrins are classified according to the dextrose equivalent. The higher value of dextrose equivalent signifies a shorter glucose chain, higher solubility, higher sweetness, and lower heat resistance. | 1. Low cost. 2. High potentiality to prevent oxidation of the encapsulated item. 3. Easily digestible in the intestine. 4. Highly soluble in water. 5. Low viscosity with a high solid content in the emulsion. 6. Heat resistance. | 1. Poor emulsifying property. 2. Poor flavour retention. 3. Sometimes offer allergenic activity. | [43] |
| Modified starch | Native starch is collected from corn (Zea mays), potato (Solanum tuberosum L.), rice (Oryza sativa), and wheat (Triticum aestivum L.) starches. | It is prepared by physical, enzymatic, or chemical treatment of native starch, which changes according to the property of the native starch. | 1. Well soluble in water. 2. Low viscosity. 3. Excellent volatile compound retention. 4. Excellent emulsifying property. 5. Provide stability in emulsion. 6. Heat stable. 7. Odourless and tasteless. 8. Low cost. | Provide allergenicity to food due to the presence of gluten. | [37,40,43] |
| Methyl cellulose | Methyl cellulose is not present in the plant cell wall. After the collection of natural cellulose from the plant cell wall, methyl cellulose is produced by the heat treatment of native cellulose with a sodium hydroxide solution and treating with methyl chloride. | Different types of methyl cellulose are produced by the substitution of different numbers of the hydroxyl group. It has an amphiphilic property. | 1. Stable viscosity over a wide range of pH (pH 3–11). 2. Heat stable. 3. Odourless and tasteless. 4. High emulsifying property due to its amphiphilic structure. 5. Satisfactory film-forming ability. | Low solubility with a higher degree of polymerization. | [37,50] |
| Whey protein | Dairy milk | 1. Whey protein is a mixture of α-lactalbumin (molecular weight: 14.2 kDa, isoelectric point: 4.2), β-globulin (molecular weight: 18.3 kDa, isoelectric point: 5.2–5.4), serum albumin (molecular weight: 66 kDa, isoelectric point: 4.9–5.1), lactoperoxidase (molecular weight: 78 kDa, isoelectric point: 9.6), lactoferrin (molecular weight: 78 kDa, isoelectric point: 8), immunoglobulin G (molecular weight: 150 kDa, isoelectric point: 6.5–9.5), immunoglobulin A (molecular weight: 320 kDa, isoelectric point: 4.5–6.5), and immunoglobulin M (molecular weight: 900 kDa, isoelectric point: 4.5–6.5). 2. All whey proteins may denature with the heat treatment ~70 °C for 20 min, but do not aggregate due to renneting or acidification of milk. | 1. High solubility in aqueous solution. 2. Satisfactory film-forming ability. 3. Efficient to protect from oxidation. 4. Good emulsifying property due to its amphiphilic structure. | 1. Coagulate at lower pH of the emulsion. 2. Heat sensitive. 3. Provide allergenicity to food. | [43] |
| Sodium caseinate | Dairy milk | Casein is a phospho protein. Different types of casein proteins, such as αs1-casein, αs2-casein, β-casein, and κ-casein are present in the casein fraction of milk. Casein is produced by the neutralisation of acid precipitated casein with sodium hydroxide. | 1. Highly soluble in aqueous solution. 2. Good film-forming ability. 3. High denaturation temperature. 4. Good emulsifying property due to the presence of hydrophilic and hydrophobic amino acids in the protein structure. | 1. Coagulate at a lower pH of the emulsion. 2. Provide allergenicity to food. | [43] |
| Vegetable proteins, such as lentil, chickpea, flaxseed, soy, pea proteins, etc. | Proteins from lentil (Lens culinaris), chickpea (Cicer arietinum), flaxseed (Linum usitatissimum), soybean (Glycine max), pea (Pisum sativum). | Proteins from different plant sources have a unique amino acid sequence. Due to that, they offer a variety of biochemical activities. | 1. Inexpensive and available throughout the year. 2. Highly soluble in aqueous solution. 3. Good film-forming ability. 4. Efficient to protect from oxidation. 5. Good emulsifying property due to its amphiphilic structure. | 1. Coagulate at a lower pH of the emulsion. 2. Heat sensitive. 3. Some vegetable proteins, such as chickpea and soy-based proteins may provide allergenicity to the food product. | [37,40,51] |
| Process | Wall Material (Matrix) | Oil Content | Emulsifier | Particle Size | Encapsulation Efficiency (%) | Moisture Content % | Oxidative Stability | References |
|---|---|---|---|---|---|---|---|---|
| Bench top spray-dryer | Combination of chickpea protein isolate and maltodextrin | 10% | - | 16.3–24.0 μm | 88.72 | 3.66–4.07 | Peroxide value 6.68–7.31 meq active O2/kg for the chickpea protein isolate. | [51] |
| 15% | 86.69 | |||||||
| 20% | 83.62 | |||||||
| Combination of lentil protein isolate and maltodextrin | 10% | 21.0–26.1 μm | 90.42 | 3.65–4.12 | Peroxide value 6.62–6.86 meq active O2/kg for the lentil protein isolate. | |||
| 15% | 87.89 | |||||||
| 20% | 85.61 | |||||||
| Spray-drying | Combination of whey protein isolate, methyl cellulose, maltodextrin, gum Arabic, and soya lecithin | >20% | Soya lecithin | 10–50 μm | ~90 | 1.8–3.1 | Rancimat induction period after 10 months (h) for gum Arabic + soya lecithin: 5.9 ± 0.1, gum Arabic + maltodextrin + soya lecithin: 2.8 ± 0.1, gum Arabic + maltodextrin + whey protein isolate + soya lecithin: 6.8 ± 0.3. | [13] |
| Coacervation, Spray-drying, Freeze-drying | Flaxseed gum, Flaxseed protein isolate | Oil-to-wall ratios 1:2, 1:3, and 1:4 | - | For liquid microcapsules 90–130 μm | Maximum value 87.60 by spray-drying and 67.06 by freeze-drying. | For spray-drying 3.20–3.70 and for freeze-drying 4.18–4.47. | Peroxidase value (meq active O2/kg) after 30 days are 2.85–5.52 for spray-drying and 3.25–8.72 for freeze-drying. | [61] |
| Spray-drying | Combination of maltodextrin and gum Arabic | 14 and 20% | - | 17.6–23.1 μm | The highest encapsulation efficiency was achieved with 14% oil. 54.6–90.7. | - | Induction time 2.83 ± 0.62 h, oxidative stability index 3.78 h for 14% oil. | [12] |
| Spray-drying | Combination of maltodextrin, whey protein concentrate, gum Arabic, modified starch, 100 Hi-Cap | 20% | - | Droplet diameter: 0.6–26 μm | The lowest value obtained for maltodextrin and whey protein concentrate is 62.3–95.7. | 1–3% | Peroxidase value (meq peroxide/kg oil) after 4 weeks for gum Arabic + maltodextrin: 138, modified starch + maltodextrin 138, Hi-Cap + maltodextrin: 124, whey protein concentrate + maltodextrin: 107. | [36] |
| Spray-drying, Freeze-drying | Zein | - | - | - | For spray-drying 93.26 ± 0.95 and for freeze-drying 59.63 ± 0.36. | For spray-drying 3.49–5.06 and freeze-drying 4.94–5.33. | - | [62] |
| Spray-drying |
| 10% 20% 30% 40% | - | 0.24–180 μm | Emulsions prepared with modified starch had the highest encapsulation efficiency, whereas emulsion prepared with whey protein concentrate had lowest encapsulation efficiency. 37–97. | For whey protein concentrate 0.36–0.78, for gum Arabic 0.89–1.74, for modified starch 0.19–0.53; Hi-Cap 100. | Peroxidase value (meg peroxide/kg oil) for modified starch. Hi-Cap 100 is 0.5–1.8, 3.1–4 for gum Arabic and 1.3–2 for whey protein concentrate. | [63] |
| Freeze-drying | Combination of lentil protein isolates and maltodextrin | 10, 20, 30% | - | 4.2–6.7 μm | Highest encapsulation efficiency ~62.8. | <6.0% | Peroxide value on day 30 for 4.0% native lentil protein isolates + 36% maltodextrin + 10% oil 25.57 and 14.75 meq of active O2/kg for free oil and entrapped oils, respectively. | [64] |
| Spray-drying | Combination of whey proteins concentrate, sodium caseinate, lactose and ascorbyl palmitate | 12.5% | - | 0.54–70.6 μm | 86.77–84.51% | 3.88–3.98 | Peroxide value after 6 months varied from 0.81 to 0.99 meq peroxides/kg. | [65] |
| Spray-drying | Modified starch | 30% | - | 0.5–100 μm | 90.9% | 3.5% | Induction period of the microcapsules exceeded 50 h for all times. | [57] |
| Spray-drying | Combination of gum Arabic, maltodextrin, skimmed milk powder, and Tween 80 | 8–22% | Tween 80 | - | 70–86% | 3.2–4.8% | Peroxide value varied from 1–1.28 meq/kg. | [56] |
| Spray-drying | Gum Arabic | 10–30% | - | 0.1–477 μm | 51–92% | - | Peroxide value 0.017–0.106 meq peroxide/kg oil. | [66] |
| Spray dried | Combination of whey protein concentrate, sodium alginate, and maltodextrin | 4.5–5% | - | 1–10 μm | 30.69–84.39% | - | Peroxidase value (meq/kg oil) 3.46–6.84. | [67] |
| Freeze-drying | Combination of whey protein isolate, maltodextrin, and low density sodium alginate | 10% | - | 27.01–95.44% | - | Peroxide value for the emulsion with 20.24 total solids content (g/100 g emulsion) was increased from 1.5 to 46.5 meq/kg oil after freeze-drying. | [68] | |
| Freeze-drying | Combination of tertiary conjugate of gelatin, flaxseed mucilage, and oxidized tannic acid | 15, 30, and 50% | - | - | >90% | - | Peroxide value increased from 3.0–5.3 meqO2/kg. | [60] |
| Spray-drying | Combination of maltodextrin and pea protein isolate | 20% and 40% | - | Particle size distribution ~24 μm | For 20% oil 35.2–95.6% and for 40% oil 22.3–93.6%. | - | - | [69] |
| Spray-drying | Combination of maltodextrin and whey protein concentrate | 20% | - | 5.47–7.09 µm | Ranged between 81.3–95.3% | 3.16–4.91% (weight basis) | - | [70] |
| Spray-drying | Different combinations of soy protein isolate, pea protein isolate, wheat dextrin soluble fiber, and trehalose | 35% | - | Mean diameter of particles 18–40 μm | Microcapsules with the protein-trehalose matrix 98–94%. Microcapsules with the protein-soluble fiber matrix 81–62%. | 1.5–2.3% | Peroxide value of microencapsulated oil before storage: 1.80–7.90 meqO2/kg and after 12 weeks 4–27 meqO2/kg. | [71] |
| Spray-drying | Gum Arabic | 10% | Droplets mean diameter 1.854 μm | ~92% | - | Peroxide value ~0.032 meq/kg oil. | [72] | |
| 20% | Droplets mean diameter 2.191 μm | ~75% | Peroxide value ~0.036 meq/kg oil. | |||||
| 30% | Droplets mean diameter 2.479 μm | ~52% | Peroxide value ~0.036 meq/kg oil. | |||||
| 40% | Droplets mean diameter 3.464 μm | ~40% | Peroxide value ~0.04 meq/kg oil. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakdhane, A.; Labidi, S.; Chaabane, D.; Tolnay, A.; Nath, A.; Koris, A.; Vatai, G. Microencapsulation of Flaxseed Oil—State of Art. Processes 2021, 9, 295. https://doi.org/10.3390/pr9020295
Yakdhane A, Labidi S, Chaabane D, Tolnay A, Nath A, Koris A, Vatai G. Microencapsulation of Flaxseed Oil—State of Art. Processes. 2021; 9(2):295. https://doi.org/10.3390/pr9020295
Chicago/Turabian StyleYakdhane, Asma, Sabrine Labidi, Donia Chaabane, Anita Tolnay, Arijit Nath, András Koris, and Gyula Vatai. 2021. "Microencapsulation of Flaxseed Oil—State of Art" Processes 9, no. 2: 295. https://doi.org/10.3390/pr9020295
APA StyleYakdhane, A., Labidi, S., Chaabane, D., Tolnay, A., Nath, A., Koris, A., & Vatai, G. (2021). Microencapsulation of Flaxseed Oil—State of Art. Processes, 9(2), 295. https://doi.org/10.3390/pr9020295








