Binary and Ternary Vanadium Oxides: General Overview, Physical Properties, and Photochemical Processes for Environmental Applications
Abstract
1. Introduction
2. Vanadium: A Versatile Element for Various Applications
2.1. Vanadium Compounds in Homogeneous Systems
2.2. Vanadium Materials in Heterogeneous Systems
3. Structures and Physical Properties of Binary and Ternary Vanadium Oxides
3.1. Case of Binary Vanadium Oxides
3.1.1. Structure of VO2, V2O5, and V2O3
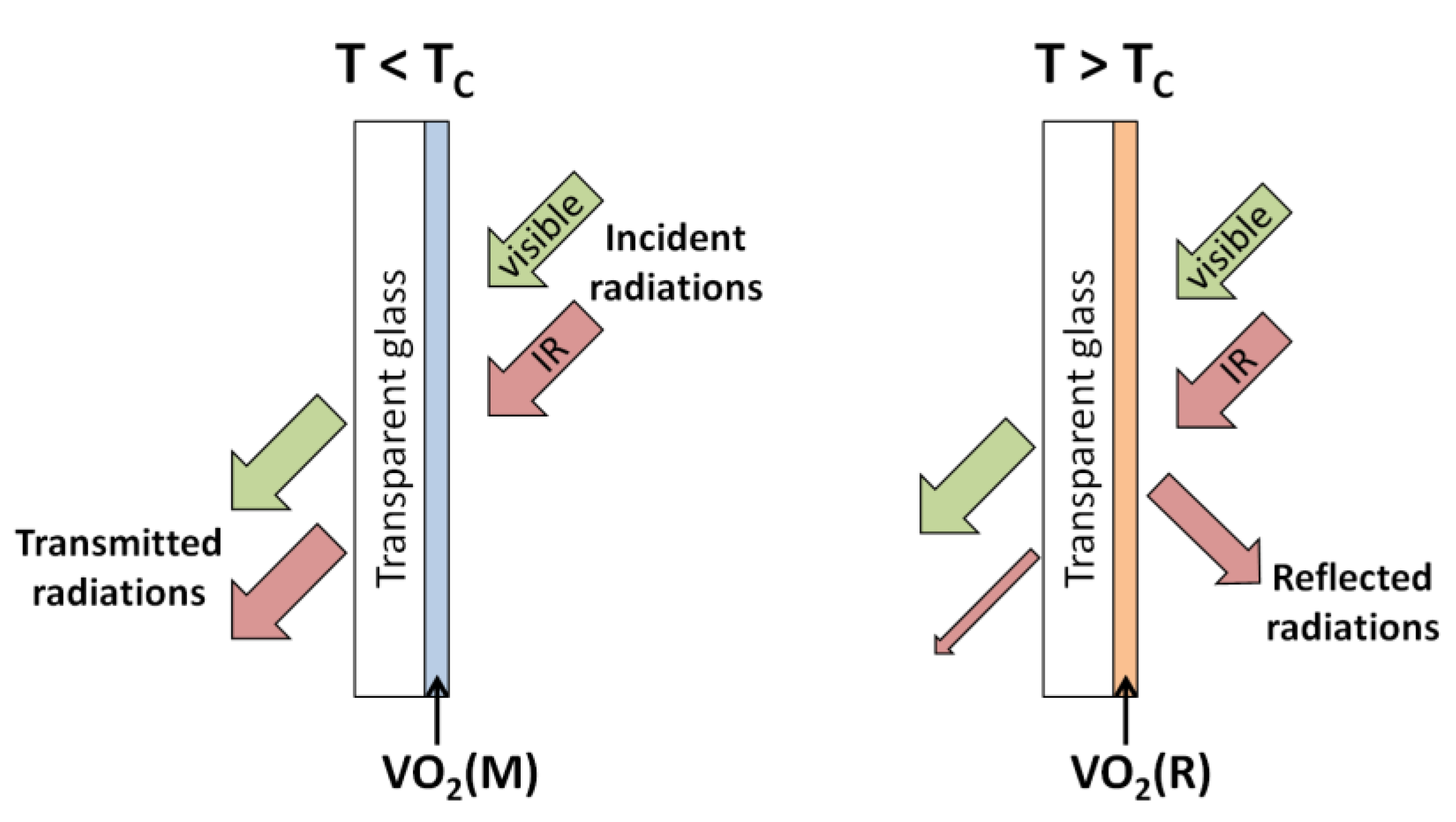
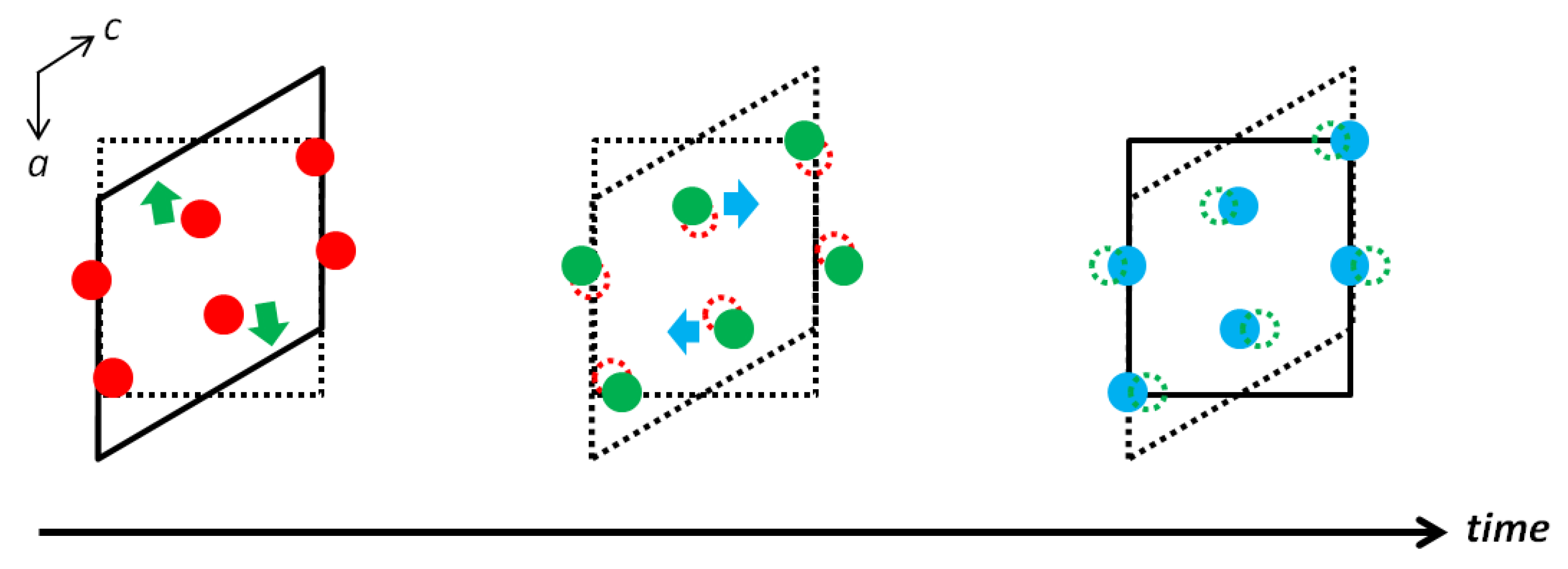
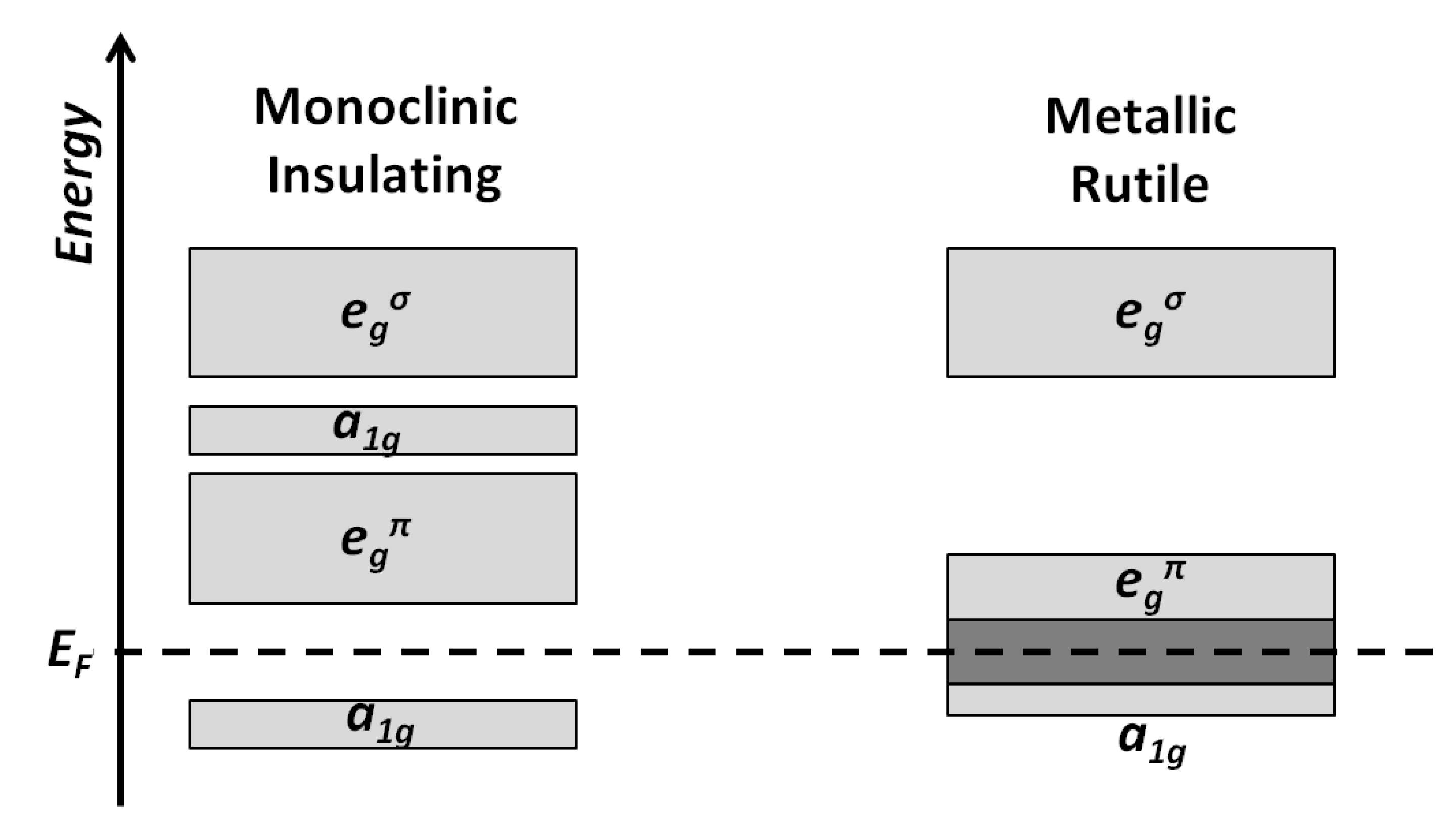
3.1.2. Properties of VO2, V2O5, and V2O3
3.1.3. Binary Vanadium Oxides with Mixed Valence States
3.2. Case of Ternary Vanadium Oxides
3.2.1. Bismuth Vanadates
3.2.2. Iron Vanadates
3.2.3. Copper Vanadates
3.2.4. Silver Vanadates
3.2.5. Manganese and Other Transition Metal Vanadates
4. Vanadium Oxides in Photochemical Processes for Environmental Applications
4.1. Production of Sustainable Energy
4.2. Photo-Induced Degradation of Organic Contaminants
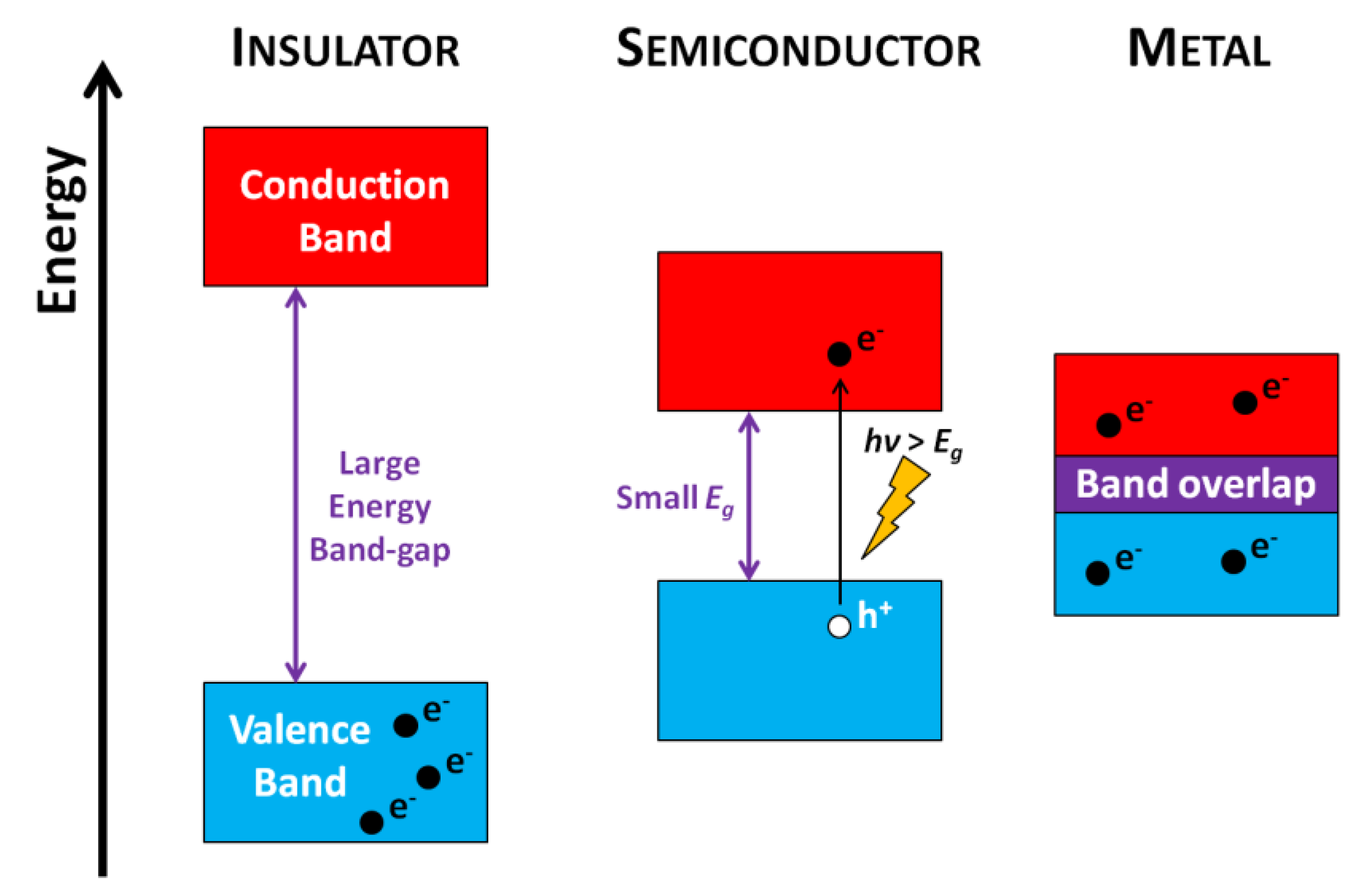
4.2.1. Generalities of Photo-Induced Processes
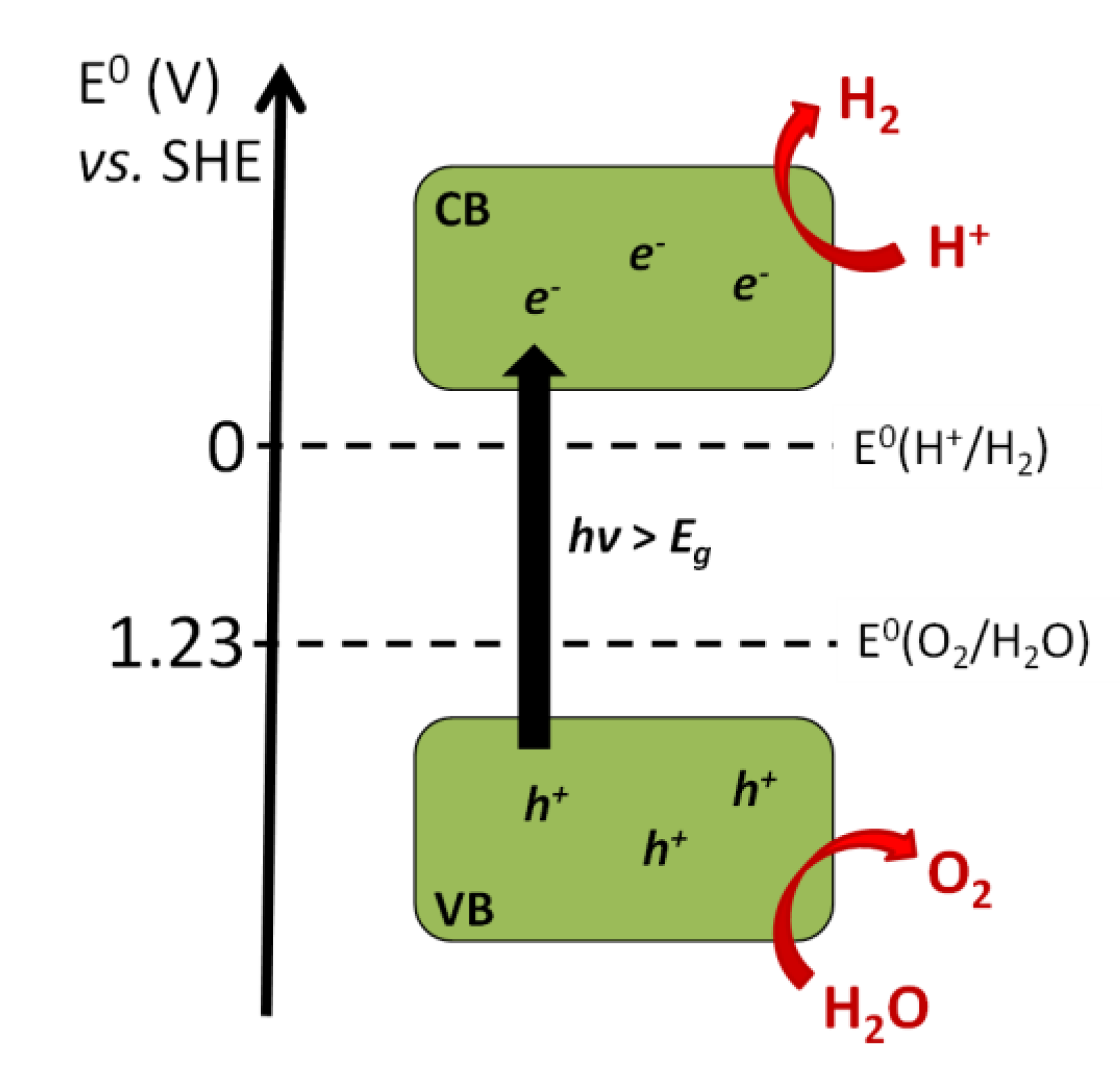
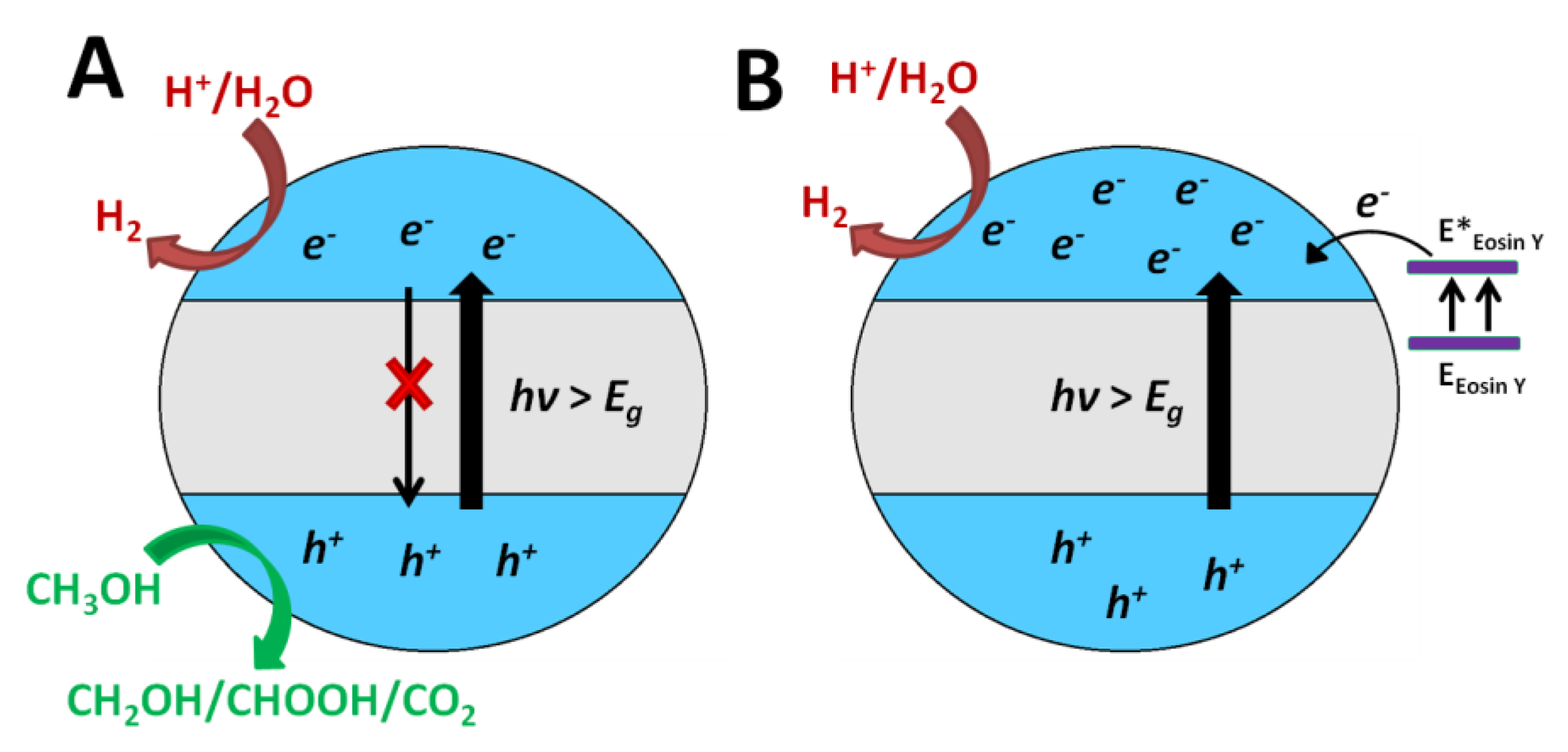
Photocatalysis
Photo-Fenton and Fenton-Based Processes
4.2.2. Binary Vanadium Oxides
V2O5
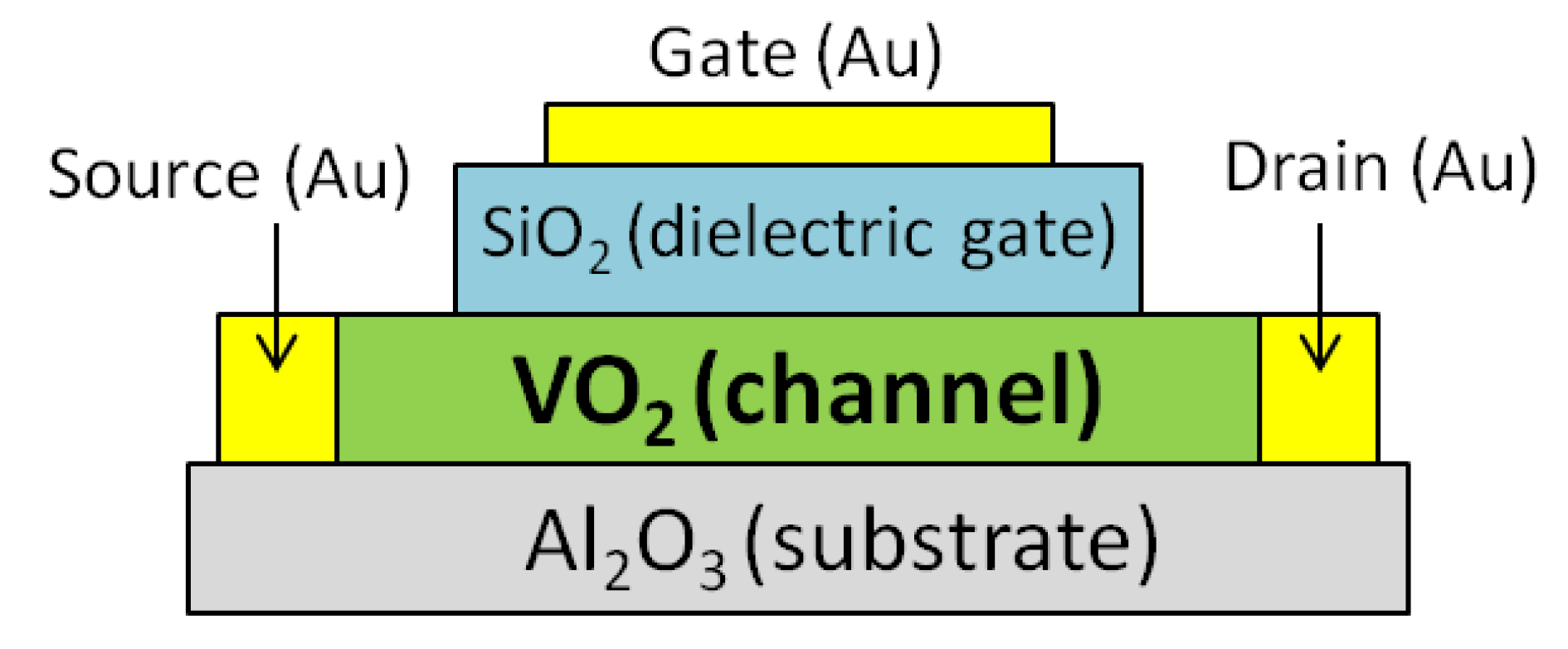
VO2
Mixed Valence Vanadium Oxides
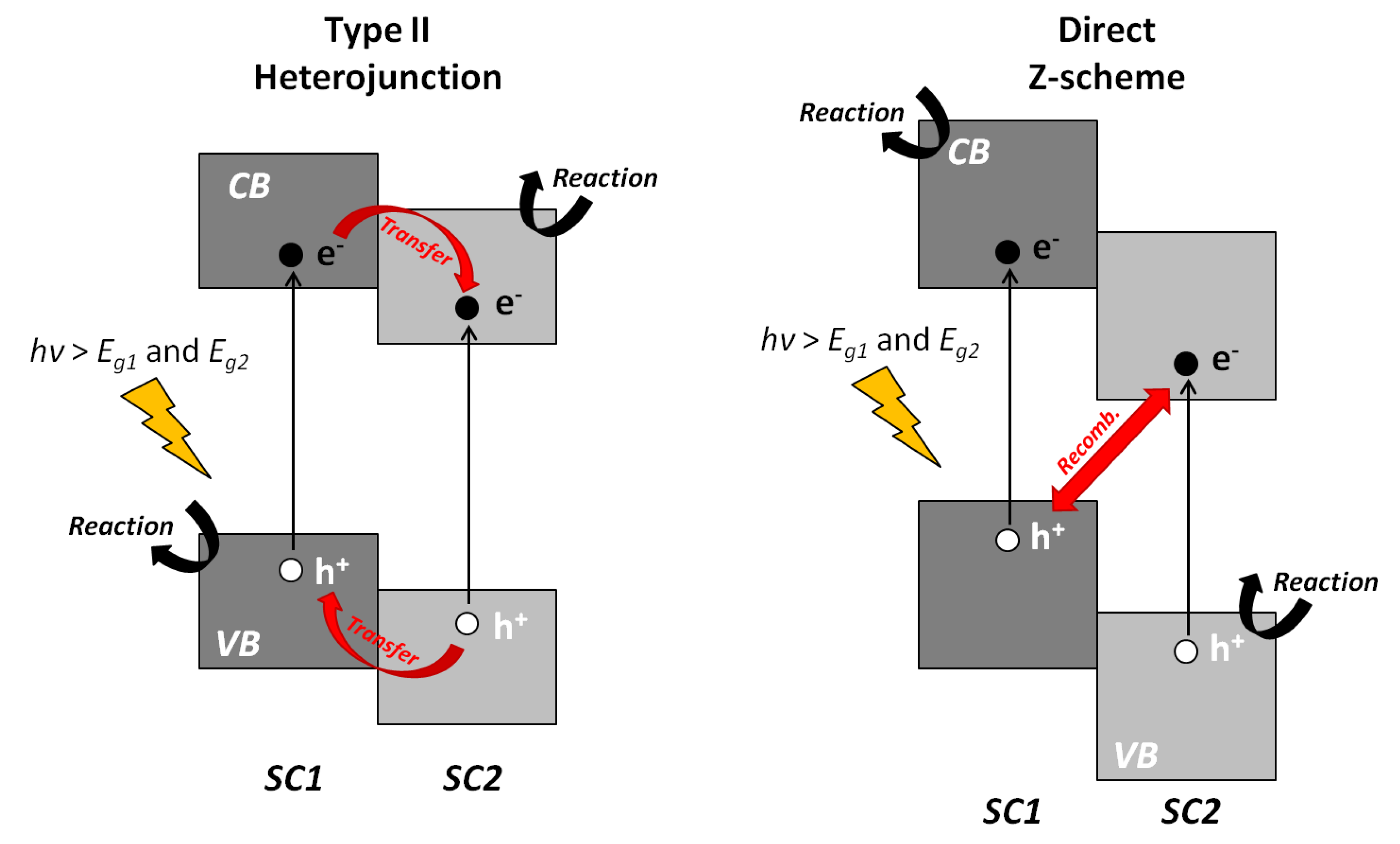
4.2.3. Ternary Vanadium Oxides
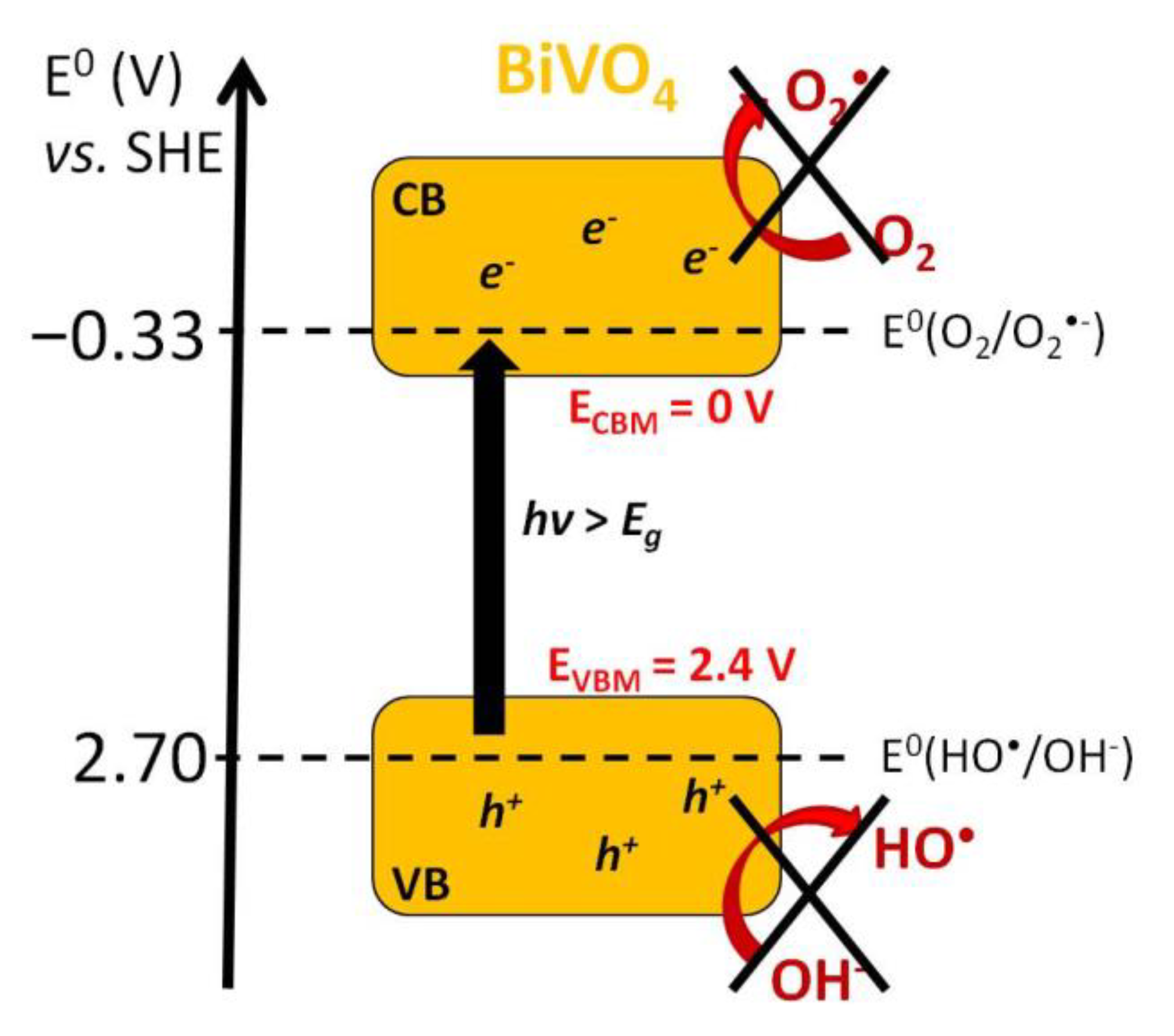
Bismuth Vanadates
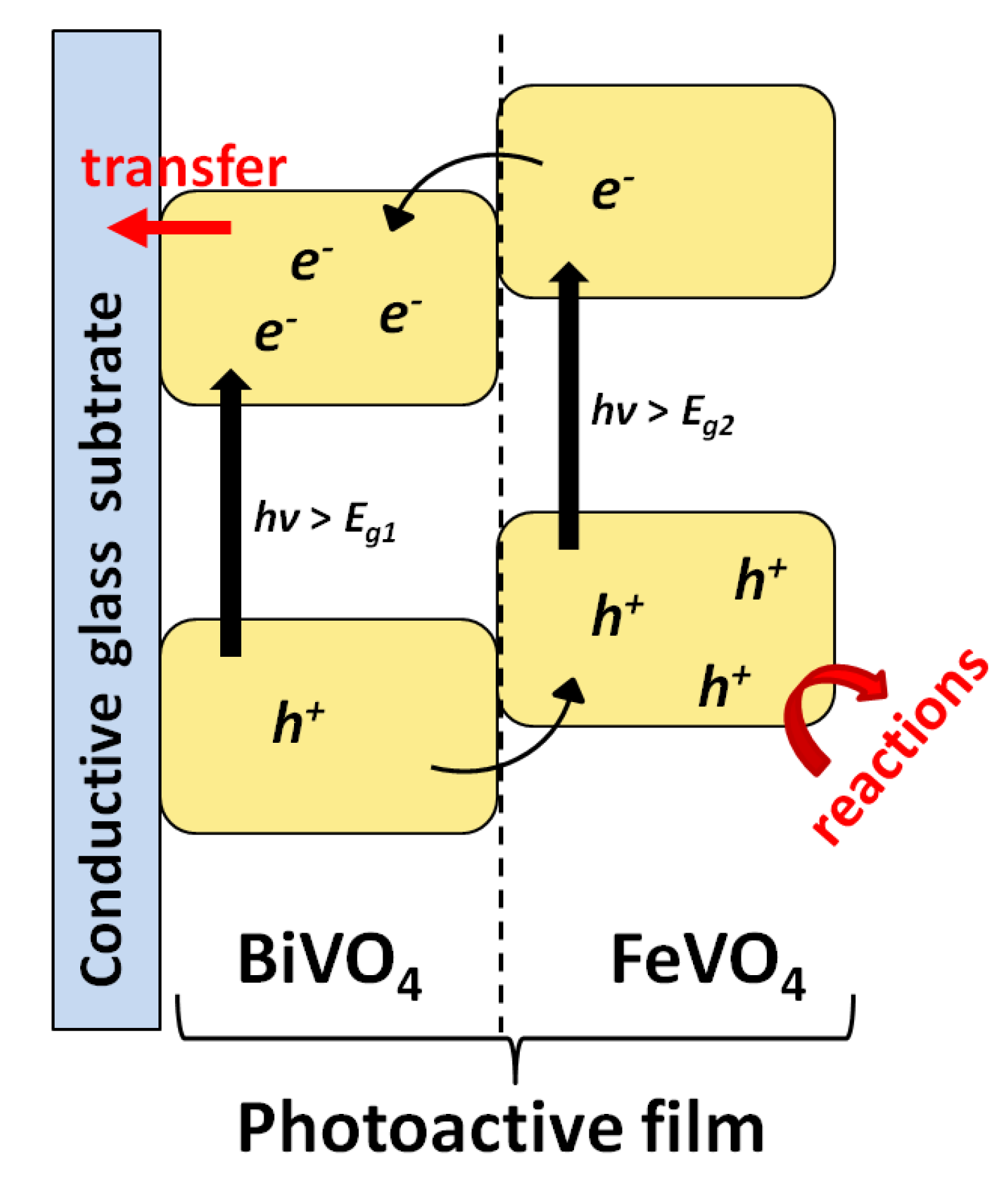
Iron Vanadates
Other Transition Metal Vanadates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weeks, M.E. The Discovery of the Elements. VII. Columbium, Tantalum, and Vanadium. J. Chem. Educ. 1932, 9, 863–884. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Vanadium geochemistry in the biogeosphere speciation, solid-solution interactions, and ecotoxicity. Appl. Geochem. 2019, 102, 1–25. [Google Scholar] [CrossRef]
- Safstrom, N.G. Sur Le Vanadium, Métal Nouveau, Trouvé Dans Du Fer En Barres De Eckersholm, Forge Qui Tire Sa Mine De Taberg, Dans Le Smaland. Ann. Chim. Phys. 1831, 46, 105–111. [Google Scholar]
- Baharum, H.; Chu, W.C.; Teo, S.S.; Ng, K.Y.; Rahim, R.A.; Ho, C.L. Molecular cloning, homology modeling and site-directed mutagenesis of vanadium-dependent bromoperoxidase (GcVBPO1) from Gracilaria changii (Rhodophyta). Phytochemistry 2013, 92, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Adriano, D.C. Trace Elements in the Terrestrial Environment; Springer: New York, NY, USA, 1986; pp. 470–501. [Google Scholar]
- Moskalyk, R.R.; Alfanti, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Baroch, E.F. Vanadium and vanadium alloys. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 2013; pp. 1–18. [Google Scholar]
- Liu, M.; Su, B.; Tang, Y.; Jiang, X.; Yu, A. Recent Advances in Nanostructured Vanadium Oxides and Composites for Energy Conversion. Adv. Energy Mater. 2017, 7, 1700885. [Google Scholar] [CrossRef]
- Rehder, D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012, 4, 1823. [Google Scholar] [PubMed]
- World Health Organization. International Programme on Chemical Safety, Environmental Health, Criteria 61, Vanadium. Available online: http://www.inchem.org/documents/ehc/ehc/ehc81.htm (accessed on 26 December 2020).
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Biochemical and medical importance of vanadium compounds. Acta Biochim. Pol. 2012, 59, 195. [Google Scholar] [CrossRef]
- Barceloux, D.G. Vanadium. J. Toxicol. Clin. Toxicol. 1999, 37, 265–278. [Google Scholar] [CrossRef]
- Rehder, D. Vanadium. Its role for humans. Met. Ions Life Sci. 2013, 13, 139–169. [Google Scholar] [PubMed]
- Wright, M.T.; Belitz, K. Factors controlling the regional distribution of vanadium in groundwater. Groundwater 2010, 48, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Vouk, V.B.; Piver, W.T. Metallic elements in fossil fuel combustion products. Environ. Health Perspect. 1983, 47, 2111–2225. [Google Scholar]
- Miramand, P.; Fowler, S.W. Bioaccumulation and transfer of vanadium in marine organisms. In Vanadium in the Environment: Part 1. Chemistry and Biochemistry; Nriagu, S.W., Ed.; Wiley: New York, NY, USA, 1998; pp. 167–169. [Google Scholar]
- Kiss, T.; Kiss, E.; Garribba, E.; Sakurai, H. Speciation of insulin-mimetic VO (IV)-containing drugs in blood serum. J. Inorg. Biochem. 2000, 80, 65–73. [Google Scholar] [CrossRef]
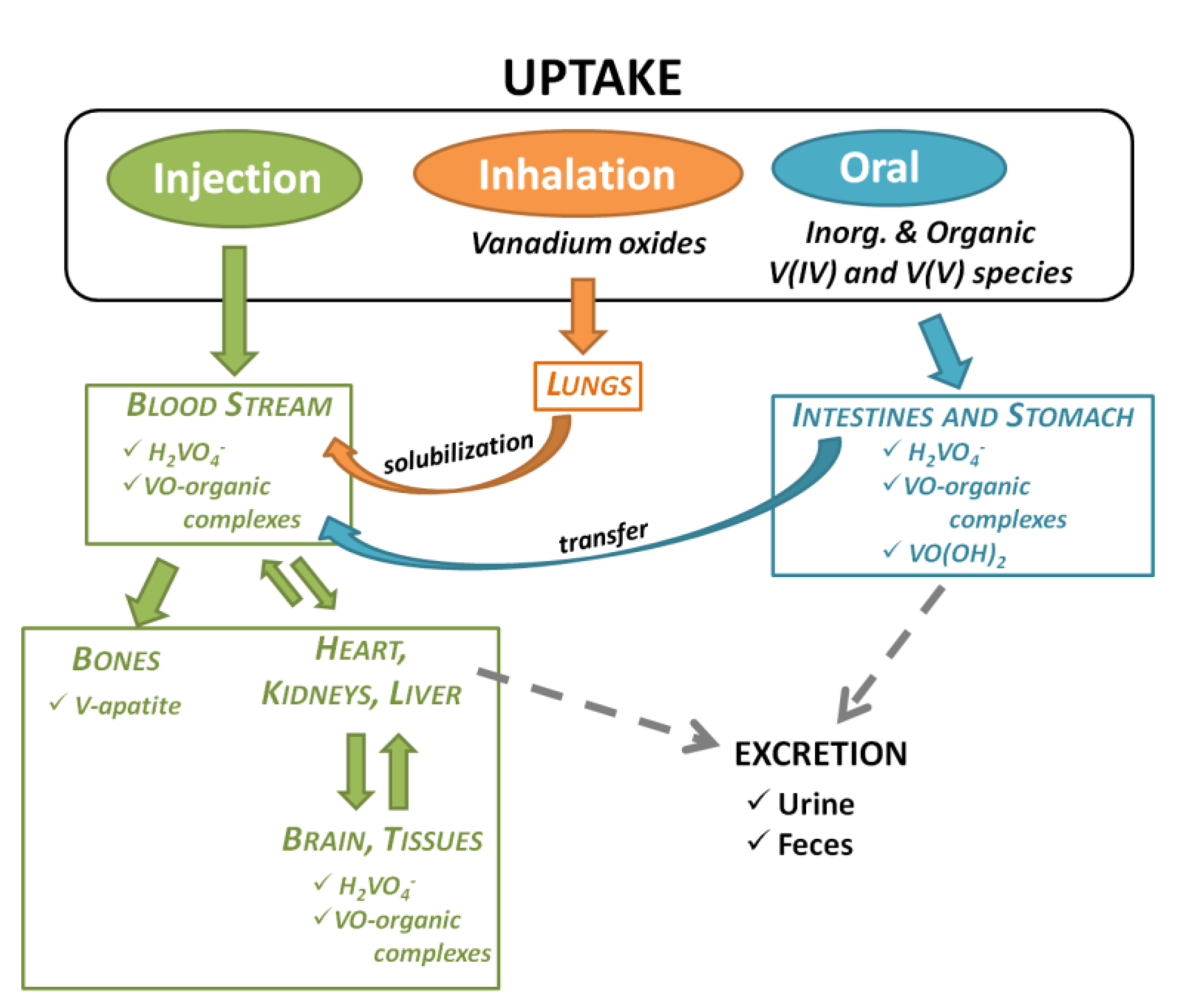
- Hansen, T.V.; Aaseth, J.; Alexander, J. The effect of chelating agents on vanadium distribution in the rat body and on uptake by human erythrocytes. Arch. Toxicol. 1982, 50, 195–202. [Google Scholar] [CrossRef]
- Rehder, D. The bioinorganic chemistry of vanadium. Angew. Chem. Int. Ed. Engl. 1991, 30, 148–167. [Google Scholar]
- Granqvist, C.G. Spectrally selective coatings for energy efficiency and solar applications. Phys. Scr. 1985, 32, 401–407. [Google Scholar] [CrossRef]
- Lamsal, C.; Ravindra, N.M. Vanadium Oxides: Synthesis, Properties, and Applications. In Semiconductors; Pech-Canul, M., Ravindra, N., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 127–218. [Google Scholar]
- Parker, J.C., Jr.; Geiser, U.W.; Lam, D.J.; Xu, Y.; Ching, W.Y. Optical properties of the vanadium oxides VO2 and V2O5. J. Am. Ceram. Soc. 1990, 73, 3206–3208. [Google Scholar] [CrossRef]
- Chang, T.C.; Cao, X.; Bao, S.H.; Ji, S.D.; Luo, H.J.; Jin, P. Review on thermochromic vanadium dioxide based smart coatings: From lab to commercial application. Adv. Manuf. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Azenha, E.; Romeiro, A.; Sarakha, M. Photodegradation of pesticides and photocatalysis in the treatment of water and waste. In Applied Photochemistry; Evans, R.C., Douglas, P., Burrows, H.D., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 247–266. [Google Scholar]
- Gaya, U.I. Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Hinojosa-Reyes, L.; Guzman-Mar, J.L.; Villanueva-Rodriguez, M. Semiconductormaterials for photocatalytic oxidation of organic pollutants in wastewater. In Photocatalytic Semiconductors; Hernandez-Ramirez, A., Medina-Ramirez, I., Eds.; Springer: Cham, Switzerland, 2015; pp. 187–228. [Google Scholar]
- Larsson, D.G.J. Pollution from drug manufacturing: Review and perspectives. Philos. Trans. R. Soc. B 2014, 369, 71–78. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in watewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Monfort, O.; Plesch, G. Bismuth vanadate-based semiconductor photocatalysts: A short critical review on the efficiency and the mechanism of photodegradation of organic pollutants. Environ. Sci. Pollut. Res. Int. 2018, 25, 19362–19379. [Google Scholar] [CrossRef]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Baess, C.F.; Mesmer, R.E. Hydrolysis of cations. Ber. Bunsen. Phys. Chem. 1976, 81, 245–246. [Google Scholar]
- Livage, J. Hydrothermal Synthesis of Nanostructured Vanadium Oxides. Materials 2010, 3, 4175–4195. [Google Scholar] [CrossRef]
- Sadoc, A.; Messaoudi, S.; Furet, E.; Gautier, R.; Le Fur, E.; Le Pollès, L.; Pivan, J.Y. Structure and stability of VO2+ in aqueous solution: A car-parrinello and static ab initio study. Inorg. Chem. 2007, 46, 4835–4843. [Google Scholar] [CrossRef]
- Bouhedja, L.; Steunou, N.; Maquet, J.; Livage, J. Synthesis of polyoxovanadates from aqueous solutions. J. Solid State Chem. 2001, 162, 315–321. [Google Scholar] [CrossRef]
- Thompson, K.H.; Orvig, C. Metal complexes in medicinal chemistry: New vistas and challenges in drug design. Dalton Trans. 2006, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. The coordination chemistry of vanadium as related to its biological functions. Coord. Chem. Rev. 1999, 182, 297–322. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2005, 301–302, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, K.; Tsuge, K. Inorganic Chemistry of Vanadium. In Vanadium Biochemical and Molecular Biological Approaches; Michibata, H., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 3–31. [Google Scholar]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium—An element of atypical biological significance. Toxicol. Lett. 2004, 150, 135–143. [Google Scholar] [CrossRef]
- Almeida, M.; Filipe, S.; Humanes, M.; Maia, M.F.; Melo, R.; Severino, N.; Silva, J.A.L.; Fraústo da Silva, J.J.R.; Wever, R. Vanadium haloperoxidases from brown algae of the Laminariaceae family. Photochemistry. 2001, 57, 633–642. [Google Scholar] [CrossRef]
- Menon, A.S.; Rau, M.; Ramasarma, T.; Crane, F.L. Vanadate inhibits mevalonate synthesis and activates NADH oxidation in microsomes. FEBS Lett. 1980, 114, 139–141. [Google Scholar] [CrossRef]
- Golden, M.H.; Golden, B.E. Trace Elements: Potential Importance in Human Nutrition with Particular Reference to Zinc and Vanadium. Br. Med. Bull. 1981, 37, 3731–3736. [Google Scholar] [CrossRef]
- Byrne, A.R.; Kosta, L. Vanadium in foods and in human body fluids and tissues. Sci. Total Environ. 1978, 10, 17–30. [Google Scholar] [CrossRef]
- Heinemann, G.; Fichtl, B.; Vogt, W. Pharmacokinetics of vanadium in humans after intravenous administration of a vanadium containing albumin solution. Br. J. Clin. Pharmacol. 2003, 55, 241–245. [Google Scholar] [CrossRef]
- Willsky, G.R.; Chi, L.H.; Godzala, M.; Kostyniak, P.J.; Smee, J.J.; Trujillo, A.M.; Alfano, J.A.; Ding, W.J.; Hu, Z.H.; Crans, D.C. Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord. Chem. Rev. 2011, 255, 2258–2269. [Google Scholar] [CrossRef]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeil, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.S.; Basu, M.; Ghosh, B.; Samanta, K.; Chatterjee, M. Vanadium, a Versatile Biochemical Effector in Chemical Rat Mammary Carcinogenesis. Nutr. Cancer. 2005, 51, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.S.; Rana, B.; Swami, B.; Venu, V.; Chatterjee, M. Vanadium mediated apoptosis and cell cycle arrest in MCF7 cell line. Chem.-Biol. Interact. 2006, 163, 239–247. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, M.T.; Menictas, C.; Kazacos, M.S. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar]
- Swette, L.; Jalan, V. Development of Electrodes for the NASA Iron/Chromium Redox System and Factors Affecting Their Performance; Giner Inc.: Waltham, MA, USA, 1984. Available online: https://ntrs.nasa.gov/api/citations/19850027158/downloads/19850027158.pdf (accessed on 26 December 2020).
- Savinell, R.F.; Liu, C.C.; Galasco, R.T.; Chiang, S.H.; Coetzee, J.F. Discharge characteristics of a soluble Iron–Titanium battery system. J. Electrochem. Soc. 1979, 126, 357. [Google Scholar] [CrossRef]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301–302, 87–105. [Google Scholar] [CrossRef]
- Schwendt, P.; Tatiersky, J.; Krivosudsky, L.; Simunekova, M. Peroxido complexes of vanadium. Coord. Chem. Rev. 2016, 318, 135–157. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of Vanadium Complex Catalysts for Precise Olefin Polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, W. (Imido)vanadium(V)-alkyl, -alkylidene complexes exhibiting unique reactivity towards olefins and alcohols. Chem. Sci. 2010, 1, 161–173. [Google Scholar] [CrossRef]
- Wischang, D.; Brucher, O.; Hartung, J. Bromoperoxidases and Functional Enzyme Mimics as Catalysts for Oxidative Bromination-A Sustainable Synthetic Approach. Coord. Chem. Rev. 2011, 255, 2204–2217. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Ichikawa, T.; Kojima, Y.; Dey, G.K. Development of vanadium based hydrogen storage material: A review. Renew. Sust. Energy Rev. 2017, 72, 791–800. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Marban, G.; Solis, T.V. Towards the hydrogen economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Ma, Y.D.; Dai, Y.; Guo, M.; Niu, C.W.; Zhu, Y.T.; Huang, B.B. Evidence of the existence of magnetism in pristine VX2 monolayers (X = S, Se) and their strain-induced tunable magnetic properties. ACS Nano 2012, 6, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Chmiola, J.; Largeot, C.; Taberna, P.L.; Simon, P.; Gogotsi, Y. Monolithic Carbide-Derived Carbon Films for Micro-Supercapacitors. Science 2010, 328, 480. [Google Scholar] [CrossRef]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene Double-Layer Capacitor with ac Line-Filtering Performance. Science 2010, 329, 1637. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, R.H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Keller, D.E. Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis. Catal. Today 2003, 78, 25–46. [Google Scholar] [CrossRef]
- Dummer, N.F.; Bartley, J.K.; Hutchings, G.J. Vanadium Phosphate Materials as Selective Oxidation Catalysts. Adv. Catal. 2011, 54, 189–247. [Google Scholar]
- Khodakov, A.; Olthof, B.; Bell, A.T.; Iglesia, E. Structure and catalytic properties of supported vanadium oxides: Support effects on oxidative dehydrogenation reactions. J. Catal. 1999, 181, 205–216. [Google Scholar] [CrossRef]
- Wu, C.; Feng, F.; Xie, Y. Design of vanadium oxide structures with controllable electrical properties for energy applications. Chem. Soc. Rev. 2013, 42, 5157–5183. [Google Scholar] [CrossRef] [PubMed]
- Prasadam, V.P.; Bahlawane, N.; Mattelaer, F.; Rampelberg, G.; Detavernier, C.; Fang, L.; Jiang, Y.; Martens, K.; Parkin, I.P.; Papakonstantinou, I. Atomic layer deposition of vanadium oxides: Process and application review. Mater. Today Chem. 2019, 12, 396–423. [Google Scholar] [CrossRef]
- Delmas, C.; Cognacauradou, H.; Cocciantelli, J.M.; Menetrier, M.; Doumerc, J.P. The LixV2O5 system—An overview of the structure modifications induced by the lithium intercalation. Solid State Ion. 1994, 69, 257–264. [Google Scholar] [CrossRef]
- Ostreng, E.; Gandrud, K.B.; Hu, Y.; Nilsen, O.; Fjellvag, H. High power nanostructured V2O5 thin film cathodes by atomic layer deposition. J. Mater. Chem. 2014, 2, 15044–15051. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, C.Z. Design of nanoarchitectured electrode materials applied in new-generation rechargeable lithium ion batteries. Dalton Trans. 2007, 5235, 5235. [Google Scholar] [CrossRef]
- Wan, Z.; Zou, Z.; Wang, J.; Long, F.; Wu, Y. Synthesis and Electrochemical Properties of Flower-like Na-doped V6O13 Cathode Materials for Li-ion Batteries. Int. J. Electrochem. Sci. 2018, 13, 6565–6576. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Niklewski, T. Accurate X-ray determination of the lattice parameters and the thermal expansion coefficients of VO2 near the transition temperature. J. Appl. Cryst. 1979, 12, 370–373. [Google Scholar] [CrossRef]
- Jerominek, H.; Picard, F.; Vincent, D. Vanadium oxide films for optical switching and detection. Opt. Eng. 1993, 32, 2092–2099. [Google Scholar] [CrossRef]
- Cavalleri, A.; Dekorsy, T.; Chong, H.H.W.; Kieffer, J.C.; Schoenlein, R.W. Evidence for a structurally-driven insulator-to-metal transition in VO2: A view from the ultrafast timescale. Phys. Rev. B 2004, 70, 161102. [Google Scholar] [CrossRef]
- Granqvist, C.G. Recent progress in thermochromics and electrochromics: A brief survey. Thin Solid Films 2016, 614, 90–96. [Google Scholar] [CrossRef]
- Gupta, A.; Aggarwal1, R.; Gupta, P.; Dutta, T.; Narayan, R.J.; Narayan, J. Semiconductor to metal transition characteristics of VO2 thin films grown epitaxially on Si (001). Appl. Phys. Lett. 2009, 95, 111915. [Google Scholar] [CrossRef]
- Darling, R.B.; Iwanaga, S. Structure, properties, and MEMS and microelectronic applications of vanadium oxides. Sadhana 2009, 34, 531–542. [Google Scholar] [CrossRef]
- Niklaus, F.; Decharat, A.; Jansson, C.; Stemme, G. Performance model for uncooled infrared bolometer arrays and performance predictions of bolometers operating at atmospheric pressure. Infrared Phys. Technol. 2008, 51, 168–177. [Google Scholar] [CrossRef]
- Wood, R.A.; Han, C.J.; Kruse, P.W. Integrated uncooled infrared detector imaging arrays. IEEE Workshop Solid-State Sencor Actuator 1992. [Google Scholar] [CrossRef]
- Mattelaer, F.; Geryl, K.; Rampelberg, G.; Dobbelaere, T.; Dendooven, J.; Detavernier, C. Atomic layer deposition of vanadium oxides for thin-film lithium-ion battery applications. RSC Adv. 2006, 6, 114658–114665. [Google Scholar] [CrossRef]
- Mattelaer, F.; Geryl, K.; Rampelberg, G.; Dendooven, J.; Detavernier, C. Amorphous and crystalline vanadium oxides as high-energy and high-power cathodes for three-dimensional thin-film lithium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 13121–13131. [Google Scholar] [CrossRef]
- Cao, A.M.; Hu, J.S.; Liang, H.P.; Wan, L.J. Self-Assembled Vanadium Pentoxide (V2O5) Hollow Microspheres from Nanorods and Their Application in Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2005, 44, 4391. [Google Scholar] [CrossRef]
- Adler, D. Mechanisms for metal-nonmetal transitions in transition-metal oxides and sulfides. Rev. Mod. Phys. 1968, 40, 714–736. [Google Scholar] [CrossRef]
- Muhr, H.J.; Krumeich, F.; Schonholzer, U.P.; Bieri, F.; Niederberger, M.; Gauckler, L.J.; Nesper, R. Vanadium Oxide Nanotubes—A New Flexible Vanadate Nanophase. Adv. Mater. 2000, 12, 231. [Google Scholar] [CrossRef]
- Pinna, N.; Wild, U.; Urban, J.; Schlogl, R. Divanadium Pentoxide Nanorods. Adv. Mater. 2003, 15, 329. [Google Scholar] [CrossRef]
- Eyert, V.; Hoch, K.-H. Electronic structure of V2O5: Role of octahedral deformations. Phys. Rev. B 1998, 57, 12727–12737. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, K.X.; Wei, X.; Chen, J.S. Carbon-Coated V2O5 Nanocrystals as High Performance Cathode Material for Lithium Ion Batteries. Chem. Mater. 2011, 23, 5290. [Google Scholar] [CrossRef]
- Hermann, K.; Chakrabarti, A.; Druzinic, R.; Witko, M. Ab initio density functional theory studies of hydrogen adsorption at the V2O5(010) surface. Phys. Stat. Sol. 1999, 173, 195–208. [Google Scholar] [CrossRef]
- Liu, L.; Cao, F.; Yao, T.; Xu, Y.; Zhou, M.; Qu, B.; Pan, B.; Wu, C.; Wei, S.; Xie, Y. New-phase VO2 micro/nanostructures: Investigation of phase transformation and magnetic property. New J. Chem. 2012, 36, 619. [Google Scholar] [CrossRef]
- Xie, J.; Wu, C.; Hu, S.; Dai, J.; Zhang, N.; Feng, J.; Yang, J.; Xie, Y. Ambient rutile VO2(R) hollow hierarchitectures with rich grain boundaries from new-state nsutite-type VO2, displaying enhanced hydrogen adsorption behavior. Phys. Chem. Chem. Phys. 2012, 14, 4810. [Google Scholar] [CrossRef] [PubMed]
- Cavalleri, A.; Toth, C.; Siders, C.W.; Squier, J.A.; Raksi, F.; Forget, P.; Kieffer, J.C. Femtosecond structural dynamics in VO2 during an ultra fast solid-solid phase transition. Phys. Rev. Lett. 2001, 87, 237401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shang, B.; Yang, J.; Yan, W.; Wei, S.; Xie, Y. From VO2 (B) to VO2 (A) nanobelts: First hydrothermal transformation, spectroscopic study and first principles calculation. Phys. Chem. Chem. Phys. 2011, 13, 15873. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hwang, H.Y.; Tao, H.; Strikwerda, A.C.; Fan, K.B.; Keiser, G.R.; Sternbach, A.J.; West, K.G.; Kittiwatanakul, S.; Lu, J.W.; et al. Terahertz-field-induced insulator-to-metal transition in vanadium dioxide metamaterial. Nature 2012, 487, 345–348. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley VCH: Weinheim, Germany, 2003. [Google Scholar]
- Yu, P.; Cardona, M. Fundamentals of Semiconductors: Physics and Materials Properties; Springer: Heidelberg, Germany, 2010; pp. 17–106. [Google Scholar]
- Batista, C.; Ribeiro, R.M.; Teixeira, V. Synthesis and characterization of VO2-based thermochromic thin films for energy-efficient windows. Nanoscale Res. Lett. 2011, 6, 301. [Google Scholar] [CrossRef]
- Parker, J.C.; Lam, D.J.; Xu, Y.N.; Ching, W.Y. Optical properties of vanadium pentoxide determined from ellipsometry and band-structure calculations. Phys. Rev. B 1990, 42, 5289–5293. [Google Scholar] [CrossRef]
- Wang, Y.; Takahashi, K.; Shang, H.; Cao, G. Synthesis and Electrochemical Properties of Vanadium Pentoxide Nanotube Arrays. J. Phys. Chem. B Lett. 2005, 109, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Alsawafta, M.; Almoabadi, A.; Badilescu, S.; Truong, V.V. Improved Electrochromic Properties of Vanadium Pentoxide Nanorods Prepared by Thermal Treatment of Sol-Gel Dip-Coated Thin Films. J. Electrochem. Soc. 2015, 162, H466–H472. [Google Scholar] [CrossRef]
- Talledo, A.; Andersson, A.M.; Granqvist, C.G. Structure and optical absorption of LiyV2O5 thin films. J. Appl. Phys. 1991, 69, 3261–3265. [Google Scholar] [CrossRef]
- Nadkarni, G.S.; Shirodkar, V.S. Experiment and theory for switching in Al/V2O5/Al devices. Thin Solid Films 1983, 105, 115–129. [Google Scholar] [CrossRef]
- Chain, E.E. Optical properties of vanadium dioxide and vanadium pentoxide thin films. Appl. Opt. 1991, 30, 2782–2787. [Google Scholar] [CrossRef]
- Livage, J. Optical and electrical properties of vanadium oxides synthesized from alkoxides. Coord. Chem. Rev. 1999, 190, 391–403. [Google Scholar] [CrossRef]
- Xu, G.; Jin, P.; Tazawa, M.; Yoshimura, K. Thickness dependence of optical properties of VO2 thin films epitaxially grown on sapphire (0001). Appl. Surf. Sci. 2005, 244, 449–452. [Google Scholar] [CrossRef]
- Borisov, B.S.; Koretkaya, S.T.; Mokerov, V.G.; Rakov, A.V.; Solovev, S.G. Electrical and optical properties of VO2 near semiconductor-semimetal transition point. Sov. Phys. Solid State (Engl. Transl.) 1971, 12, 1763–1766. [Google Scholar]
- Biermann, S.; Poteryaev, A.; Lichtenstein, A.I.; Georges, A. Dynamical singlets and correlation-assisted peierls transition in VO2. Phys. Rev. Lett. 2005, 94, 026404. [Google Scholar] [CrossRef]
- Adler, D.; Brooks, H. Theory of semicondutor to metal transitions. Phys. Rev. 1967, 155, 826–840. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier: Amsterdam, The Netherlands, 1995; pp. 237–295. [Google Scholar]
- Zylbersztejn, A.; Mott, N.F. Metal-insulator transition in vanadium dioxide. Phys. Rev. B 1975, 11, 4383–4395. [Google Scholar] [CrossRef]
- Becker, M.; Buckman, A.B.; Walser, R.M. Femtosecond laser excitation dynamics of the semiconductor-metal phase transition in VO2. Appl. Phys. Lett. 1996, 79, 2404–2408. [Google Scholar] [CrossRef]
- Shao, Z.; Luo, H.; Jin, P. Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials. NPG Asia Mater. 2018, 10, 581–605. [Google Scholar] [CrossRef]
- Aetukuri, N.B.; Aetukuri, N.B.; Gray, A.X.; Drouard, M.; Cossale, M.; Gao, L.; Reid, L.; Kukreja, R.; Ohldag, H.; Jenkins, C.A.; et al. Control of the metal-insulator transition in vanadium dioxide by modifying orbital occupancy. Nat. Phys. 2013, 9, 661–666. [Google Scholar] [CrossRef]
- Ardakani, A.H.; Nie, A.; Marley, P.M.; Zhu, Y.; Phillips, P.J.; Singh, S.; Mashayek, M.; Sambandamurthy, G.; Low, K.; Klie, R.B.; et al. Atomic origins of monoclinic-tetragonal (rutile) phase transition in doped VO2 nanowires. Nano Lett. 2015, 15, 7179–7188. [Google Scholar] [CrossRef]
- Booth, J.M.; Casey, P.S. Anisotropic structure deformation in the VO2 metalinsulator transition. Phys. Rev. Lett. 2009, 103, 086402. [Google Scholar] [CrossRef]
- Marezio, M.; McWhan, D.B.; Remeika, J.P.; Dernier, P.D. Structural aspects of the metal-insulator transitions in Cr-doped VO2. Phys. Rev. B 1972, 5, 2541. [Google Scholar] [CrossRef]
- Brückner, W.; Gerlach, U.; Thuss, B. Phase diagram of V1-xAlxO2. Phys. Status Solidi 1977, 40, K131–K134. [Google Scholar] [CrossRef]
- Thomas, G.A.; Rapkine, D.H.; Carter, S.A.; Millis, A.J.; Rosenbaum, T.F.; Metcalf, P.; Honig, J.M. Observation of the gap and kinetic energy in a correlated insulator. Phys. Rev. Lett. 1994, 73, 1529–1532. [Google Scholar] [CrossRef]
- Saha-Dasgupta, T.; Andersen, O.K.; Nuss, J.; Poteryaev, A.I.; Georges, A.; Lichtenstein, A.I. Electronic structure of V2O3: Wannier orbitals from LDA-NMTO calculations. arXiv 2009, arXiv:0907.2841. [Google Scholar]
- Surnev, S.; Ramsey, M.G.; Netzer, F.P. Vanadium oxide surface studies. Prog. Surf. Sci. 2003, 73, 117–165. [Google Scholar] [CrossRef]
- Yamazaki, S.; Li, C.; Ohoyama, K.; Nishi, M.; Ichihara, M.; Ueda, H.; Ueda, Y. Synthesis, structure and magnetic properties of V4O9—A missing link in binary vanadium oxides. J. Solid State Chem. 2010, 183, 1496–1503. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Xie, G.; Yu, L.; Yi, S.; Hu, M.; Huang, C. Hydrothermal synthesis, characterization, formation mechanism and electrochemical property of V3O7·H2O single-crystal nanobelts. Mater. Sci. Eng. B 2010, 175, 164–171. [Google Scholar] [CrossRef]
- Zakharova, G.S.; Volkov, V.L.; Täschner, C.; Hellmann, I.; Leonhardt, A.; Klingeler, R.; Büchner, B. Synthesis and characterization of V3O7.H2O nanobelts. Solid State Commun. 2009, 149, 814–817. [Google Scholar] [CrossRef]
- Cristopher, M.; Karthick, P.; Sivakumar, R.; Gopalakrishnan, C.; Sanjeeviraja, C.; Jeyadheepan, K. On the preparation of Tri-vanadium hepta-oxide thin films for electrochromic applications. Vacuum 2019, 160, 238–245. [Google Scholar] [CrossRef]
- Mjejri, I.; Rougier, A. Color Switch in V3O7.H2O cycled in Li and Na based electrolytes: Novel vanadium oxide based electrochromic material. J. Mater. Chem. C 2020, 8, 3631–3638. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, M. Nanocrystalline iron vanadate: Facile morphology-controlled preparation, characterization and investigation of optical and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2017, 28, 5866–5871. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M.; Naseh, S. Enhanced photodegradation of dye in waste water using iron vanadate nanocomposite; ultrasound-assisted preparation and characterization. Ultrason. Sonochem. 2017, 39, 494–503. [Google Scholar] [CrossRef]
- Heydari, A.; Sheykhan, M.; Sadeghi, M.; Radfar, I. Nano-Rods of FeVO4: An Efficient Heterogeneous Catalyst for Chemo-Selective Oxidation of Benzylic Alcohols. Inorg. Nano-Metal Chem. 2016, 47, 248–255. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Li, C.; Ci, L. Cu3V2O8 hollow spheres in photocatalysis and primary lithium batteries. Solid State Sci. 2013, 25, 15–21. [Google Scholar] [CrossRef]
- Kumada, N.; Takei, T.; Haramoto, R.; Yonesaki, Y.; Dong, Q.; Kinomura, N.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Preparation and crystal structure of a new bismuth vanadate, Bi3.33(VO4)2O2. Mater. Res. Bull. 2011, 46, 962–965. [Google Scholar] [CrossRef]
- Gan, J.; Lu, X.; Tong, Y. Towards highly efficient photoanodes: Boosting sun light-driven semiconductor nanomaterials for water oxidation. Nanoscale 2014, 6, 7142. [Google Scholar] [CrossRef] [PubMed]
- Tolod, K.R.; Hernandez, S.; Russo, N. Recent advances in the BiVO4 photo catalyst for sun-driven water oxidation: Top-performing photoanodes and scale-up challenges. Catalysts 2017, 7, 13. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photo anodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Scott, J.A.; Amal, R. Progress in heterogeneous photocatalysis: From classical radical chemistry to engineering nanomaterials and solar reactors. J. Phys. Chem. Lett. 2012, 3, 629–639. [Google Scholar] [CrossRef]
- Tachikawa, T.; Ochi, T.; Kobori, Y. Crystal-face-dependent charge dynamics on a BiVO4 photocatalyst revealed by single-particle spectroelectrochemistry. ACS Catal. 2016, 6, 2250–2256. [Google Scholar] [CrossRef]
- Tan, H.L.; Wen, X.; Amal, R.; Ng, Z.H. BiVO4 {010} and {110} relative exposure extent: Governing factor of surface charge population and photocatalytic activity. J. Phys. Chem. Lett. 2016, 7, 1400–1405. [Google Scholar] [CrossRef]
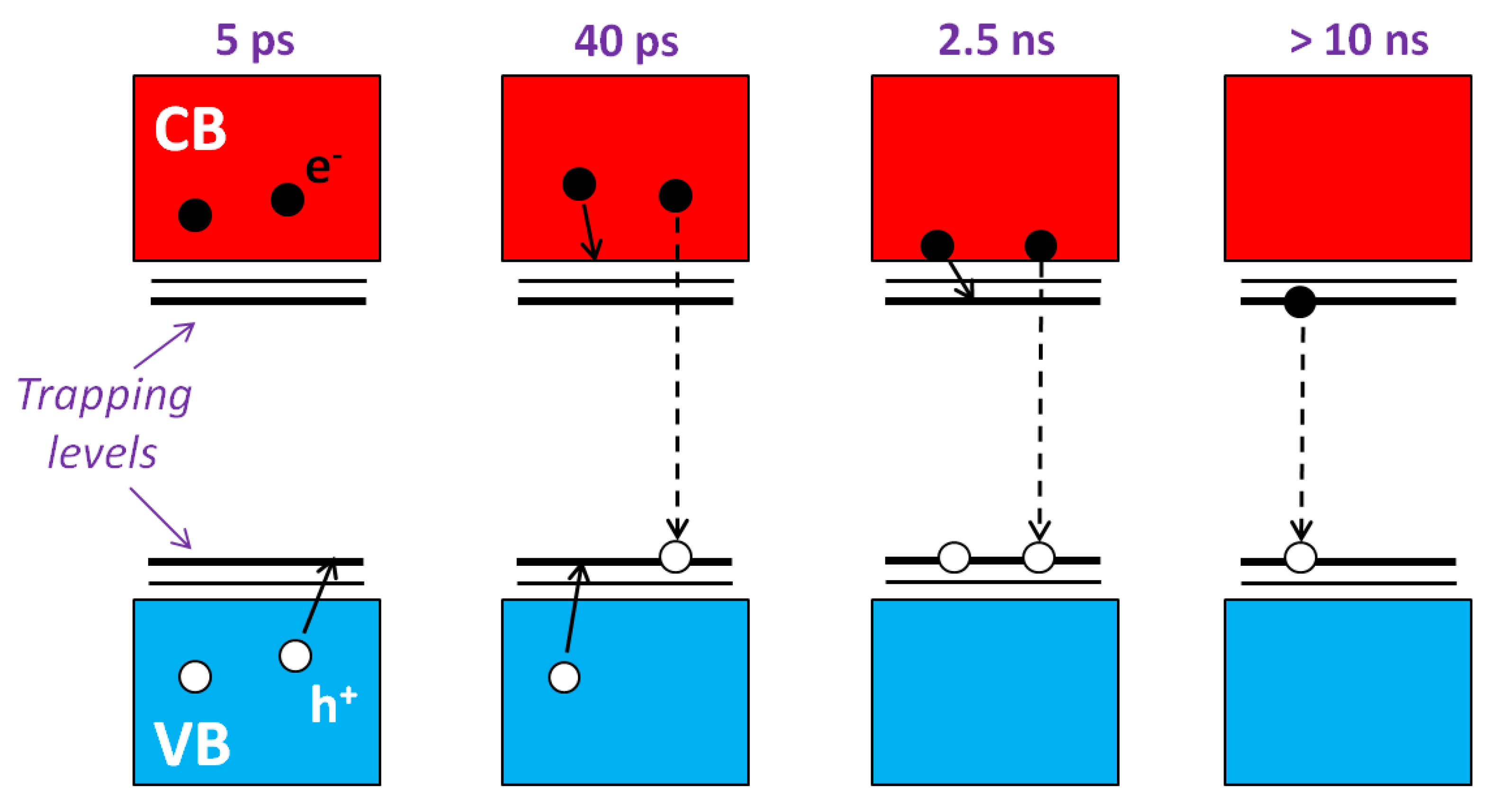
- Ma, Y.; Pendlebury, S.R.; Reynal, A.; Le Formal, F.; Durrant, J.R. Dynamics of photogenerated holes in undoped BiVO4 photoanodes for solar water oxidation. Chem. Sci. 2014, 5, 2964–2973. [Google Scholar] [CrossRef]
- Ravensbergen, J.; Abdi, F.F.; Santen, J.H.; Frese, R.N.; Dam, B.; Krol, R.; Kennis, J.T.M. Unraveling the Carrier Dynamics of BiVO4: A Femtosecond to Microsecond Transient Absorption Study. J. Phys. Chem. C 2014, 118, 27793–27800. [Google Scholar] [CrossRef]
- Vannier, N.; Pernot, E.; Anne, M.; Isnard, O.; Nowogrocki, G.; Mairesse, G. Bi4V2O11 polymorph crystal structures related to their electrical properties. Solid State Ion. 2003, 157, 147–153. [Google Scholar] [CrossRef]
- Mairesse, G.; Roussel, P.; Vannier, R.N.; Anne, M.; Pirovano, C.; Nowogrocki, G. Crystal structure determination of α, β and γ-Bi4V2O11 polymorphs. Part I: γ and β-Bi4V2O11. Solid State Sci. 2003, 5, 851–859. [Google Scholar] [CrossRef]
- Mairesse, G.; Roussel, P.; Vannier, R.N.; Anne, M.; Nowogrocki, G. Crystal structure determination of α, β and γ-Bi4V2O11 polymorphs. Part II: Crystal structure of α-Bi4V2O11. Solid State Sci. 2003, 5, 861–869. [Google Scholar] [CrossRef]
- Lakkepally, S.; Kalegowda, Y.; Ramarao, V.; Hanumantharayappa, E.; Siddaramanna, A. Room temperature synthesis of amorphous Bi4V2O11 as cathode material for Li secondary batteries. Mater. Res. Express 2018, 5, 115501. [Google Scholar] [CrossRef]
- Liang, M.; Yang, Z.; Mei, Y.; Zhou, H.; Yang, S. Dye-Sensitized-Assisted, Enhanced Photocatalytic Activity of TiO2/Bi4V2O11. NANO Brief Rep. Rev. 2018, 13, 1850028. [Google Scholar] [CrossRef]
- Nithya, V.D.; Selvan, R.K.; Sanjeeviraja, C.; Radheep, D.M.; Arumugam, S. Synthesis and characterization of FeVO4 nanoparticles. Mater. Res. Bull. 2011, 46, 461654–461658. [Google Scholar] [CrossRef]
- Baeis, M.G.; Mousavi, S.H.; Jeddy, M.R. Controlled synthesis and characterization of iron vanadate magnetic nanoparticles: Investigation it’s photodegradation of Rhodamine B. J. Mater. Sci. Mater. Electron. 2017, 28, 1480–1484. [Google Scholar] [CrossRef]
- Deng, J.H.; Jiang, J.Y.; Zhang, Y.Y.; Lin, X.P.; Du, C.M.; Xiong, Y. FeVO4 as a highly active heterogeneous Fenton-like catalyst towards the degradation of Orange II. Appl. Catal. B 2008, 84, 468–473. [Google Scholar] [CrossRef]
- Ozturk, B.; Soylu, G.S.P. Synthesis of surfactant-assisted FeVO4 nanostructure: Characterization and photocatalytic degradation of phenol. J. Mol. Catal. A Chem. 2015, 398, 65–71. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Friedrich, D.; Krol, R.; Wong, L.H.; Abdi, F.F. Elucidation of opto-electronic and photoelectrochemical properties of FeVO4 photoanodes for solar water oxidation. J. Mater. Chem. A 2018, 6, 548–555. [Google Scholar] [CrossRef]
- Arunachalam, M.; Yun, G.; Ahn, K.S.; Kang, S.H. Revealing the beneficial effects of FeVO4 nanoshell layer on the BiVO4 inverse opal core layer for photoelectrochemical water oxidation. J. Phys. Chem. C 2017, 121, 7625–7634. [Google Scholar] [CrossRef]
- Dutta, D.P.; Ramakrishnan, M.; Roy, M.; Kumar, A. Effect of transition metal doping on the photocatalytic properties of FeVO4 nanoparticles. J. Photochem. Photobiol. A 2017, 335, 102–111. [Google Scholar] [CrossRef]
- Lakkepally, S.; Kalegowda, Y.; Ganganagappa, N.; Siddaramanna, A. A new and effective approach for Fe2V4O13 nanoparticles synthesis: Evaluation of electrochemical performance as cathode for lithium secondary batteries. J. Alloy. Compd. 2018, 737, 665–671. [Google Scholar] [CrossRef]
- Tang, D.; Rettie, A.J.E.; Mabayoje, O.; Wygant, B.R.; Lai, Y.; Liu, Y.; Mullins, C.B. Facile Growth of Porous Fe2V4O13 Films for Photoelectrochemical Water Oxidation. J. Mater. Chem. A 2016, 4, 3034–3042. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Deng, J.H.; He, C.; Huang, S.S.; Tian, S.H.; Xiong, Y. Application of Fe2V4O13 as a new multi-metal heterogeneous Fenton-like catalyst for the degradation of organic pollutants. Environ. Technol. 2010, 31, 145–154. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Y.; Li, H.; Xu, Q.; Meng, X.; Wang, X.; Xiao, M.; Zou, Z. All-solid-state Z-scheme system arrays of Fe2V4O13/RGO/CdS for visible light-driving photocatalytic CO2 reduction into renewable hydrocarbon fuel. Chem. Commun. 2015, 51, 800–803. [Google Scholar] [CrossRef]
- Maggay, I.V.B.; Juan, L.M.Z.; Lu, J.S.; Nguyen, M.T.; Yonezawa, T.; Chan, T.S.; Liu, W.R. Electrochemical properties of novel FeV2O4 as an anode for Na-ion batteries. Sci. Rep.-UK 2018, 8, 8839. [Google Scholar] [CrossRef]
- Mandal, H.; Shyamal, S.; Hajra, P.; Bera, A.; Sariket, D.; Kundu, S.; Bhattacharya, C. Development of ternary iron vanadium oxide semiconductors for their applications in Photoelectrochemical Water Oxidation. RSC Adv. 2016, 6, 4992–4999. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhou, J.; Zhang, C.Y. pH-controlled growth of ultrathin iron vanadium oxide (FeV3O8) nanoplatelets with high visible-light photo-catalytic activity. J. Mater. Chem. A 2014, 2, 14903. [Google Scholar] [CrossRef]
- Hassan, A.; Iqbal, T.; Tahir, M.B.; Afsheen, S. A review on copper vanadate-based nanostructures for photocatalysis energy production. Int. J. Energy Res. 2019, 43, 9–28. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Masjedi-Arani, M.; Salavati-Niasari, M. Facile synthesis, characterization and optical properties of copper vanadate nanostructures for enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2016, 27, 4871–4878. [Google Scholar] [CrossRef]
- Jiang, C.M.; Farmand, M.; Wu, C.H.; Liu, Y.S.; Guo, J.; Drisdell, W.S.; Cooper, J.K.; Sharp, I.D. Electronic Structure, Optoelectronic Properties, and Photoelectrochemical Characteristics of γ-Cu3V2O8 Thin Films. Chem. Mater. 2017, 29, 3334–3345. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Hanh, N.T.; Nguyen, M.V.; Chi, N.T.P.L.; Noi, N.V.; Tran, D.T.; Ha, M.N.; Trung, D.Q.; Pham, T.D. Novel direct Z-scheme Cu2V2O7/g-C3N4 for visible light photocatalytic conversion of CO2 into valuable fuels. Appl. Surf. Sci. 2018, 457, 968–974. [Google Scholar] [CrossRef]
- Guo, W.; Chemelewski, W.D.; Mabayoje, O.; Xiao, P.; Zhang, Y.; Mullins, C.B. Synthesis and Characterization of CuV2O6 and Cu2V2O7: Two Photoanode Candidates for Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2015, 119, 27220–27227. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, C.; Zhang, C. Silver vanadate nanowires: Photocatalytic properties and theoretical calculations. Res. Chem. Intermed 2015, 41, 7725–7737. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Assis, M.; Teixeira, M.M.; Silva, M.D.P.; Li, M.S.; Andres, J.; Gracia, L.; Longo, E. An Experimental and Computational Study of β-AgVO3: Optical Properties and Formation of Ag Nanoparticles. J. Phys. Chem. C 2016, 120, 12254–12264. [Google Scholar] [CrossRef]
- Hu, X.; Hu, C.; Qu, J. Preparation and visible-light activity of silver vanadate for the degradation of pollutants. Mater. Res. Bull. 2008, 43, 2986–2997. [Google Scholar] [CrossRef]
- McNulty, D.; Ramasse, Q.; O’Dwyer, C. The Structural Conversion from α-AgVO3 to β-AgVO3: Ag Nanoparticle Decorated Nanowires with Application as Cathode Materials for Li-ion Batteries. Nanoscale 2016, 8, 16266–16275. [Google Scholar] [CrossRef]
- Xu, J.; Hu, C.; Xi, Y.; Wan, B.; Zhang, C.; Zhang, Y. Synthesis and visible light photocatalytic activity of b-AgVO3 nanowires. Solid State Sci. 2012, 14, 535–539. [Google Scholar] [CrossRef]
- Cao, X.; Zhan, H.; Xie, J.; Zhou, Y. Synthesis of Ag2V4O11 as a cathode material for lithium battery via a rheological phase method. Mater. Lett. 2006, 60, 435–438. [Google Scholar] [CrossRef]
- Shi, H.; Li, Z.; Kou, J.; Ye, J.; Zou, Z. Facile Synthesis of Single-Crystalline Ag2V4O11 Nanotube Material as a Novel Visible-Light-Sensitive Photocatalyst. J. Phys. Chem. C 2011, 115, 145–151. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Sun, G.; Xia, J.; Wu, C.; Ye, Z.; Zhang, Q. Photocatalytic activity of La2O3-modified silver vanadates catalyst for Rhodamine B dye degradation under visible light irradiation. Chem. Eng. J. 2010, 160, 33–41. [Google Scholar] [CrossRef]
- Ren, C.; Fan, J.; Liu, S.; Li, W.; Wang, F.; Li, H.; Liu, X.; Chang, Y. One-step hydrothermal synthesis of the novel Ag3VO4/Ag4V2O7 composites for enhancing visible-light photocatalytic performance. RSC Adv. 2016, 6, 95156–95164. [Google Scholar] [CrossRef]
- Xia, D.; Xu, S.; Wang, W.; Wang, D.; Wu, M.; Gong, F. Pure-phase β-Mn2V2O7 interconnected nanospheres as high performance lithium ion battery anode. Chem. Commun. 2020, 56, 8043–8046. [Google Scholar] [CrossRef]
- Pei, L.Z.; Lin, N.; Wei, T.; Yu, H.Y. Synthesis of manganese vanadate nanobelts and their visible light photocatalytic activity for methylene blue. J. Exp. Nanosci. 2016, 11, 197–214. [Google Scholar] [CrossRef]
- Yan, Q.; Yu, J.; Suram, S.K.; Zhou, L.; Shinde, A.; Newhouse, P.F.; Chen, W.; Li, G.; Persson, K.A.; Gregoire, J.M.; et al. Solar fuels photoanode materials discovery by integrating high-throughput theory and experiment. Proc. Natl. Acad. Sci. 2017, 114, 3040–3043. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, A.; Nasiri, M. Manganese vanadate nanostructure: Facile precipitation preparation, characterization, and investigation of their photocatalyst activity. J. Mater. Sci. Mater. Electron. 2017, 28, 9096–9101. [Google Scholar] [CrossRef]
- Tsai, Y.C. Desalination plants and renewables combined to solve power and water issues. Energy 2016, 113, 1018–1030. [Google Scholar] [CrossRef]
- Yaqoot, M.; Diwan., P.; Kandpal, T.C. Review of barriers to the dissemination of decentralized renewable energy systems. Renew. Sustain. Energy Rev. 2016, 58, 477–490. [Google Scholar] [CrossRef]
- Benedek, J.; Sebestyén, T.T.; Bartók, B. Evaluation of renewable energy sources in peripheral areas and renewable energy-based rural development. Renew. Sustain. Energy Rev. 2018, 90, 516–535. [Google Scholar] [CrossRef]
- Arnaut, L.G.; Barroso, M.; Serpa, C. Solar energy conversion. In Applied Photochemistry; Giacomo, B., Serena, S., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 267–304. [Google Scholar]
- Chiarello, G.L.; Selli, E. Photocatalytic hydrogen production. Recent Pat. Eng. 2010, 4, 155. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85. [Google Scholar] [CrossRef]
- Gholipour, M.R.; Dinh, C.-T.; Beland, F.; Do, T.-O. Nanocomposite hetero junctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187. [Google Scholar] [CrossRef]
- Liao, C.-H.; Huang, C.-W.; Wu, J.C.C. Hydrogen production from semicon ductor-based photocatalysis via water splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semicon ductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Shena, T.F.-R.; Lai, M.H.; Yang, T.C.K.; Fu, I.P.; Liang, N.Y.; Chen, W.T. Photocatalytic production of hydrogen by vanadium oxides under visible light irradiation. J. Taiwan Inst. Chem. Eng. 2012, 43, 95–101. [Google Scholar] [CrossRef]
- Monfort, O.; Lianos, O.; Plesch, G. Design of Bismuth Vanadate-Based Materials: New Advanced Photoanodes for Solar Hydrogen Generation. In Photoelectrochemical Solar Cells; Sankir, N.D., Sankir, M., Eds.; Wiley-Scrivener: Beverly, MA, USA, 2019; pp. 219–249. [Google Scholar]
- Wang, Y.; Zhang, Z.; Zhu, Z.; Li, Z.; Vajtai, R.; Ci, L.; Ajayan, P.M. Nanostructured VO2 Photocatalysts for Hydrogen Production. ACS Nano 2008, 2, 1492–1496. [Google Scholar] [CrossRef]
- Puangpetch, T.; Chavadej, S.; Sreethawong, T. Mesoporous-assembled V2O5 nanosheet synthesized via a surfactant-modified sol–gel technique and its photocatalytic H2 production activity under visible light irradiation. Powder Technol. 2011, 208, 37–41. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Ma, X.; Zhang, Y.; Wang, H. Property of iron vanadate over CdS nanorod for efficient photocatalytic hydrogen production. New J. Chem. 2019, 43, 3609–3618. [Google Scholar] [CrossRef]
- Tan, H.L.; Amal, R.; Ng, Y.H. Alternative Strategies in Improving the Photocatalytic and Photoelectrochemical Activities of Visible Light-driven BiVO4: A Review. J. Mater. Chem. A 2017, 5, 16498–16521. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; Wang, X. High Efficient Photodegradation and photocatalytic Hydrogen Production of CdS/BiVO4 Heterostructure through Z-Scheme Process. ACS Sustain. Chem. Eng. 2017, 5, 303–309. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Wang, M.; Huang, K.; He, J.; Ma, W.; Chen, H.; Li, Y.; Feng, S. Facile preparation of BiVO4/FeVO4 heterostructure for efficient water-splitting applications. Int. J. Hydrogen Energy 2019, 44, 23046–23053. [Google Scholar] [CrossRef]
- Abdi, F.F.; Han, L.; Smets, A.H.M.; Zeman, M.; Dam, B.; Krol, R. Efficient solar water splitting by enhanced charge separation in a bismuth vanadate-silicon tandem photoelectrode. Nat. Commun. 2013, 4, 2195. [Google Scholar] [CrossRef]
- Monfort, O.; Pop, L.-C.; Sfaelou, S.; Plecenik, T.; Roch, T.; Dracopoulos, V.; Stathatos, E.; Plesch, G.; Lianos, P. Photoelectrocatalytic hydrogen production by water splitting using BiVO4 photoanodes. Chem. Eng. J. 2016, 286, 91. [Google Scholar] [CrossRef]
- Biswas, S.K.; Baeg, J.O. Enhanced photoactivity of visible light responsive W incorporated FeVO4 photoanode for solar water splitting. Int. J. Hydrogen Energy 2013, 38, 14451–14457. [Google Scholar] [CrossRef]
- Morton, C.D.; Slipper, I.J.; Thomas, M.J.K.; Alexander, B.D. Synthesis and characterisation of Fe-V-O thin film photoanodes. Photochem. Photobiol. 2010, 216, 209–214. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Wang, L.; Bi, Y. Facile synthesis of Fe3+/Fe2+ self-doped nanoporous FeVO4 photoanodes for efficient solar water splitting. J. Mater. Chem. A 2017, 5, 2478–2482. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Zhu, J.; Tang, J. 1D Co-Pi modified BiVO4/ZnO junction cascade for efficient photoelectrochemical water cleavage. Adv. Energy Mater. 2014, 4, 1301590. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Yu, J.C. Ordered Mesoporous BiVO4 through Nanocasting: A Superior Visible Light-Driven Photocatalyst. Chem. Mater. 2008, 20, 3983. [Google Scholar] [CrossRef]
- Monfort, O.; Sfaelou, S.; Satrapinskyy, L.; Plecenik, T.; Roch, T.; Plesch, G.; Liano, P. Comparative study between pristine and Nb-modified BiVO4 films employed for photoelectrocatalytic production of H2 by water splitting and for photocatalytic degradation of organic pollutants under simulated solar light. Catal. Today 2017, 280, 51–57. [Google Scholar] [CrossRef]
- Gunes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, K.; Trost, S.; Meyer, J.; Kahn, A.; Behrendt, A.; Lützenkirchen-Hecht, D.; Frahm, R.; Riedl, T. Inverted Organic Solar Cells with Sol–Gel Processed High Work-Function Vanadium Oxide Hole-Extraction Layers. Adv. Funct. Mater. 2011, 21, 4776. [Google Scholar] [CrossRef]
- Sun, H.; Hou, X.; Wei, Q.; Liu, H.; Yang, K.; Wang, W.; An, Q.; Rong, Y. Low-temperature solution-processed p-type vanadium oxide for perovskite solar cells. Chem. Commun. 2016, 52, 8099. [Google Scholar]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jafari, A.J.; Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP Technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of tartaric acid in the presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Pouran, S.R.; Aziz, A.R.A.; Daud, W.M.A.W. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Niu, Y.; Xu, Y. Heterogeneous Fenton-like magnetic nanosphere coated with vanadium oxide quantum dots for enhanced organic dyes decolorization. J. Colloid Interface Sci. 2020, 579, 269–281. [Google Scholar] [CrossRef]
- Le, T.K.; Kang, M.; Kim, S.W. A review on the optical characterization of V2O5 micro-nanostructures. Ceram. Int. 2019, 45, 15781–15798. [Google Scholar] [CrossRef]
- Shahid, M.; Rhen, D.S.; Shakir, I.; Patole, S.P.; Yoo, J.B.; Yang, S.J.; Kang, D.J. Facile synthesis of single crystalline vanadium pentoxide nanowires and their photocatalytic behavior. Mater. Lett. 2010, 64, 2458–2461. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Zhao, Q.; Liu, J.; Liu, S.; Wang, S.; Tade, M.O. Insight into the Mechanism of Photocatalytic Degradation of Gaseous o-dichlorobenzene over Flower-Type V2O5 Hollow Spheres. J. Mater. Chem. A 2015, 3, 15163–15170. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Zhou, J.; Liu, X.; Chen, Z.; Cao, C.; Luo, H.; Kanehira, M. Growth of oriented vanadium pentaoxide nanostructures on transparent conducting substrates and their applications in photocatalysis. J. Solid State Chem. 2014, 214, 79–85. [Google Scholar] [CrossRef]
- Jayaraj, S.K.; Sadishkumar, V.; Arun, T.; Thangadurai, P. Enhanced photocatalytic activity of V2O5 nanorods for the photodegradation of organic dyes: A detailed understanding of the mechanism and their antibacterial activity. Mater. Sci. Semicon. Proc. 2018, 85, 122–133. [Google Scholar] [CrossRef]
- Sajid, M.M.; Shad, N.A.; Javed, Y.; Khan, S.B.; Zhang, Z.; Amin, N.; Zhai, H. Preparation and characterization of Vanadium pentoxide (V2O5) for photocatalytic degradation of monoazo and diazo dyes. Surfaces Interfaces 2020, 19, 100502. [Google Scholar] [CrossRef]
- Aslam, M.; Ismail, I.M.I.; Salah, N.; Chandrasekaran, S.; Qamar, M.T.; Hameed, A. Evaluation of Sunlight Induced Structural Changes and Their Effect on the Photocatalytic Activity of V2O5 for the Degradation of Phenols. J. Hazard. Mater. 2015, 286, 127–135. [Google Scholar] [CrossRef]
- Sahraeian, N.; Esmaeilzadeh, F.; Mowla, D. Hydrothermal synthesis of V2O5 nanospheres as catalyst for hydrogen sulfide removal from sour water. Ceram. Int. 2021, 47, 923–934. [Google Scholar] [CrossRef]
- Monfort, O.; Roch, T.; Satrapinskyy, L.; Gregor, M.; Plecenik, T.; Plecenik, A.; Plesch, G. Reduction of V2O5 thin films deposited by aqueous sol–gel method toVO2(B) and investigation of its photocatalytic activity. Appl. Surf. Sci. 2014, 322, 21–27. [Google Scholar] [CrossRef]
- Monfort, O.; Roch, T.; Gregor, M.; Satrapinskyy, L.; Plecenik, T.; Plecenik, A.; Plesch, G. Formation of vanadium oxide thin films prepared from aqueous sol-gel system. Key Eng. Mater. 2014, 605, 79–82. [Google Scholar] [CrossRef]
- Saini, M.; Dehiya, B.S.; Umar, A. VO2(M)@CeO2 core-shell nanospheres for thermochromic smart Windows and photocatalytic applications. Ceram. Int. 2020, 46, 986–995. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, C.; Chen, S.; Luo, H.; Gao, Y. Crystallised mesoporous TiO2(A)–VO2(M/R) nanocomposite films with self-cleaning and excellent thermochromic properties. Mater. Chem. A 2014, 2, 11874. [Google Scholar] [CrossRef]
- Li, W.; Ji, S.; Sun, G.; Ma, Y.; Guo, H.; Jin, P. Novel VO2(M)–ZnO heterostructured dandelions with combined thermochromic and photocatalytic properties for application in smart coatings. New J. Chem. 2016, 40, 2592–2600. [Google Scholar] [CrossRef]
- Tao, X.; Hang, Q.; Wu, T.; Liao, F. Quasi-Hexagonal VO2/Ag3VO4 Microcrystals for Photo-Catalytic Degradation of Rhodamine B. Asian J. Chem. 2014, 26, 8291–8294. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Gao, Y.; Luo, H.; Kanehira, M. Core-shell VO2@TiO2 nanorods that combine thermochromic and photocatalytic properties for application as energy-saving smart coatings. Sci. Rep. 2013, 3, 1370. [Google Scholar] [CrossRef]
- Moshfegh, A.Z.; Ignatiev, A. Photo-Enhanced Catalytic Decomposition Of Isopropanol On V205. Catal. Lett. 1990, 4, 113–122. [Google Scholar] [CrossRef]
- Jiang, B.; Peng, X.; Qu, Y.; Wang, H.; Tian, C.; Pan, Q.; Li, M.; Zhou, W.; Fu, H. A New Combustion Route to Synthesize Mixed Valence Vanadium Oxide Heterojunction Composites as Visible-Light-Driven Photocatalysts. ChemCatChem 2014, 6, 2553–2559. [Google Scholar] [CrossRef]
- Zavahir, S.; Xiao, Q.; Sarina, S.; Zhao, J.; Bottle, S.; Wellard, M.; Jia, J.; Jing, L.; Huang, Y.; Blinco, J.P.; et al. Selective Oxidation of Aliphatic Alcohols using Molecular Oxygen at Ambient Temperature: Mixed-Valence Vanadium Oxide Photocatalysts. ACS Catal. 2016, 6, 3580–3588. [Google Scholar] [CrossRef]
- Arunadevi, R.; Kavitha, B.; Rajarajan, M.; Suganthi, A. Synthesis of Ce/Mo-V4O9 nanoparticles with superior visible light photocatalytic activity for Rhodamine-B degradation. J. Environ. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A 2018, 555, 47–74. [Google Scholar]
- Samsudin, M.F.R.; Sufian, S.; Hameed, B.H. Epigrammatic progress and perspective on the photocatalytic properties of BiVO4-based photocatalyst in photocatalytic water treatment technology: A review. J. Mol. Liq. 2018, 268, 438–459. [Google Scholar] [CrossRef]
- de la Martinez Cruz, A.; Perez, U.M.G. Photocatalytic properties of BiVO4 prepared by the co-precipitation method:Degradation of rhodamine B and possible reaction mechanisms under visible Irradiation. Mater. Res. Bull. 2010, 45, 135–141. [Google Scholar] [CrossRef]
- Li, F.; Kang, Y.; Chen, M.; Liu, G.; Lv, W.; Yao, K.; Chen, P.; Huang, H. Photocatalytic degradation and removal mechanism of ibuprofen via monoclinic BiVO4 under simulated solar light. Chemosphere 2016, 150, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yan, Y.; Yan, X. Microwave-assisted synthesis of nano-scale BiVO4 photocatalysts and their excellent visible-light-driven photocatalytic activity for the degradation of ciprofloxacin. Chem. Eng. J. 2013, 215–216, 740–746. [Google Scholar] [CrossRef]
- Kohtani, S.; Koshiko, M.; Kudo, A.; Tokumura, K.; Ishigaki, Y.; Toriba, A.; Hayakawa, K.; Nakagaki, R. Photodegradation of 4-alkylphenols using BiVO4 photocatalyst under irradiation with visible light from a solar simulator. Appl. Catal. B 2003, 46, 573–586. [Google Scholar] [CrossRef]
- Huang, C.-M.; Pan, G.-T.; Peng, P.-Y.; Yang, T.C.K. In situ DRIFT study of photocatalytic degradation of gaseous isopropanol over BiVO4 under indoor illumination. J. Mol. Catal. A Chem. 2010, 327, 38–44. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Song, W.; Wang, H.; Li, Y.; Liu, J.; Zhao, Z.; Tan, J.; Duan, Z.; Deng, J. Experimental and DFT insights of BiVO4 as an effective photocatalytic catalyst for N2O decomposition. Chem. Eng. J. 2019, 366, 504–513. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, H.; Cai, P.; Qiu, R.; Xiong, Y. Simultaneous photocatalytic reduction of Cr(VI) and oxidation of phenol over monoclinic BiVO4 under visible light irradiation. Chemosphere 2006, 63, 956–963. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, D.; Qin, L.; Liang, J.; Sun, X.; Huang, Y. Enhanced photocatalytic activity of hydrogenated BiVO4 with rich surface-oxygen-vacancies for remarkable degradation of tetracycline hydrochloride. J. Alloys Compd. 2019, 783, 10–18. [Google Scholar] [CrossRef]
- Saison, T.; Chemin, N.; Chaneac, C.; Durupthy, O.; Mariey, L.; Mauge, F.; Brezova, V.; Jolivet, J.-P. New Insights Into BiVO4 Properties as Visible Light Photocatalyst. J. Phys. Chem. C 2015, 119, 12967–12977. [Google Scholar] [CrossRef]
- Monfort, O.; Roch, T.; Gregor, M.; Satrapinskyy, L.; Raptis, D.; Lianos, P.; Plesch, G. Photooxidative properties of various BiVO4/TiO2 layered composite films and study of their photocatalytic mechanism in pollutant degradation. J. Environ. Chem. Eng. 2017, 5, 5143–5149. [Google Scholar] [CrossRef]
- Li, J.; Lu, P.; Deng, W.; Zeng, Z.; Lin, L.; Zhao, G. Facile synthesis of sheet-like BiVO4/Bi4V2O11 composite for enhanced photocatalytic properties. Mater. Chem. Phys. 2020, 254, 123489. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, T.; Huang, Y.; Chen, C.; Kim, S.I.; Seo, H.J. Optical properties and visible-light-driven photocatalytic activity of Bi8V2O17 nanoparticles. J. Nanopart. Res. 2015, 17, 202. [Google Scholar] [CrossRef]
- Ghiyasiya-Arani, M.; Salavati-Niasari, M.; Masjedi-Arani, M.; Mazloom, F. An easy sonochemical route for synthesis, characterization and photocatalytic performance of nanosized FeVO4 in the presence of aminoacids as green capping agents. J. Mater. Sci. Mater. Electron. 2018, 29, 474–485. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Q.; Wei, M.; Guo, E. FeVO4 nanobelts: Controllable synthesis by electrospinning and visible-light photocatalytic properties. J. Sol-Gel Sci. Technol. 2017, 82, 67–74. [Google Scholar] [CrossRef]
- Tan, G.; Xu, C.; Ren, H.; Yang, W.; Zhao, C.; Xia, A. Synthesis and Photocatalytic Activities of Bamboo-Like FeVO4 Nanocrystalline. J. Nano Res. 2017, 46, 123–134. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Li, Z.; Lu, G.; Zhu, M. Visible-light-assisted peroxymonosulfate activation over Fe(II)/V(IV) selfdoped FeVO4 nanobelts with enhanced sulfamethoxazole degradation: Performance and mechanism. Chem. Eng. J. 2021, 403, 126384. [Google Scholar] [CrossRef]
- Rahimpour, R.; Chaibakhsh, N.; Zanjanchi, M.A.; Moradi-Shoeili, Z. Fabrication of ZnO/FeVO4 heterojunction nanocomposite with high catalytic activity in photo-Fenton-like process. J. Alloys Compd. 2020, 817, 152702. [Google Scholar] [CrossRef]
- Eshaq, G.H.; Wang, S.; Sun, H.; Sillanpaa, M. Superior performance of FeVO4@CeO2 uniform core-shell nanostructures in heterogeneous Fenton-sonophotocatalytic degradation of 4-nitrophenol. J. Hazard. Mater. 2020, 382, 121059. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.M.; Khan, S.B.; Shad, N.A.; Amin, N.; Zhang, Z. Visible light assisted photocatalytic degradation of crystal violet dye and electrochemical detection of ascorbic acid using a BiVO4/FeVO4 heterojunction composite. RSC Adv. 2018, 8, 23489. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.; Lu, Q.; Guo, E.; Wei, M. Fabrication of Direct Z-scheme a-Fe2O3/FeVO4 Nanobelts with Enhanced Photoelectrochemical Performance. ChemistrySelect 2018, 3, 809–815. [Google Scholar] [CrossRef]
- Marikkani, S.; Vinoth Kumar, J.; Muthuraj, V. Design of novel solar-light driven sponge-like Fe2V4O13 photocatalyst: A unique platform for the photoreduction of carcinogenic hexavalent chromium. Sol. Energy 2019, 188, 849–856. [Google Scholar] [CrossRef]
- Gowthami, K.; Krishnakumar, B.; Thirunarayanan, G.; Swaminathan, M.; Muthuvel, I. Novel Fe2V4O13/ZnO nano-heterojunction: Effective decomposition of methyl orange under solar light irradiation. Mater. Today-Proc. 2020, 29, 1199–1203. [Google Scholar] [CrossRef]
- Tahir, M. Hierarchical 3D VO2/ZnV2O4 microspheres as an excellent visible light photocatalyst for CO2 reduction to solar fuels. Appl. Surf. Sci. 2019, 467–468, 1170–1180. [Google Scholar] [CrossRef]
- Wang, R.; Cao, L. Facile synthesis of a novel visible-light-driven AgVO3/BiVO4 heterojunction photocatalyst and mechanism insight. J. Alloys Compd. 2017, 722, 445–451. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Z.; Li, G.; Zhang, M.; Song, Y.; Wang, J. Self-generating CeVO4 as conductive channel within CeO2/CeVO4/V2O5 to induce Z-scheme charge-transfer driven photocatalytic degradation coupled with hydrogen production. Int. J. Hydrogen Energy 2019, 44, 23921–23935. [Google Scholar] [CrossRef]
- Hu, Y.; Fan, J.; Pu, C.; Li, H.; Liu, E.; Hu, X. Facile synthesis of double cone-shaped Ag4V2O7/BiVO4 nanocomposites with enhanced visible light photocatalytic activity for environmental purification. J. Photochem. Photobiol. A 2017, 337, 172–183. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.; Wang, D.; Chen, A.; Dai, W.L.; Zhao, X.; Yang, J.; Wang, X. Activation of Kagome lattice-structured Cu3V2O7(OH)2·2H2O volborthite via hydrothermal crystallization for boosting visible light-driven water oxidation. Phys. Chem. Chem. Phys. 2018, 20, 24561–24569. [Google Scholar] [CrossRef]
- Min, W.; Qiong, L. Synthesis and Photocatalytic Property of Cu3V2O8 Prepared by Liquid Phase Precipitation. Adv. Mater. Res. 2011, 236–238, 1675–1678. [Google Scholar]
- Kalal, S.; Pandey, A.; Ameta, R.; Punjabi, P.B. Heterogeneous photo-Fenton-like catalysts Cu2V2O7 and Cr2V4O13 for an efficient removal of azo dye in water. Cogent Chem. 2016, 2, 1143344. [Google Scholar] [CrossRef]








| Phase | Measured (eV) | Calculated (eV) | Photocurrent (mA·cm−2) | ||
|---|---|---|---|---|---|
| Direct Eg | Indirect Eg | Direct Eg | Indirect Eg | ||
| Cr2V4O13 | 2.56 | 2.55 | 2.52 | 2.30 | 0.139 |
| α-CoV2O6 | 2.17 | 2.16 | 2.25 | 2.25 | 0.015 |
| Co3V2O8 | 2.06 | 2.03 | 2.34 | 2.22 | 0.006 |
| Ni2V2O7 | 2.75 | 2.72 | 2.73 | 2.50 | 0.003 |
| Ni3V2O8 | 2.55 | 2.54 | 2.66 | 2.50 | 0.003 |
| Cu11V6O26 | 1.41 | 1.38 | 2.49 | 1.87 | 0.950 |
| α-Ag3VO4 | 2.07 | 1.70 | 2.38 | 2.14 | 0.062 |
| β-Ag3VO4 | 1.91 | 1.61 | 2.51 | 2.51 | 0.410 |
| System | Eg (eV) | Pollutant | Incident Irradiation | Degradation Extent | Ref. |
|---|---|---|---|---|---|
| ZnV2O4 | 2.1 | CO2 | Visible light | ~80% conversion into CO | [262] |
| AgVO3/BiVO4 | 2.32–2.43 | Rhodamine B | Visible light | 93% after 120 min | [263] |
| CeO2/CeVO4/V2O5 | 2.26–2.93 | Methylene blue | Visible light | 93% after 4 h (189 µmol of produced H2) | [264] |
| Ag4V2O7/BiVO4 | 2.36–2.41 | Methylene blue and NO | Visible light | 98% for MB and 53% for NO after 30 min | [265] |
| Cu3V2O7(OH)2·2H2O | 2.1 | Methylene blue | Visible light | ~90% after 150 min | [266] |
| Cu3V2O8 | - | Methyl orange | UVA light | 72% after 100 min | [267] |
| Cu2V2O7 (+H2O2) | 2.17 | Evans blue | UVA light | 77% after 120 min | [268] |
| Cu2V2O7/g-C3N4 | 2.0–2.7 | CO2 | Visible light | - | [167] |
| Cu3V2O8 | 2.05–2.10 | Methyl orange | UVA light | 78% after 120 min | [135] |
| Mn(VO3)2 | 3.05 | Methyl orange | UVA light | 84% after 80 min | [181] |
| Mn2V2O7 | 2.79 | Methylene blue | Solar light | 90% after 4 h | [179] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monfort, O.; Petrisková, P. Binary and Ternary Vanadium Oxides: General Overview, Physical Properties, and Photochemical Processes for Environmental Applications. Processes 2021, 9, 214. https://doi.org/10.3390/pr9020214
Monfort O, Petrisková P. Binary and Ternary Vanadium Oxides: General Overview, Physical Properties, and Photochemical Processes for Environmental Applications. Processes. 2021; 9(2):214. https://doi.org/10.3390/pr9020214
Chicago/Turabian StyleMonfort, Olivier, and Patrícia Petrisková. 2021. "Binary and Ternary Vanadium Oxides: General Overview, Physical Properties, and Photochemical Processes for Environmental Applications" Processes 9, no. 2: 214. https://doi.org/10.3390/pr9020214
APA StyleMonfort, O., & Petrisková, P. (2021). Binary and Ternary Vanadium Oxides: General Overview, Physical Properties, and Photochemical Processes for Environmental Applications. Processes, 9(2), 214. https://doi.org/10.3390/pr9020214






