Dry Mesophilic Anaerobic Digestion of Separately Collected Organic Fraction of Municipal Solid Waste: Two-Year Experience in an Industrial-Scale Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Industrial AD Plant Characteristics
2.2. Sampling
2.3. Monitoring and Control Methods
2.4. Feeding Characteristics
2.5. Organic Loading Rate (OLR)
3. Results and Discussion
3.1. Biogas and Methane Yield
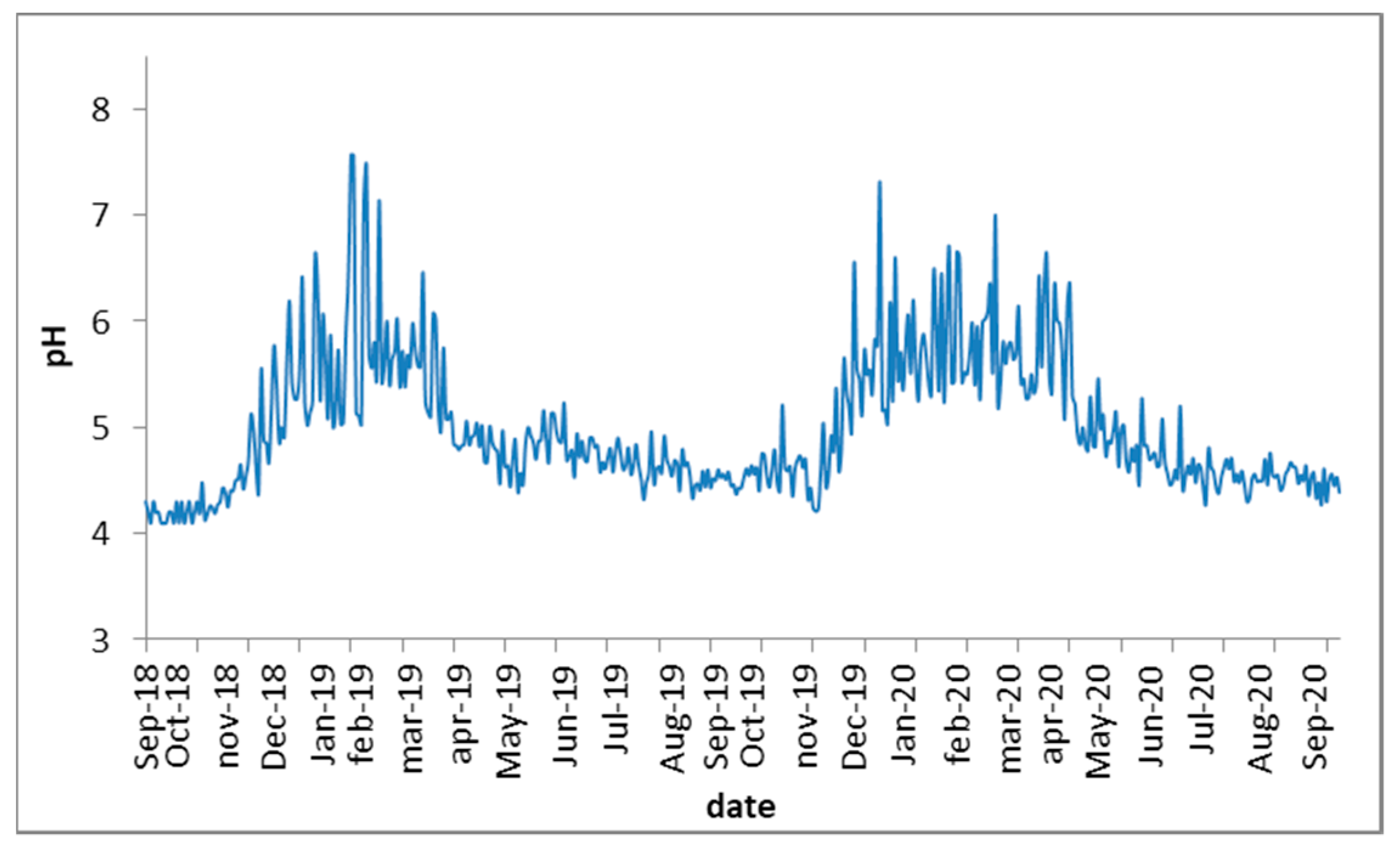
3.2. Control Parameters Values
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Soria, B.Y.M. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic process to its perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Eur-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2008/98/2018-07-05 (accessed on 21 December 2020).
- US EPA. Available online: https://www.epa.gov/sustainable-management-food (accessed on 21 December 2020).
- Rada, E.C. Biological Treatment of Solid Waste, Enhancing Sustainability, 1st ed.; Apple Academic Press, Inc.: Oakville, ON, Canada, 2015; pp. 3–103. [Google Scholar] [CrossRef]
- Kaparaju, P.; Rintala, J. Generation of heat and power from biogas for stationary applications: Boilers, gas engines and turbines, combined heat and power (CHP) plants and fuel cells. In The Biogas Handbook, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 404–427. [Google Scholar] [CrossRef]
- Svensson, M. Biomethane for transport applications. In The Biogas Handbook, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 428–443. [Google Scholar] [CrossRef]
- Guilayn, F.; Rouez, M.; Crest, M.; Patureau, D.; Jimenez, J. Valorization of digestates from urban or centralized biogas plants: A critical review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 419–462. [Google Scholar] [CrossRef]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Karagiannidis, A.; Perkoulidis, G. A multi-criteria ranking of different technologies for the anaerobic digestion for energy recovery of the organic fraction of municipal solid wastes. Bioresour. Technol. 2009, 100, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.D.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Performance evaluation of anaerobic digestion technology for energy recovery from organic fraction of municipal solid waste: A review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Lissens, G.; Vandevivere, P.; De Baere, L.; Biey, E.M.; Verstraete, W. Solid waste digestors: Process performance and practice for municipal solid waste digestion. Water Sci. Technol. 2001, 44, 91–102. [Google Scholar] [CrossRef]
- Rapport, J.L.; Zhang, R.; Williams, R.B.; Jenkins, B.M. Anaerobic digestion technologies for the treatment of municipal solid waste. Int. J. Environ. Waste Manag. 2012, 9, 100–122. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V.; Naddeo, V. Comparative technology assessment of anaerobic digestion of organic fraction of MSW. Trans. Ecol. Environ. 2010, 142, 355–366. [Google Scholar] [CrossRef]
- Angelonidi, E.; Smith, S.R. A comparison of wet and dry anaerobic digestion processes for the treatment of municipal solid waste and food waste. Water Environ. J. 2015, 29, 549–557. [Google Scholar] [CrossRef]
- Orhorhoro, E.K.; Ebunilo, P.O.; Sadjere, G.E. Experimental determination of effect of total solid (TS) and volatile solid (VS) on biogas yield. Am. J. Mod. Energy 2017, 3, 131–135. [Google Scholar] [CrossRef]
- Motte, J.-C.; Trably, E.; Escudié, R.; Hamelin, J.; Steyer, J.-P.; Bernet, N.; Delgenes, J.-P.; Dumas, C. Total solids content: A key parameter of metabolic pathways in dry anaerobic digestion. Biotechnol. Biofuels 2013, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Poquet, V.; Papirio, S.; Trably, E.; Rintala, J.; Escudié, R.; Esposito, G. High-solids anaerobic digestion requires a trade-off between total solids, inoculum-to-substrate ratio and ammonia inhibition. Int. J. Environ. Sci. Technol. 2019, 16, 7011–7024. [Google Scholar] [CrossRef]
- Wang, A.; Jiang, Y.; Wang, S.; Zhang, Y.; Hu, Y.; Hu, Z.; Wu, G.; Zhan, X. Impact of total solids content on anaerobic co-digestion of pig manure and food waste: Insights into shifting of the methanogenic pathway. Waste Manag. 2020, 114, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Jansson, A.T.; Patinvoh, R.J.; Horváth, I.S.; Taherzadeh, M.J. Dry anaerobic digestion of food and paper industry wastes at different solid contents. Fermentation 2019, 5, 40. [Google Scholar] [CrossRef]
- Cho, S.-K.; Im, W.T.; Kim, D.H.; Kim, M.H.; Shin, H.S.; Oh, S.E. Dry anaerobic digestion of food waste under mesophilic conditions: Performance and methanogenic community analysis. Bioresour. Technol. 2013, 131, 210–217. [Google Scholar] [CrossRef]
- Forster-Carneiro, T.; Pérez, M.; Romero, L. Influence of total solid and inoculum contents on performance of anaerobic reactors treating food waste. Bioresour. Technol. 2008, 99, 6994–7002. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, D.-H.; Yun, Y.-M. Effect of operation temperature on anaerobic digestion of food waste: Performance and microbial analysis. Fuel 2017, 209, 598–605. [Google Scholar] [CrossRef]
- Singh, G.; Jain, V.K.; Singh, A. Effect of temperature and other factors on anaerobic digestion process, responsible for bio gas production. Int. J. Theor. Appl. Mech. 2017, 12, 637–657. [Google Scholar]
- Paramaguru, G.; Kannan, M.; Senthilkumar, N.; Lawrence, P. Effect of temperature on biogas production from food waste through anaerobic digestion. Desalin. Water Treat. 2017, 85, 68–72. [Google Scholar] [CrossRef]
- Mata-Alvarez, J. Biomethanization of the Organic Fraction of Municipal Solid Wastes, 1st ed.; IWA Publishing: London, UK, 2002. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Zou, D.; Yuan, H.; Zhu, B.; Li, X.; Pang, Y. Influence of temperature on hydrolysis acidification of food waste. Procedia Environ. Sci. 2012, 16, 85–94. [Google Scholar] [CrossRef]
- Jiang, J.; He, S.; Kang, X.; Sun, Y.; Yuan, Z.; Xing, T.; Guo, Y.; Li, L. Effect of Organic Loading Rate and Temperature on the anaerobic digestion of municipal solid waste: Process performance and energy recovery. Front. Energy Res. 2020, 8, 89. [Google Scholar] [CrossRef]
- Christensen, T.H. Anaerobic Digestion: Process. In Solid Waste Technology & Management, 1st ed.; Blackwell Publishing Ltd.: Chichester, UK, 2011; p. 596. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Riggio, S.; Hernandéz-Shek, M.A.; Torrijos, M.; Vives, G.; Esposito, G.; van Hullebusch, E.D.; Steyer, J.P.; Escudié, R. Comparison of the mesophilic and thermophilic anaerobic digestion of spent cow bedding in leach-bed reactors. Bioresour. Technol. 2017, 234, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Koniuszewska, I. Inhibitors of the methane fermentation process with particular emphasis on the microbiological aspect: A review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef]
- Ryue, J.; Lin, L.; Kakar, F.L.; Elbeshbishy, E.; Al-Mamun, A.; Dhar, B.R. A critical review of conventional and emerging methods for improving process stability in thermophilic anaerobic digestion. Energy Sustain. Dev. 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Goux, X.; Calusinska, M.; Lemaigre, S.; Marynowska, M.; Klocke, M.; Udelhoven, T.; Benizri, E.; Delfosse, P. Microbial community dynamics in replicate anaerobic digesters exposed sequentially to increasing organic loading rate, acidosis, and process recovery. Biotechnol. Biofuels 2015, 8, 122. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Vavilin, V.A.; Qu, X.; Mazéas, L.; Lemunier, M.; Duquennoi, C.; He, P.; Bouch, T. Methanosarcina as the dominant aceticlastic methanogens during mesophilic anaerobic digestion of putrescible waste. Antonie van Leeuwenhoek 2008, 94, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Rattanapan, C.; Sinchai, L.; Suksaroj, T.T.; Kantachote, D.; Ounsaneha, W. Biogas production by co-digestion of canteen food waste and domestic wastewater under organic loading rate and temperature optimization. Environments 2019, 6, 16. [Google Scholar] [CrossRef]
- Grangeiro, L.C.; de Almeida1, S.G.C.; de Mello, B.S.; Fuess, L.T.; Sarti, A.; Dussan, K.J. New trends in biogas production and utilization. In Sustainable Bioenergy, 1st ed.; Rai, M., Ingle, A.P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 199–223. [Google Scholar] [CrossRef]
- Moestedt, J.; Müller, B.; Westerholm, M.; Schnürer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2016, 9, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, H.; Touhami, Y.; Cheikh, R.B.; Hamdi, M. Bioreactor performance in anaerobic digestion of fruit and vegetable wastes. Process Biochem. 2005, 40, 989–995. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Belmonte, M.; Hsieh, C.-F.; Figueroa, C.; Campos, J.L.; Vidal, G. Effect of free ammonia nitrogen on the methanogenic activity of swine wastewater. Electron. J. Biotechnol. 2011, 14, 2. [Google Scholar] [CrossRef]
- Liu, Y.; Ngo, H.H.; Guo, W.; Peng, L.; Wang, D.; Ni, B. The roles of free ammonia (FA) in biological wastewater treatment processes: A review. Environ. Int. 2019, 123, 10–19. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, A.; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sustain. Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Westerholm, M.; Liu, T.; Schnürer, A. Comparative study of industrial-scale high-solid biogas production from food waste: Process operation and microbiology. Bioresour. Technol. 2020, 304, 122981. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A review of the role of critical parameters in the design and operation of biogas production plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhao, X.; Peng, Y.Y. Anaerobic digestion: A review on process monitoring. Renew. Sustain. Energy Rev. 2019, 103, 1–12. [Google Scholar] [CrossRef]
- Kumar, S. Biogas, 1st ed.; InTech: Rijeka, Crotia, 2012; p. 158. [Google Scholar] [CrossRef]
- Charles, W.; Carnaje, N.P.; Cord-Ruwisch, R. Methane conversion efficiency as a simple control parameter for an anaerobic digester at high loading rates. Water Sci. Technol. 2011, 64, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Latrille, E.; Harmand, J.; Robles, A.; Ferrer, J.; Gaida, D.; Wolf, C.; Mairet, F.; Bernard, O.; Alcaraz-Gonzalez, V.; et al. Instrumentation and control of anaerobic digestion processes: A review and some research challenges. Rev. Environ. Sci. Bio/Technol. 2015, 14, 615–648. [Google Scholar] [CrossRef]
- Migliori, M.; Catizzone, E.; Giordano, G.; Le Pera, A.; Sellaro, M.; Lista, A.; Zanardi, G.; Zoia, L. Pilot plant data assessment in anaerobic digestion of organic fraction of municipal waste solids. Processes 2019, 7, 54. [Google Scholar] [CrossRef]
- Ministero delle Politiche Agricole, Alimentari e Forestali. Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/10087 (accessed on 21 December 2020).
- Ministero dello Sviluppo Economico. Available online: https://www.mise.gov.it/images/stories/normativa/decreto_ministeriale_18_maggio_2018_gas_aggiornamento_%20regola_tecnica.pdf (accessed on 21 December 2020).
- Baird, R.B.; Eaton, A.D.; Rice, E.W. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Hach. Available online: https://www.hach.com/asset-get.download.jsa?id=29641556309 (accessed on 21 December 2020).
- Campuzano, R.; González-Martínez, S. Characteristics of the organic fraction of municipal solid waste and methane production: A review. Waste Manag. 2016, 54, 3–12. [Google Scholar] [CrossRef]
- Fisgativa, H.; Tremier, A.; Dabert, P. Characterizing the variability of food waste quality: A need for efficient valorisation through anaerobic digestion. Waste Manag. 2016, 50, 264–274. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Romero Garcia, L.I.; Ng, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Giordano, A.; Liotta, F.; Panico, A.; Pirozzi, F. Anaerobic co-digestion of organic wastes. Rev. Environ. Sci. Bio/Technol. 2012, 11, 325–341. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Macé, S.; Astals, S. Codigestion of solid wastes: A review of its uses and perspectives including modeling. Crit. Rev. Biotechnol. 2011, 31, 99–111. [Google Scholar] [CrossRef]
- Westerholm, M.; Castillo, M.d.P.; Andersson, A.C.; Nilsen, P.J.; Schnürer, A. Effects of thermal hydrolytic pre-treatment on biogas process efficiency and microbial community structure in industrial- and laboratory-scale digesters. Waste Manag. 2019, 95, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Pognani, M.; Barrena, R.; Font, X.; Sánchez, A. A complete mass balance of a complex combined anaerobic/aerobic municipal source-separated waste treatment plant. Waste Manag. 2012, 32, 799–805. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Wilk, M. Thermophilic co-digestion of the organic fraction of municipal solid wastes—The influence of food industry wastes addition on biogas production in full-scale operation. Molecules 2018, 23, 3146. [Google Scholar] [CrossRef] [PubMed]
- Kübler, H.; Hoppenheidt, K.; Hirsch, P.; Kottmair, A.; Nimmrichter, R.; Nordsieck, H.; Mücke, W.; Swerev, M. Full scale co-digestion of organic waste. Water Sci. Technol. 2000, 41, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Chesshire, M.; Heaven, S.; Arnold, R. Anaerobic digestion of source-segregated domestic food waste: Performance assessment by mass and energy balance. Bioresour, Technol. 2011, 102, 612–620. [Google Scholar] [CrossRef] [PubMed]
- de Laclos, H.F.; Desbois, S.; Saint-Joly, C. Anaerobic digestion of municipal solid organic waste: Valorga full-scale plant in Tilburg, the Netherlands. Water Sci. Tech. 1997, 36, 457–462. [Google Scholar] [CrossRef]
- Sposob, M.; Moon, H.-S.; Lee, D.; Kim, T.-H.; Yun, Y.-M. Comprehensive analysis of the microbial communities and operational parameters of two full-scale anaerobic digestion plants treating food waste in South Korea: Seasonal variation and effect of ammonia. J. Hazard. Mater. 2020, 398, 122975. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Seruga, A.; Niedźwiecki, Ł.; Pawlak-Kruczek, H.; Urbanowska, A. Anaerobic Digestion Performance: Separate Collected vs. Mechanical Segregated Organic Fractions of Municipal Solid Waste as Feedstock. Energies 2020, 13, 3768. [Google Scholar] [CrossRef]
- Gallert, C.; Henning, A.; Winter, J. Scale-up of anaerobic digestion of the biowaste fraction from domestic wastes. Water Res. 2003, 37, 1433–1441. [Google Scholar] [CrossRef]
- Bolzonella, D.; Pavan, P.; Mace, S.; Cecchi, F. Dry anaerobic digestion of differently sorted organic municipal solid waste: A full-scale experience. Water Sci. Technol. 2006, 53, 23–32. [Google Scholar] [CrossRef]
- de Laclos, H.F.; Thiebaut, E.; Saint-Joly, C. Anaerobic digestion of residual municipal solid waste using biological–mechanical pre-treatment: The plant of Varennes Jarcy. Water Sci. Technol. 2008, 58, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Saint-Joly, C.; Desbois, S.; Lotti, J.-P. Determinant impact of waste collection and composition on anaerobic digestion performance: Industrial results. Water Sci. Technol. 2000, 41, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.H. Anaerobic Digestion: Mass Balances and Products. In Solid Waste Technology & Management, 1st ed.; Blackwell Publishing Ltd.: Chichester, UK, 2011; p. 623. [Google Scholar] [CrossRef]
- Romero-Guiza, M.S.; Peces, M.; Astals, S.; Benavent, J.; Valls, J.; Mata-Alvarez, J. Implementation of a prototypal optical sorter as core of the new pre-treatment configuration of a mechanical–biological treatment plant treating OFMSW through anaerobic digestion. Appl. Energy 2014, 135, 63–70. [Google Scholar] [CrossRef]
- Challen Urbanic, J.M.; VanOpstal, B.; Parker, W. Anaerobic digestion of the organic fraction of municipal solid waste (OFMSW)—full scale vs laboratory results. J. Solid Waste Technol. Manag. 2011, 37, 33–39. [Google Scholar] [CrossRef]
- Qian, M.Y.; Li, R.H.; Li, J.; Wedwitschka, H.; Nelles, M.; Stinner, W.; Zhou, H.J. Industrial scale garage-type dry fermentation of municipal solid waste to biogas. Bioresour. Technol. 2016, 217, 82–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Banks, C.J.; Heaven, S. Anaerobic digestion of two biodegradable municipal waste streams. J. Environ. Manag. 2012, 104, 166–174. [Google Scholar] [CrossRef]
- Hartmann, H.; Ahring, B.K. Strategies for the anaerobic digestion of the organic fraction of municipal solid waste: An overview. Water Sci. Technol. 2006, 53, 7–22. [Google Scholar] [CrossRef]
- Menardo, S.; Airoldi, G.; Balsari, P. The effect of particle size and thermal pre-treatment on the methane yield of four agricultural by-products. Bioresour. Technol. 2012, 104, 708–714. [Google Scholar] [CrossRef]
- Dai, X.; Hua, Y.; Dai, L.; Cai, C. Particle size reduction of rice straw enhances methane production under anaerobic digestion. Bioresour. Technol. 2019, 293, 122043. [Google Scholar] [CrossRef]
- Armah, E.K.; Chetty, M.; Deenadayalu, N. Effect of particle size on biogas generation from sugarcane bagasse and corn silage. Chem. Eng. Trans. 2019, 76, 1471–1476. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mishra, I.M.; Sharma, M.P.; Saini, J.S. Effect of particle size on biogas generation from biomass residues. Biomass 1988, 17, 251–263. [Google Scholar] [CrossRef]
- Vigueras-Carmona, S.E.; Trujillo, M.A.M.; Rivero, M.G.; Venegas, I.M.; Jiménez, G.Z. Effect of particle size on mesophilic anaerobic digestion of thermally pre-treated waste activated sludge. J. Biotech. Res. 2016, 7, 11–19. [Google Scholar]
- Silvestre, G.; Bonmatí, A.; Fernández, B. Optimisation of sewage sludge anaerobic digestion through co-digestion with OFMSW: Effect of collection system and particle size. Waste Manag. 2015, 43, 137–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Banks, C.J. Impact of different particle size distributions on anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2013, 33, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Okishio, Y.-K.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T.T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Rosato, M.A. Managing Biogas Plants; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–48. [Google Scholar] [CrossRef]
- Yan, M.; Fotidis, I.A.; Tian, H.; Khoshnevisan, B.; Treu, L.; Tsapekos, P.; Angelidaki, I. Acclimatization contributes to stable anaerobic digestion of organic fraction of municipal solid waste under extreme ammonia levels: Focusing on microbial community dynamics. Bioresour. Technol. 2019, 286, 121376. [Google Scholar] [CrossRef]
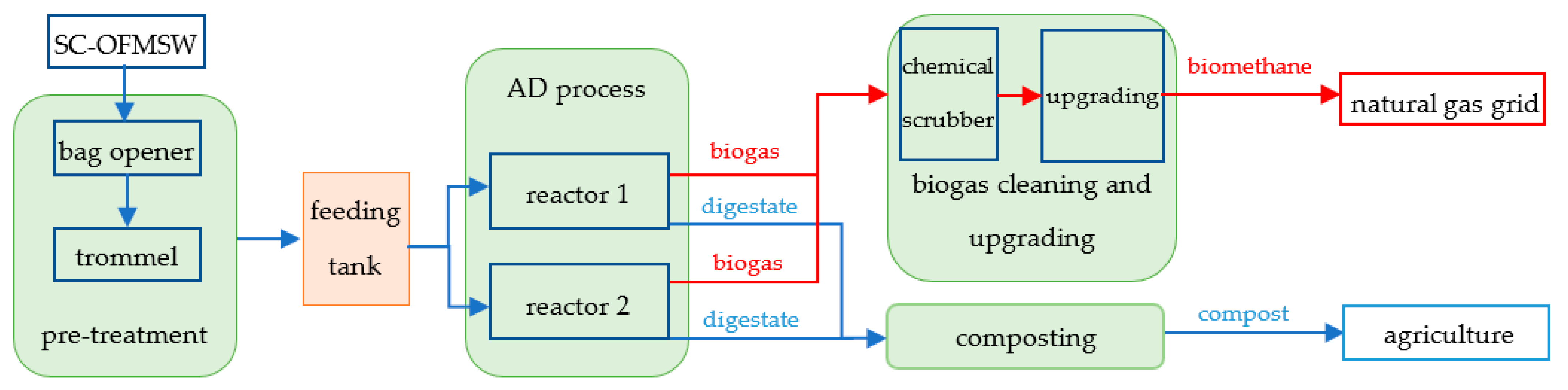

| Process Type | Process Characteristics |
|---|---|
| BTA® | One- or two-stage, wet (TS = 10%), mesophilic (35 °C) or thermophilic (55 °C) continuous process able to producing biogas volumes of around 150 m3/t of input waste or 511 m3/t TVS and methane volumes of 332 m3/t TVS. |
| Dranco® | Single-stage, dry (TS = 20–50%), thermophilic (50–58 °C) continuous process able to achieve biogas productions between 100 and 200 m3/t of input materials or 468–622 m3/t TVS and biogas production rate between 4–9.2 m3/m3 in around 14–20 days. |
| Kompogas® | Single-stage, dry (TS = 23–30%), thermophilic (55–60 °C) continuous process able to reach biogas yield of 130–150 m3/t of input waste and methane yield 390–580 m3/t TVS operating at a 15–29 days HRT. |
| Valorga® | Single-stage, dry (TS = 25–35%), mesophilic continuous process able to producing biogas volumes between 80–180 m3/t of input waste and methane volumes of 220–300 m3/t TVS operating at 18–23 days HRT. |
| Waasa® | Single-stage, wet (TS = 10–15%), mesophilic or thermophilic continuous process able to generating a biogas production of 100–150 m3/t of input materials or also a methane production of 170–320 Nm3/t TVS with a retention time of 10–20 days. |
| Garage-type (Bekon®, Gicon®) | Wet or dry, thermophilic or mesophilic (37–55 °C), batch process able to achieve methane productions between 170–370 m3/t TVS in around 28–35 days. |
| BioFerm® | Dry (TS = 25%), mesophilic (37 °C), batch process able to achieve methane productions of 210–350 Nm3/t TVS in 28 days. |
| Biocel® | Dry (TS = 35–40%), mesophilic (35–40 °C), batch process producing 50 m3 of biogas every t of input materials in 21 days. |
| Sebac® | Dry (TS = 30%), thermophilic (55 °C), batch process running 25–40 days and producing 220–530 m3 of methane every t of TVS. |
| Reactor 1 | Reactor 2 | |||
|---|---|---|---|---|
| Biogas | Methane | Biogas | Methane | |
| CH4 (%) | 59.22 | 58.96 | ||
| Yield (Sm3/d) 1 | 9661 | 5709 | 9865 | 5829 |
| Specific production (Sm3/t TVS) 1 | 798.5 | 471.9 | 815.4 | 481.8 |
| Specific production (Sm3/t input material) 1 | 177.3 | 104.8 | 181.0 | 107.0 |
| Production rate (Sm3/m3) 1,2 | 8.05 | 4.76 | 8.22 | 4.86 |
| Plant Location | Process Characteristics | Operating Parameters | Biogas Yield | Methane Yield | Ref. |
|---|---|---|---|---|---|
| South Shropshire (UK) | Wet | 42 °C; OLR = 2.7 kg TVS/m3 d; HRT = 80 d | 156 m3/t TS; 642 m3/t TVS; 1.59 m3/m3 | 98 m3/t TS; 402 m3/t TVS; 1.00 m3/m3 | [69] |
| Tilburg (NL) | Semicontinous one-stage, dry (Valorga) | 40 °C; HRT = 20–55d; TS = 30% | 210–290 m3/t TVS | [70] | |
| (KR) | Wet | (a) mesophilic; OLR = 3.9–5.4 kg TVS/m3 d (b) mesophilic; OLR = 5.3–6.1 kg TVS/m3 d | (a) 340–430 m3/t TVS (b) 220–310 m3/t TVS | [71] | |
| Oława (PL) | Dry (Kompogas) | 54 °C; (a) HRT = 32.2 d; (b) HRT = 31; TS = 32–35% | (a) 102.3 m3/t w; 2.79 m3/m3 (feed = 30 t w, operating vol. = 1,100 m3) (b) 111.1 m3/t w | (a) 53.2 m3/t w d (calc.CH4 = 52%); (b) 66.7 m3/t w d (calc.CH4 = 60%) | [72] |
| Karlsruhe (DE) | Wet (BTA) | 37 °C; OLR = 8.3–8.5 kg COD/m3 d; HRT = 12 d | 511 m3/t TVS; 2.6 m3/m3 | 332.2 m3/t TVS (calc., CH4 = 65%) | [73] |
| Bassano (I) | Dry (Valorga) | 36.7 °C; OLR = 4–6 kg TVS/m3 d; HRT = 40–60 d; TS = 30% | 400 m3/t TVS; 3.2 m3/m3 | [74] | |
| Varennes Jarcy (FR) | One-stage, dry, (Valorga) | TS = 30% | 140 Sm3/t w | 240 Sm3/t TVS | [75] |
| Amiens (FR) | One-stage, dry, (Valorga) | 37 °C; TS = 30% | 145 m3/t w | 205 m3/t TVS | [76] |
| Engelskirchen (DE) | One-stage, dry, (Valorga) | 37 °C; TS = 30% | 126 m3/t w | 280 m3/t TVS | [76] |
| Müster (DE) | One-step, wet | thermophilic | 90 Nm3/t w | 40.5–58.5 m3/t w (calc. CH4 = 45–65%) | [77] |
| Kirchstockach (DE) | Two-step, wet | mesophilic | 60–100 Nm3/t w | 36–60 m3/t w (calc. CH4 = 60–65%) | [77] |
| Barcelona (ES) | Wet | 37 °C; OLR = 0.8–1.7 kg TVS/m3 d; HRT = 20–40 d | 440–580 m3/t TVS | [78] | |
| Toronto (CA) | Wet | 37 °C; OLR = 5 kg TVS/m3 d; HRT = 17 d | 377 m3/t TVS | [79] | |
| Berlin (DE) | Semidry | mesophilic; OLR = 3–7 kg TVS/m3 d; HRT = 15–17 d; TS = 10–15% | 100–150 m3/t TVS | 50–105 m3/t TVS (calc. CH4 = 50–70%) | [15] |
| Harbin (CN) | Batch, dry (garage-type) | 37 °C; RT = 35 d; TS = 43.5% | 411 m3/t TVS; 0.72–2.22 m3/m3 | 270 m3/t TVS | [80] |
| pH Mean (Min–Max) | VFA/TA Mean (Min–Max) | TVS %Mean (Min–Max) | TAN g/L Mean (Min–Max) | |
|---|---|---|---|---|
| IR1 | 7.90 (7.40–8.13) | 0.381 (0.295–0.452) | 8.99 (6.78–9.95) | 3.79 (2.55–4.51) |
| IR2 | 7.89 (7.38–8.15) | 0.388 (0.292–0.449) | 8.98 (7.24–9.97) | 3.76 (2.78–4.39) |
| D1 | 8.01 (7.81–8.28) | 0.319 (0.232–0.358) | 8.38 (6.20–9.09) | 3.55 (2.02–4.27) |
| D2 | 8.02 (7.78–8.37) | 0.314 (0.249–0.350) | 8.42 (6.92–8.98) | 3.44 (2.18–4.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Pera, A.; Sellaro, M.; Migliori, M.; Bianco, M.; Zanardi, G. Dry Mesophilic Anaerobic Digestion of Separately Collected Organic Fraction of Municipal Solid Waste: Two-Year Experience in an Industrial-Scale Plant. Processes 2021, 9, 213. https://doi.org/10.3390/pr9020213
Le Pera A, Sellaro M, Migliori M, Bianco M, Zanardi G. Dry Mesophilic Anaerobic Digestion of Separately Collected Organic Fraction of Municipal Solid Waste: Two-Year Experience in an Industrial-Scale Plant. Processes. 2021; 9(2):213. https://doi.org/10.3390/pr9020213
Chicago/Turabian StyleLe Pera, Adolfo, Miriam Sellaro, Massimo Migliori, Micaela Bianco, and Giuseppe Zanardi. 2021. "Dry Mesophilic Anaerobic Digestion of Separately Collected Organic Fraction of Municipal Solid Waste: Two-Year Experience in an Industrial-Scale Plant" Processes 9, no. 2: 213. https://doi.org/10.3390/pr9020213
APA StyleLe Pera, A., Sellaro, M., Migliori, M., Bianco, M., & Zanardi, G. (2021). Dry Mesophilic Anaerobic Digestion of Separately Collected Organic Fraction of Municipal Solid Waste: Two-Year Experience in an Industrial-Scale Plant. Processes, 9(2), 213. https://doi.org/10.3390/pr9020213






