Modeling and Flowsheet Simulation of Vibrated Fluidized Bed Dryers
Abstract
1. Introduction
1.1. Background on Fluidized Bed Modeling
1.1.1. Modeling of Hydrodynamics
1.1.2. Modeling of Dying Kinetics
1.2. Flowsheet Simulation
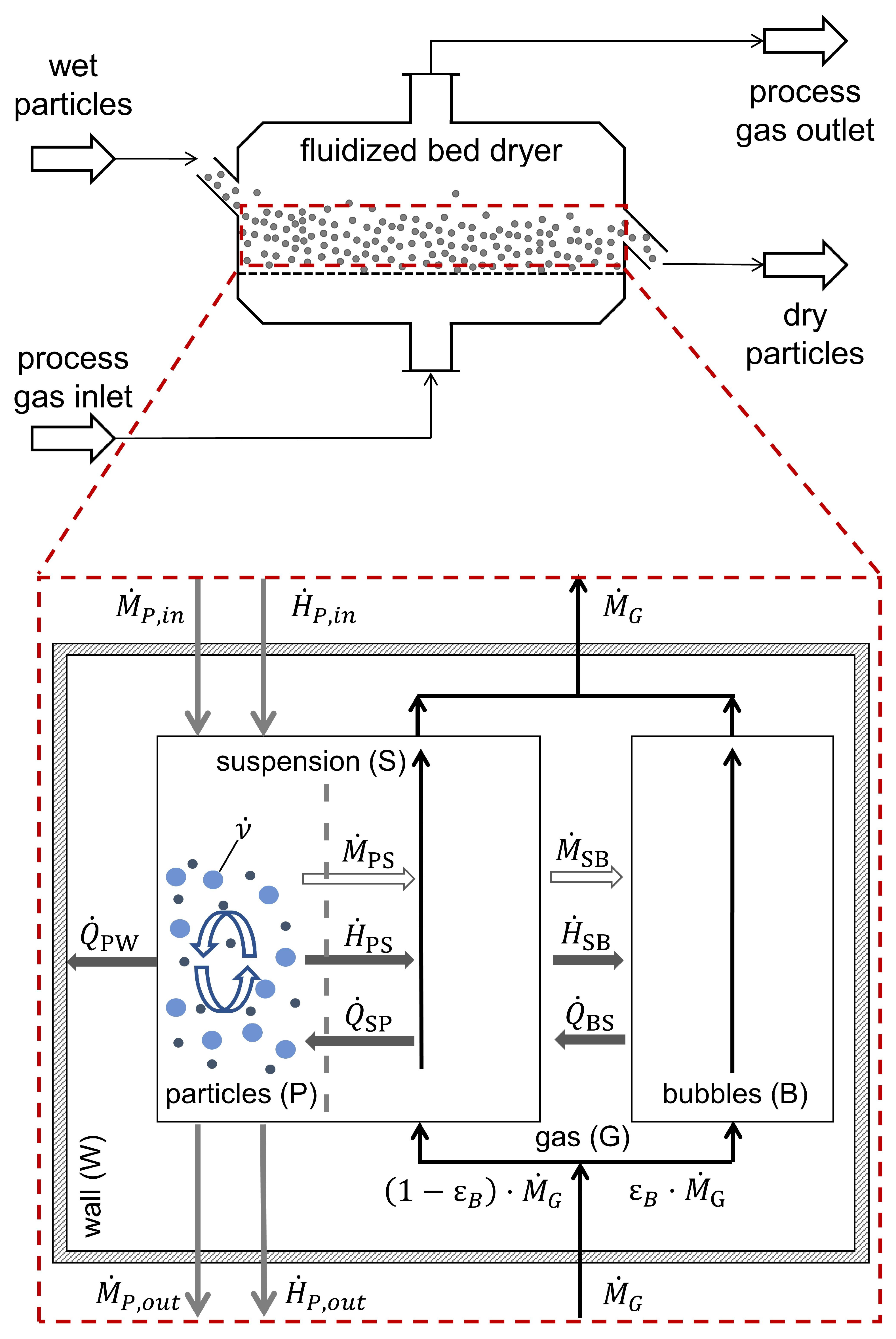
2. Model
2.1. Structure and Assumptions
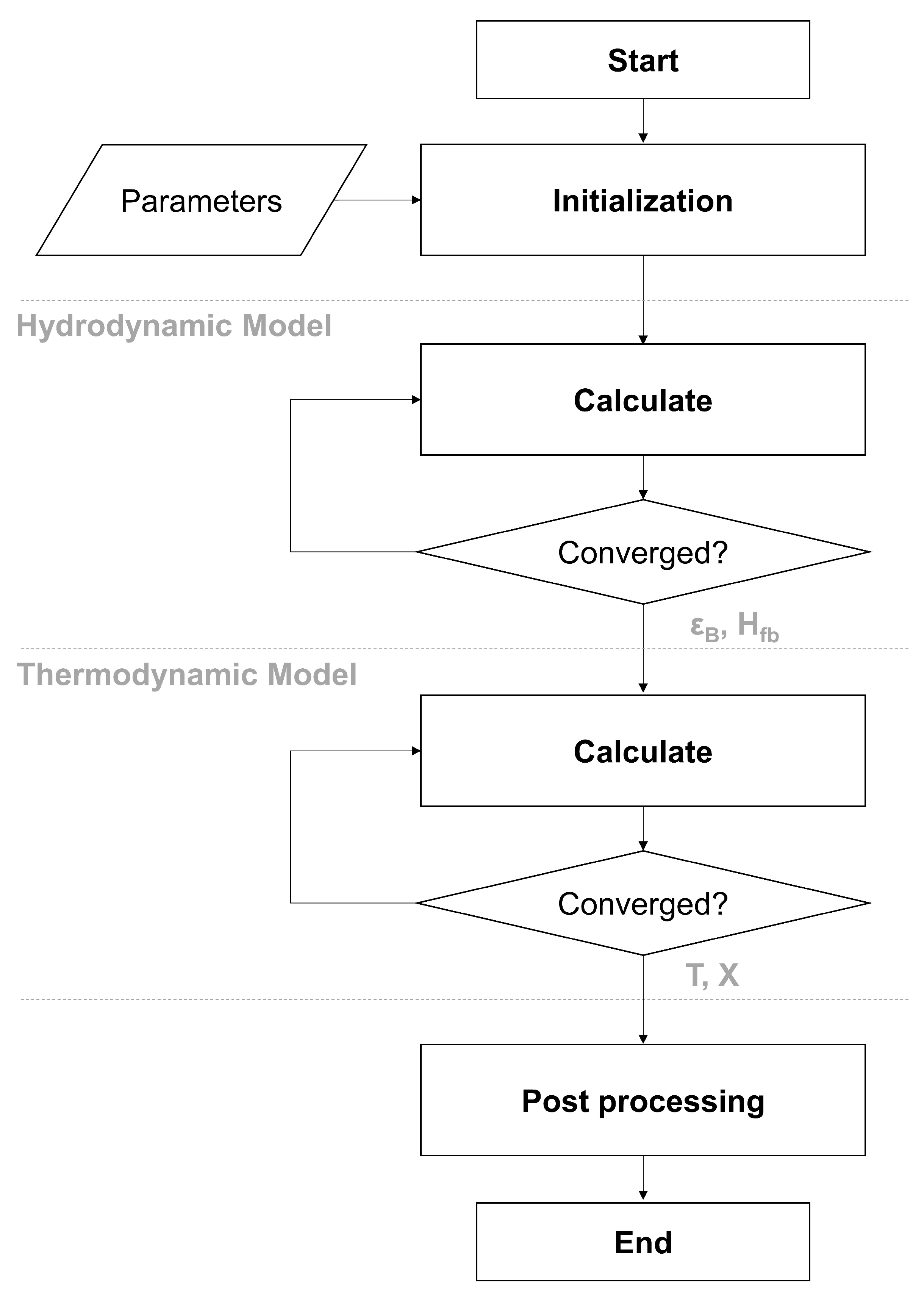
2.2. Hydrodynamics Model
2.3. Thermodynamic Model
2.4. Implementation
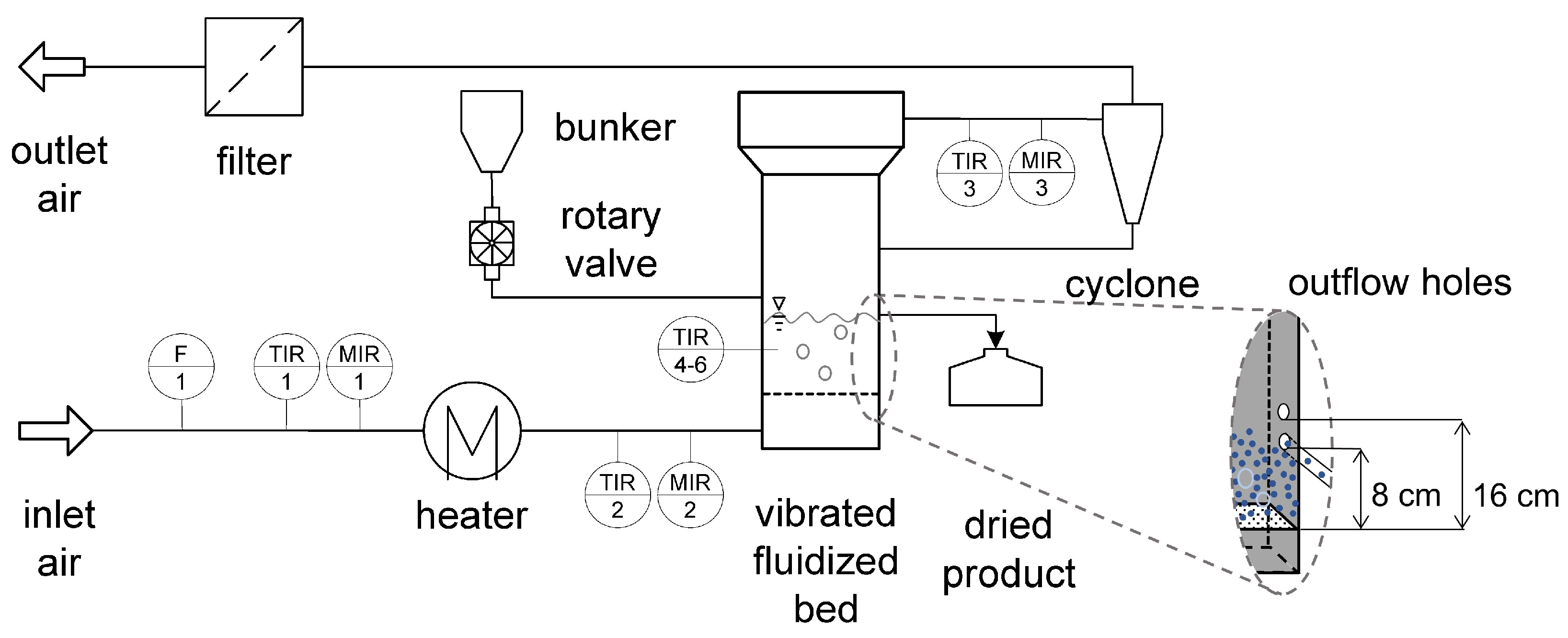
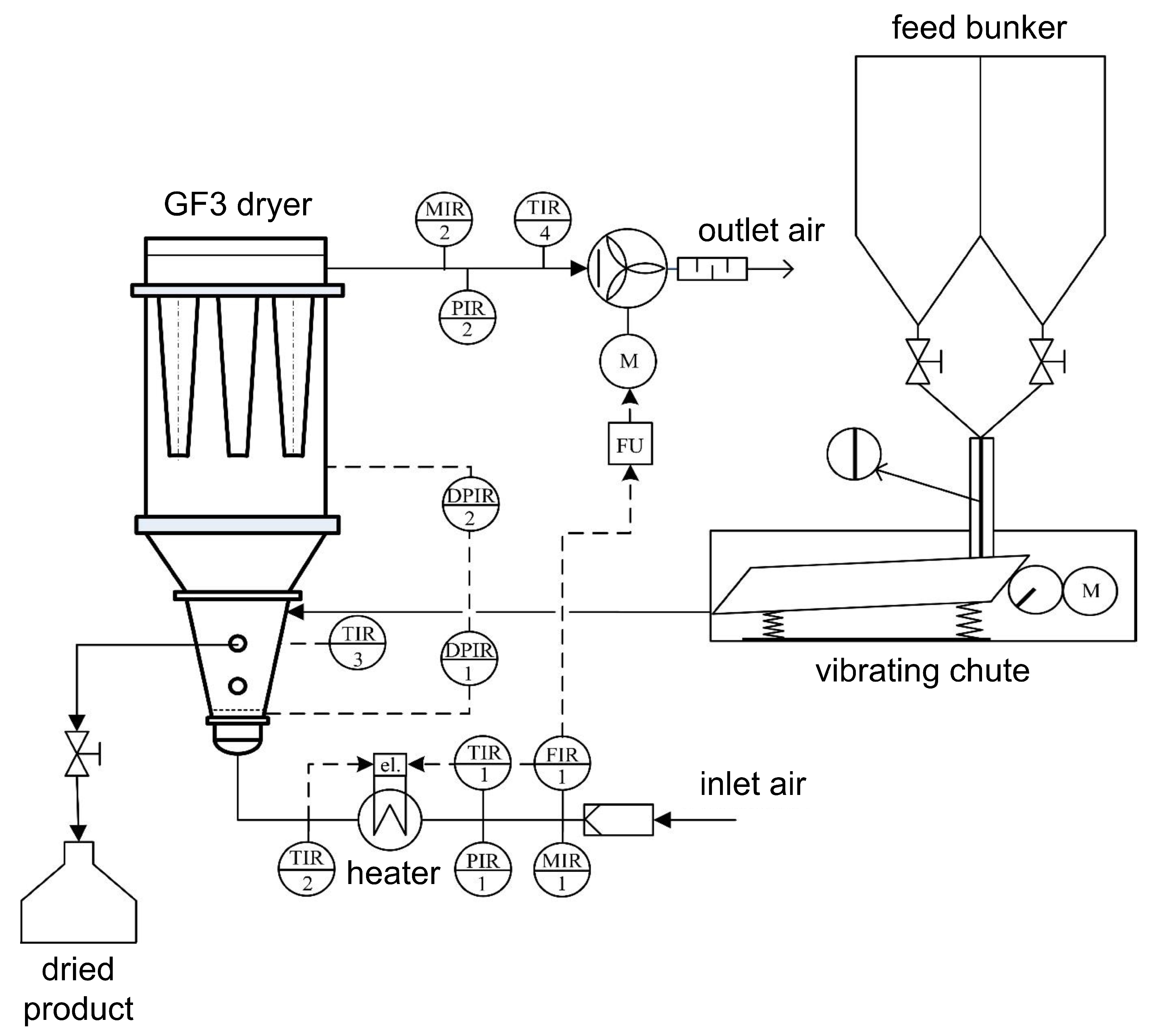
3. Materials and Methods
3.1. Materials
3.2. Drying Experiments
4. Results and Discussion
4.1. Validation
4.1.1. Validation for Geldart D Particles
4.1.2. Validation for Geldart B Particles
4.1.3. Validation for Dryer Geometries and Vibration
4.1.4. Validation for Geldart A
4.1.5. Validation of Model Assumptions
4.2. Sensitivity Analysis
4.2.1. Heat Transfer between Particles and Dryer Wall
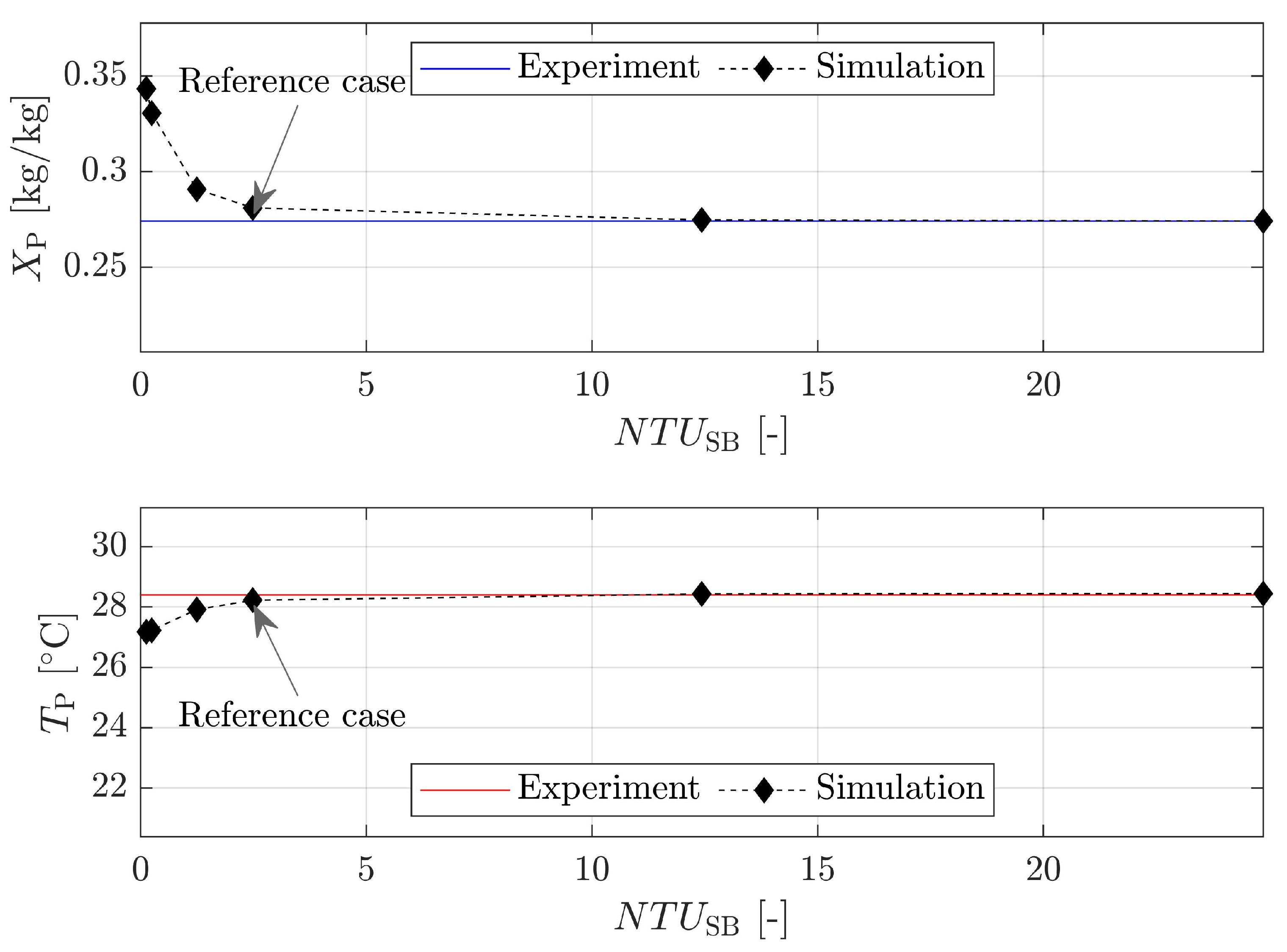
4.2.2. Number of Transfer Units
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Roman letters | ||
| A | transfer surface area | m2 |
| amplitude of vibration | m | |
| CSTR | continuous stirred tank reactor | - |
| CFD | computational fluid dynamics | - |
| heat capacity | /kg−1/k−1 | |
| d | diameter | m |
| DEM | discrete element modeling | - |
| activation energy | ||
| evaporation enthalpy | /kg−1 | |
| f | frequency of vibration | Hz |
| FCC | fluid catalytic cracking | - |
| g | gravitational acceleration | /s−2 |
| h | specific enthalpy | /kg−1 |
| H | height | m |
| enthalpy flow | W | |
| n | number density distribution of residence time | - |
| mass flux | /s−1/m−2 | |
| M | mass | |
| mass flow | /s−1 | |
| NCDC | Normalized Characteristic Drying Curve | - |
| NTU | number of transfer units | - |
| PFTR | plug flow tank reactor | - |
| heat flow | W | |
| REA | Reaction Engineering Approach | - |
| T | temperature | |
| u | gas velocity | /s−1 |
| VFB | vibrated fluidized bed | - |
| X | moisture content of solids | /kg−1 |
| Y | gas moisture content | /kg−1 |
| Greek symbols | ||
| heat transfer coefficient | /kg−1/k−1 | |
| mass transfer coefficient | /s−1 | |
| bed porosity | - | |
| normalized bed height | - | |
| vibration intensity | - | |
| material specific drying curve | - | |
| normalized moisture content | - | |
| density | /m−3 | |
| residence time | ||
| relative humidity | - | |
| Subscripts | ||
| amb | ambient | |
| B | bubble phase | |
| d | discretized particle size class | |
| cr | critical | |
| eq | equilibrium | |
| exp | experiment | |
| f | discretized particle moisture class | |
| fb | fluidized bed under operation conditions | |
| G | gas phase | |
| in | inlet | |
| mf | minimum fluidization | |
| max | maximum | |
| out | outlet | |
| P | particle phase | |
| S | suspension phase | |
| sim | simulation | |
| v | vapor/gaseous | |
| W | wall |
Appendix A. Heat and Mass Transfer Streams
Appendix B. Material Properties and Investigated Vibration Parameters

| frequency f | [Hz] | 0 | 4 | 6 | 8 | 10 |
| amplitude | [mm] | 0 | 5 | 4 | 3.5 | 3 |
| vibration intensity | [-] | 0 | 0.32 | 0.59 | 0.9 | 1.21 |

Appendix C. Summary of Experimental and Simulation Results
| Exp. Name | [cm] | [cm] | [%] | [K] | [K] | [%] | [kg kg] | [kg kg] | [%] |
|---|---|---|---|---|---|---|---|---|---|
| GF3_A_01 | 13.0 | 12.4 | 4.47 | 295.60 | 294.95 | 0.22 | 0.170 | 0.144 | 15.16 |
| GF3_A_02 | 13.0 | 12.4 | 4.35 | 301.55 | 301.37 | 0.06 | 0.291 | 0.281 | 3.51 |
| GF3_A_03 | 13.0 | 12.4 | 4.27 | 308.68 | 314.67 | −1.94 | 0.141 | 0.139 | 1.45 |
| GF3_A_04 | 13.0 | 12.4 | 4.32 | 308.02 | 312.53 | −1.47 | 0.172 | 0.182 | −5.68 |
| GF3_A_05 | 13.0 | 12.4 | 4.28 | 308.98 | 313.53 | −1.47 | 0.150 | 0.150 | 0.03 |
| GF3_A_06 | 8.0 | 7.7 | 4.19 | 307.33 | 318.81 | −3.74 | 0.138 | 0.132 | 4.38 |
| GF3_C_01 | 8.1 | 8.2 | −2.20 | 301.62 | 303.56 | −0.64 | 0.087 | 0.060 | 31.23 |
| GF3_C_02 | 8.1 | 8.2 | −2.24 | 301.25 | 302.40 | −0.38 | 0.073 | 0.069 | 5.19 |
| GF3_C_03 | 7.2 | 7.4 | −3.24 | 306.52 | 311.91 | −1.76 | 0.081 | 0.064 | 21.04 |
| GF3_C_04 | 7.2 | 7.4 | −3.24 | 302.91 | 306.55 | −1.20 | 0.124 | 0.092 | 25.45 |
| GF3_C_05 | 8.1 | 8.3 | −3.14 | 315.69 | 318.16 | −0.78 | 0.061 | 0.054 | 10.87 |
| GF3_C_06 | 8.1 | 8.3 | −3.05 | 311.69 | 312.85 | −0.37 | 0.068 | 0.070 | −3.75 |
| GF3_C_07 | 7.2 | 7.4 | −3.84 | 325.43 | 334.32 | −2.73 | 0.046 | 0.046 | −1.05 |
| GF3_C_08 | 8.1 | 8.4 | −3.88 | 336.58 | 336.53 | 0.02 | 0.044 | 0.052 | −18.06 |
| GF3_C_09 | 8.1 | 8.4 | −3.83 | 326.78 | 330.95 | −1.28 | 0.062 | 0.056 | −8.70 |
| VFB_C_01 | 6.8 | 6.4 | 6.06 | 296.79 | 296.60 | −0.12 | 0.104 | 0.114 | −10.17 |
| VFB_C_02 | 7.2 | 6.3 | 12.6 | 294.91 | 295.26 | −1.95 | 0.157 | 0.169 | −7.78 |
| VFB_C_03 | 6.9 | 7.0 | −0.93 | 307.60 | 313.59 | 0.34 | 0.056 | 0.064 | −13.62 |
| VFB_C_04 | 6.9 | 7.0 | −0.56 | 303.90 | 307.04 | −1.03 | 0.081 | 0.090 | −11.82 |
| VFB_C_05 | 7.0 | 6.9 | 1.73 | 298.05 | 298.70 | −0.22 | 0.111 | 0.102 | 7.99 |
| VFB_C_06 | 7.0 | 7.2 | −2.55 | 303.72 | 306.53 | −0.93 | 0.105 | 0.101 | 3.67 |
| VFB_C_07 | 7.0 | 6.9 | 1.81 | 309.55 | 313.03 | −1.12 | 0.063 | 0.070 | −11.33 |
| VFB_F_01 | 13.0 | 11.45 | 11.93 | 300.22 | 302.50 | −0.76 | 0.031 | 0.034 | −7.66 |
| VFB_F_02 | 13.0 | 11.45 | 11.93 | 303.15 | 302.70 | 0.15 | 0.028 | 0.034 | −21.40 |
| VFB_F_03 | 15.0 | 13.96 | 6.94 | 298.32 | 299.21 | −0.30 | 0.037 | 0.041 | −10.96 |
| VFB_F_04 | 15.0 | 13.96 | 6.94 | 302.76 | 302.76 | −0.20 | 0.026 | 0.020 | 24.80 |
References
- Kunii, D.; Levenspiel, O.; Brenner, H. Fluidization Engineering, 2nd ed.; Elsevier Science: Burlington, MA, USA, 2013. [Google Scholar]
- Sivakumar, R.; Saravanan, R.; Elaya Perumal, A.; Iniyan, S. Fluidized bed drying of some agro products—A review. Renew. Sustain. Energy Rev. 2016, 61, 280–301. [Google Scholar] [CrossRef]
- Palzer, S. The effect of glass transition on the desired and undesired agglomeration of amorphous food powders. Chem. Eng. Sci. 2005, 60, 3959–3968. [Google Scholar] [CrossRef]
- Van Ommen, J.R. Reshaping the Structure of Fluidized Beds. CEP 2009, 105, 49–57. [Google Scholar]
- Pisecký, J. Handbook of Milk Powder Manufacture, 2nd ed.; GEA Niro: Soeborg, Danmark, 2012. [Google Scholar]
- Lehmann, S.E.; Hartge, E.U.; Jongsma, A.; deLeeuw, I.M.; Innings, F.; Heinrich, S. Fluidization characteristics of cohesive powders in vibrated fluidized bed drying at low vibration frequencies. Powder Technol. 2019, 357, 54–63. [Google Scholar] [CrossRef]
- Lee, J.R.; Lee, K.S.; Hasolli, N.; Park, Y.O.; Lee, K.Y.; Kim, Y.H. Fluidization and mixing behaviors of Geldart groups A, B and C particles assisted by vertical vibration in fluidized bed. Chem. Eng. Process. Process. Intensif. 2020, 149, 107856. [Google Scholar] [CrossRef]
- Brennan, J.G. DRYING|Fluidized-bed Drying. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Oxford, UK, 2003; pp. 1922–1929. [Google Scholar] [CrossRef]
- Kieckhefen, P.; Pietsch, S.; Dosta, M.; Heinrich, S. Possibilities and Limits of Computational Fluid Dynamics-Discrete Element Method Simulations in Process Engineering: A Review of Recent Advancements and Future Trends. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Skorych, V.; Dosta, M.; Hartge, E.U.; Heinrich, S. Novel system for dynamic flowsheet simulation of solids processes. Powder Technol. 2017, 314, 665–679. [Google Scholar] [CrossRef]
- Skorych, V.; Dosta, M.; Heinrich, S. Dyssol—An open-source flowsheet simulation framework for particulate materials. SoftwareX 2020, 12, 100572. [Google Scholar] [CrossRef]
- Daud, W.R.W. Fluidized Bed Dryers—Recent Advances. Adv. Powder Technol. 2008, 19, 403–418. [Google Scholar] [CrossRef]
- Davidson, J.F.; Harrison, D. The behaviour of a continuously bubbling fluidised bed. Chem. Eng. Sci. 1966, 21, 731–738. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Bubbling Bed Model. Model for Flow of Gas through a Fluidized Bed. Ind. Eng. Chem. Fundam. 1968, 7, 446–452. [Google Scholar] [CrossRef]
- Hilligardt, K.; Werther, J. Local bubble gas hold-up and expansion of gas-solid fluidized beds. Ger. Chem. Eng. 1986, 9, 215–221. [Google Scholar]
- Groenewold, H.; Tsotsas, E. Predicting apparent Sherwood numbers for fluidized beds. Dry. Technol. 1999, 17, 1557–1570. [Google Scholar] [CrossRef]
- Burgschweiger, J.; Tsotsas, E. Experimental investigation and modelling of continuous fluidized bed drying under steady-state and dynamic conditions. Chem. Eng. Sci. 2002, 57, 5021–5038. [Google Scholar] [CrossRef]
- Alaathar, I.; Hartge, E.U.; Heinrich, S.; Werther, J. Modeling and flowsheet simulation of continuous fluidized bed dryers. Powder Technol. 2013, 238, 132–141. [Google Scholar] [CrossRef]
- Abbasi, M.R.; Shamiri, A.; Hussain, M.A.; Kaboli, S.H.A. Dynamic process modeling and hybrid intelligent control of ethylene copolymerization in gas phase catalytic fluidized bed reactors. J. Chem. Technol. Biotechnol. 2019, 94, 2433–2451. [Google Scholar] [CrossRef]
- Agu, C.E.; Tokheim, L.A.; Eikeland, M.; Moldestad, B.M. Improved models for predicting bubble velocity, bubble frequency and bed expansion in a bubbling fluidized bed. Chem. Eng. Res. Des. 2019, 141, 361–371. [Google Scholar] [CrossRef]
- Bakshi, A.; Altantzis, C.; Glicksman, L.R.; Ghoniem, A.F. Gas-flow distribution in bubbling fluidized beds: CFD-based analysis and impact of operating conditions. Powder Technol. 2017, 316, 500–511. [Google Scholar] [CrossRef]
- Haghgoo, M.R.; Bergstrom, D.J.; Spiteri, R.J. Effect of particle stress tensor in simulations of dense gas–particle flows in fluidized beds. Particuology 2018, 38, 31–43. [Google Scholar] [CrossRef]
- Karimipour, S.; Pugsley, T. A critical evaluation of literature correlations for predicting bubble size and velocity in gas–solid fluidized beds. Powder Technol. 2011, 205, 1–14. [Google Scholar] [CrossRef]
- Kemp, I.C.; Oakley, D.E. Modelling of particulate drying in theory and practice. Dry. Technol. 2002, 20, 1699–1750. [Google Scholar] [CrossRef]
- Lehmann, S.E.; Oesau, T.; Jongsma, A.; Innings, F.; Heinrich, S. Material specific drying kinetics in fluidized bed drying under mechanical vibration using the reaction engineering approach. Adv. Powder Technol. 2020. [Google Scholar] [CrossRef]
- van Meel, D.A. Adiabatic convection batch drying with recirculation of air. Chem. Eng. Sci. 1958, 9, 36–44. [Google Scholar] [CrossRef]
- Tsotsas, E. From single particle to fluid bed drying kinetics. Dry. Technol. 1994, 12, 1401–1426. [Google Scholar] [CrossRef]
- Chen, K.; Bachmann, P.; Bück, A.; Jacob, M.; Tsotsas, E. Experimental study and modeling of particle drying in a continuously-operated horizontal fluidized bed. Particuology 2017, 34, 134–146. [Google Scholar] [CrossRef]
- Langrish, T.; Kockel, T.K. The assessment of a characteristic drying curve for milk powder for use in computational fluid dynamics modelling. Chem. Eng. J. 2001, 84, 69–74. [Google Scholar] [CrossRef]
- Burgschweiger, J.; Groenewold, H.; Hirschmann, C.; Tsotsas, E. From hygroscopic single particle to batch fluidized bed drying kinetics. Can. J. Chem. Eng. 1999, 77, 333–341. [Google Scholar] [CrossRef]
- Chen, X.D. The Basics of a Reaction Engineering Approach to Modeling Air-Drying of Small Droplets or Thin-Layer Materials. Dry. Technol. 2008, 26, 627–639. [Google Scholar] [CrossRef]
- Chen, X.D.; Xie, G.Z. Fingerprints of the Drying Behaviour of Particulate or Thin Layer Food Materials Established Using a Reaction Engineering Model. Food Bioprod. Process. 1997, 75, 213–222. [Google Scholar] [CrossRef]
- Chen, X.D.; Mujumdar, A.S. Drying Technologies in Food Processing; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Chen, X.D.; Putranto, A. Modelling Drying Processes: A Reaction Engineering Approach; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- Putranto, A.; Chen, X.D.; Webley, P.A. Infrared and convective drying of thin layer of polyvinyl alcohol (PVA)/glycerol/water mixture—The reaction engineering approach (REA). Chem. Eng. Process. Process. Intensif. 2010, 49, 348–357. [Google Scholar] [CrossRef]
- Alaathar, I. Fließschema-Simulation der Kontinuierlichen Wirbelschichttrocknung mit Verteilten Parametern, 1st ed.; SPE-Schriftenreihe; Cuvillier Verlag: Göttingen, Germany, 2017; Volume 9. [Google Scholar]
- Groenewold, H.; Tsotsas, E. A new model for fluid bed drying. Dry. Technol. 1997, 15, 1687–1698. [Google Scholar] [CrossRef]
- Burgschweiger, J. Modellierung des Statischen und Dynamischen Verhaltens von Kontinuierlich Betriebenen Wirbelschichttrocknern: Zugl.: Magdeburg, Univ., Fak. für Verfahrens- und Systemtechnik, Diss., 2000; Fortschritt-Berichte/VDI Reihe 3, Verfahrenstechnik; VDI-Verl.: Düsseldorf, Germany, 2000; Volume 665. [Google Scholar]
- SUNDIALS: SUite of Nonlinear and DIfferential/ALgebraic Equation Solvers. Available online: https://computation.llnl.gov/projects/sundials (accessed on 28 December 2020).
- Anderson, D.G. Iterative Procedures for Nonlinear Integral Equations. J. ACM 1965, 12, 547–560. [Google Scholar] [CrossRef]
- Martin, H. Heat Transfer in Fluidized Beds. In VDI Heat Atlas; VDI-Buch, Springer: Berlin/Heidelberg, Germany, 2010; pp. 1300–1309. [Google Scholar]
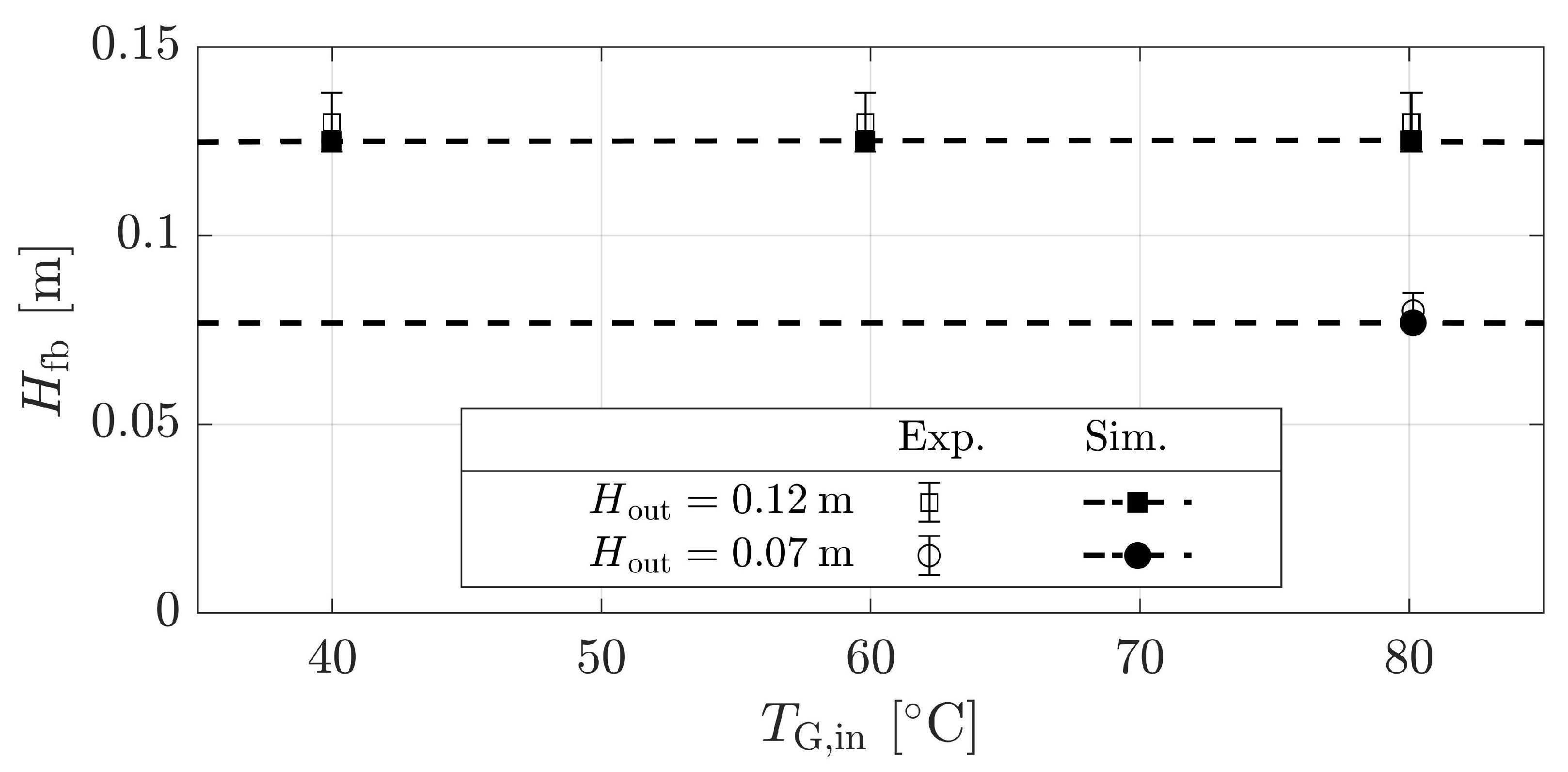

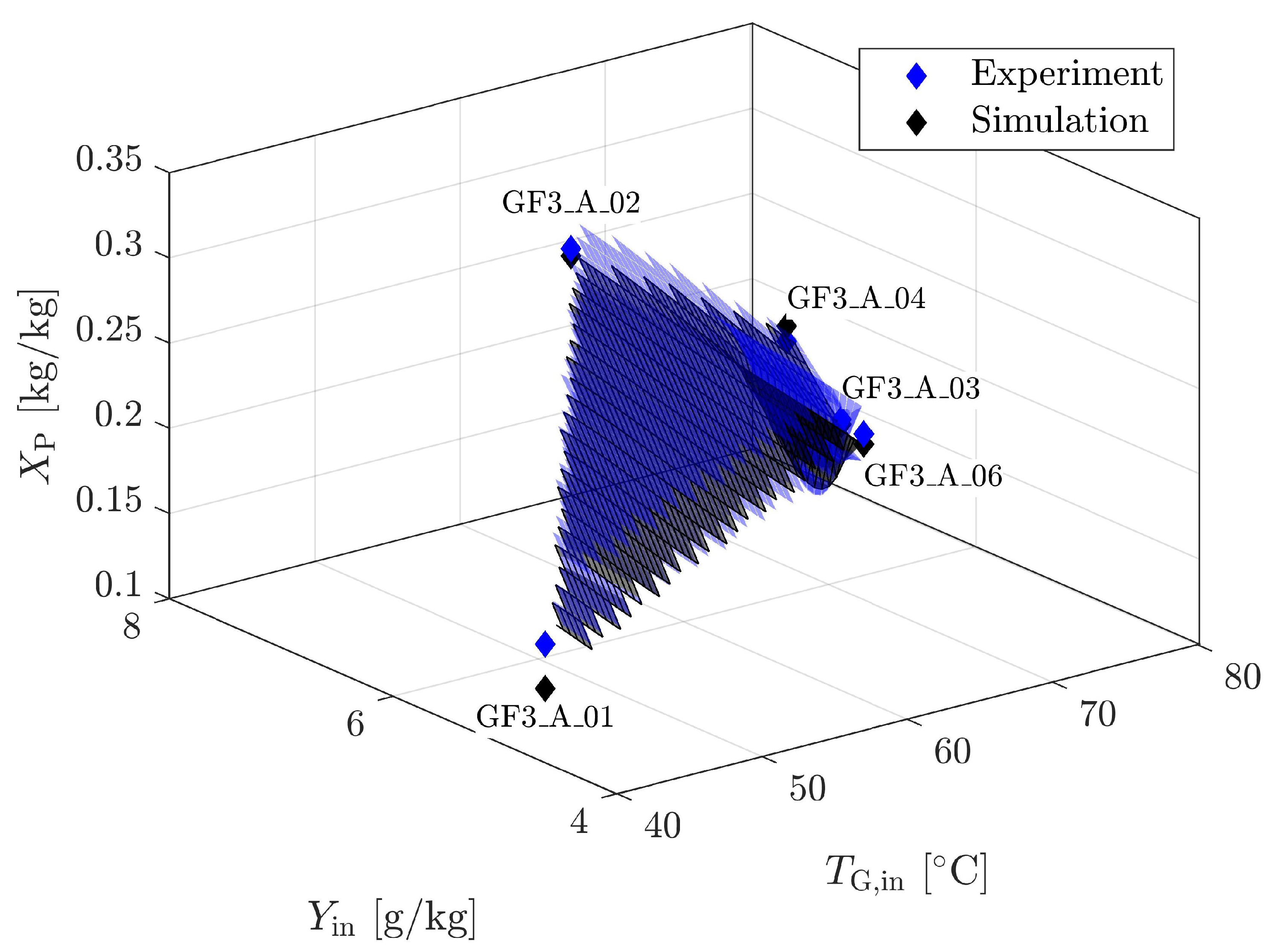

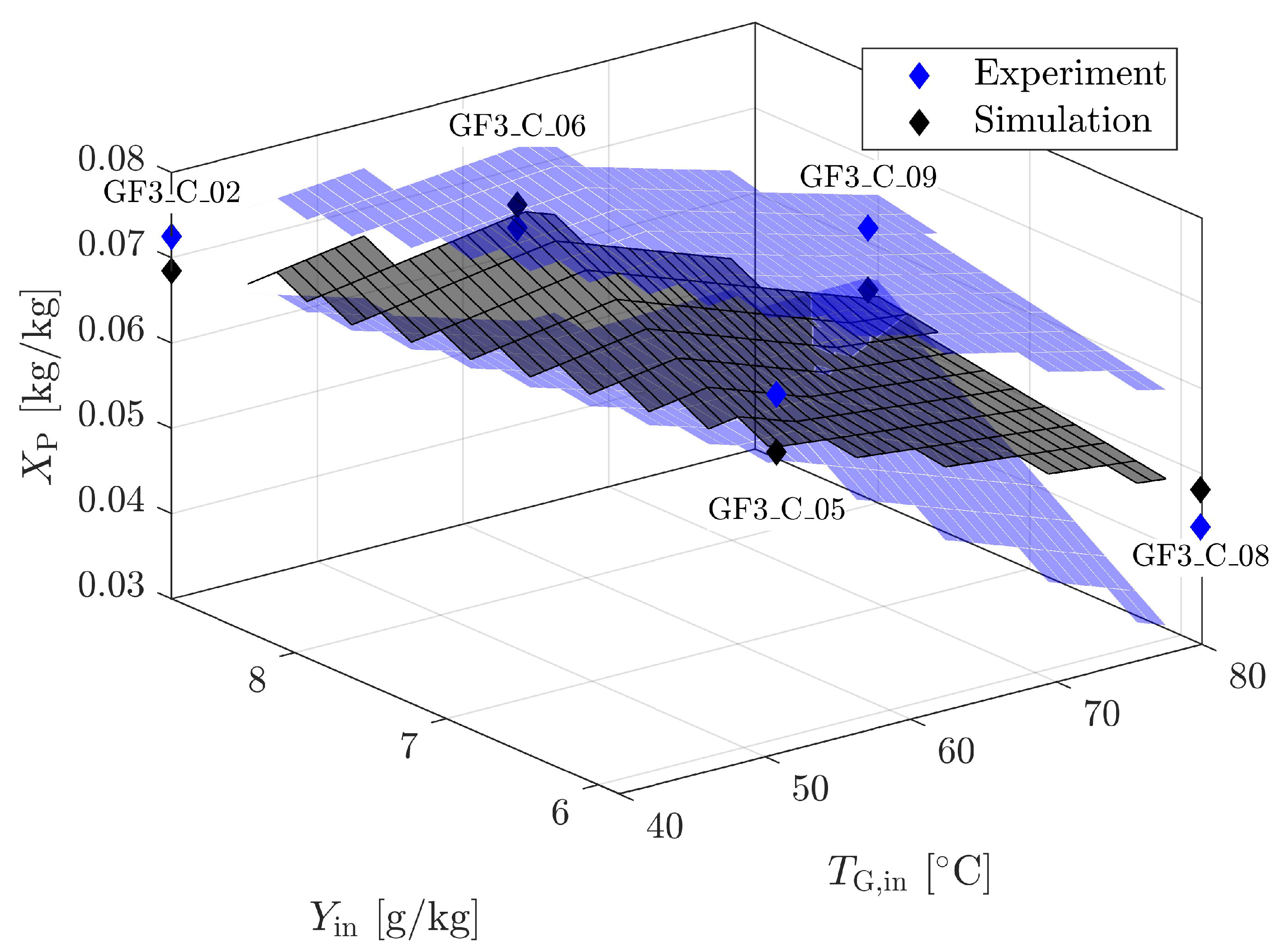
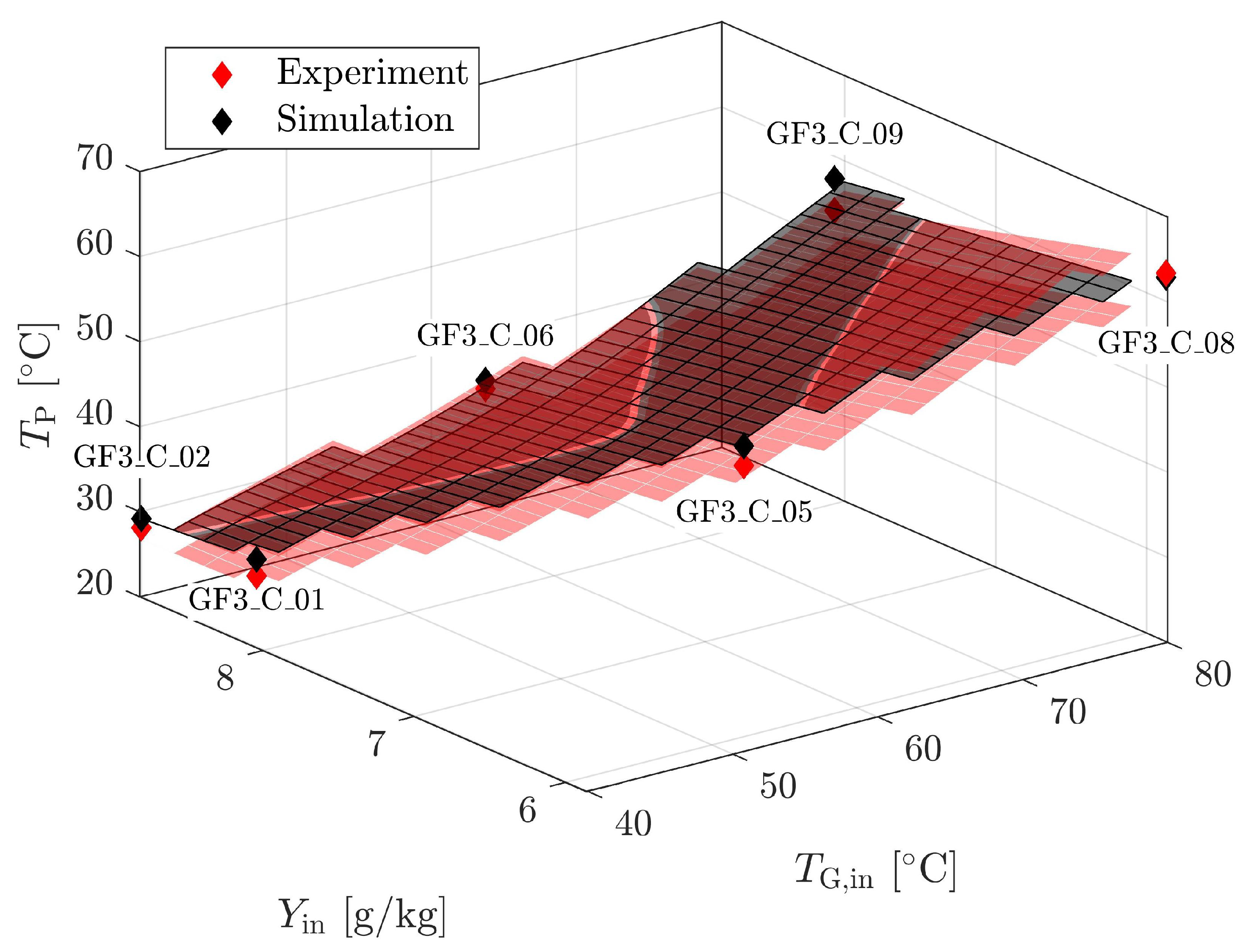


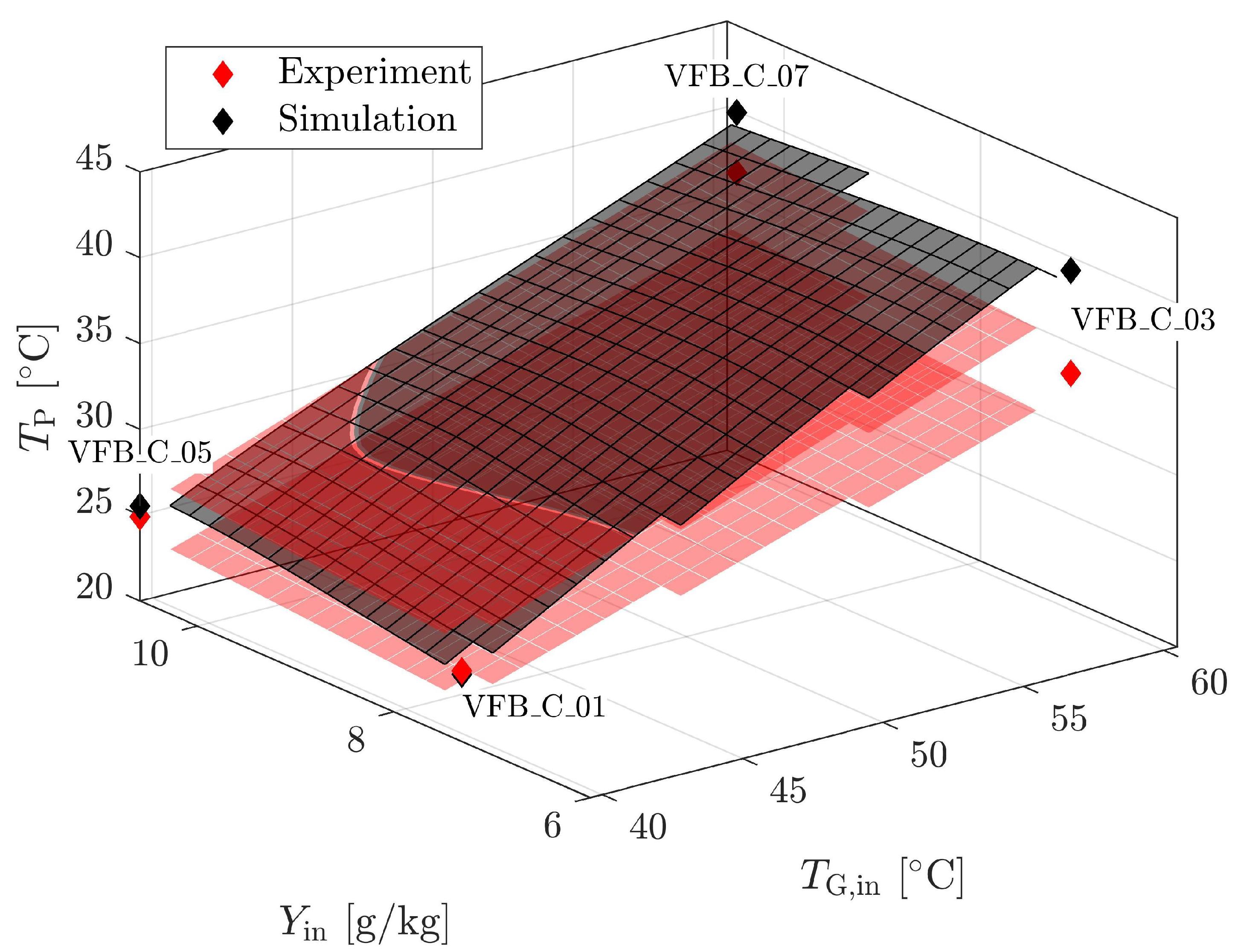

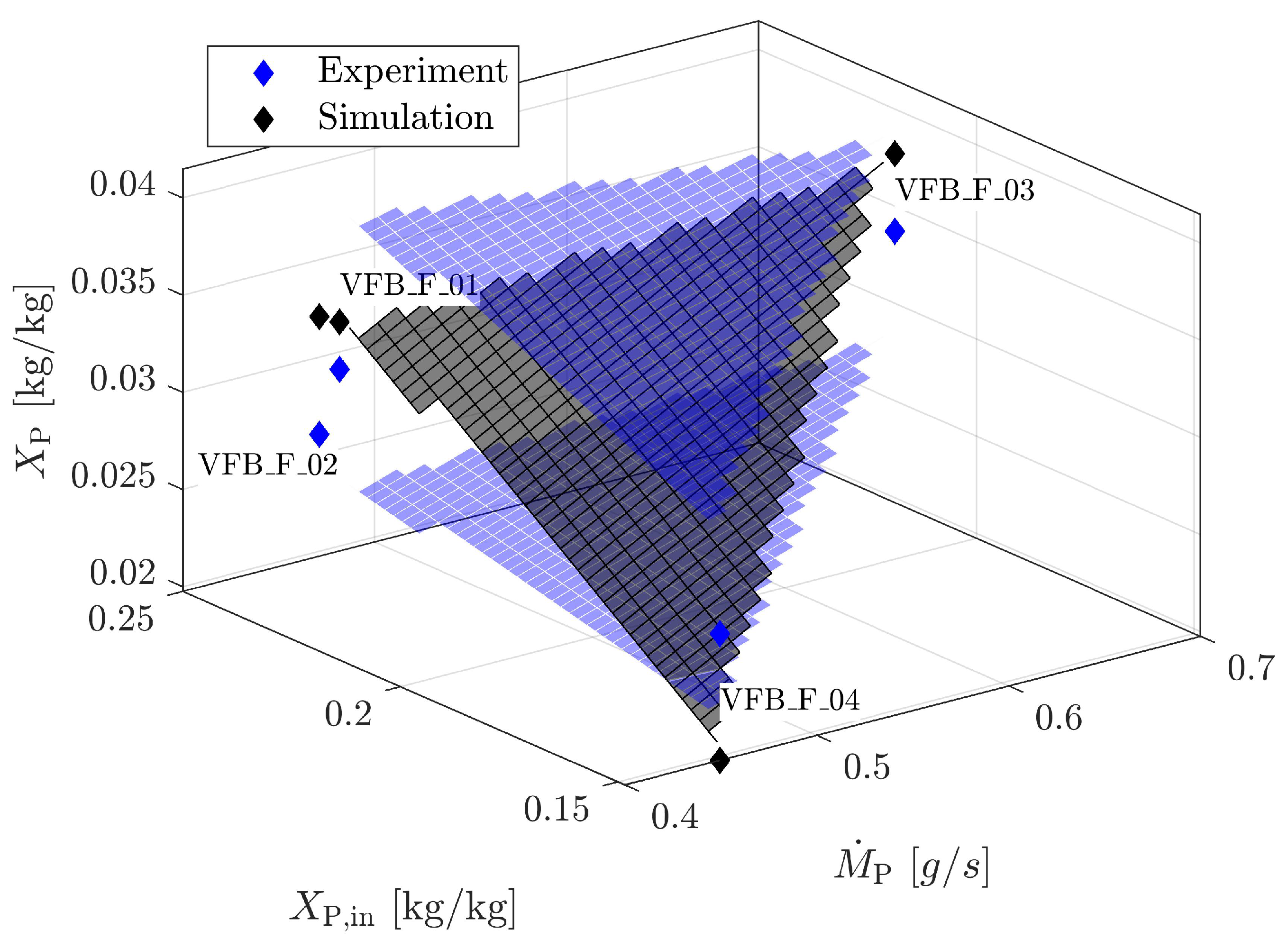
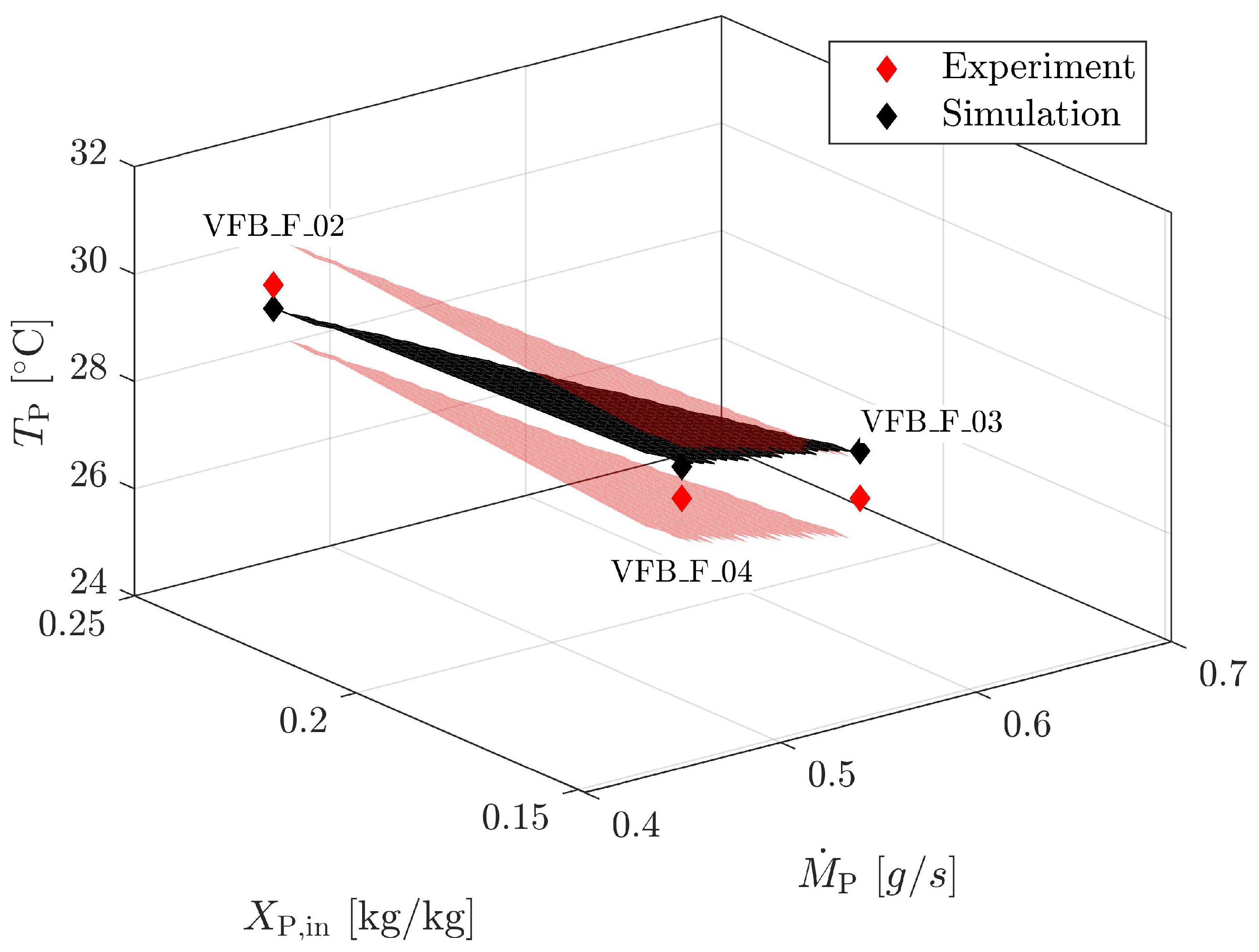


| Material | Skeletal Density [kg m] | Apparent Density [kg m] | Sauter Diameter [m] | Sphericity [m g] | Specific Surface Area [-] | Geldart Group [-] | * [m s] |
|---|---|---|---|---|---|---|---|
| - | 3261.2 | 1006.4 | 1701.4 | 0.97 | 206.3 | D | 0.51 |
| Cellets 500 | 1464.6 | 1113.6 | 631.8 | 0.85 | 63.6 | B | 0.2 |
| FCC catalyst | 2729.2 | 1632.5 | 41.6 | 0.863 | 93.9 | A | 0.015 |
| Exp. Name | Dryer | Material | [C] | [m s] | [g s] | [kg kg] | [cm] | [-] | * [g kg] | [C] |
|---|---|---|---|---|---|---|---|---|---|---|
| GF3_A_01 | GF3 | - | 40 | 1.3 | 1.5 | 0.33 | 12 | 0 | 4.64 | 20 |
| GF3_A_02 | GF3 | - | 60 | 1.3 | 2.0 | 0.50 | 12 | 0 | 6.98 | 21 |
| GF3_A_03 | GF3 | - | 80 | 1.3 | 1.6 | 0.49 | 12 | 0 | 7.19 | 24 |
| GF3_A_04 | GF3 | - | 80 | 1.3 | 1.6 | 0.54 | 12 | 0 | 7.79 | 25 |
| GF3_A_05 | GF3 | - | 80 | 1.3 | 1.9 | 0.45 | 12 | 0 | 10.13 | 21 |
| GF3_A_06 | GF3 | - | 80 | 1.3 | 1.0 | 0.59 | 7 | 0 | 7.00 | 23 |
| GF3_C_01 | GF3 | Cellets | 40 | 1.1 | 0.7 | 0.21 | 12 | 0 | 8.04 | 23 |
| GF3_C_02 | GF3 | Cellets | 40 | 1.1 | 0.7 | 0.24 | 12 | 0 | 8.81 | 22 |
| GF3_C_03 | GF3 | Cellets | 60 | 0.5 | 0.9 | 0.20 | 12 | 0 | 5.51 | 24 |
| GF3_C_04 | GF3 | Cellets | 60 | 0.5 | 1.2 | 0.23 | 12 | 0 | 10.28 | 22 |
| GF3_C_05 | GF3 | Cellets | 60 | 1.1 | 1.0 | 0.20 | 12 | 0 | 6.76 | 21 |
| GF3_C_06 | GF3 | Cellets | 60 | 1.1 | 1.1 | 0.26 | 12 | 0 | 8.45 | 22 |
| GF3_C_07 | GF3 | Cellets | 80 | 0.5 | 0.5 | 0.24 | 12 | 0 | 7.40 | 22 |
| GF3_C_08 | GF3 | Cellets | 80 | 1.1 | 0.9 | 0.24 | 12 | 0 | 5.86 | 22 |
| GF3_C_09 | GF3 | Cellets | 80 | 1.1 | 1.3 | 0.23 | 12 | 0 | 8.07 | 22 |
| VFB_C_01 | VFB | Cellets | 40 | 0.3 | 2.7 | 0.24 | 8 | 0.59 | 7.43 | 22 |
| VFB_C_02 | VFB | Cellets | 40 | 0.3 | 5.0 | 0.24 | 8 | 0 | 7.19 | 23 |
| VFB_C_03 | VFB | Cellets | 60 | 0.3 | 2.1 | 0.25 | 8 | 0 | 6.77 | 21 |
| VFB_C_04 | VFB | Cellets | 60 | 0.3 | 3.3 | 0.25 | 8 | 0 | 8.81 | 21 |
| VFB_C_05 | VFB | Cellets | 40 | 0.4 | 3.0 | 0.23 | 8 | 0.59 | 10.58 | 28 |
| VFB_C_06 | VFB | Cellets | 60 | 0.4 | 5.3 | 0.23 | 8 | 0 | 10.72 | 28 |
| VFB_C_07 | VFB | Cellets | 60 | 0.4 | 3.2 | 0.24 | 8 | 0.59 | 10.48 | 28 |
| VFB_F_01 | VFB | FCC | 60 | 0.07 | 0.44 | 0.23 | 16 | 0 | 1.06 | 18 |
| VFB_F_02 | VFB | FCC | 60 | 0.07 | 0.43 | 0.24 | 16 | 0 | 1.07 | 18 |
| VFB_F_03 | VFB | FCC | 60 | 0.07 | 0.66 | 0.20 | 16 | 0 | 1.02 | 17 |
| VFB_F_04 | VFB | FCC | 60 | 0.07 | 0.45 | 0.15 | 16 | 0 | 1.04 | 17 |
| Parameter | GF3_A_02 | GF3_C_03 |
|---|---|---|
| Anderson acceleration factor | 10 | 10 |
| Number of height classes | 100 | 100 |
| Number of residence time classes | 200 | 200 |
| Maximum residence time | 0.95 | 0.95 |
| Geldart group | D | B |
| [] | 0.53 | 0.2 |
| [] | 1.7 | 1.1 |
| [] | 2.05 | 1.13 |
| [] | 294 | 295 |
| [] | 0.50 | 0.26 |
| [] | 0.033 | 0.03 |
| [] | 333 | 333 |
| [] | 6.98 | 8.45 |
| [−] | 0 | 0 |
| [] | 0.05 | 0.05 |
| [] | n.a. | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmann, S.E.; Buchholz, M.; Jongsma, A.; Innings, F.; Heinrich, S. Modeling and Flowsheet Simulation of Vibrated Fluidized Bed Dryers. Processes 2021, 9, 52. https://doi.org/10.3390/pr9010052
Lehmann SE, Buchholz M, Jongsma A, Innings F, Heinrich S. Modeling and Flowsheet Simulation of Vibrated Fluidized Bed Dryers. Processes. 2021; 9(1):52. https://doi.org/10.3390/pr9010052
Chicago/Turabian StyleLehmann, Soeren E., Moritz Buchholz, Alfred Jongsma, Fredrik Innings, and Stefan Heinrich. 2021. "Modeling and Flowsheet Simulation of Vibrated Fluidized Bed Dryers" Processes 9, no. 1: 52. https://doi.org/10.3390/pr9010052
APA StyleLehmann, S. E., Buchholz, M., Jongsma, A., Innings, F., & Heinrich, S. (2021). Modeling and Flowsheet Simulation of Vibrated Fluidized Bed Dryers. Processes, 9(1), 52. https://doi.org/10.3390/pr9010052







