In Vitro Bioadsorption of Cd2+ Ions: Adsorption Isotherms, Mechanism, and an Insight to Mycoremediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Used
2.2. Sample Collection and Isolation of Fungi
2.3. Screening and Identification of Efficient Cd2+-Tolerant Fungal Isolate
2.4. Optimization of Batch Cultures
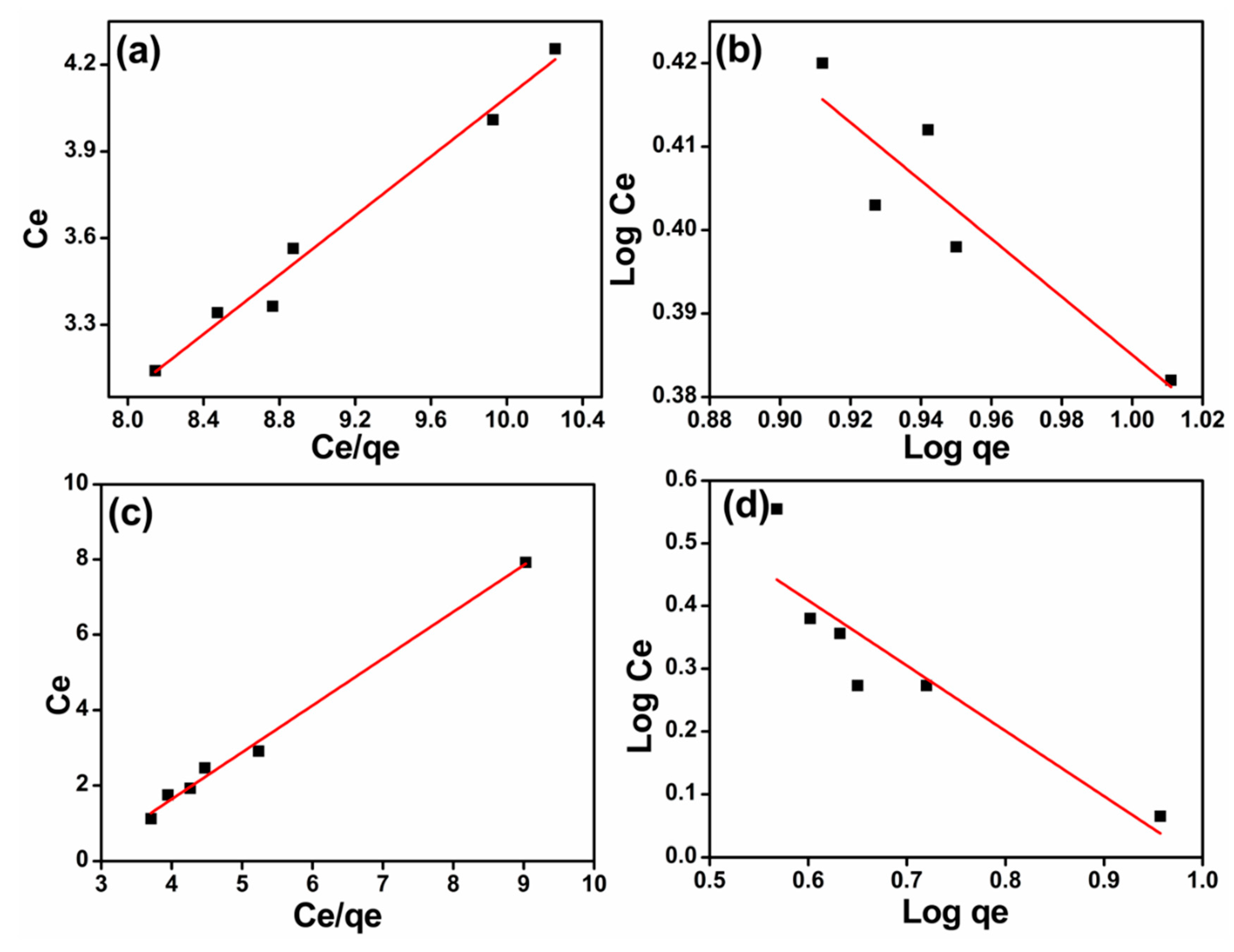
2.5. Equilibrium Isotherms
2.5.1. Langmuir Isotherm
2.5.2. Freundlich Isotherm
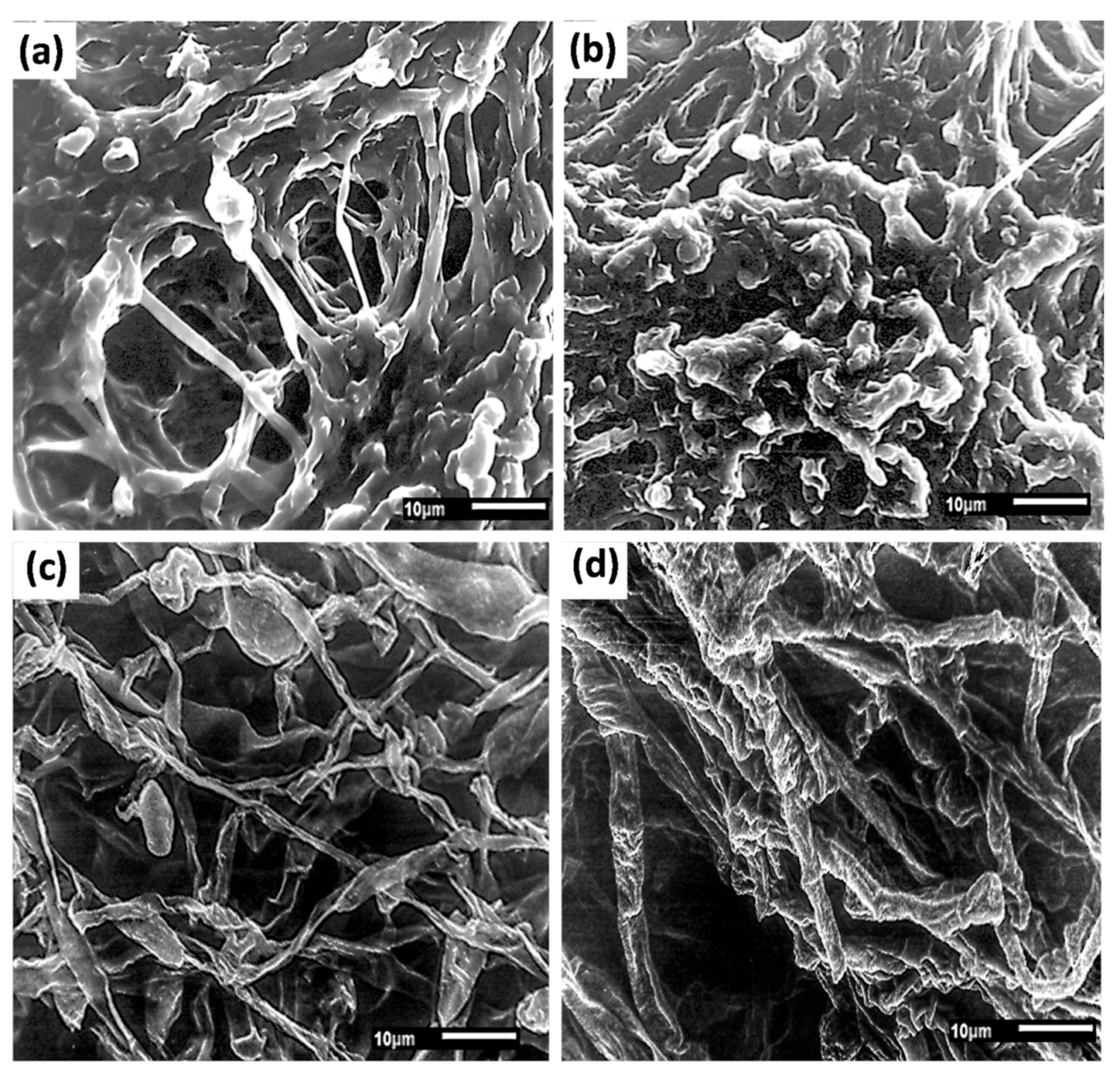
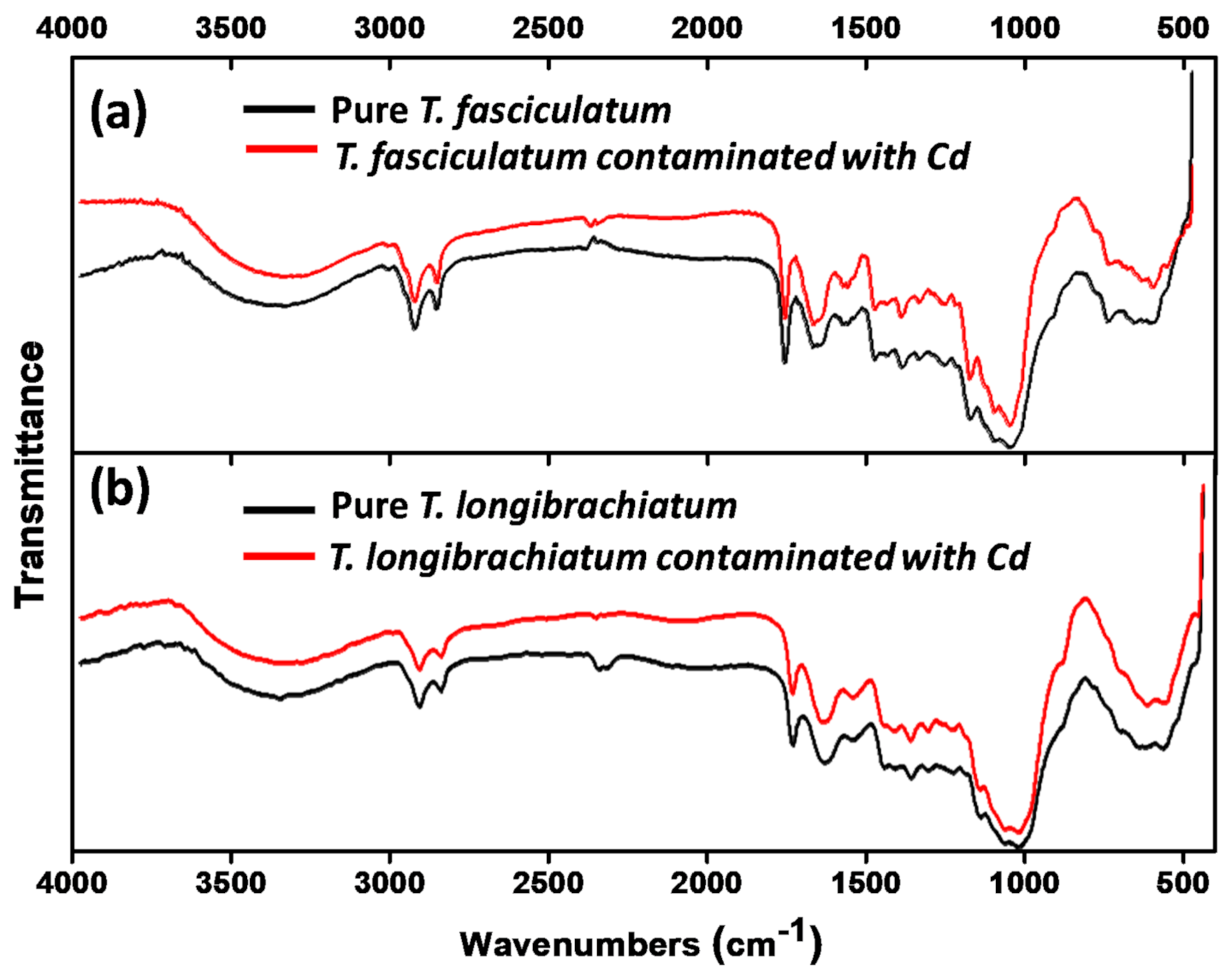
2.6. Scanning Electron Microscopy (SEM) and Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3. Results and Discussion
3.1. Isolation, Screening, and Identification of Cd2+-Resistant Fungal Biomass
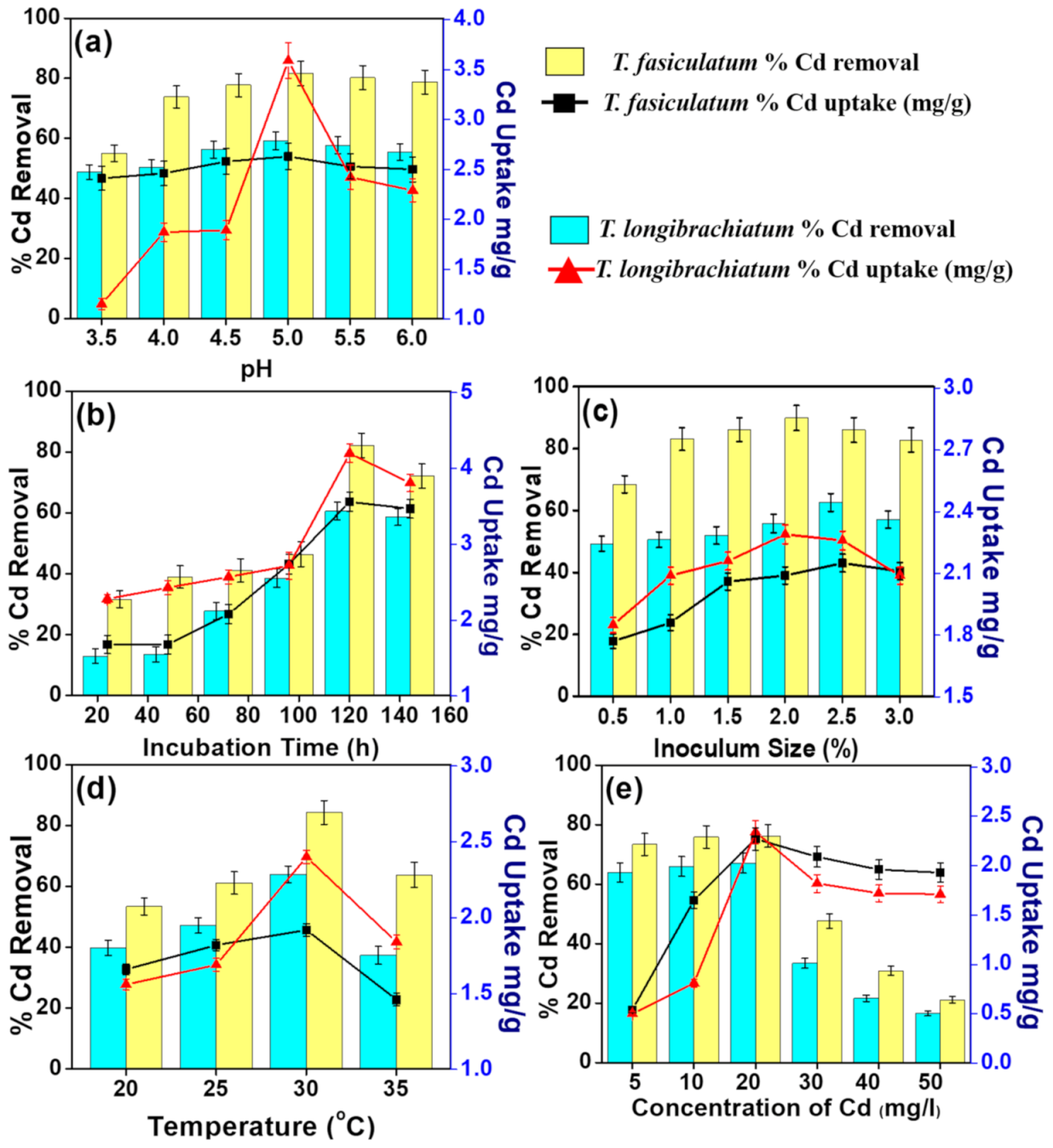
3.2. Optimization of Process Parameters
3.2.1. pH Effect
3.2.2. Incubation Time Effect
3.2.3. Inoculum Size Effect
3.2.4. Temperature Effect
3.2.5. Initial Metal Concentration Effect
3.3. Adsorption Isotherms
3.4. SEM and FTIR Analysis
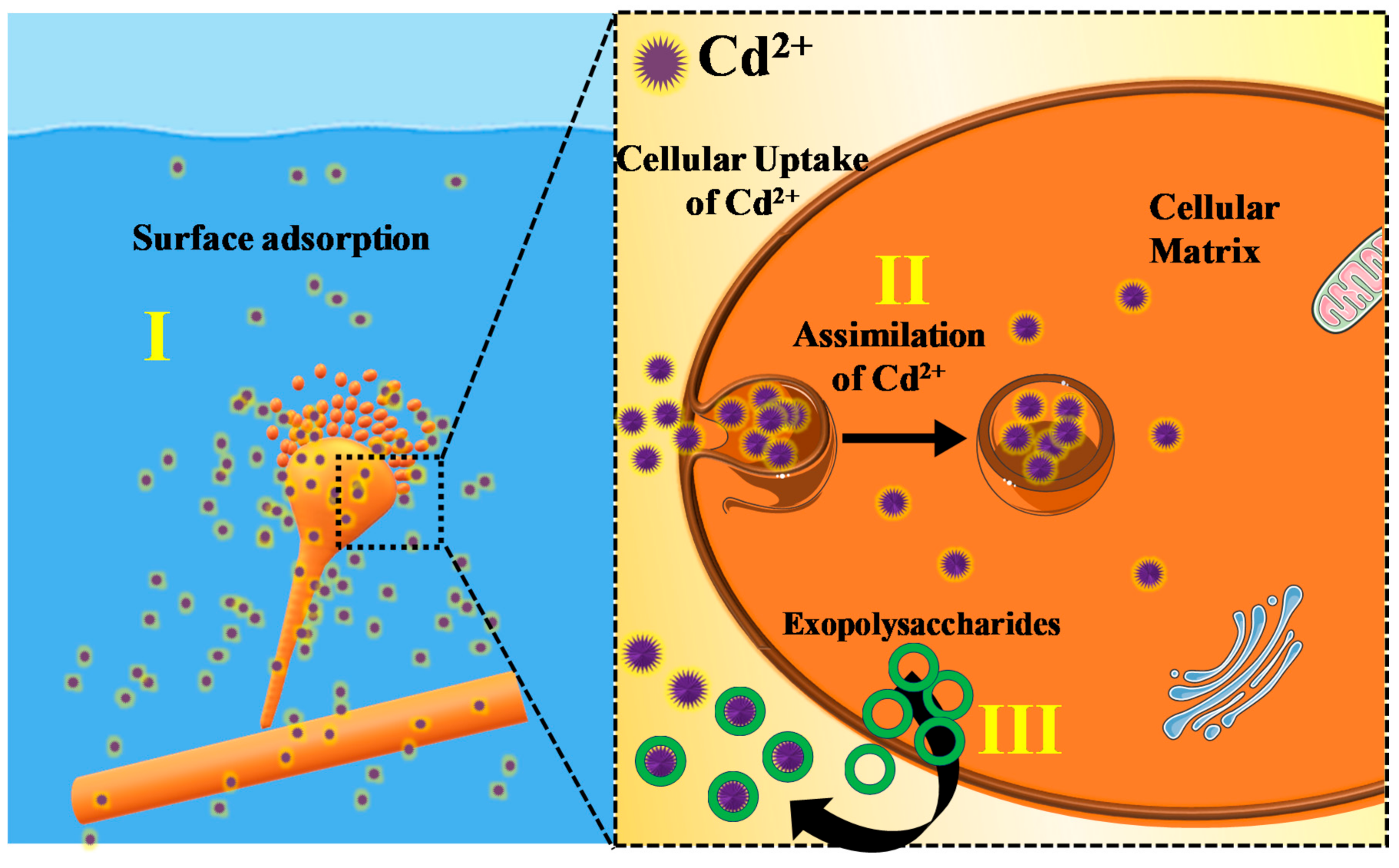
3.5. Proposed Mechanism for Cd2+ Removal
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, H.-Z.; Meng, L.-F.; Song, W.-G. Glutathione-Stabilized Silver Nanoparticles and Magnetic Nanoparticles Combination for Determination of Lead and Cadmium in Environmental Waters. Sci. Adv. Mater. 2019, 11, 1133–1139. [Google Scholar] [CrossRef]
- Hoang, H.-G.; Lin, C.; Tran, H.-T.; Chiang, C.-F.; Bui, X.-T.; Cheruiyot, N.K.; Shern, C.-C.; Lee, C.-W. Heavy metal contamination trends in surface water and sediments of a river in a highly-industrialized region. Environ. Technol. Innov. 2020, 20, 101043. [Google Scholar] [CrossRef]
- Song, T.; Li, R.; Li, N.; Gao, Y. Research Progress on the Application of Nanometer TiO2 Photoelectrocatalysis Technology in Wastewater Treatment. Sci. Adv. Mater. 2019, 11, 158–165. [Google Scholar] [CrossRef]
- Tan, K.; Ma, W.; Chen, L.; Wang, H.; Du, Q.; Du, P.; Yan, B.; Liu, R.; Li, H. Estimating the Distribution Trend of Soil Heavy Metals in Mining Area from HyMap Airborne Hyperspectral Imagery Based on Ensemble Learning. J. Hazard. Mater. 2020, 401, 123288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Y.; Wang, X.-R. Impact of industrial activities on heavy metal contamination in soils in three major urban agglomerations of China. J. Clean. Prod. 2019, 230, 1–10. [Google Scholar] [CrossRef]
- Yao, S.; Li, Q.; Kong, N.; Li, C.; Wei, M.; Song, R.; Ren, X.; Pu, X.; Li, W. Influence of Initial Cu/(Zn+Sn) Concentration Ratio in Cu–Zn–Sn–S Composites on Their Microstructures, Adsorption and Visible-Light-Sensitive Photocatalytic Activities. Sci. Adv. Mater. 2018, 10, 1381–1388. [Google Scholar] [CrossRef]
- Gulan, L.; Milenkovic, B.; Zeremski, T.; Milic, G.; Vuckovic, B. Persistent organic pollutants, heavy metals and radioactivity in the urban soil of Priština City, Kosovo and Metohija. Chemosphere 2017, 171, 415–426. [Google Scholar] [CrossRef]
- Hassen, A.; Saidi, N.; Cherif, M.; Boudabous, A. Effects of heavy metals on Pseudomonas aeruginosa and Bacillus thuringiensis. Bioresour. Technol. 1998, 65, 73–82. [Google Scholar] [CrossRef]
- Di Cesare, A.; Pjevac, P.; Eckert, E.; Curkov, N.; Šparica, M.M.; Corno, G.; Orlić, S.; Andrea, D.C.; Petra, P.; Ester, M.E.; et al. The role of metal contamination in shaping microbial communities in heavily polluted marine sediments. Environ. Pollut. 2020, 265, 114823. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elements Med. Boil. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Guo, J.; Xie, J.; Zhou, B.; Găman, M.-A.; Varkaneh, H.K.; Clark, C.C.; Salehi-Sahlabadi, A.; Li, Y.; Han, X.; Hao, Y.; et al. The influence of zinc supplementation on IGF-1 levels in humans: A systematic review and meta-analysis. J. King Saud Univ. Sci. 2020, 32, 1824–1830. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Piñón-Gimate, A.; Jakes-Cota, U.; Tripp-Valdez, A.; Casas-Valdez, M.; Almendarez-Hernández, L.C.; Alejandra, P.-G.; Ulianov, J.-C.; Arturo, T.-V.; Margarita, C.-V.; Cesar, A.-H.L. Assessment of human health risk: Copper and lead concentrations in Stone Scorpionfish (Scorpaena mystes) from the coastal region of Santa Rosalia in the Gulf of California, Mexico. Reg. Stud. Mar. Sci. 2020, 34, 101003. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, D.; Huang, M. Preparation and Characterization of Nano Hydroxyapatite and Its Adsorption Behavior toward Lead Ions. Sci. Adv. Mater. 2018, 10, 896–903. [Google Scholar] [CrossRef]
- Hosseini, S.; Alibakhshi, H.; Jashni, E.; Parvizian, F.; Shen, J.; Taheri, M.; Ebrahimi, M.; Rafiei, N. A novel layer-by-layer heterogeneous cation exchange membrane for heavy metal ions removal from water. J. Hazard. Mater. 2020, 381, 120884. [Google Scholar] [CrossRef]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Lv, H.; Mao, H.; Wang, D.; Zheng, Y.; Peng, C.; Hou, S. Synthesis, Characterization and Adsorption Behavior of Cd(II) Ion-Imprinted Mesoporous Materials. Sci. Adv. Mater. 2018, 10, 324–330. [Google Scholar] [CrossRef]
- Ya, V.; Martin, N.; Chou, Y.-H.; Chen, Y.; Choo, K.-H.; Chen, S.-S.; Li, C.-W. Electrochemical treatment for simultaneous removal of heavy metals and organics from surface finishing wastewater using sacrificial iron anode. J. Taiwan Inst. Chem. Eng. 2018, 83, 107–114. [Google Scholar] [CrossRef]
- Azarian, G.; Rahmani, A.R.; Khoram, M.M.; Atashzaban, Z.; Nematollahi, D. New batch electro-coagulation process for treatment and recovery of high organic load and low volume egg processing industry wastewater. Process. Saf. Environ. Prot. 2018, 119, 96–103. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Chien, S.-K.; Jhang, S.-R.; Lin, Y.-C. Chemical leaching, precipitation and solvent extraction for sequential separation of valuable metals in cathode material of spent lithium ion batteries. J. Taiwan Inst. Chem. Eng. 2019, 100, 151–159. [Google Scholar] [CrossRef]
- Jantunen, N.; Virolainen, S.; Latostenmaa, P.; Salminen, J.; Haapalainen, M.; Sainio, T. Removal and recovery of arsenic from concentrated sulfuric acid by solvent extraction. Hydrometallurgy 2019, 187, 101–112. [Google Scholar] [CrossRef]
- Xia, Y.; Tsai, F.-C.; Ma, N.; Zhang, K.-D.; Liu, H.-L.; Jiang, T.; Chiang, T.-C. Green Synthesis of H3O40PW12@MIL-100(Fe): Enhanced and Selective Adsorption of Methylene Blue from Aqueous Solution. Sci. Adv. Mater. 2018, 10, 172–180. [Google Scholar] [CrossRef]
- Hao, J.; Ji, L.; Li, C.; Hu, C.; Wu, K. Rapid, efficient and economic removal of organic dyes and heavy metals from wastewater by zinc-induced in-situ reduction and precipitation of graphene oxide. J. Taiwan Inst. Chem. Eng. 2018, 88, 137–145. [Google Scholar] [CrossRef]
- Kim, T.; Kim, T.-K.; Zoh, K.-D. Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J. Water Process. Eng. 2020, 33, 101109. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Y.; Kang, D.; Deng, L.; Li, C. Oil-Tea Shell Derived N-Doped Porous Carbon for Selective Separation of CO2, CH4, and N2. Sci. Adv. Mater. 2019, 11, 1146–1155. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C. Efficient removal of heavy metal ions based on the optimized dissolution-diffusion-flow forward osmosis process. Chem. Eng. J. 2018, 334, 1128–1134. [Google Scholar] [CrossRef]
- Sadeghi, M.H.; Tofighy, M.A.; Mohammadi, T. One-dimensional graphene for efficient aqueous heavy metal adsorption: Rapid removal of arsenic and mercury ions by graphene oxide nanoribbons (GONRs). Chemosphere 2020, 253, 126647. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Wu, W.; Usman, M.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Adsorptive removal of heavy metal ions using polystyrene-poly(N-isopropylmethacrylamide-acrylic acid) core/shell gel particles: Adsorption isotherms and kinetic study. J. Mol. Liq. 2019, 277, 522–531. [Google Scholar] [CrossRef]
- Gola, D.; Dey, P.; Bhattacharya, A.; Mishra, A.; Malik, A.; Namburath, M.; Ahammad, S.Z. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour. Technol. 2016, 218, 388–396. [Google Scholar] [CrossRef]
- Talukdar, D.; Jasrotia, T.; Sharma, R.; Jaglan, S.; Kumar, R.; Vats, R.; Kumar, R.; Mahnashi, M.H.; Umar, A. Evaluation of novel indigenous fungal consortium for enhanced bioremediation of heavy metals from contaminated sites. Environ. Technol. Innov. 2020, 20, 101050. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. Characteristics, performances, equilibrium and kinetic modeling aspects of heavy metal removal using algae. Bioresour. Technol. Rep. 2019, 5, 261–279. [Google Scholar] [CrossRef]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-Q.; Zhao, Y.; Zhao, X.; Gao, X.; Zheng, Y.; Zuo, H.; Wei, Z. Roles of different humin and heavy-metal resistant bacteria from composting on heavy metal removal. Bioresour. Technol. 2019, 296, 122375. [Google Scholar] [CrossRef]
- Bano, A.; Hussain, J.; Akbar, A.; Mehmood, K.; Anwar, M.; Hasni, M.S.; Ullah, S.; Sajid, S.; Ali, I. Biosorption of heavy metals by obligate halophilic fungi. Chemosphere 2018, 199, 218–222. [Google Scholar] [CrossRef]
- El Hameed, A.H.A.; Eweda, W.E.; Abou-Taleb, K.A.; Mira, H. Biosorption of uranium and heavy metals using some local fungi isolated from phosphatic fertilizers. Ann. Agric. Sci. 2015, 60, 345–351. [Google Scholar] [CrossRef]
- Visoottiviseth, P.; Panviroj, N. Selection of fungi capable of removing toxic arsenic compounds from liquid medium. Sci. Asia 2011, 27, 83–92. [Google Scholar] [CrossRef]
- Say, R.; Yılmaz, N.; Denizli, A. Biosorption of Cadmium, Lead, Mercury, and Arsenic Ions by the Fungus Penicillium purpurogenum. Sep. Sci. Technol. 2003, 38, 2039–2053. [Google Scholar] [CrossRef]
- Goksungur, Y. Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour. Technol. 2005, 96, 103–109. [Google Scholar] [CrossRef]
- Dönmez, G.; Aksu, Z. Bioaccumulation of copper(II) and nickel(II) by the non-adapted and adapted growing Candida SP. Water Res. 2001, 35, 1425–1434. [Google Scholar] [CrossRef]
- Prakasham, R.; Merrie, J.; Sheela, R.; Saswathi, N.; Ramakrishna, S. Biosorption of chromium VI by free and immobilized Rhizopus arrhizus. Environ. Pollut. 1999, 104, 421–427. [Google Scholar] [CrossRef]
- Kumar, R.; Negi, S.; Sharma, P.; Prasher, I.; Chaudhary, S.; Dhau, J.S.; Umar, A. Wastewater cleanup using Phlebia acerina fungi: An insight into mycoremediation. J. Environ. Manag. 2018, 228, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sharma, P.; Kaur, A.; Kumar, R.; Mehta, S.K. Surfactant Coated Silica Nanoparticles as Smart Scavengers for Adsorptive Removal of Naphthalene. J. Nanosci. Nanotechnol. 2018, 18, 3218–3229. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sharma, P.; Singh, D.; Umar, A.; Kumar, R. Chemical and Pathogenic Cleanup of Wastewater Using Surface-Functionalized CeO2 Nanoparticles. ACS Sustain. Chem. Eng. 2017, 5, 6803–6816. [Google Scholar] [CrossRef]
- Sharma, Y. Cr(VI) removal from industrial effluents by adsorption on an indigenous low-cost material. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 215, 155–162. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Duvnjak, Z. Adsorption of copper and chromium by Aspergillus carbonarius. Biotechnol. Prog. 1995, 11, 638–642. [Google Scholar] [CrossRef]
- Juang, R.-S.; Wu, F.-C.; Tseng, R.-L. Adsorption Isotherms of Phenolic Compounds from Aqueous Solutions onto Activated Carbon Fibers. J. Chem. Eng. Data 1996, 41, 487–492. [Google Scholar] [CrossRef]
- Manna, A.; Sundaram, E.; Amutha, C.; Vasantha, V.S. Efficient Removal of Cadmium Using Edible Fungus and Its Quantitative Fluorimetric Estimation Using (Z)-2-(4H-1,2,4-Triazol-4-yl)iminomethylphenol. ACS Omega 2018, 3, 6243–6250. [Google Scholar] [CrossRef]
- Naumann, A.; Navarro-González, M.; Peddireddi, S.; Kües, U.; Polle, A. Fourier transform infrared microscopy and imaging: Detection of fungi in wood. Fungal Genet. Biol. 2005, 42, 829–835. [Google Scholar] [CrossRef]
- Jilkine, K.; Gough, K.M.; Julian, R.; Kaminskyj, S.G. A sensitive method for examining whole-cell biochemical composition in single cells of filamentous fungi using synchrotron FTIR spectromicroscopy. J. Inorg. Biochem. 2008, 102, 540–546. [Google Scholar] [CrossRef]
- Akar, T.; Tunali, S. Biosorption performance of Botrytis cinerea fungal by-products for removal of Cd(II) and Cu(II) ions from aqueous solutions. Miner. Eng. 2005, 18, 1099–1109. [Google Scholar] [CrossRef]
- Morley, G.F.; Gadd, G.M. Sorption of toxic metals by fungi and clay minerals. Mycol. Res. 1995, 99, 1429–1438. [Google Scholar] [CrossRef]
- Krämer, U.; Talke, I.N.; Hanikenne, M.; Krämer, U. Transition metal transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
| Sr. No. | Fungal Isolate Code | Control | Cd2+ Ion Concentration (mg/L) | ||
|---|---|---|---|---|---|
| 25 | 50 | 75 | |||
| 1 | FS1 | ++++ | ++ | ++ | + |
| 2 | FS2 | ++++ | ++ | ++ | + |
| 3 | FS3 | ++++ | ++ | ++ | + |
| 4 | FS4 | ++++ | ++++ | +++ | ++ |
| 5 | FS5 | ++++ | ++ | ++ | + |
| 6 | FS6 | ++++ | ++ | ++ | + |
| 7 | FS7 | ++++ | ++++ | ++++ | +++ |
| 8 | FS8 | ++++ | − | − | − |
| 9 | FS9 | ++++ | ++++ | +++ | ++ |
| 10 | FS10 | ++++ | ++++ | +++ | ++ |
| 11 | FS11 | ++++ | − | − | − |
| 12 | FS12 | ++++ | ++++ | +++ | +++ |
| 13 | FS13 | ++++ | − | − | − |
| 14 | FS14 | ++++ | ++ | + | − |
| 15 | FS15 | ++++ | − | − | − |
| 16 | FS16 | ++++ | ++++ | ++++ | +++ |
| 17 | FS17 | ++++ | + | − | − |
| 18 | FS18 | ++++ | + | − | − |
| 19 | FS19 | ++++ | - | − | - |
| 20 | FS20 | ++++ | +++ | ++ | + |
| 21 | FS21 | ++++ | +++ | ++ | + |
| 22 | FS22 | ++++ | ++++ | +++ | ++ |
| 23 | FS23 | ++++ | +++ | ++ | + |
| 24 | FS24 | ++++ | − | − | − |
| 25 | FS25 | ++++ | ++ | + | − |
| Isotherms | Parameters | T. fasciculatum | T. longibrachiatum |
|---|---|---|---|
| Langmuir | qmax (mg/g) | 1.90 | 0.80 |
| b (L/mg) | −2.23 | −2.75 | |
| R2 | 0.99 | 0.99 | |
| Freundlich | Kf (L/g) | −0.15 | 0.02 |
| n | −3.11 | −0.95 | |
| R2 | 0.84 | 0.84 |
| Sr. no. | T. fasciculatum | T. longibrachiatum | Assigned Groups | ||
|---|---|---|---|---|---|
| Control | Cd2+ Addition | Control | Cd2+ Addition | ||
| 1 | 3332.6 | 3320.1 | 3363.5 | 3325.2 | -OH, -NH |
| 2 | 2923.6 | 2924.3 | 2923.3 | 2923.3 | -CH, -OH |
| 3 | 2853.3 | 2853.2 | 2854.6 | 2854.9 | -CH |
| 4 | 1745.4 | 1746.0 | 1744.9 | 1745.1 | -C=O, of ester group |
| 5 | 1654.8 | 1654.3 | 1645.5 | 1648.1 | -C=O, COO |
| 6 | 1560.2 | 1563.0 | 1554.5 | 1558.8 | -NH |
| 7 | 1373.8 | 1375.6 | 1372.3 | 1373.8 | -CH |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Sharma, P.; Umar, A.; Kumar, R.; Singh, N.; Joshi, P.K.; A. Alharthi, F.; Ali Alghamdi, A.; Al-Zaqri, N. In Vitro Bioadsorption of Cd2+ Ions: Adsorption Isotherms, Mechanism, and an Insight to Mycoremediation. Processes 2020, 8, 1085. https://doi.org/10.3390/pr8091085
Kumar R, Sharma P, Umar A, Kumar R, Singh N, Joshi PK, A. Alharthi F, Ali Alghamdi A, Al-Zaqri N. In Vitro Bioadsorption of Cd2+ Ions: Adsorption Isotherms, Mechanism, and an Insight to Mycoremediation. Processes. 2020; 8(9):1085. https://doi.org/10.3390/pr8091085
Chicago/Turabian StyleKumar, Raman, Priyanka Sharma, Ahmad Umar, Rajeev Kumar, Namita Singh, P. K. Joshi, Fahad A. Alharthi, Abdulaziz Ali Alghamdi, and Nabil Al-Zaqri. 2020. "In Vitro Bioadsorption of Cd2+ Ions: Adsorption Isotherms, Mechanism, and an Insight to Mycoremediation" Processes 8, no. 9: 1085. https://doi.org/10.3390/pr8091085
APA StyleKumar, R., Sharma, P., Umar, A., Kumar, R., Singh, N., Joshi, P. K., A. Alharthi, F., Ali Alghamdi, A., & Al-Zaqri, N. (2020). In Vitro Bioadsorption of Cd2+ Ions: Adsorption Isotherms, Mechanism, and an Insight to Mycoremediation. Processes, 8(9), 1085. https://doi.org/10.3390/pr8091085







