Cu(II) and As(V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan
Abstract
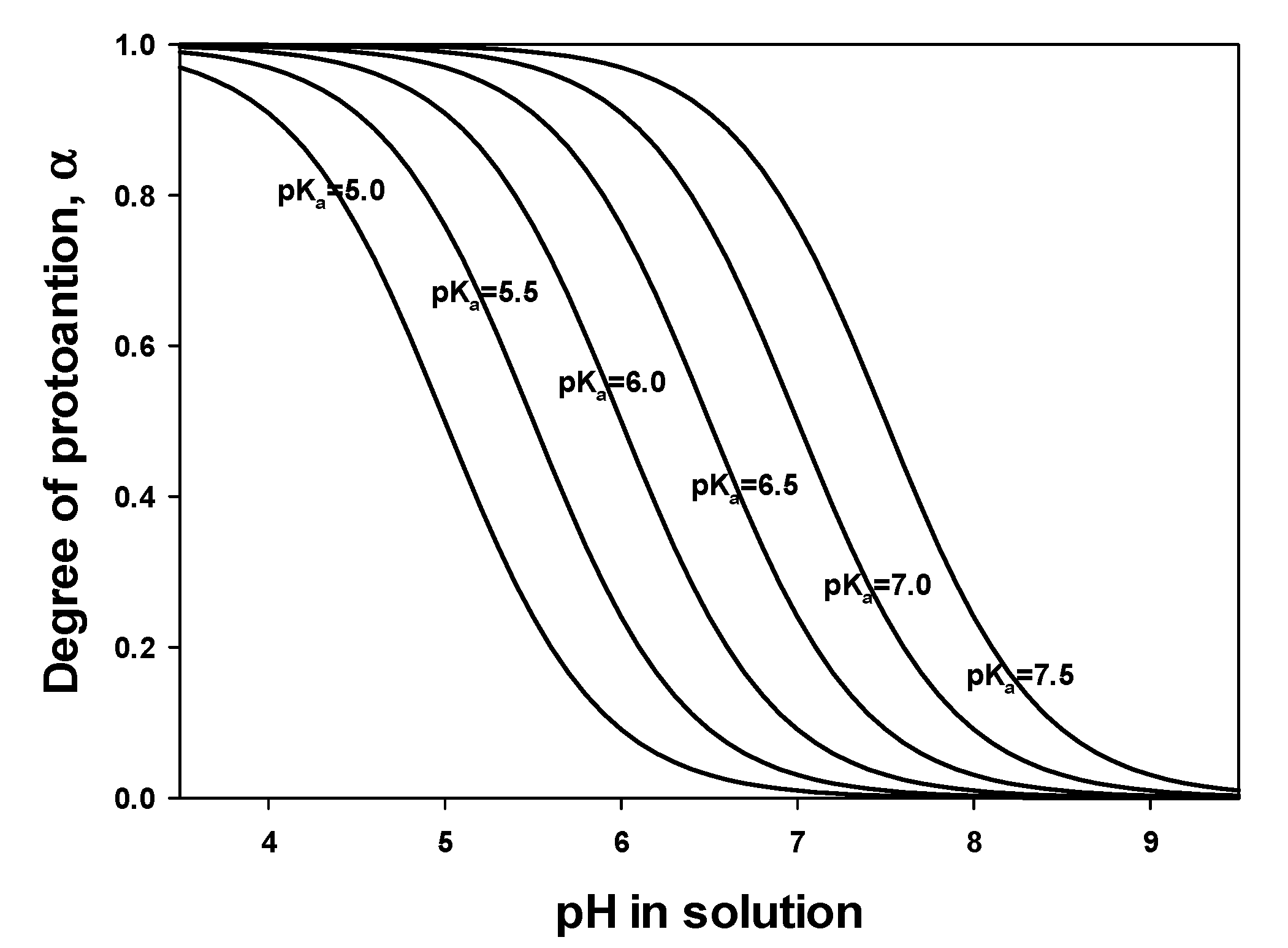
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Preparation of Chitosan Beads
2.3. Batch Adsorption Tests
2.4. Adsorption Reaction Model
2.4.1. Pseudo First-Order Model
2.4.2. Pseudo Second-Order Model
2.5. Adsorption Diffusion Model
2.5.1. Intraparticle Diffusion
2.5.2. External Mass Transfer
3. Results and Discussion
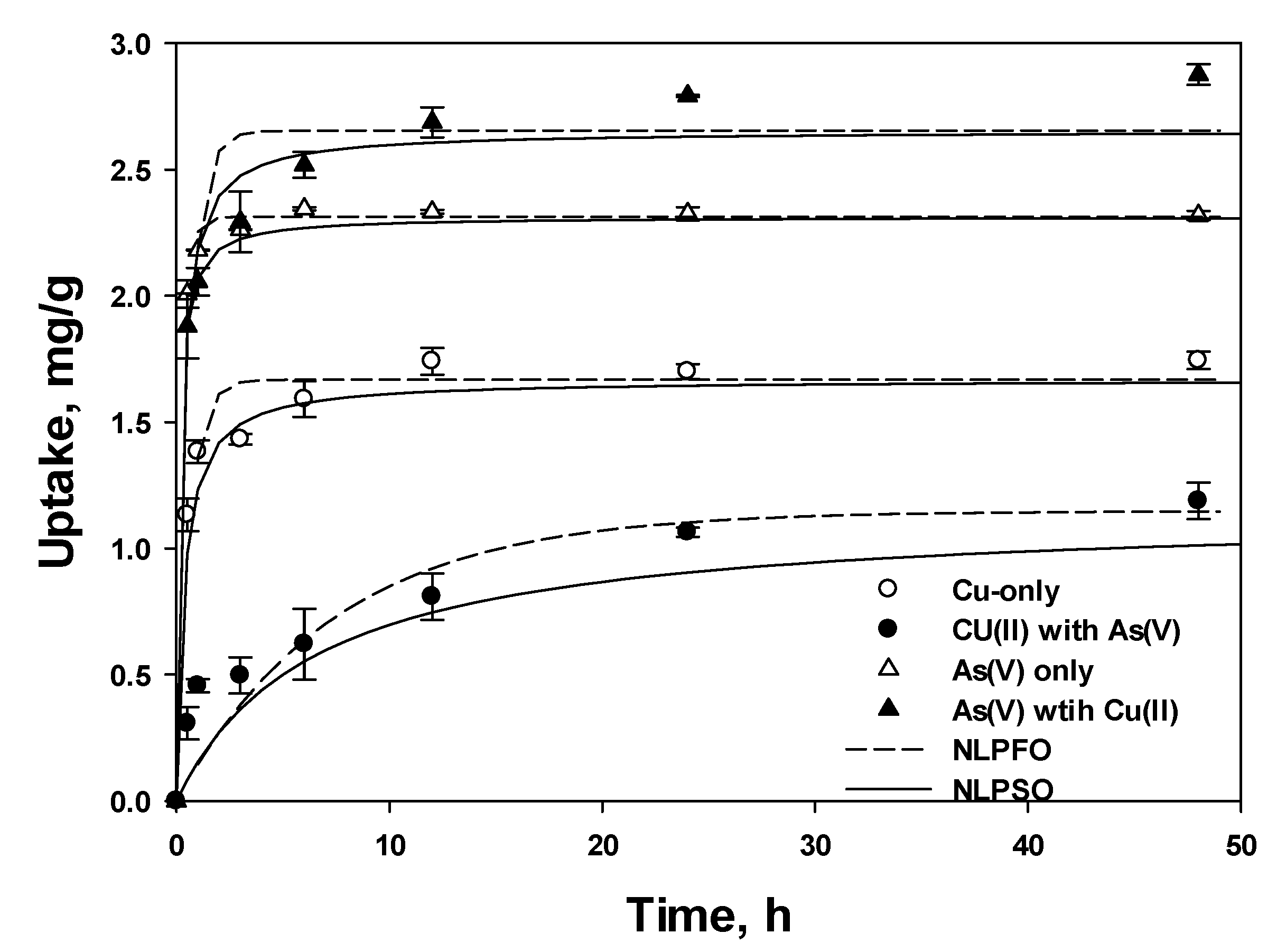
3.1. Batch Test
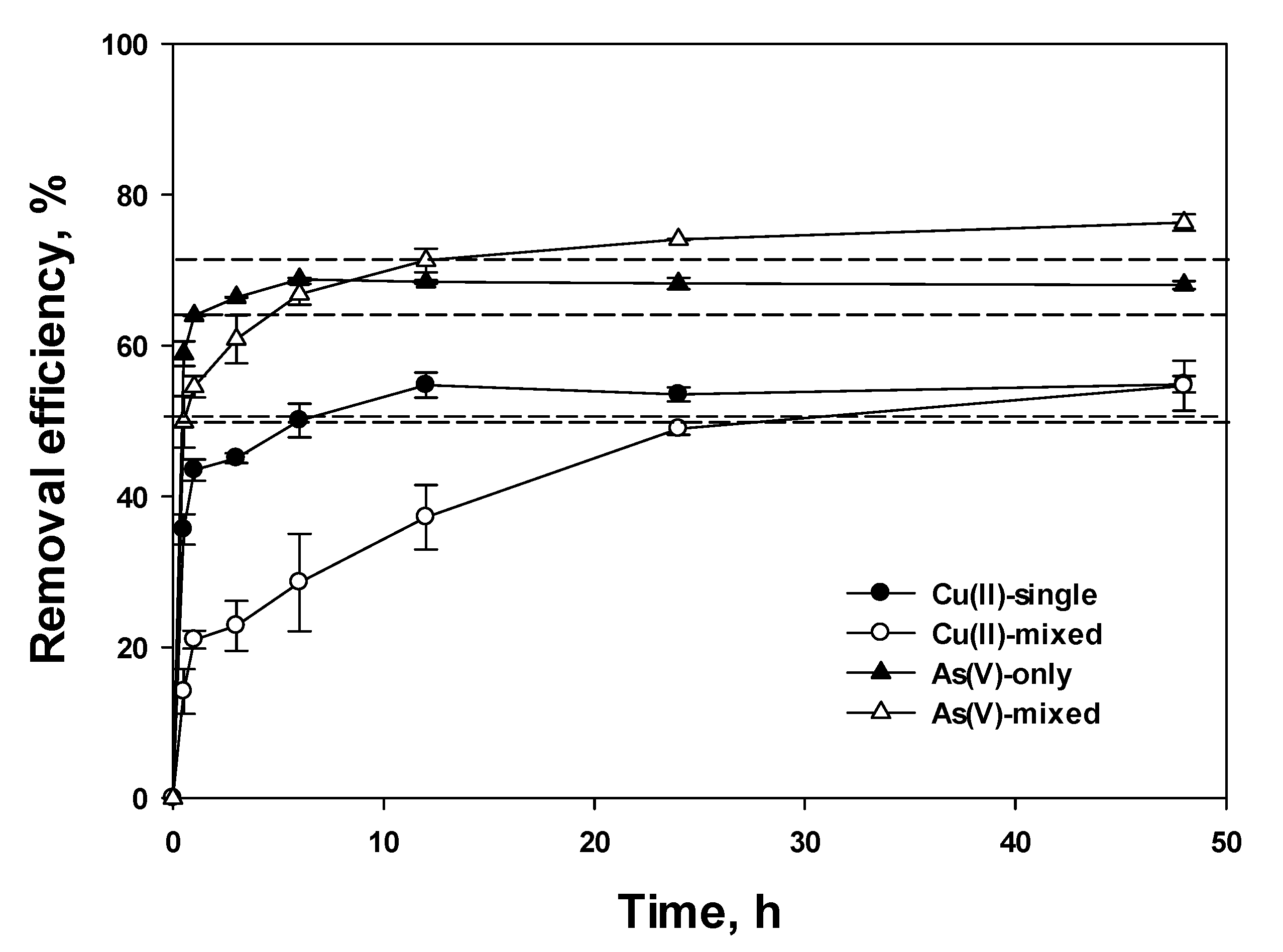
3.2. Removal Efficiency
3.3. Adsorption Reaction Kinetics
3.3.1. Nonlinear Pseudo First-Order (PFO) and Second-Order (PSO) Models
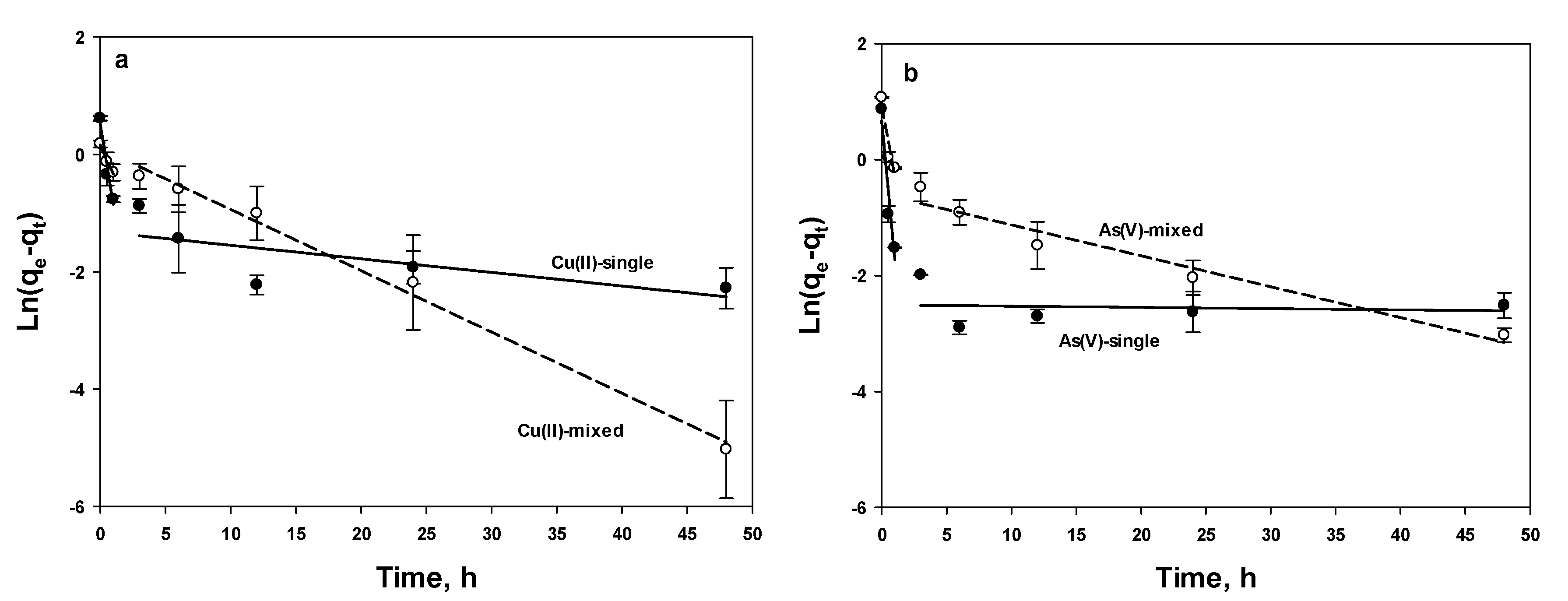
3.3.2. Linear PFO and PSO
3.4. Diffusion Model
3.4.1. Intraparticle Diffusion
3.4.2. External Mass Transfer
4. Conclusions
Funding
Conflicts of Interest
Nomenclature
| A | Surface area of adsorbent (m2) |
| C0 | Initial concentration in the solution, mg/L |
| Ct | Liquid phase concentration at any time, mg/L |
| K1 | Sorption rate constant (min−1) at PFO |
| K2 | Sorption rate constant (g/mg∙min) at PSO |
| Ki | Intraparticle diffusion rate constant (mg/g∙ min1/2) |
| Kn | n order diffusion rate constant |
| Ks | The liquid-film mass transfer coefficient (m2/s) |
| M | Mass of adsorbent (g) |
| n | Kinetic order |
| qe | Adsorbate uptake at equilibrium (mg/g) |
| qt | Adsorbate uptake at any time (mg/g) |
| t | Reaction time (min) |
| V | Volume of batch experiment (L) |
References
- An, B.; Son, H.; Chung, J.; Choi, J.W.; Lee, S.H.; Hong, S. Calcium and hydrogen effects during sorption of copper onto an alginate-based ion exchanger: Batch and fixed-bed column studies. Chem. Eng. J. 2013, 232, 51–58. [Google Scholar] [CrossRef]
- Sessarego, S.; Rodrigues, S.C.G.; Xiao, Y.; Lu, Q.; Hill, J.M. Phosphonium-enhanced chitosan for Cr(VI) adsorption in wastewater treatment. Carbohydr. Polym. 2019, 211, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Morisi, R.E.; Alsabagh, A.M.; Nasr, S.; Zaki, M.M. Multifunctional nanocomposites of chitosan, silver nanoparticles, copper nanoparticles and carbon nanotubes for water treatment: Antimicrobial characteristics. Int. J. Biol. Macromol. 2017, 97, 264–269. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Ionic conductivity of chitosan membranes. Polymer 2003, 44, 1057–1065. [Google Scholar] [CrossRef]
- Philippova, O.E.; Korchagina, E.V. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polym. ScI. Ser. A 2012, 54, 552–572. [Google Scholar] [CrossRef]
- Wang, Z.; Nie, J.; Qin, W.; Hu, Q.; Tang, B.Z. Gelation process visualized by aggregation-induced emission fluorogens. Nat. Commun. 2016, 7, 12033. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Endud, C.S.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Kwok, K.C.M.; Koong, L.F.; Al Ansari, T.; Mckay, G. Adsorption/desorption of arsenite and arsenate on chitosan and nanochitosan. Environ. Sci. Pollut. Res. 2018, 25, 14734–14742. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Zhaong, Y.; Yu, Z.; Qi, C. Preparation of a novel chitosan based bioplymer dye and application in wood dyeing. Polymers 2016, 8, 338. [Google Scholar] [CrossRef]
- Mohamed, M.; Ouki, S. Removal mechanism of toluene from aqueous solutions by chitin and chitosan. Ind. Eng. Chem. Res. 2011, 50, 9557. [Google Scholar] [CrossRef]
- An, B.; Jung, K.Y.; Zhao, D.; Lee, S.H.; Choi, J.W. Preparation and characterization of polymeric ligand exchanger based on chitosan hydrogel for selective removal phosphate. React. Funct. Polym. 2014, 85, 45–53. [Google Scholar] [CrossRef]
- An, B.; Choi, J.W. An experimental application of four types of chitosan bead for removal of cationic and anionic pollutants. Water Air Soil pollut. 2019, 230, 314. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, W.; Guo, Q.; Jin, L.; Liua, Z.; Hu, S. On-site separation of Cr(VI) and Cr(III) in natural waters by parallel cartridge ion-exchange columns. RSC Adv. 2017, 7, 50657. [Google Scholar] [CrossRef]
- Dou, Q.; Liu, L.; Yang, B.; Lang, J.; Yan, X. Silica-grafted ionic liquids for revealing the respective charging behaviors of cations and anions in supercapacitors. Nat. Commun. 2017, 8, 2188. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, F. Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater. Environ. Sci.Technol. 2005, 39, 6808–6818. [Google Scholar] [CrossRef] [PubMed]
- Wahba, M.I. Porous chitosan beads of superior mechanical properties for the covalent immobilization of enzymes. Int. J. Biol. Macromol. 2017, 105, 894–904. [Google Scholar] [CrossRef]
- Li, N.; Bai, R. Copper adsorption on chitosan–cellulose hydrogel beads: Behaviors and mechanisms. Sep. Purif. Technol. 2005, 42, 237–247. [Google Scholar] [CrossRef]
- Magdy, Y.H.; Altaher, H. Kinetic analysis of the adsorption of dyes from high strength wastewater on cement kiln dust. J. Environ. Chem. Eng. 2018, 6, 834–841. [Google Scholar] [CrossRef]
- McKay, G. The Adsorption of basic dye onto silica from aqueous solution-solid diffusion model. Chem. Eng. Sci. 1984, 39, 129–138. [Google Scholar] [CrossRef]
- Altaher, H.; Khalil, T.E.; Abubeah, R. The effect of dye chemical structure on adsorption on activated carbon: A comparative study. Color. Technol. 2014, 30, 205–214. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Malachite green adsorption by rattan sawdust: Isotherm, kinetic and mechanism modeling. J. Hazad. Mater. 2008, 159, 574–579. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study. S. Afr. J. Chem. Eng. 2020, 32, 39–55. [Google Scholar] [CrossRef]
- Huang, Y.; Farooq, M.U.; Lai, S.; Feng, X.; Sampranpiboon, P.; Wang, X.; Huang, W. Model fitting of sorption kinetics data: Misapplications overlooked and their rectifications. AIChE 2018, 64, 1793–1805. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press, Oxford Science Publications: London, UK, 1975. [Google Scholar]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar]
- Spahn, H.; Schlunder, U. The scale-up of activated carbon columns for water purification based on results from batch test. I. Theoretical and experimental determination of adsorption rates of single organic solutes in batch test. Chem. Eng. Sci. 1975, 30, 529–537. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics. J. am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Kim, T.; An, B. Adsorption characteristic of Cu(II) and phosphate using non-linear and linear isotherm equation for chitosan bead. J. Korean Soc. Water Wastewater 2020, 34, 201–210. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Zhrani, G.; Kosa, S.A. Removal of heavy metal ions from aqueous solution by multi-walled carbon nanotubes modified with 8-hydroxyquinoline: Kinetic study. J. Ind. Eng. Chem. 2014, 20, 572–580. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Lagergreen, S. Zur theorie der sogenannten adsorption geloster stoffe. Z. fur Chem. Ind. der Kolloide 1907, 2, 15. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazar. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Katchalsky, A. Problems in the physical chemistry of polyelectrolytes. J. Polym. Sci. 1954, 12, 159–184. [Google Scholar] [CrossRef]
- Domard, A. Determination, of N-acetyl content in chitosan samples by c.d. measurements. Int. J. Biol. Macromol. 1987, 9, 333–336. [Google Scholar] [CrossRef]
- Kannamba, B.; Laxma Reddy, K.L.; AppaRao, B.V. Removal of Cu(II) from aqueous solutions using chemically modified chitosan. J. Hazad. Mater. 2010, 175, 939–948. [Google Scholar] [CrossRef]
- Gérente, C.; Andrès, Y.; McKay, G.; Le Cloirec, P. Removal of arsenic(V) onto chitosan: From sorption mechanism explanation to dynamic water treatment process. Chem. Eng. J. 2010, 158, 593–598. [Google Scholar] [CrossRef]
- Klapiszewski, L.; Siwińska-Stefańska, K.; Kołodyńska, D. Development of lignin based multifunctional hybrid materials for Cu(II) and Cd(II) removal from the aqueous system. Chem. Eng. J. 2017, 330, 518–530. [Google Scholar] [CrossRef]
- Özer, A. Removal of Pb(II) ions from aqueous solutions by sulphuric acid- treated wheat bran. J. Hazard Mater. 2007, 141, 753–761. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. J. Chem. Eng. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Konggidinata, M.I.; Chao, B.; Lian, Q.; Subramaniam, R.; Zappi, M.; Gang, D.D. Equilibrium, kinetic and thermodynamic studies for adsorption of BTEX onto ordered mesoporous carbon (OMC). J. Hazad. Mater. 2017, 336, 249–259. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A Review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, G.; Manuaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Kumar, K.V. Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J. Hazard. Mater. 2006, B137, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Moussout, H.; Ahlafi, J.; Aazza, M.; Maghat, H. Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L. Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front. Environ. Sci. En. 2009, 3, 320–324. [Google Scholar] [CrossRef]
- Na, C.K.; Park, H.J. Applicability of theoretical adsorption models for studies on adsorption properties of adsorbents (II). J. Korean Soc. Environ. Eng. 2011, 33, 804–811. [Google Scholar] [CrossRef]
- Guthrie, W. Engineering Statistics Handbook. Section 4.4 Model Validation; 2013. Available online: https://www.itl.nist.gov/div898/handbook/ (accessed on 12 October 2019).
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interf. Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mangwandi, C.; Al-Muhtaseb, A.A.H.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Kinetic and thermodynamics of chromium ions adsorption onto low-cost dolomite adsorbent. Chem. Eng. J. 2012, 179, 193–202. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—An agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Wang, W.; Maimaiti, A.; Shi, H.; Wu, R.; Wang, R.; Li, Z.; Qi, Q.; Yu, G.; Deng, S. Adsorption behavior and mechanism of emerging perfluoro-2- propoxypropanoic acid (GenX) on activated carbons and resins. Chem. Eng. J. 2019, 364, 132–138. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Doke, K.M.; Khan, E.M. Equilibrium, kinetic and diffusion mechanism of Cr(VI) adsorption onto activated carbon derived from wood apple shell. Arab. J. Chem. 2017, 10, s252–s260. [Google Scholar] [CrossRef]
- Hamdaoui, O. Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J. Hazard. Mater. 2006, B135, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Fernadez, N.A.; Chacin, E.; Gutierrez, E.; Alastre, N. Adsorption of lauryl benzyl sulphonate on algae. Bioresour. Technol. 1995, 54, 111. [Google Scholar] [CrossRef]

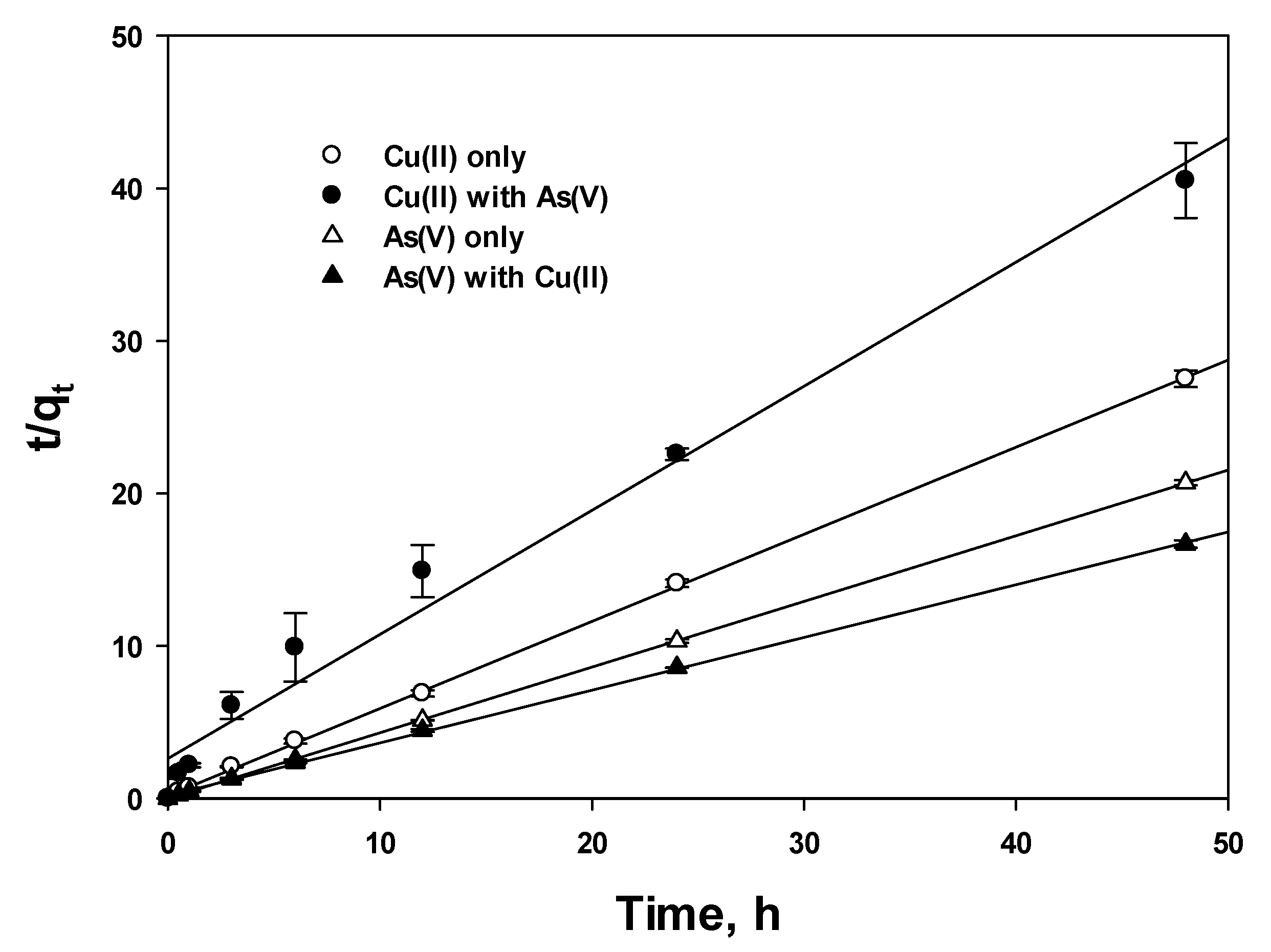
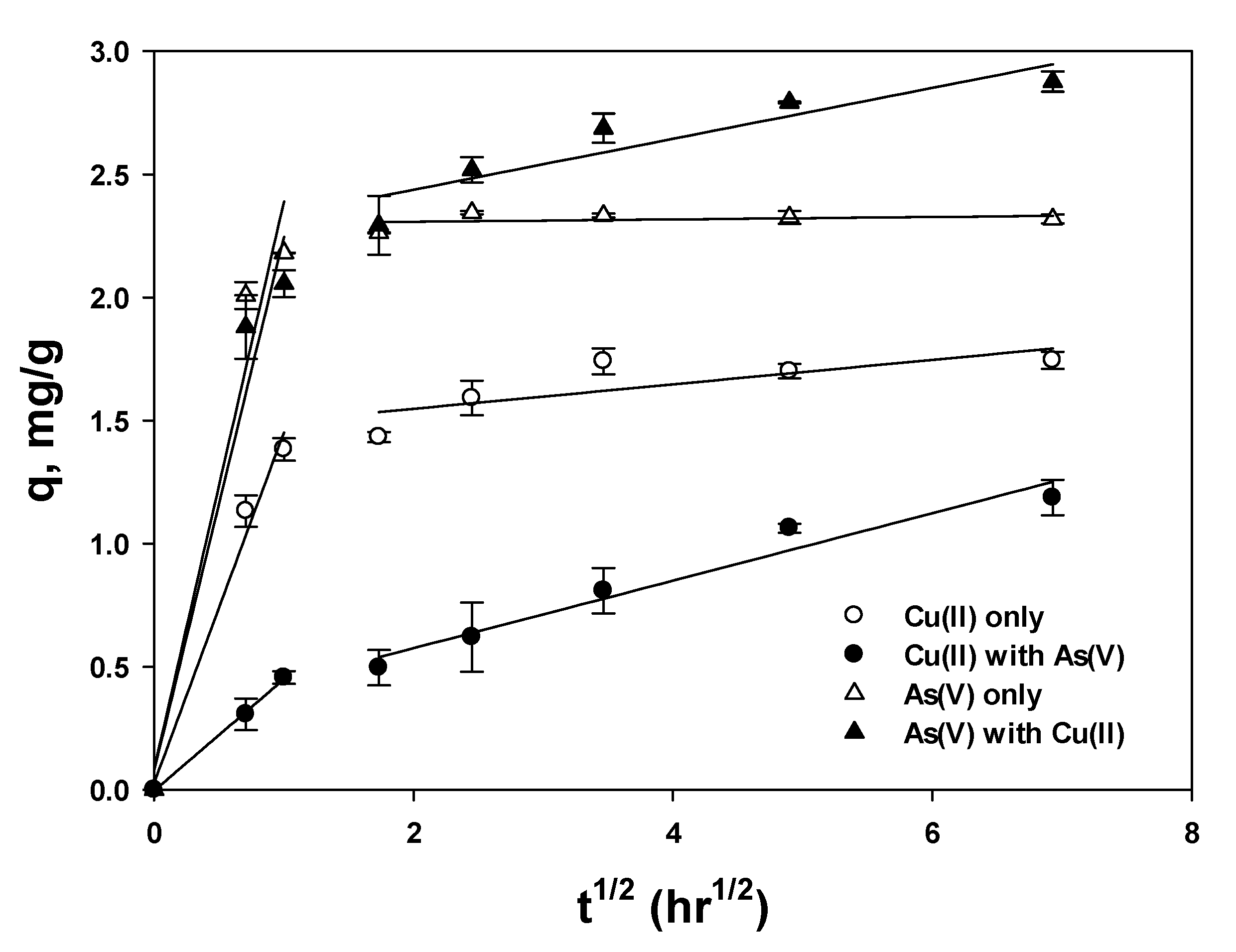
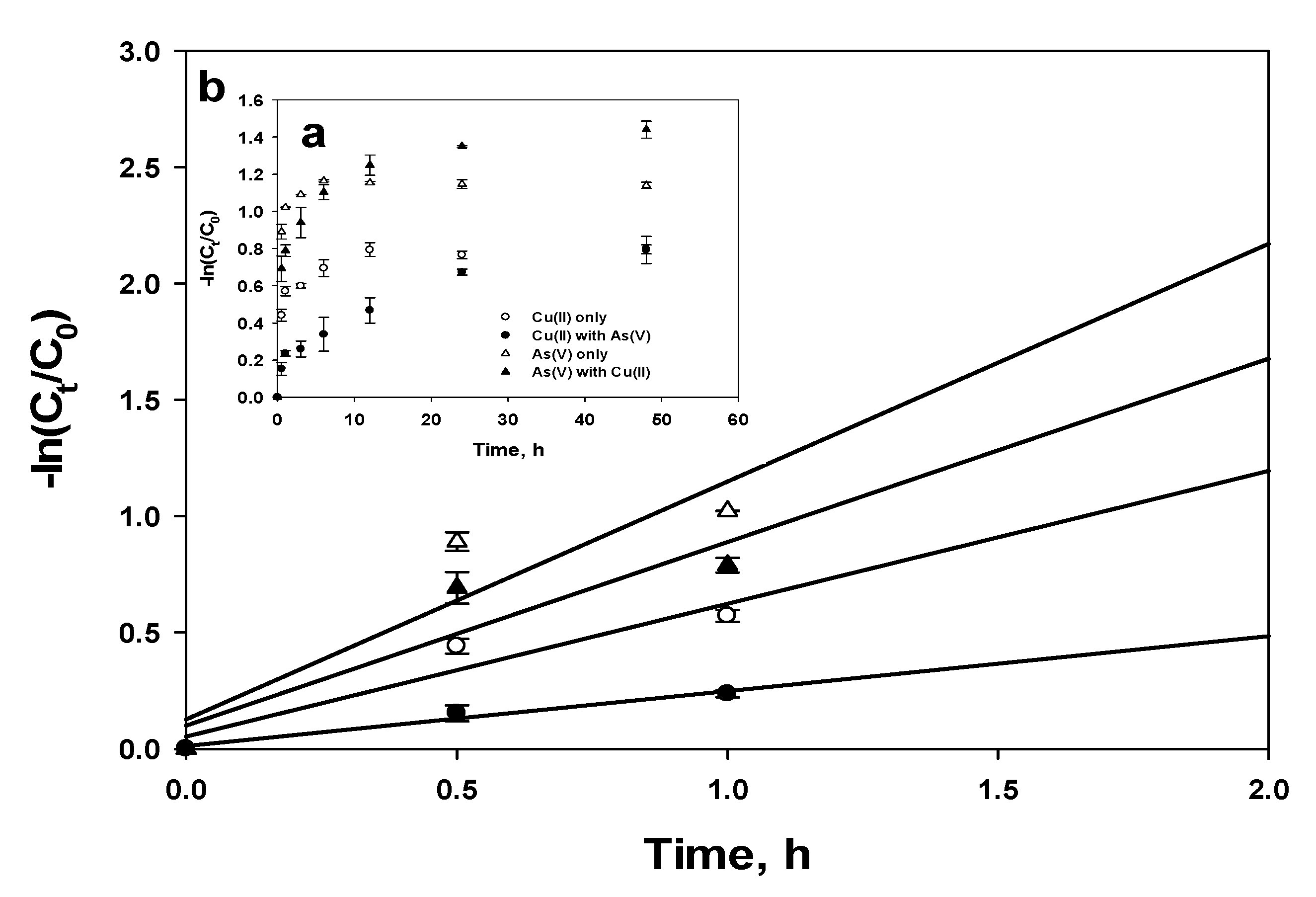
| Cu(II)-Single | Cu(II)-Mixed | As(V)-Single | As(V)-Mixed | ||
|---|---|---|---|---|---|
| qeexp. (b) | 1.85 | 1.25 | 2.4 | 2.92 | |
| PFO | qecal. (c) | 1.64 | 1.07 | 2.30 | 2.62 |
| K | 2.16 | 0.187 | 3.96 | 2.10 | |
| R2 | 0.969 | 0.819 | 0.997 | 0.942 | |
| PSO | qecal. | 1.71 | 1.17 | 2.34 | 2.74 |
| K | 2.19 | 0.258 | 5.28 | 1.28 | |
| R2 | 0.986 | 0.891 | 0.999 | 0.979 | |
| PNO (a) | qecal. | 1.45 | 0.813 | 2.03 | 2.32 |
| K | 15.7 | 4.04 | 36.3 | 12.4 | |
| n | 1.98 | 1.84 | 2.01 | 1.98 |
| PFO | PSO | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without Separation | t ≤ 1 | t ≥ 3 | |||||||||||
| qe-exp. | qe-cal. | K1 | R2 | qe-cal. | K1 | R2 | qe-cal. | K1 | R2 | qe-cal. | K2 | R2 | |
| Cu(II)single | 1.85 | 0.529 | 0.0437 | 0.526 | 1.69 | 1.38 | 0.952 | 0.268 | 0.0231 | 0.514 | 1.75 | 5.37 | 0.999 |
| Cu(II)mixed | 1.25 | 1.03 | 0.102 | 0.990 | 1.17 | 0.489 | 0.979 | 1.11 | 0.104 | 0.992 | 1.23 | 1.86 | 0.981 |
| As(V)single | 2.4 | 0.259 | 0.0371 | 0.240 | 1.95 | 2.39 | 0.918 | 0.0816 | 0.00212 | 0.0129 | 2.32 | 12.5 | 0.999 |
| As(V)mixed | 2.92 | 0.959 | 0.0700 | 0.820 | 2.54 | 1.21 | 0.860 | 0.552 | 0.0530 | 0.954 | 2.89 | 24.2 | 0.999 |
| First Section | Second Section | Without Separation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | K | R2 | C | K | R2 | C | K | R2 | |
| Cu(II)-single | 0.0286 | 1.42 | 0.986 | 1.45 | 0.0497 | 0.605 | 2.23 | 0.103 | 0.850 |
| Cu(II)-mixed | −0.00285 | 0.452 | 0.999 | 0.301 | 0.137 | 0.954 | 1.41 | 0.00916 | 0.482 |
| As(V)-single | 0.0861 | 2.30 | 0.953 | 2.30 | 0.00483 | 0.0994 | 2.21 | 0.00345 | 0.238 |
| As(V)-mixed | 0.0787 | 2.17 | 0.956 | 2.23 | 0.103 | 0.850 | 0.470 | 0.0175 | 0.852 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, B. Cu(II) and As(V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan. Processes 2020, 8, 1194. https://doi.org/10.3390/pr8091194
An B. Cu(II) and As(V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan. Processes. 2020; 8(9):1194. https://doi.org/10.3390/pr8091194
Chicago/Turabian StyleAn, Byungryul. 2020. "Cu(II) and As(V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan" Processes 8, no. 9: 1194. https://doi.org/10.3390/pr8091194
APA StyleAn, B. (2020). Cu(II) and As(V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan. Processes, 8(9), 1194. https://doi.org/10.3390/pr8091194





