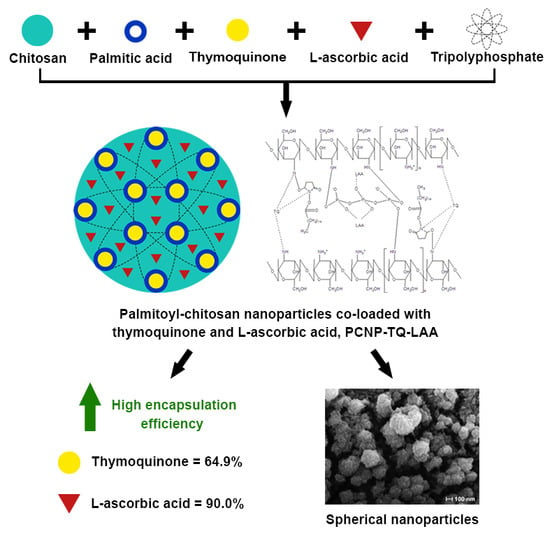
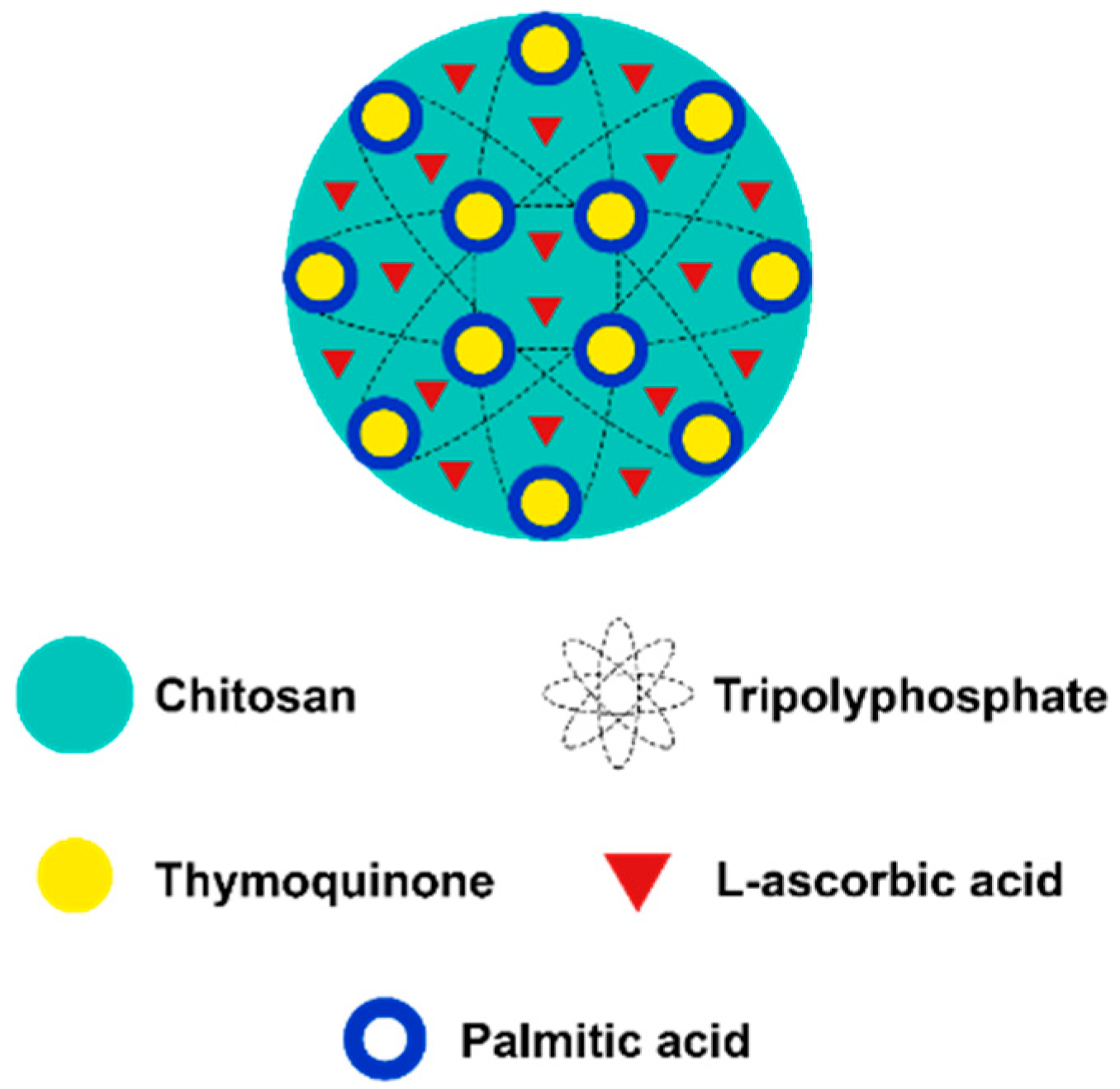
l-Ascorbic Acid and Thymoquinone Dual-Loaded Palmitoyl-Chitosan Nanoparticles: Improved Preparation Method, Encapsulation and Release Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
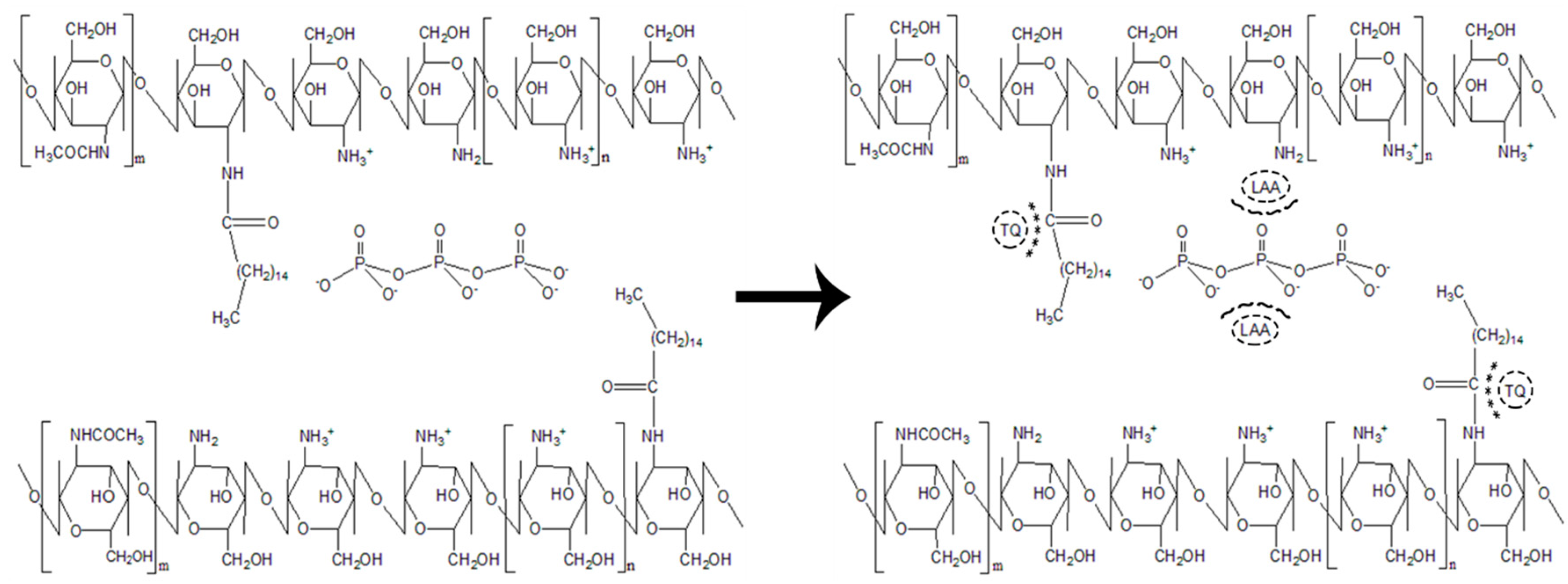
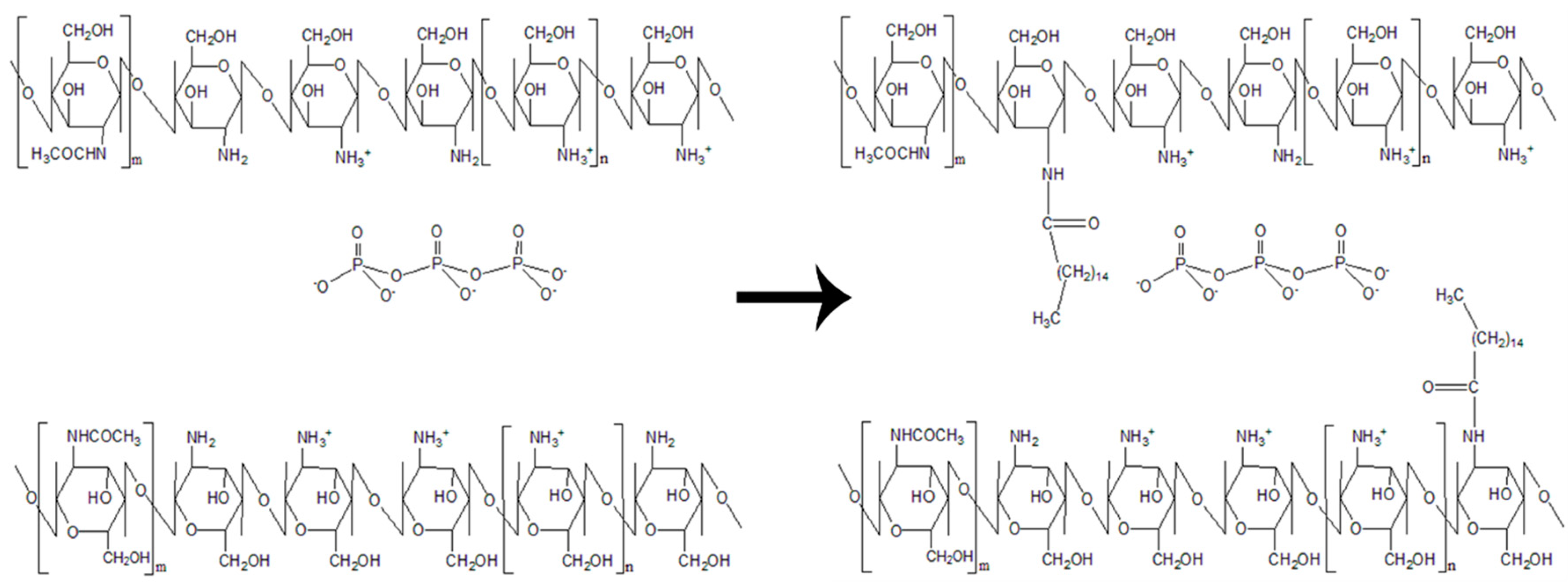
2.2. Synthesis of Palmitic Acid-Modified Chitosan, PCS
2.3. Synthesis of Palmitic Acid-Modified Chitosan Nanoparticles, PCNP
2.4. Encapsulation of l-Ascorbic Acid and Thymoquinone into PCNP
2.5. Quantification of Primary Amine Content in CNP and PCNP
2.6. Physicochemical Characterizations of PCNP Samples
2.6.1. Detection of Functional Groups in PCNP Samples
2.6.2. Particle Size Distribution, PSD Study
2.6.3. Surface Morphology of PCNP Samples by Field-Emission Scanning Electron Microscopy (FESEM)
2.6.4. Surface Morphology of PCNP Samples by High-Resolution Transmission Electron Microscopy (HRTEM)
2.6.5. Encapsulation Efficiency Study of Thymoquinone and l-Ascorbic Acid into PCNP
2.6.6. Preliminary Study of Thymoquinone and l-Ascorbic Acid In Vitro Release from PCNP
3. Results and Discussion
3.1. Quantification of Hydrophobic Palmitic Acid Functionalization on Chitosan by (TNBS) Assay
3.2. Functional Groups Determination by Fourier-Transform Infrared Spectroscopy (FTIR)
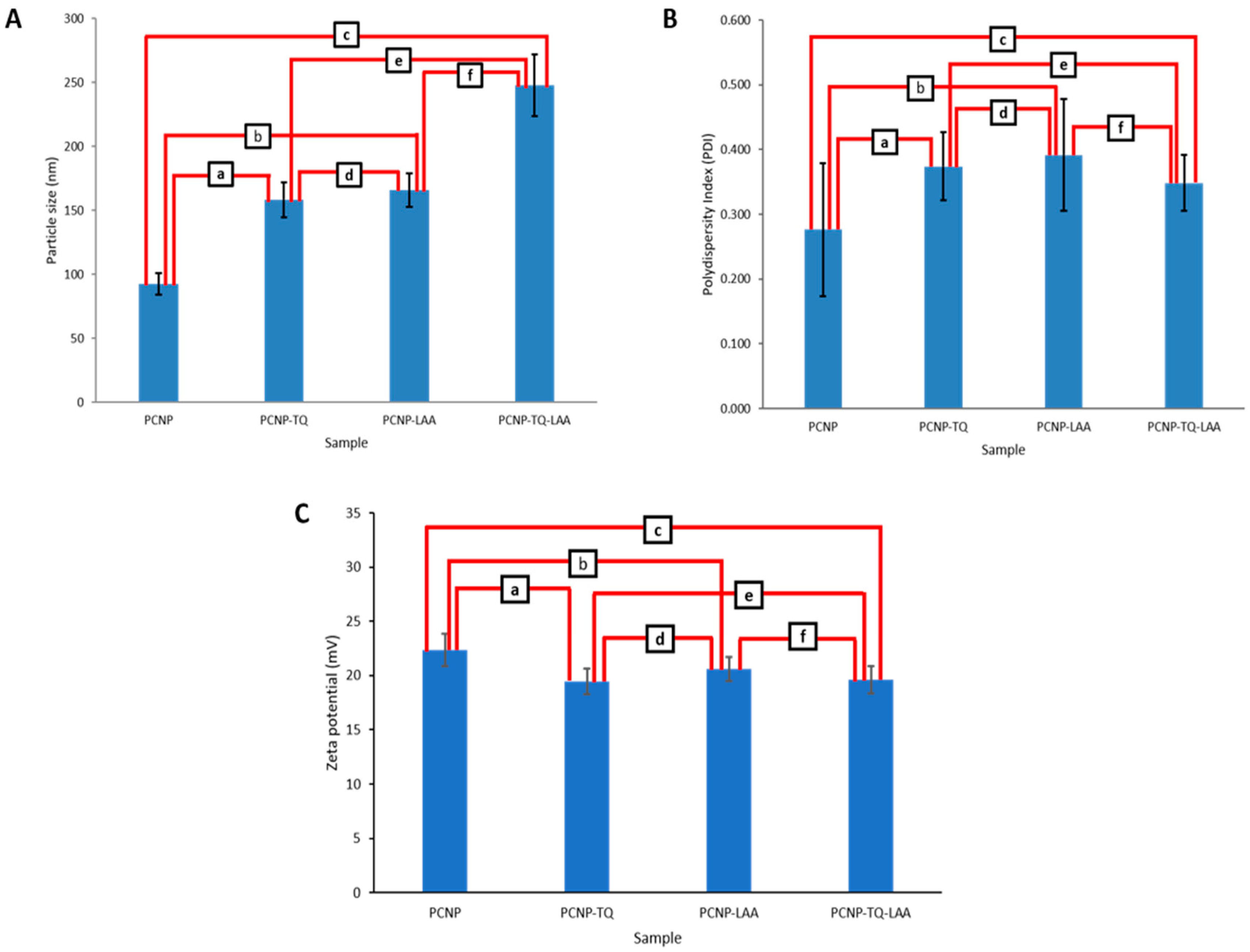
3.3. Particle Size Distribution by Zetasizer
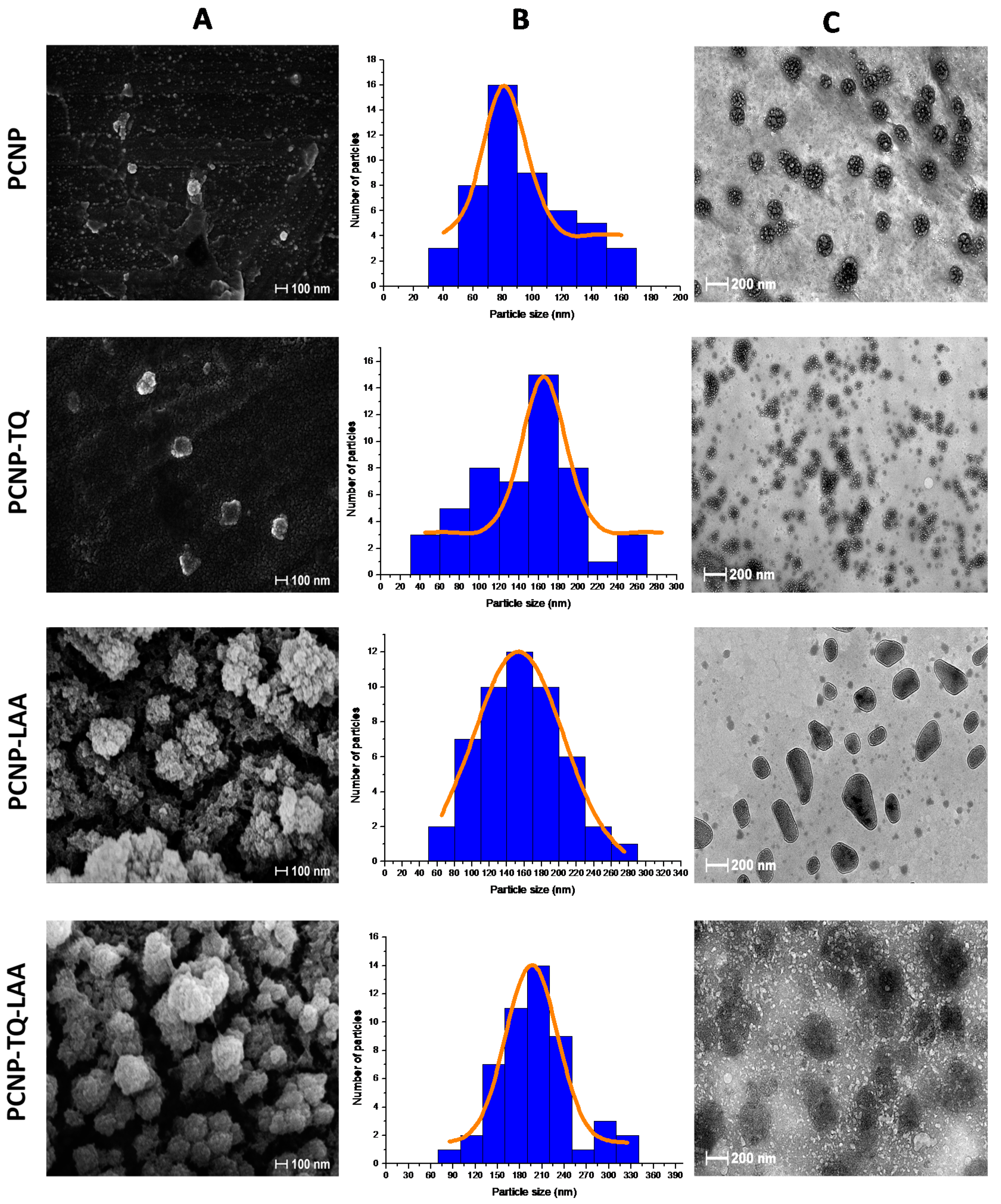
3.4. Surface Morphologies of Nanoparticles
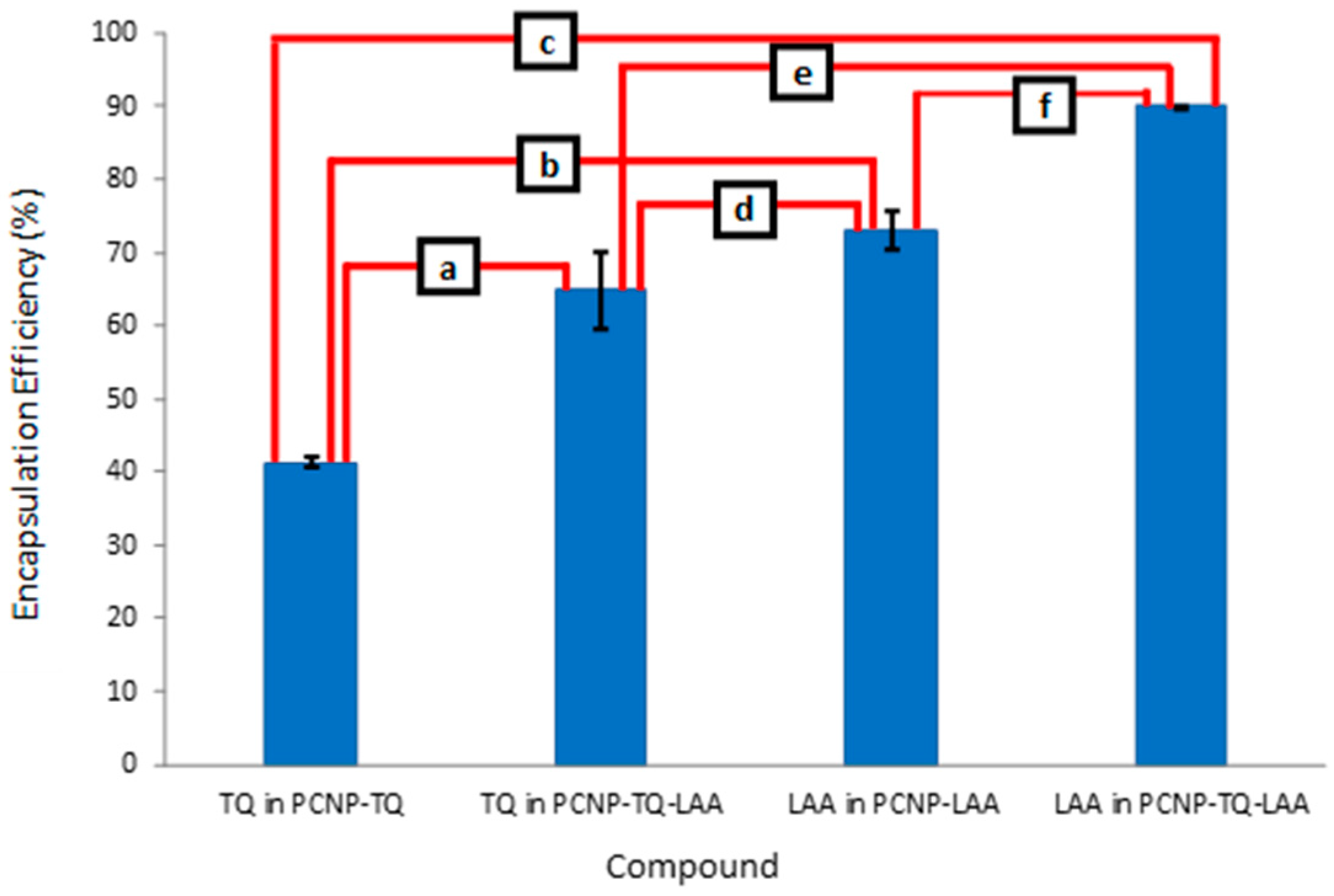
3.5. Encapsulation Efficiency (EE)
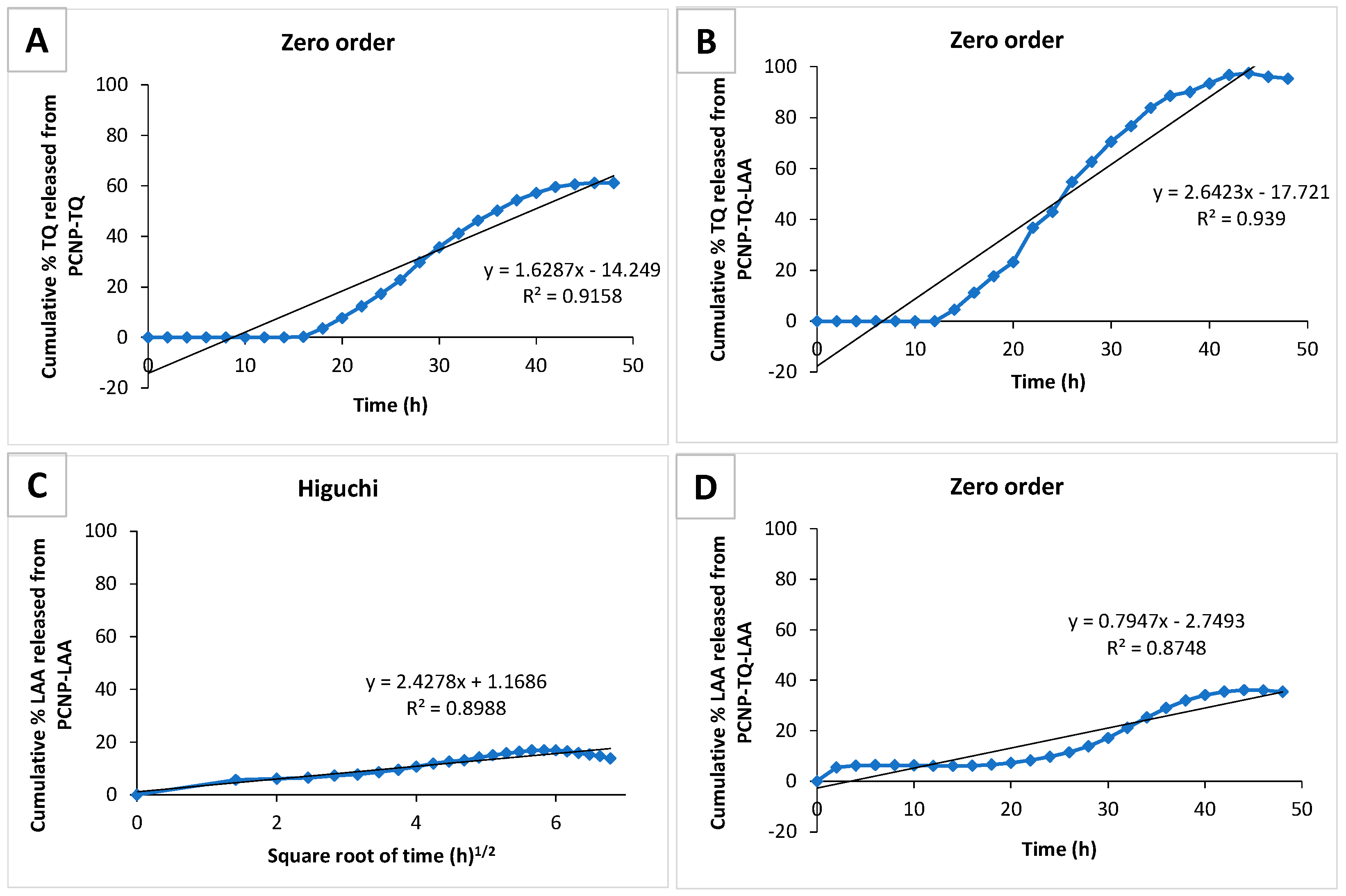
3.6. Preliminary Study of Thymoquinone and l-Ascorbic Acid In Vitro Release from PCNP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Wang, Z.; Huang, Y.; Barr, S.A.O.; Wong, R.A.; Yeung, S.; Chow, M.S.S. Ginseng and Anticancer Drug Combination to Improve Cancer Chemotherapy: A Critical Review. Hindawi 2014, 2014. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Q.; Zhou, Y.; Wang, J.; Liu, Q.; Xu, H. A systematic analysis of FDA-approved anticancer drugs. BMC Syst. Biol. 2017, 11. [Google Scholar] [CrossRef]
- Wong, C.C.; Cheng, K.; Rigas, B. Preclinical Predictors of Anticancer Drug Efficacy: Critical Assessment with Emphasis on Whether Nanomolar Potency Should Be Required of Candidate Agents. J. Pharm. Exp. Ther. 2012, 341, 572–578. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, J.; Liao, S.; Sun, Z. Treating tuberculosis with high doses of anti-TB drugs: Mechanisms and outcomes. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, Y.; Hu, L.; Yang, X.M.; He, Z. Molecular mechanism of the synergistic activity of ethambutol and isoniazid against Mycobacterium tuberculosis. J. Biol. Chem. 2018, 293, 16741–16750. [Google Scholar] [CrossRef]
- Rey-jurado, E.; Tudó, G.; Antonio, J.; González-martín, J. Synergistic effect of two combinations of antituberculous drugs against Mycobacterium tuberculosis. Tuberculosis 2012, 92, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J.; Sun, W.; Xie, Q.R.; Xia, W.; Gu, H. Delivering hydrophilic and hydrophobic chemotherapeutics simultaneously by magnetic mesoporous silica nanoparticles to inhibit cancer cells. Int. J. Nanomed. 2012, 7, 999–1013. [Google Scholar]
- Ma, Y.; Liu, D.; Wang, D.; Wang, Y.; Fu, Q.; Fallon, J.K.; Yang, X.; He, Z.; Liu, F. Combinational delivery of hydrophobic and hydrophilic anticancer drugs in single nanoemulsions to treat MDR in cancer. Mol. Pharm. 2014, 11, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Jingou, J.; Shilei, H.; Weiqi, L.; Danjun, W.; Tengfei, W.; Yi, X. Preparation, characterization of hydrophilic and hydrophobic drug in combine loaded chitosan/cyclodextrin nanoparticles and in vitro release study. Colloids Surf. B Biointerfaces 2011, 83, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Naderinezhad, S.; Amoabediny, G.; Haghiralsadat, F. Co-delivery of hydrophilic and hydrophobic anticancer drugs using biocompatible pH-sensitive lipid-based nano-carriers for multidrug-resistant cancers. RSC Adv. 2017, 7, 30008–30019. [Google Scholar] [CrossRef]
- Hao, S.; Wang, B.; Wang, Y. Porous hydrophilic core/hydrophobic shell nanoparticles for particle size and drug release control. Mater. Sci. Eng. C 2015, 49, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Mehta, S.K.; Kumar, S.; Bhanjana, G.; Dilbaghi, N. Coencapsulation of Hydrophobic and Hydrophilic Antituberculosis Drugs in Synergistic Brij 96 Microemulsions: A Biophysical Characterization. J. Pharm. Sci. 2015, 104, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Dini, E.; Alexandridou, S.; Kiparissides, C. Synthesis and characterization of cross-linked chitosan microspheres for drug delivery applications. J. Microencapsul. 2003, 20, 375–385. [Google Scholar] [CrossRef]
- Liu, H.; He, J. Simultaneous release of hydrophilic and hydrophobic drugs from modified chitosan nanoparticles. Mater. Lett. 2015, 161, 415–418. [Google Scholar] [CrossRef]
- Jamil, A.; Lim, H.N.; Yusof, N.A.; Ahmad Tajudin, A.; Huang, N.M.; Pandikumar, A.; Moradi Golsheikh, A.; Lee, Y.H.; Andou, Y. Preparation and characterization of silver nanoparticles-reduced graphene oxide on ITO for immunosensing platform. Sens. Actuators B Chem. 2015, 221, 1423–1432. [Google Scholar] [CrossRef]
- Vijayan, V.; Reddy, K.R.; Sakthivel, S.; Swetha, C. Optimization and charaterization of repaglinide biodegradable polymeric nanoparticle loaded transdermal patchs: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2013, 111, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Islam, R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology based approaches in cancer therapeutics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Emeje, M.O.; Obidike, I.C.; Akpabio, E.I.; Ofoefule, S.I. Nanotechnology in Drug Delivery; IntechOpen: London, UK, 2012. [Google Scholar]
- Ahad, A.; Al-Saleh, A.A.; Akhtar, N.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Transdermal delivery of antidiabetic drugs: Formulation and delivery strategies. Drug Discov. Today 2015, 20, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Nam, J.S.; Doss, G.P.C.; Lee, S.S.; Chakraborty, C. Nanoparticle based insulin delivery system: The next generation efficient therapy for Type 1 diabetes. J. Nanobiotechnol. 2015, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Control. Release 2017, 264, 247–275. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, B.; Hojjat-farsangi, M.; Mohammadi, H.; Anvari, E. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef]
- Grobmyer, S.R.; Zhou, G.; Gutwein, L.G.; Iwakuma, N.; Sharma, P.; Hochwald, S.N. Nanoparticle delivery for metastatic breast cancer. Nanomed. Nanotechnol. Biol. Med. 2012, 8, S21–S30. [Google Scholar] [CrossRef]
- Li, C.; Kuo, T.; Su, H.; Lai, W.; Yang, P.-C.; Chen, J.-S.; Wang, D.-Y.; Wu, Y.C.; Chen, C.-C. Fluorescence-Guided Probes of Aptamer-Targeted Gold Nanoparticles with Computed Tomography Imaging Accesses for in Vivo Tumor Resection. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Zhao, L.M.; Shi, L.E.; Zhang, Z.L.; Chen, J.M.; Shi, D.D.; Yang, J.; Tang, Z.X. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng. 2011, 28, 353–362. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Uddin, S.; Ferdosh, S.; Adam, A.; Jalal, A.; Chowdhury, K.; Islam, Z. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2014, 23, 619–629. [Google Scholar] [CrossRef]
- Szymanska, E.; Winnicka, K. Stability of Chitosan—A Challange for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, K.; Qian, C.; Nishimura, M.; Sugiyama, I.; Murata, Y.; Kawashima, S. Relationship between physicochemical characteristics and functional properties of chitosan. Eur. Polym. J. 2005, 41, 2784–2791. [Google Scholar] [CrossRef]
- Banquy, X.; Suarez, F.; Argaw, A.; Rabanel, J.; Grutter, P.; Giasson, S. Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake. Soft Matter 2009, 5, 3984–3991. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef]
- Kuen, C.; Fakurazi, S.; Othman, S.; Masarudin, M. Increased Loading, Efficacy and Sustained Release of Silibinin, a Poorly Soluble Drug Using Hydrophobically-Modified Chitosan Nanoparticles for Enhanced Delivery of Anticancer Drug Delivery Systems. Nanomaterials 2017, 7, 379. [Google Scholar] [CrossRef]
- Othman, N.; Masarudin, M.J.; Kuen, C.Y.; Dasuan, N.A.; Abdullah, L.C.; Md. Jamil, S.N.A. Synthesis and Optimization of Chitosan Nanoparticles Loaded with L -Ascorbic Acid and Thymoquinone. Nanomaterials 2018, 8, 920. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N. Recent modifications of chitosan for adsorption applications: A critical and systematic review. Mar. Drugs 2015, 13, 312–337. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, J. Synthesis and Characterization of Fatty Acid Grafted Chitosan Polymer and Their Nanomicelles for Nonviral Gene Delivery Applications. Bioconjug. Chem. 2017, 28, 2772–2783. [Google Scholar] [CrossRef] [PubMed]
- Thotakura, N.; Dadarwal, M.; Kumar, R.; Singh, B.; Sharma, G.; Kumar, P.; Prakash, O.; Raza, K. Chitosan-palmitic acid based polymeric micelles as promising carrier for circumventing pharmacokinetic and drug delivery concerns of tamoxifen. Int. J. Biol. Macromol. 2017, 102, 1220–1225. [Google Scholar] [CrossRef]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymoquinone—Encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic potential and pharmaceutical development of thymoquinone: A multitargeted molecule of natural origin. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Libr. Cochrane Rev. 2017. [Google Scholar] [CrossRef]
- Gonzalez, M.J.; Miranda-Massari, J.R.; Berdiel, M.J.; Duconge, J.; Rodriguez-Lopez, J.L.; Hunninghake, R.; Cobas-Rosario, V.J. High Dose Intraveneous Vitamin C and Chikungunya Fever: A Case Report. J. Orthomol. Med. 2015, 29, 154–156. [Google Scholar]
- Hemilä, H. Vitamin C and Infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef]
- Ullah, I.; Badshah, H.; Naseer, M.I.; Lee, H.Y.; Kim, M.O. Thymoquinone and Vitamin C Attenuates Pentylenetetrazole- Induced Seizures Via Activation of GABA B1 Receptor in Adult Rats Cortex and Hippocampus. Neuromol. Med. 2014. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, S.A.; El-din, H.M.A.; Elberry, M.H.; Allam, N.G.; Hasanin, M.T.M.; Abdellah, A.M. Synthesis, characterization and cytotoxic evaluation of chitosan nanoparticles: In vitro liver cancer model. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Review Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Subri, N.N.S.; Cormack, P.A.G.; Md. Jamil, S.N.A.; Abdullah, L.C.; Daik, R. Synthesis of poly(acrylonitrile-co-divinylbenzene-co-vinylbenzyl chloride)-derived hypercrosslinked polymer microspheres and a preliminary evaluation of their potential for the solid-phase capture of pharmaceuticals. J. Appl. Polym. Sci. 2017, 135, 1–9. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, A.; Shi, Y.; Zhang, Y.; Chen, Y. Experimental and mathematical studies on the drug release properties of aspirin loaded chitosan nanoparticles. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric nanoparticles containing diazepam: Preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016, 3. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained—Action Medication. J. Pharm. Sci. 1963. [Google Scholar]
- Hixson, A.W.; Crowell, J.H. Dependence of Reaction Velocity upon Surface and Agitation. Ind. Eng. Chem. 1931, 23, 1160–1168. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Balan, V.; Redinciuc, V.; Tudorachi, N.; Verestiuc, L. Biotinylated N-palmitoyl chitosan for design of drug loaded self-assembled nanocarriers. Eur. Polym. J. 2016, 81, 284–294. [Google Scholar] [CrossRef]
- Reusch, W. Virtual Textbook of Organic Chemistry. Available online: https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/Spectrpy/InfraRed/infrared.htm#ir1 (accessed on 11 February 2020).
- Ramalingam, P.; Ko, Y.T. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2015, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, W.; Wu, H.; Wu, M.; Liu, Z.; Dong, S. Chitosan/sodium tripolyphosphate nanoparticles as efficient vehicles for enhancing the cellular uptake of fish—Derived peptide. J. Food Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-I.; Lee, H.G. Stability of Chitosan Nanoparticles for L-Ascorbic Acid during Heat Treatment in Aqueous Solution. J. Agric. Food Chem. 2008, 56, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Wang, J.L.; Iqbal, S.; Li, H.J.; Cao, Z.T.; Wang, Y.C.; Wang, J. The Effect of Surface Charge on Oral Absorption of Polymeric Nanoparticles. Biomater. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Ong, Y.S.; Yazan, L.S.; Ng, W.K.; Noordin, M.M.; Sapuan, S.; Foo, J.B.; Tor, Y.S. Acute and subacute toxicity profiles of thymoquinone-loaded nanostructured lipid carrier in BALB/c mice. Int. J. Nanomed. 2016, 5905–5915. [Google Scholar] [CrossRef] [PubMed]
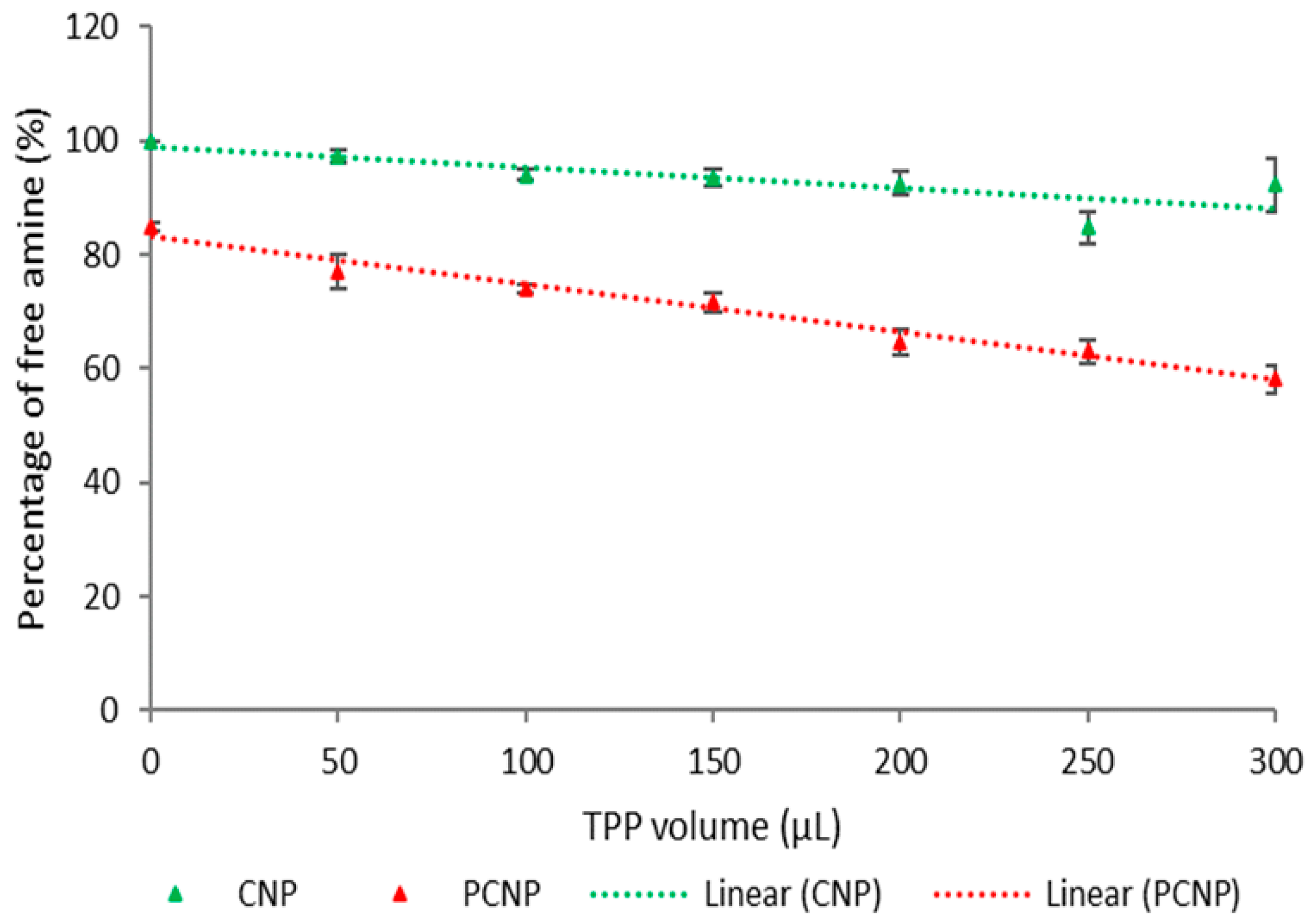

| Label | Volume of TPP (µL) | Percentage of Free Amine (%) | t-Test | ||||
|---|---|---|---|---|---|---|---|
| CNP | Standard Deviation | PCNP | Standard Deviation | p-Value | Significance between CNP and PCNP Reading at Different TPP Volume | ||
| a | 0 | 100.00 | 0 | 84.76 | 0.77 | <0.0001 | **** |
| b | 50 | 97.41 | 1.15 | 77.11 | 2.93 | 0.0004 | *** |
| c | 100 | 94.05 | 0.94 | 74.04 | 0.79 | <0.0001 | **** |
| d | 150 | 93.47 | 1.59 | 71.61 | 1.78 | <0.0001 | **** |
| e | 200 | 92.54 | 2.16 | 64.72 | 2.14 | <0.0001 | **** |
| f | 250 | 84.87 | 2.81 | 62.99 | 2.18 | 0.0004 | *** |
| g | 300 | 92.38 | 4.70 | 58.17 | 2.43 | 0.0004 | *** |
| Peak | Functional Group | Wavenumber (cm−1) | Percent Transmittance (%) | |||
|---|---|---|---|---|---|---|
| PCNP | PCNP-TQ | PCNP-LAA | PCNP-TQ-LAA | |||
| a | Alcohol OH (H bonded) and NH stretch (2° amine) | 3399 | 7.47 | 4.15 | 3.17 | 6.18 |
| b | Amine NH2 scissor (1° amine), carboxylic acid C=O (amide) and alkene C=C stretch | 1647 | 24.20 | 20.24 | 12.46 | 21.96 |
| c | Amine NH2 scissor (1° amine) | 1563 | 26.91 | - | 10.89 | 18.99 |
| d | Carboxylic acid NH (amide) bend | 1542 | - | 62.19 | - | - |
| e | Alkane CH2 and CH3 deformation and carboxylic acid C-O-H bend | 1414 | 42.41 | - | 24.14 | 36.45 |
| f | Alkane CH2 and CH3 deformation | 1379 | - | 56.40 | - | - |
| g | Amine C-N and carboxylic acid C-O stretch, P=O stretch | 1106 | 3.19 | 9.01 | 8.52 | 7.02 |
| h | Alkene =C-H and =CH2 bend | 903 | 12.77 | 28.42 | 26.88 | 18.32 |
| i | Amine NH2 and N-H wagging (shifts on H-bonding) | 718 | 64.79 | 63.52 | 77.46 | 86.57 |
| Sample | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| PCNP | 92.6 ± 8.6 | 0.277 ± 0.103 | 22.35 ± 1.48 |
| PCNP-TQ | 158.3 ± 13.9 | 0.374 ± 0.052 | 19.45 ± 1.20 |
| PCNP-LAA | 165.8 ± 12.9 | 0.392 ± 0.087 | 20.60 ± 1.13 |
| PCNP-TQ-LAA | 247.7 ± 24.0 | 0.348 ± 0.043 | 19.60 ± 1.27 |
| Compound | Loaded Compound Concentration (µM) | EE (%) | Standard Deviation | Encapsulated Compound Concentration (µM) |
| TQ in PCNP-TQ | 150 | 41.3 | 0.6 | 62.0 |
| TQ in PCNP-TQ-LAA | 64.9 | 5.3 | 97.3 | |
| LAA in PCNP-LAA | 160 | 73.0 | 2.6 | 116.8 |
| LAA in PCNP-TQ-LAA | 90.0 | 0.0 | 143.9 |
| Label | Release of | Released | ||
|---|---|---|---|---|
| Percentage (%) | Concentration (µM) | Total Time (h) | ||
| A | TQ from PCNP-TQ | 60.6 | 37.6 | 43.9 |
| B | TQ from PCNP-TQ-LAA | 97.5 | 94.9 | 43.9 |
| C | LAA from PCNP-LAA | 17.0 | 19.8 | 33.7 |
| D | LAA from PCNP-TQ-LAA | 36.1 | 52.0 | 33.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, N.; Md. Jamil, S.N.A.; Masarudin, M.J.; Abdullah, L.C.; Daik, R.; Sarman, N.S. l-Ascorbic Acid and Thymoquinone Dual-Loaded Palmitoyl-Chitosan Nanoparticles: Improved Preparation Method, Encapsulation and Release Efficiency. Processes 2020, 8, 1040. https://doi.org/10.3390/pr8091040
Othman N, Md. Jamil SNA, Masarudin MJ, Abdullah LC, Daik R, Sarman NS. l-Ascorbic Acid and Thymoquinone Dual-Loaded Palmitoyl-Chitosan Nanoparticles: Improved Preparation Method, Encapsulation and Release Efficiency. Processes. 2020; 8(9):1040. https://doi.org/10.3390/pr8091040
Chicago/Turabian StyleOthman, Nurhanisah, Siti Nurul Ain Md. Jamil, Mas Jaffri Masarudin, Luqman Chuah Abdullah, Rusli Daik, and Nor Syazwani Sarman. 2020. "l-Ascorbic Acid and Thymoquinone Dual-Loaded Palmitoyl-Chitosan Nanoparticles: Improved Preparation Method, Encapsulation and Release Efficiency" Processes 8, no. 9: 1040. https://doi.org/10.3390/pr8091040
APA StyleOthman, N., Md. Jamil, S. N. A., Masarudin, M. J., Abdullah, L. C., Daik, R., & Sarman, N. S. (2020). l-Ascorbic Acid and Thymoquinone Dual-Loaded Palmitoyl-Chitosan Nanoparticles: Improved Preparation Method, Encapsulation and Release Efficiency. Processes, 8(9), 1040. https://doi.org/10.3390/pr8091040