Functional Properties of Biopolymer-Based Films Modified with Surfactants: A Brief Review
Abstract
1. Introduction
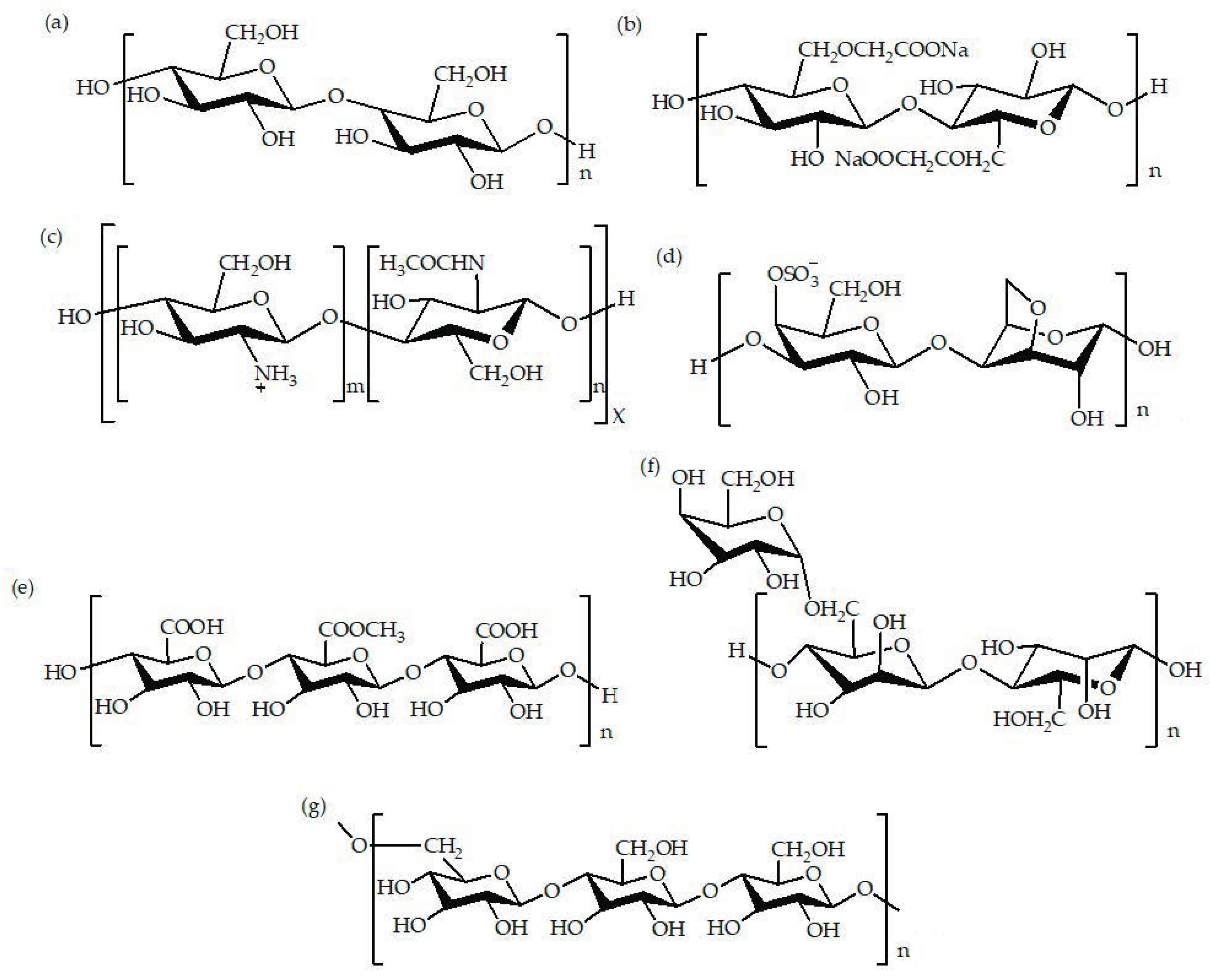
2. Types of Biopolymer-Based Films
2.1. Solution-Based Films
2.2. Emulsion-Based Films
3. Modification by Different Types of Surfactants
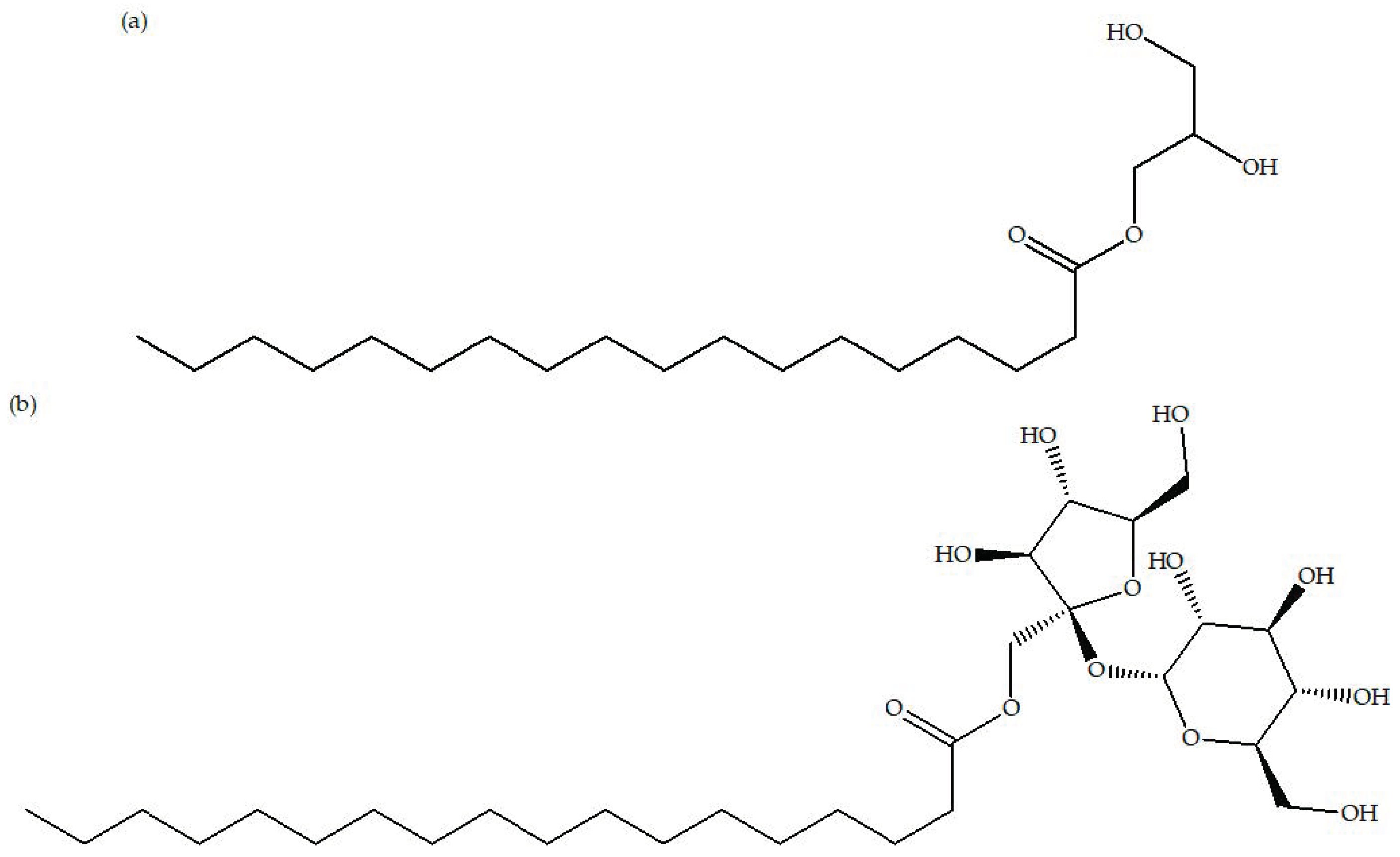
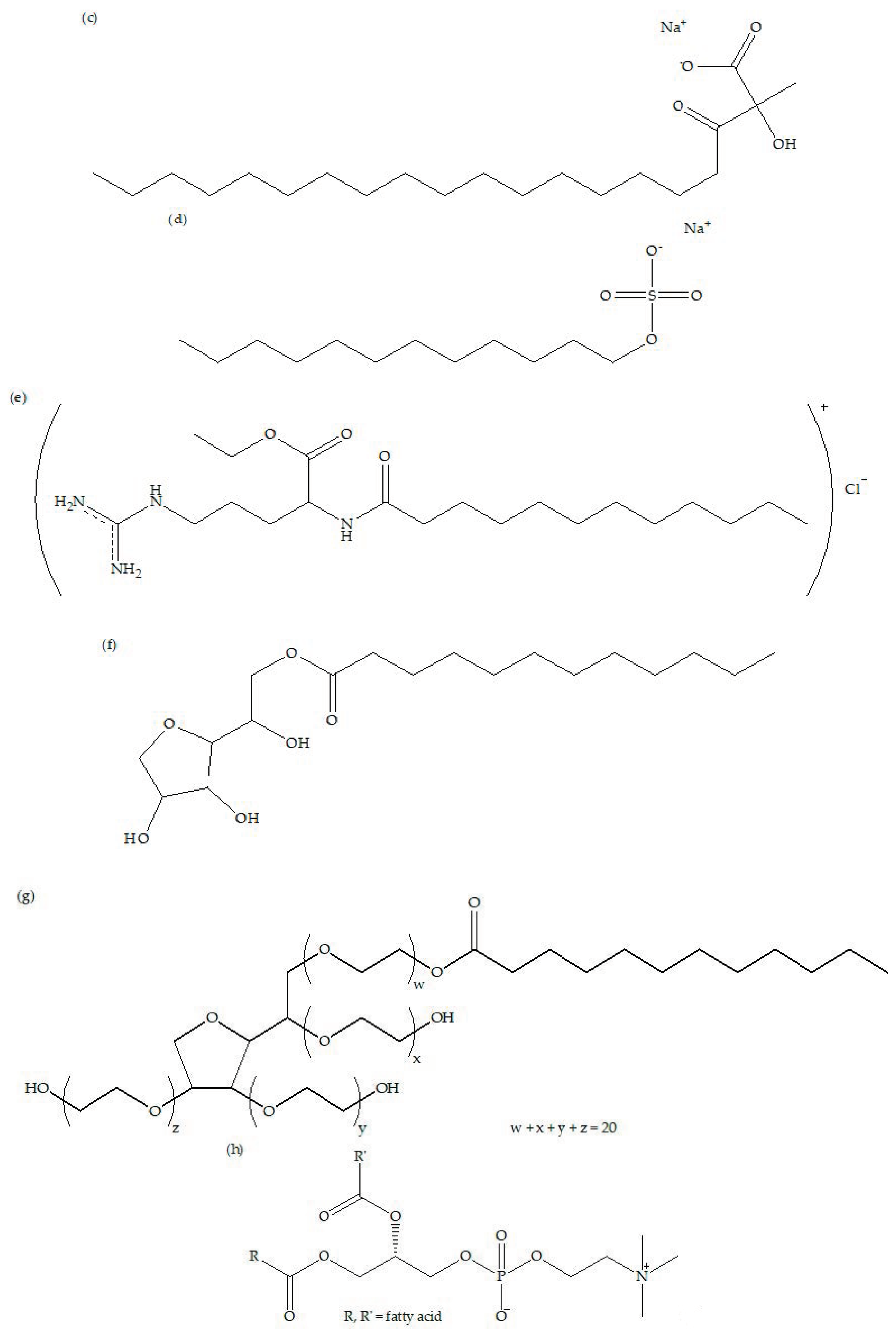
3.1. Types of Surfactants
3.2. Modification by Non-Ionic Surfactants
3.3. Modification by Ionic Surfactants
3.4. Modification by Amphoteric Surfactant
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.H.; Kuo, W.S.; Lai, L.S. Effect of surfactants on water barrier and physical properties of tapioca starch/decolorized hsian-tsao leaf gum films. Food Hydrocoll. 2009, 23, 714–721. [Google Scholar] [CrossRef]
- Rodríguez, M.; Oses, J.; Ziani, K.; Mate, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res. Int. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Bravin, B.; Peressini, D.; Sensidoni, A. Influence of emulsifier type and content on functional properties of polysaccharide lipid-based edible films. J. Agric. Food Chem. 2004, 52, 6448–6455. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.C.; del Carmen Nuñez-Santiago, M.; Romero-Bastida, C.A.; Martinez-Bustos, F. Effects of coconut oil concentration as a plasticizer and Yucca schidigera extract as a surfactant in the preparation of extruded corn starch films. Starch-Staerke 2014, 66, 1079–1088. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Effect of the incorporation of surfactants on the physical properties of corn starch films. Food Hydrocoll. 2014, 38, 66–75. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Biendl, M. Physicochemical and antioxidant properties of biopolymer/candelilla wax emulsion films containing hop extract–A comparative study. Food Hydrocoll. 2016, 60, 384–392. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Lei, L.; Zhao, G. Combined effects of octenylsuccination and oregano essential oil on sweet potato starch films with an emphasis on water resistance. Int. J. Biol. Macromol. 2018, 115, 547–553. [Google Scholar] [CrossRef]
- Santacruz, S.; Rivadeneira, C.; Castro, M. Edible films based on starch and chitosan. Effect of starch source and concentration, plasticizer, surfactant’s hydrophobic tail and mechanical treatment. Food Hydrocoll. 2015, 49, 89–94. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Laroque, D.A.; de Andrade, L.M.; Carciofi, B.A.M.; Tenório, J.A.S.; de Andrade, C.J. Production of active cassava starch films; effect of adding a biosurfactant or synthetic surfactant. React. Funct. Polym. 2019, 144, 104368–104374. [Google Scholar] [CrossRef]
- Porras, D.P.N.; Suárez, M.G.; Umaña, J.H.; Perdomo, L.G.P. Optimization of physical, optical and barrier properties of films made from cassava starch and rosemary oil. J. Polym. Environ. 2019, 27, 127–140. [Google Scholar] [CrossRef]
- Chen, C.H.; Kuo, W.S.; Lai, L.S. Water barrier and physical properties of starch/decolorized hsian-tsao leaf gum films: Impact of surfactant lamination. Food Hydrocoll. 2010, 24, 200–207. [Google Scholar] [CrossRef]
- Trezza, T.A.; Krochta, J.M. The gloss of edible coatings as affected by surfactants, lipids, relative humidity, and time. J. Food Sci. 2000, 65, 658–662. [Google Scholar] [CrossRef]
- Rodríguez-Castellanos, W.; Martínez-Bustos, F.; Jiménez-Arévalo, O.; González-Núñez, R.; Galicia-García, T. Functional properties of extruded and tubular films of sorghum starch-based glycerol and Yucca Schidigera extract. Ind. Crop. Prod. 2013, 44, 405–412. [Google Scholar] [CrossRef]
- Cao, T.L.; Song, K.B. Effects of gum karaya addition on the characteristics of loquat seed starch films containing oregano essential oil. Food Hydrocoll. 2019, 97, 105198–105206. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Y. Effects of surfactants on the functional and structural properties of kudzu (Pueraria lobata) starch/ascorbic acid films. Carbohydr. Polym. 2011, 85, 622–628. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Development of biocomposite films incorporated with different amounts of shellac, emulsifier, and surfactant. Food Hydrocoll. 2017, 72, 174–184. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Martelli, S.M.; Motta, C.; Caon, T.; Alberton, J.; Bellettini, I.C.; do Prado, A.C.P.; Barreto, P.L.M.; Soldi, V. Edible carboxymethyl cellulose films containing natural antioxidant and surfactants: α-tocopherol stability, in vitro release and film properties. LWT 2017, 77, 21–29. [Google Scholar] [CrossRef]
- Lian, H.; Peng, Y.; Shi, J.; Wang, Q. Effect of emulsifier hydrophilic-lipophilic balance (HLB) on the release of thyme essential oil from chitosan films. Food Hydrocoll. 2019, 97, 105213–105220. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zivanovic, S.; Zhong, Q. Physical, mechanical, and antimicrobial properties of chitosan films with microemulsions of cinnamon bark oil and soybean oil. Food Hydrocoll. 2016, 52, 533–542. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Q.; Shi, J.; Chen, Y.; Zhang, X. Optimization and release evaluation for tea polyphenols and chitosan composite films with regulation of glycerol and Tween. Food Sci. Technol. 2019, 40, 162–170. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Ziani, K.; Oses, J.; Coma, V.; Maté, J.I. Effect of the presence of glycerol and Tween 20 on the chemical and physical properties of films based on chitosan with different degree of deacetylation. LWT-Food Sci. Technol. 2008, 41, 2159–2165. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Galicia-García, T.; Martinez-Bustos, F.; González-Nuñez, R.; Grosso, C.R. Functional properties of gelatin-based films containing Yucca schidigera extract produced via casting, extrusion and blown extrusion processes: A preliminary study. J. Food Eng. 2012, 113, 33–40. [Google Scholar] [CrossRef]
- Bahram, S.; Rezaei, M.; Soltani, M.; Kamali, A.; Ojagh, S.M.; Abdollahi, M. Whey protein concentrate edible film activated with cinnamon essential oil. J. Food Process. Preserv. 2014, 38, 1251–1258. [Google Scholar] [CrossRef]
- Carpiné, D.; Dagostin, J.L.A.; Bertan, L.C.; Mafra, M.R. Development and characterization of soy protein isolate emulsion-based edible films with added coconut oil for olive oil packaging: Barrier, mechanical, and thermal properties. Food Bioprocess Technol. 2015, 8, 1811–1823. [Google Scholar] [CrossRef]
- Dias, T.P.; Grosso, C.R.; Andreuccetti, C.; Carvalho, R.A.D.; Galicia-García, T.; Martinez-Bustos, F. Effect of the addition of soy lecithin and Yucca schidigera extract on the properties of gelatin and glycerol based biodegradable films. Polímeros 2013, 23, 339–345. [Google Scholar] [CrossRef]
- Limpisophon, K.; Tanaka, M.; Osako, K. Characterisation of gelatin–fatty acid emulsion films based on blue shark (Prionace glauca) skin gelatin. Food Chem. 2010, 122, 1095–1101. [Google Scholar] [CrossRef]
- Pereira, G.V.D.S.; Pereira, G.V.D.S.; Neves, E.M.P.X.; Joele, M.R.S.P.; Lourenço, L.D.F.H. Effect of adding fatty acids and surfactant on the functional properties of biodegradable films prepared with myofibrillar proteins from acoupa weakfish (Cynoscion acoupa). Food Sci. Technol. 2019, 39, 287–294. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Structural, morphological and thermal behaviour characterisations of fish gelatin film incorporated with basil and citronella essential oils as affected by surfactants. Food Hydrocoll. 2014, 41, 33–43. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Galicia-García, T.; Martínez-Bustos, F.; Grosso, C.R. Effect of surfactants on the functional properties of gelatin-based edible films. J. Food Eng. 2011, 103, 129–136. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Zięba, E.; Kazimierczak, W.; Mężyńska, M.; Basiura-Cembala, M.; Wiącek, A.E. Edible films made from blends of gelatin and polysaccharide-based emulsifiers—A comparative study. Food Hydrocoll. 2019, 96, 555–567. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Properties, microstructure and heat seal ability of bilayer films based on fish gelatin and emulsified gelatin films. Food Biophys. 2017, 12, 234–243. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Pisuchpen, S.; Osako, K. Mechanical, thermal and heat sealing properties of fish skin gelatin film containing palm oil and basil essential oil with different surfactants. Food Hydrocoll. 2016, 56, 93–107. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, W.; Wang, K.; Liu, Y.; Liu, A.; Zhang, S.; Zhao, Y. Impact of melting point of palm oil on mechanical and water barrier properties of gelatin-palm oil emulsion film. Food Hydrocoll. 2016, 60, 243–251. [Google Scholar] [CrossRef]
- Hanani, Z.N.; Husna, A.A. Effect of different types and concentrations of emulsifier on the characteristics of kappa-carrageenan films. Int. J. Biol. Macromol. 2018, 114, 710–716. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef]
- Chambi, H.; Grosso, C. Effect of surfactants on the functional properties of gelatin—Polysaccharide-based films. Eur. Food Res. Technol. 2011, 232, 63–69. [Google Scholar] [CrossRef]
- Saurabh, C.K.; Gupta, S.; Variyar, P.S.; Sharma, A. Effect of addition of nanoclay, beeswax, tween-80 and glycerol on physicochemical properties of guar gum films. Ind. Crop. Prod. 2016, 89, 109–118. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, T.; Gao, C.; Liu, X.; Zhang, N.; Feng, X.; Liu, X.; Shen, X.; Tang, X. Evaluations of physicochemical and biological properties of pullulan-based films incorporated with cinnamon essential oil and Tween 80. Int. J. Biol. Macromol. 2019, 122, 388–394. [Google Scholar] [CrossRef]
- Davanco, T.; Tanada-Palmu, P.; Grosso, C. Composite films made with gelatin, tracetin, stearic and caproic acids: Effect of pH and surfactants addition on the functionality of films. Cienc. E Tecnol. Aliment. 2007, 27, 408–416. [Google Scholar]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Emulsion stability and properties of fish gelatin-based films as affected by palm oil and surfactants. J. Sci. Food Agric. 2016, 96, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Carpiné, D.; Dagostin, J.L.A.; de Andrade, E.F.; Bertan, L.C.; Mafra, M.R. Effect of the natural surfactant Yucca schidigera extract on the properties of biodegradable emulsified films produced from soy protein isolate and coconut oil. Ind. Crop. Prod. 2016, 83, 364–371. [Google Scholar] [CrossRef]
- Panzarasa, G.; Osypova, A.; Sicher, A.; Bruinink, A.; Dufresne, E.R. Controlled formation of chitosan particles by a clock reaction. Soft Matter 2018, 14, 6415–6418. [Google Scholar] [CrossRef] [PubMed]
- Debeaufort, F.; Voilley, A. Effect of surfactants and drying rate on barrier properties of emulsified edible films. Int. J. Food Sci. Technol. 1995, 30, 183–190. [Google Scholar] [CrossRef]
- Villalobos-Carvajal, R.; Hernández-Muñoz, P.; Albors, A.; Chiralt, A. Barrier and optical properties of edible hydroxypropyl methylcellulose coatings containing surfactants applied to fresh cut carrot slices. Food Hydrocoll. 2009, 23, 526–535. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; Aran, H.; Cutter, C.N. Optimization of formulations for pullulan films containing lauric arginate and nisin Z. LWT-Food Sci. Technol. 2015, 63, 1110–1120. [Google Scholar] [CrossRef]
- Haghighi, H.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative analysis of blend and bilayer films based on chitosan and gelatin enriched with LAE (lauroyl arginate ethyl) with antimicrobial activity for food packaging applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Peng, Y.; Yin, L.; Li, Y. Combined effects of lemon essential oil and surfactants on physical and structural properties of chitosan films. Int. J. Food Sci. Technol. 2013, 48, 44–50. [Google Scholar] [CrossRef]
- Villalobos, R.; Hernández-Muñoz, P.; Chiralt, A. Effect of surfactants on water sorption and barrier properties of hydroxypropyl methylcellulose films. Food Hydrocoll. 2006, 20, 502–509. [Google Scholar] [CrossRef]
- Ali, M.; Khan, N.R.; Basit, H.M.; Mahmood, S. Physico-chemical based mechanistic insight into surfactant modulated sodium Carboxymethylcellulose film for skin tissue regeneration applications. J. Polym. Res. 2020, 27, 20. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Grosso, C.R. Effect of hydrophobic plasticizers on functional properties of gelatin-based films. Food Res. Int. 2009, 42, 1113–1121. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tajik, S.; Ghasemlou, M.; Shojaee-Aliabadi, S.; Noghabi, M.S.; Khaksar, R. Characterization of soluble soybean polysaccharide film incorporated essential oil intended for food packaging. Carbohydr. Polym. 2013, 98, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Baraniak, B. Effect of candelilla wax on functional properties of biopolymer emulsion films—A comparative study. Food Hydrocoll. 2014, 41, 195–209. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kordowska-Wiater, M.; Sołowiej, B.; Baraniak, B. Physicochemical and antimicrobial properties of biopolymer-Candelilla wax emulsion films containing potassium sorbate—A comparative study. Food Bioprocess Technol. 2015, 8, 567–579. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Quality changes of shrimp cracker covered with fish gelatin film without and with palm oil incorporated during storage. Int. Aquat. Res. 2016, 8, 227–238. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of chitosan films incorporated with lauric arginate, cinnamon oil, and ethylenediaminetetraacetate. LWT-Food Sci. Technol. 2016, 65, 173–179. [Google Scholar] [CrossRef]
- Villalobos, R.; Chanona, J.; Hernández, P.; Gutiérrez, G.; Chiralt, A. Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocoll. 2005, 19, 53–61. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Effects of soy lecithin levels and microfluidization conditions on properties of fish gelatin-based film incorporated with palm oil. Int. J. Food Eng. 2016, 12, 647–660. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Characteristics and antioxidant activity of leaf essential oil–incorporated fish gelatin films as affected by surfactants. Int. J. Food Sci. Technol. 2013, 48, 2143–2149. [Google Scholar] [CrossRef]
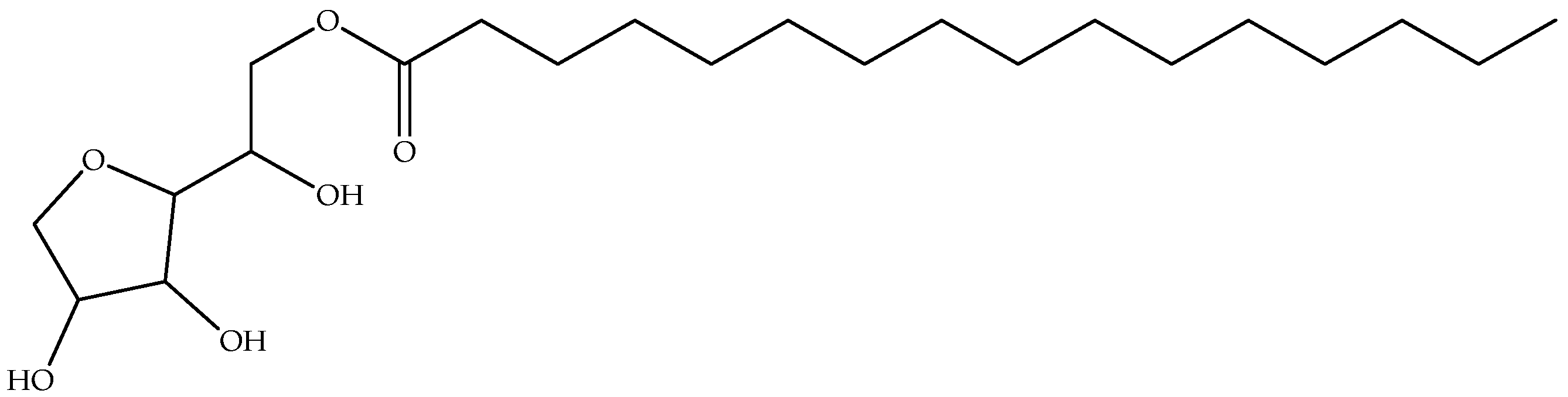
| Biopolymer | Additional Component | Processing Parameter | References |
|---|---|---|---|
| Kudzu starch | Glycerol | 3.0% (w/v) of kudzu starch, 0.9% (w/v) of glycerol, 100 mL of water, stirring at temperature of 100 °C. | [16] |
| CMC | Glycerol | 2.5 g/L of CMC, 5 g/L of glycerol, 350 mL of water, stirring at temperature of 60 °C. | [56] |
| Chitosan | Acetic acid, glycerol | 1 g of chitosan, 1 g of acetic acid, 20 g of glycerol, 100 mL of water, stirring at room temperature (25 ± 1 °C). | [24] |
| Gelatin | Glycerol | 3.5 g of gelatin, 30 wt% of glycerol, 90 mL of water, stirring at temperature of 70 °C. | [57] |
| Pectin | Glucomannan | 1% (w/v) of pectin, 0.75% (w/v) of glucomannan, 100 mL of water, stirring at room temperature (25 ± 1 °C). | [39] |
| Guar gum | Glycerol | 0.3 g of guar gum, 25 wt% of glycerol, 100 mL of water, stirring at temperature of 40 °C. | [17] |
| Pullulan | Glycerol | 2% (w/v) of pullulan, 15 wt% of glycerol, 100 mL of water, stirring at temperature of 45 °C. | [41] |
| Loquat seed starch | Sorbitol | 3.5% (w/v) of loquat seed starch, 45% (w/w) of sorbitol, 100 mL of water, stirring at temperature of 95 °C. | [15] |
| Starch/chitosan | Glycerol, glucose | 1.5% (w/v) of starch/chitosan, 1% (w/v) of glycerol or glucose, 100 mL of water, stirring at temperature of 90 °C. | [9] |
| Biopolymer | Plasticizer | Oil | Surfactant | Processing Parameter | References |
|---|---|---|---|---|---|
| Fish gelatin | Glycerol | Palm oil | Soy lecithin | 3.5% (w/v) of fish gelatin, 10% (w/w) of glycerol, 100 mL of water, 25% (w/w) of palm oil, 12.5% (w/w) of soy lecithin, homogenizing at 22,000 rpm for 3 min at 70 °C, casting the FFE onto a plastic Petri dish and air-blowing for 12 h prior to drying at 25 °C and 50% ± 5% RH for 48 h in an environmental chamber. | [34] |
| Tapioca starch/decolorized hsian-tsao gum | Glycerol | Beeswax | Sucrose stearate | 2% (w/v) of biopolymer blend, 10 wt% of glycerol, 100 mL of water, 10 wt% of beeswax, 10-wt% sucrose stearate, homogenizing at 10,000 rpm for 3 min at 95 °C, pouring the FFE onto a level circular Petri dish and drying in an environmental chamber at 50 °C and 58% RH for 1 h and sequentially at 25 °C for 96 h. | [1] |
| CMC, potato starch soy protein, pork gelatin | Sorbitol | Candelilla wax | Tween-40 | 5% (w/w) biopolymer, 3% (w/w) of sorbitol, 100 mL of water, 0.5% (w/w) of candelilla wax, 0.35% (w/w) of Tween-40, homogenizing at 20,000 rpm for 3 min at 90 °C, casting the FFE onto leveled polycarbonate tray and drying at 25 ± 1 °C for 24 h. | [56] |
| Carrageenan | Glycerol | Palm oil | Tween-20, Tween-40, Tween-80 | 1% (w/v) of carrageenan, 50% (w/w) of glycerol, 100 mL of water, 3% (v/v) of palm oil, 0.1%–0.5% (v/v) of surfactant, homogenizing at 13,500 rpm for 3 min at 80 °C, casting the FFE at the center of a circular glass plate and drying at 30 °C for 48 h. | [37] |
| Soy protein | Glycerol | Virgin coconut oil | YSE | 6.5% (w/w) of soy protein, 20% (w/w) of glycerol, 100 mL of water, 0.99%–6.54% (w/w) of virgin coconut oil, 9%–23% (w/w) of YSE, homogenizing at 20,500 rpm for 2 min at 70 ± 3 °C, dispersing the FFE on acrylic plates and drying at room temperature (25 ± 3 °C) for 24 h. | [44] |
| Chitosan | Glycerol | Cinnamon bark oil, soybean oil | Tween-80 | 2% (w/w) of chitosan, 20% (w/w) of glycerol, 100 mL of water, 1%–3% (w/w) of cinnamon or soybean oil, 40% (w/w) of Tween-80, homogenizing at 7000 rpm for 2 min at 21 °C, casting the FFE onto glass plate and drying at ambient conditions (21 °C) for 24 h. | [21] |
| Gelatin | Glycerol | Palm oil | Tween-80 | 3.9 wt% of gelatin, 33 wt% of glycerol, 100 mL of water, 36 wt% of palm oil, 20 wt% of Tween-80, homogenizing at 12,000 rpm for 3 min at 60 °C, casting the FFE evenly over a rimmed acrylic plate and drying at 25 °C in a convection chamber for 48 h. | [36] |
| Soy protein | Glycerol | Virgin coconut oil | Soy lecithin | 6.5% (w/w) of soy protein, 20% (w/w) of glycerol, 100 mL of water, 0.99%–6.54% (w/w) of virgin coconut oil, 9%–23% (w/w) of soy lecithin, homogenizing at 20,500 rpm for 2 min at 70 ± 3 °C, pouring the FFE on acrylic plate and drying at 25 ± 3 °C for 24 h. | [27] |
| Fish gelatin | Glycerol | Palm oil | Tween-20, soy lecithin, sodium dodecyl sulfate | 3.7 wt% of fish gelatin, 30 wt% of glycerol, 90 mL of water, 50 wt% of palm oil, 25 wt% of surfactant, homogenizing at 22,000 rpm for 3 min at 70 °C, casting the FFE onto a rimmed silicone resin plate and air-blowing for 12 h at 28–30 °C prior to further drying at 25 °C and 50% ± 5% RH for 24 h in an environmental chamber. | [43] |
| Surfactant | Type of Surfactant | Film-Forming | References |
|---|---|---|---|
| Span 20 to 80 | Non-ionic | Solution/Emulsion | [2,5,16,47] |
| Tween-20 to 80 | Non-ionic | Solution/Emulsion | [20,21,37,55] |
| Sucrose ester | Non-ionic | Solution/Emulsion | [1,51,59] |
| Sodium stearoyl lactate | Anionic | Solution | [39] |
| Sodium dodecyl sulfate | Anionic | Solution | [10,30] |
| Ethyl lauroyl arginate HCl | Cationic | Solution/Emulsion | [48,49,58] |
| Soy lecithin | Amphoteric | Solution/Emulsion | [2,28,35,43] |
| Non-Ionic Surfactant | HLB Value | Alkyl Chain Length | Biopolymer | Film-Forming | WVP | TS | EAB | Op | References |
|---|---|---|---|---|---|---|---|---|---|
| Span 40 | 6.7 | C16 | Corn starch | Solution | ↓ | ↓ | ↓ | ↓ | [5] |
| Span 80 | 4.3 | C18 | Corn/wheat starch | Emulsion | ↓ | ↓ | ↑ | ↑ | [6] |
| Tween-20 | 16.7 | C12 | Kudzu starch | Solution | ↓ | ↓ | ↑ | n/a | [16] |
| Tween-80 | 15.0 | C18 | Corn/wheat starch | Emulsion | ↓ | ↓ | ↓ | ↑ | [6] |
| Sucrose ester (S-1570) | 15.0 | C18 | Tapioca starch/decolorized hsian-tsao gum | Solution | ↓ | ↓ | ↓ | ↓ | [1] |
| Sucrose ester (S-1170) | 11.0 | C18 | Tapioca starch/decolorized hsian-tsao gum | Emulsion | ↓ | ↓ | ↓ | ↑ | [1] |
| Ionic Surfactant | HLB Value | Alkyl Chain Length | Biopolymer | Film-Forming | WVP | TS | EAB | Op | References |
|---|---|---|---|---|---|---|---|---|---|
| Sodium stearoyl lactate | 8.3 | C18 | Bovine skin Gelatin | Solution | ↓ | ↓ | ↑ | ↑ | [39] |
| Sodium dodecyl sulfate | 40 | C12 | Cassava starch | Solution | ↓ | ↑ | ↓ | ↑ | [10] |
| Ethyl lauroyl arginate HCl | 16 | C12 | Gelatin | Solution | ⇃↾ | ↑ | ↑ | ↓ | [49] |
| Ethyl lauroyl arginate HCl | 16 | C12 | Chitosan | Emulsion | ↑ | ↓ | ↑ | n/a | [58] |
| Biopolymer | Film-Forming | WVP | TS | EAB | Op | References |
|---|---|---|---|---|---|---|
| Soy protein | Emulsion | ↓ | ↓ | ↑ | ↑ | [27] |
| Fish gelatin | Emulsion | ↓ | ↓ | ↑ | ↑ | [43] |
| Fish gelatin | Emulsion | ↓ | ↓ | ↑ | ↑ | [61] |
| Pig hide gelatin | Solution | ↓ | ↓ | ↑ | ↑ | [53] |
| Pig hide gelatin | Solution | ↓ | ↓ | ↑ | ↑ | [28] |
| Potato starch | Solution | ↓ | ↓ | ↑ | n/a | [2] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamsuri, A.A.; Md. Jamil, S.N.A. Functional Properties of Biopolymer-Based Films Modified with Surfactants: A Brief Review. Processes 2020, 8, 1039. https://doi.org/10.3390/pr8091039
Shamsuri AA, Md. Jamil SNA. Functional Properties of Biopolymer-Based Films Modified with Surfactants: A Brief Review. Processes. 2020; 8(9):1039. https://doi.org/10.3390/pr8091039
Chicago/Turabian StyleShamsuri, Ahmad Adlie, and Siti Nurul Ain Md. Jamil. 2020. "Functional Properties of Biopolymer-Based Films Modified with Surfactants: A Brief Review" Processes 8, no. 9: 1039. https://doi.org/10.3390/pr8091039
APA StyleShamsuri, A. A., & Md. Jamil, S. N. A. (2020). Functional Properties of Biopolymer-Based Films Modified with Surfactants: A Brief Review. Processes, 8(9), 1039. https://doi.org/10.3390/pr8091039






