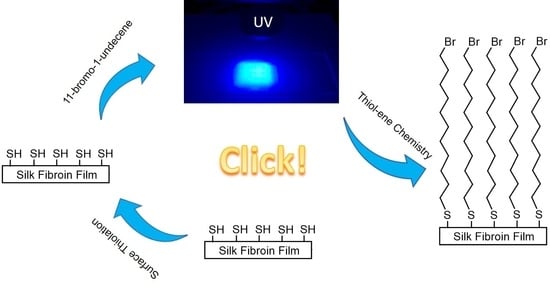
Surface Modification of Bombyx mori Silk Fibroin Film via Thiol-ene Click Chemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterization
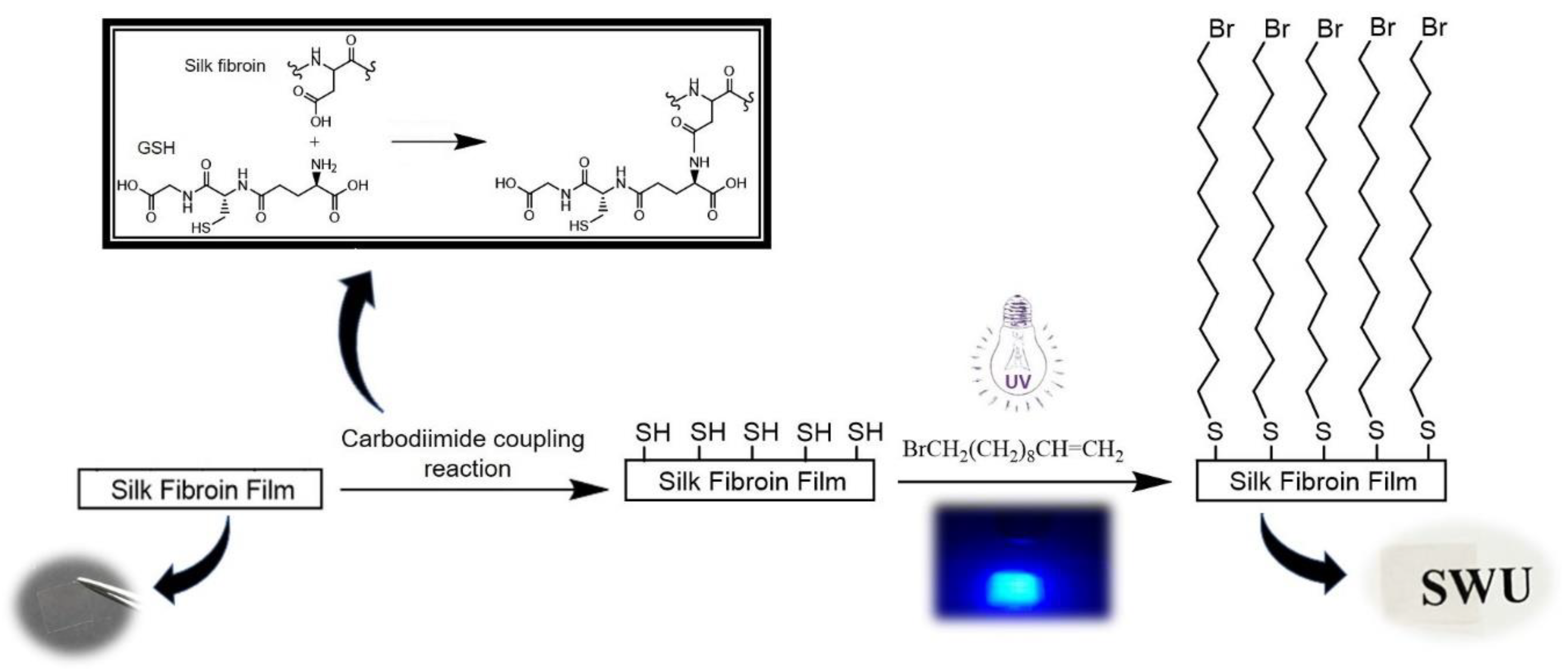
2.3. Covalent Coupling of BUD to SF Films
3. Results
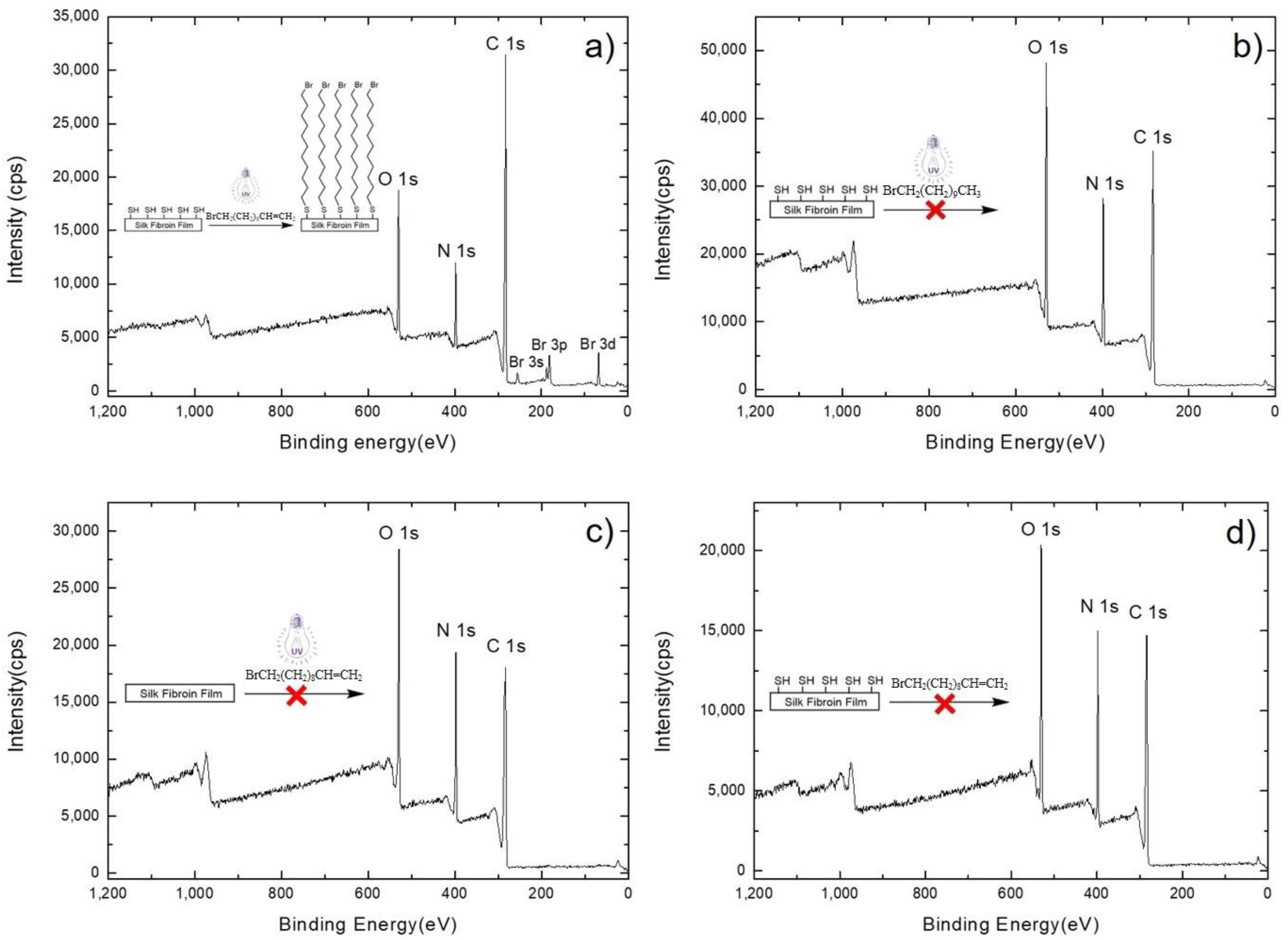
3.1. X-ray Photoelectron Spectroscopy Results
3.2. Differential Scanning Calorimetry Analysis
3.3. Static Contact Angle Measurement
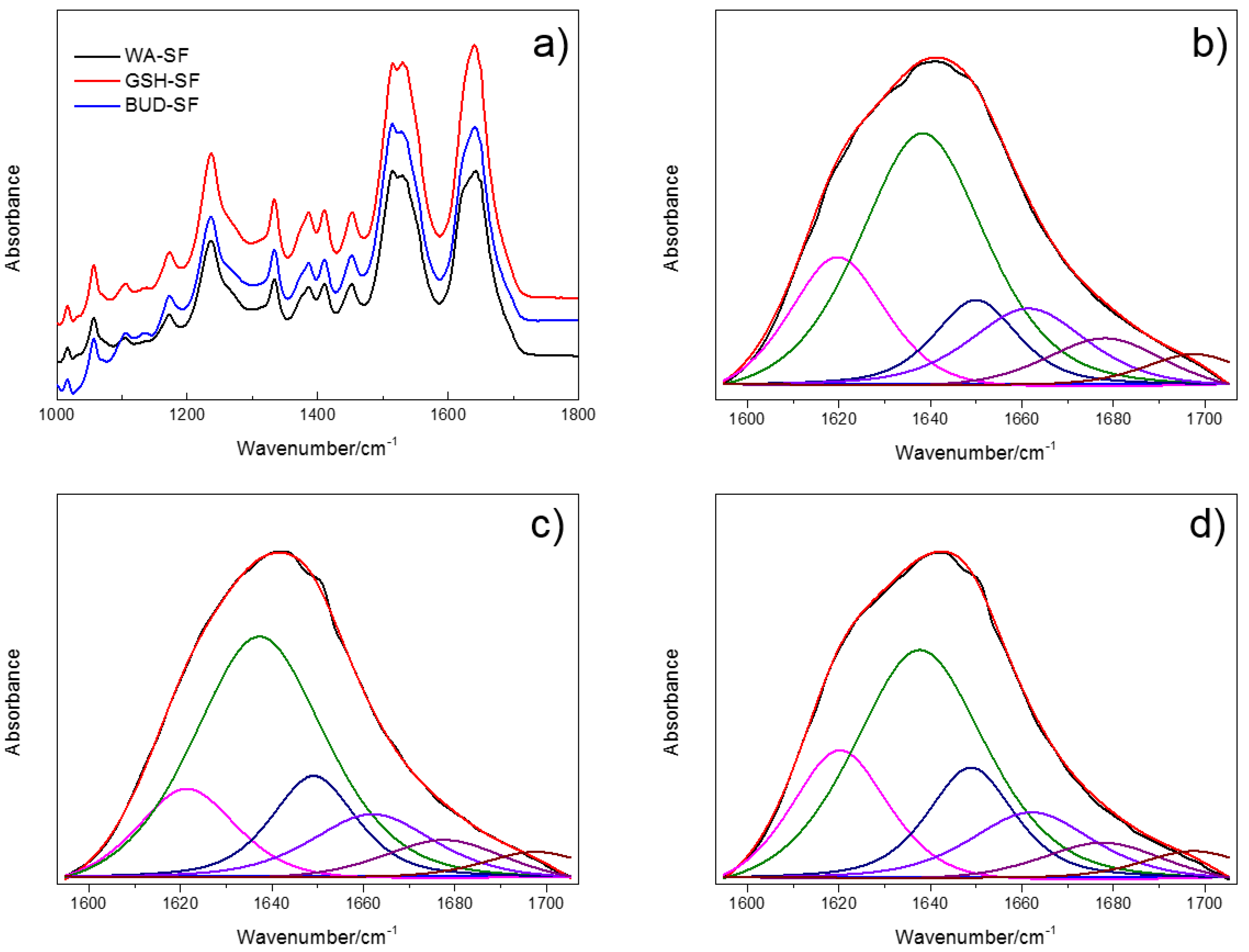
3.4. Infrared Spectroscopy Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Na2CO3 | Sodium carbonate |
| Na2HPO4 | Sodium phosphate dibasic dodecahydrate |
| NaH2PO4·2H2O | Sodium phosphate monobasic dihydrate |
| CaCl2 | Calcium chloride |
| C2H5OH | Ethanol |
| H2C=CH(CH2)8CH2Br | 11-bromo-1-undecene |
| CH3(CH2)9CH2Br | 1-bromoundecane |
References
- Kaplan, D.L.; Sears, R.; Vunjak-Novakovic, G.; Meinel, L. Silk Fibroin Materials and Use Thereof. Patents Number US8361617 B2, 12 June 2018. [Google Scholar]
- Mcgill, M.; Coburn, J.M.; Partlow, B.P.; Mu, X.; Kaplan, D.L. Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design. Acta Biomater. 2017, 63, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, Z.; Qiu, W.; Chen, F.; Meng, Z.; Hou, C.; Guo, W.; Liu, X.Y. Stretchable and heat-resistant protein-based electronic skin for human thermoregulation. Adv. Funct. Mater. 2020, 30, 1910547. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Zhang, Y.; Kaplan, D.L. Silk-based advanced materials for soft electronics. Acc. Chem. Res 2019, 52, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk materials—A road to sustainable high technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Koo, H. Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 2019, 10, 7835–7851. [Google Scholar] [CrossRef] [PubMed]
- Raynal, L.; Allardyce, B.J.; Wang, X.; Dilley, R.J.; Rajkhowa, R.; Henderson, L.C. Facile and versatile solid state surface modification of silk fibroin membranes using click chemistry. J. Mater. Chem. B 2018, 6, 8037–8042. [Google Scholar] [CrossRef] [PubMed]
- Pickens, C.J.; Johnson, S.N.; Pressnall, M.M.; Leon, M.A.; Berkland, C.J. Practical considerations, challenges, and limitations of bioconjugation via azide–alkyne cycloaddition. J. Bioconjugate. Chem. 2018, 29, 686–701. [Google Scholar] [CrossRef]
- National Research Council. Prudent Practices in the Laboratory: Handling and Disposal of Chemicals; National Academies Press: Washington, DC, USA, 1995; p. 102. [Google Scholar]
- McKenas, C.G.; Fehr, J.M.; Liu, B.; Donley, C.L.; Lockett, M.R. Mechanistic insights into UV-initiated thiol–ene reactions on amorphous carbon films. J. Phys. Chem. C 2018, 122, 21854–21860. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, H.H.; Park, Y.H.; Lin, C.C.; Um, I.C.; Ki, C.S. Dual mode gelation behavior of silk fibroin microgel embedded poly(ethylene glycol) hydrogels. J. Mater. Chem. B 2016, 4, 4574–4584. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Pan, Z.; Weber, M.D.; Kaplan, D.L.; Rosenblatt, M.I. Silk film culture system for in vitro analysis and biomaterial design. J. Visualized. Exp. 2012, 62, 3646. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.R.; Fang, Y.; Bao, H.; Wu, T.F.; Zhang, X.N.; Xu, S.; Zhu, Y. Preparation and properties of silk fibroin based bilayer dressing materials. J. Text. Res. 2020, 41, 13–19. [Google Scholar] [CrossRef]
- Sofia, S.; McCarthy, M.B.; Gronowicz, G.; Kaplan, D.L. Functionalized silk-based biomaterials for bone formation. J. Biomed. Mater. Res. 2001, 54, 139–148. [Google Scholar] [CrossRef]
- Sy Piecco, K.; Aboelenen, A.M.; Pyle, J.R.; Vicente, J.R.; Gautam, D.; Chen, J. Kinetic model under light-limited condition for photoinitiated thiol-ene coupling reactions. ACS Omega 2018, 3, 14327–14332. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Deng, C.-h.; Sun, N. Hydrophilic tripeptide-functionalized magnetic metal–organic frameworks for the highly efficient enrichment of N-linked glycopeptides. Nanoscale 2018, 10, 12149–12155. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta. Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
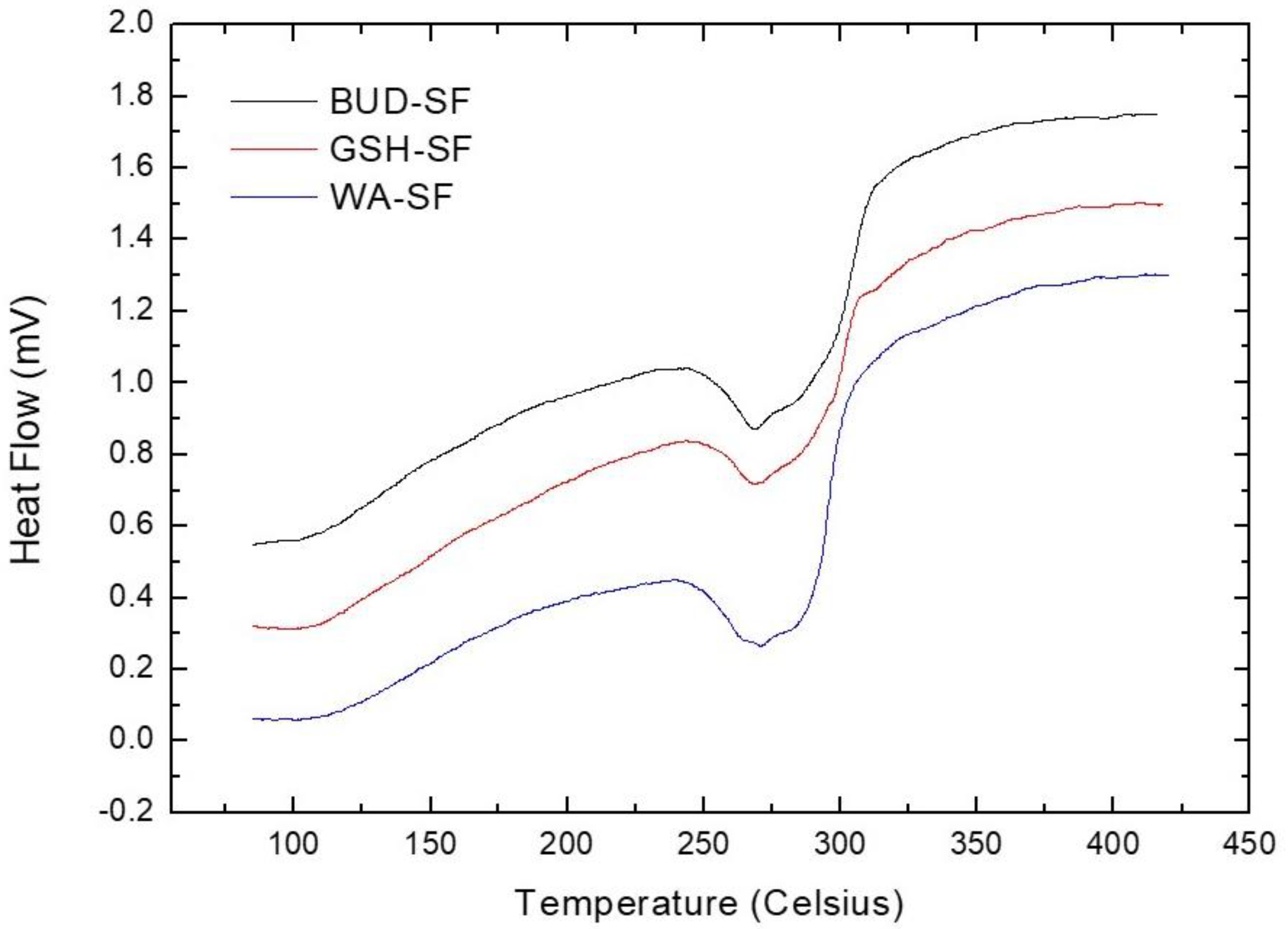
| Group | Degradation Temperatures |
|---|---|
| WA-SF | 269.1 ± 1.7 °C |
| GSH-SF | 270.5 ± 3.5 °C |
| BUD-SF | 269.7 ± 4.1 °C |
| Group | Contact Angle |
|---|---|
| WA-SF | 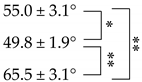 |
| GSH-SF | |
| BUD-SF |
| Assignment | WA-SF (%) | GSH-SF (%) | BUD-SF (%) |
|---|---|---|---|
| Side chains (1598–1605 cm−1) | 0.13 ± 0.23 | 0.07 ± 0.10 | 0.01 ± 0.01 |
| Silk II, β-sheet (1610–1635 cm−1, 1695–1700 cm−1) | 34.09 ± 4.37 | 29.96 ± 2.69 | 25.65 ± 2.16 |
| Random coil (1635–1645 cm−1) | 29.67 ± 4.95 | 37.31 ± 4.01 | 42.15 ± 2.01 |
| Silk I, Type II β-turn (1647–1654 cm−1) | 17.44 ± 1.84 | 13.82 ± 2.11 | 12.19 ± 1.55 |
| α-helix (1658–1664 cm−1) | 10.40 ± 1.95 | 11.19 ± 0.66 | 11.96 ± 0.52 |
| Turns and bends (1666–1695 cm−1) | 8.28 ± 0.84 | 7.69 ± 0.39 | 8.05 ± 0.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liang, J.; Chen, Z.; Donley, C.; Zhang, X.; Cheng, G. Surface Modification of Bombyx mori Silk Fibroin Film via Thiol-ene Click Chemistry. Processes 2020, 8, 498. https://doi.org/10.3390/pr8050498
Zhang X, Liang J, Chen Z, Donley C, Zhang X, Cheng G. Surface Modification of Bombyx mori Silk Fibroin Film via Thiol-ene Click Chemistry. Processes. 2020; 8(5):498. https://doi.org/10.3390/pr8050498
Chicago/Turabian StyleZhang, Xiaoning, Jianwei Liang, Zhenyu Chen, Carrie Donley, Xiaolin Zhang, and Guotao Cheng. 2020. "Surface Modification of Bombyx mori Silk Fibroin Film via Thiol-ene Click Chemistry" Processes 8, no. 5: 498. https://doi.org/10.3390/pr8050498
APA StyleZhang, X., Liang, J., Chen, Z., Donley, C., Zhang, X., & Cheng, G. (2020). Surface Modification of Bombyx mori Silk Fibroin Film via Thiol-ene Click Chemistry. Processes, 8(5), 498. https://doi.org/10.3390/pr8050498