Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction Conditions
2.4. HPLC Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microwave-Assisted Extraction (MAE)
3.2. Ultrasound-Assisted Extraction (UAE)
3.3. High Pressure-Assisted Extraction (HPAE)
3.4. Mutual Comparison of Advanced Extraction Techniques and with Conventional Heat-Reflux
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sahin, S.; Şamli, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Ben, S.M.; Abdelmelek, H. Study of phenolic composition and biological activities assessment of olive leaves from different varieties grown in Tunisia. Med. Chem. 2012, 2, 107–111. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of “Sikitita” olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents “Arbequina” and “Picual” olive leaves. LWT Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Ibañez, E.; Zarrouk, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Optimization of microwave-assisted extraction for the characterization of olive leaf phenolic compounds by using HPLC-ESI-TOF-MS/IT-MS2. J. Agric. Food Chem. 2012, 60, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Mkaouar, S.; Gelicus, A.; Bahloul, N.; Allaf, K.; Kechaou, N. Kinetic study of polyphenols extraction from olive (Olea europaea L.) leaves using instant controlled pressure drop texturing. Sep. Purif. Technol. 2016, 161, 165–171. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-ESI-QTOF-MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef]
- Bouaziz, M.; Sayadi, S. Isolation and evaluation of antioxidants from leaves of a Tunisian cultivar olive tree. Eur. J. Lipid Sci. Technol. 2005, 107, 497–504. [Google Scholar] [CrossRef]
- Mitsopoulos, G.; Hagidimitriou, M.; Papageorgiou, V.; Komaitis, M. Total phenolic content, phenolic profile and antioxidant activity in leaves and drupes of Greek olive cultivars. Acta Hortic. 2011, 924, 425–430. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer Science + Business Media: New York, NY, USA, 2013; ISBN 978-1-4614-4829-7. [Google Scholar]
- Hossain, M.; Brunton, N.; Patras, A.; Tiwari, B.; O’Donnell, C.; Martin-Diana, A.B.; Barry-Ryan, C. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason. Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef]
- Xi, J.; Shen, D.; Zhao, S.; Lu, B.; Li, Y.; Zhang, R. Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction. Int. J. Pharm. 2009, 382, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Hannachi, H.; Benmoussa, H.; Saadaoui, E.; Saanoun, I.; Negri, N.; Elfalleh, W. Optimization of ultrasound and microwave-assisted extraction of phenolic compounds from olive leaves by response surface methodology. Res. J. Biotechnol. 2019, 14, 28–37. [Google Scholar]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Microwave-Assisted extraction of phenolic compounds from olive leaves, A comparison with maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Spigno, G.; De Faveri, D.M. Microwave-Assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Buzrul, S.; Alpas, H.; Largeteau, A.; Bozoglu, F.; Demazeau, G. Compression heating of selected pressure transmitting fluids and liquid foods during high hydrostatic pressure treatment. J. Food Eng. 2008, 85, 466–472. [Google Scholar] [CrossRef]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef]
- Damtoft, S.; Franzyk, H.; Jensen, S.R. Biosynthesis of secoiridoid glucosides in Oleaceae. Phytochemistry 1993, 34, 1291–1299. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. The effect of minor constituents of olive oil on cardiovascular disease: New findings. Nutr. Rev. 1998, 56, 142–147. [Google Scholar] [CrossRef]
- Le Tutour, B.; Guedon, D. Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry 1992, 31, 1173–1178. [Google Scholar] [CrossRef]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuño, A.D.R.J.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Dekanski, D.; Janicijevic-Hudomal, S.; Tadic, V.; Markovic, G.; Arsic, I.; Mitrovic, D. Phytochemical analysis and gastroprotective activity of an olive leaf extract. J. Serb. Chem. Soc. 2009, 74, 367–377. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; De Castro, M.D.L. Multivariate optimisation of the microwave-assisted extraction of oleuropein and related biophenols from olive leaves. Anal. Bioanal. Chem. 2006, 385, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Murakami, M.; Yamaguchi, T.; Takamura, H.; Atoba, T.M. Effects of thermal treatment on radical-scavenging activity of single and mixed polyphenolic compounds. J. Food Sci. 2004, 69, FCT7–FCT10. [Google Scholar] [CrossRef]
- Ioannou, I.; Ghoul, M. Biological activities and effects of food processing on flavonoids as phenolics antioxidants. Adv. Appl. Biotechnol. 2012, 101–124. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Chimsook, T.; Wannalangka, W. Effect of ultrasonic-assisted extraction on phenolic content of freshwater macroalgae in northern Thailand. In Proceedings of the MATEC Web of Conferences, Lisbon, Portugal, 23–25 November 2015; Volume 35, p. 04002. [Google Scholar] [CrossRef]
- Elez, G.I.; Zorić, Z.; Pedisić, S.; Brnčić, M.; Dragović-Uzelac, V. UPLC-MS2 profiling of blackthorn flower polyphenols isolated by ultrasound-assisted extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef]
- Dent, M.; Dragovic-Uzelac, V.; Garofulic, I.E.; Bosiljkov, T.; Ježek, D.; Brncic, M. Comparison of conventional and ultrasound-assisted extraction techniques on mass fraction of phenolic compounds from sage (Salvia officinalis L.). Chem. Biochem. Eng. Q. 2015, 29, 475–484. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Optimization of ultrasound-assisted extraction conditions for recovery of phenolic compounds and antioxidant capacity from banana (Musa cavendish) peel. J. Food Process. Preserv. 2016, 2016, e13148. [Google Scholar] [CrossRef]
- Chen, R.; Meng, F.; Zhang, S.; Liu, Z. Effects of ultrahigh pressure extraction conditions on yields and antioxidant activity of ginsenoside from ginseng. Sep. Purif. Technol. 2009, 66, 340–346. [Google Scholar] [CrossRef]
- Butz, P.; Koller, W.D.; Tauscher, B.; Wolf, S. Ultra-High pressure processing of onions: Chemical and sensory changes. LWT Food Sci. Technol. 1994, 27, 463–467. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Zhao, M.; Wei, X.; Jiang, Y.; Chen, F. High pressure extraction of corilagin from longan (Dimocarpus longan Lour.) fruit pericarp. Sep. Purif. Technol. 2009, 70, 41–45. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Shouqin, Z.; Junjie, Z.; Changzhen, W. Novel high pressure extraction technology. Int. J. Pharm. 2004, 278, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Afaneh, I.; Yateem, H.; Al-Rimawi, F. Effect of Olive Leaves Drying on the Content of Oleuropein. Am. J. Anal. Chem. 2015, 6, 246–252. [Google Scholar] [CrossRef]
- Briante, R.; La Cara, F.; Febbraio, F.; Barone, R.; Piccialli, G.; Carolla, R.; Mainolfi, P.; De Napoli, L.; Patumi, M.; Fontanazza, G.; et al. Hydrolysis of oleuropein by recombinant b-glycosidase from hyperthermophilic archaeon. J. Biotechnol. 2000, 77, 275–286. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef]
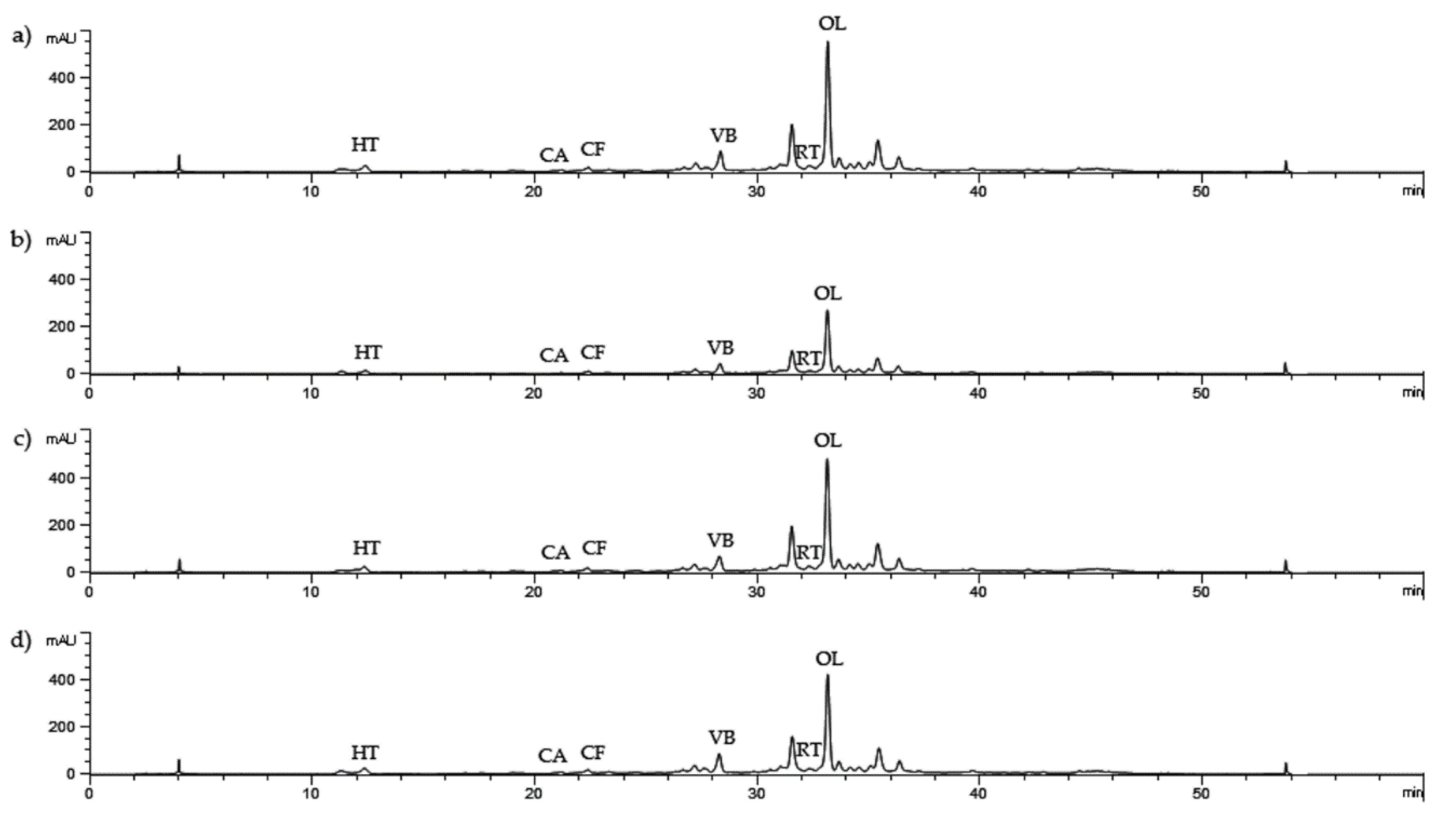
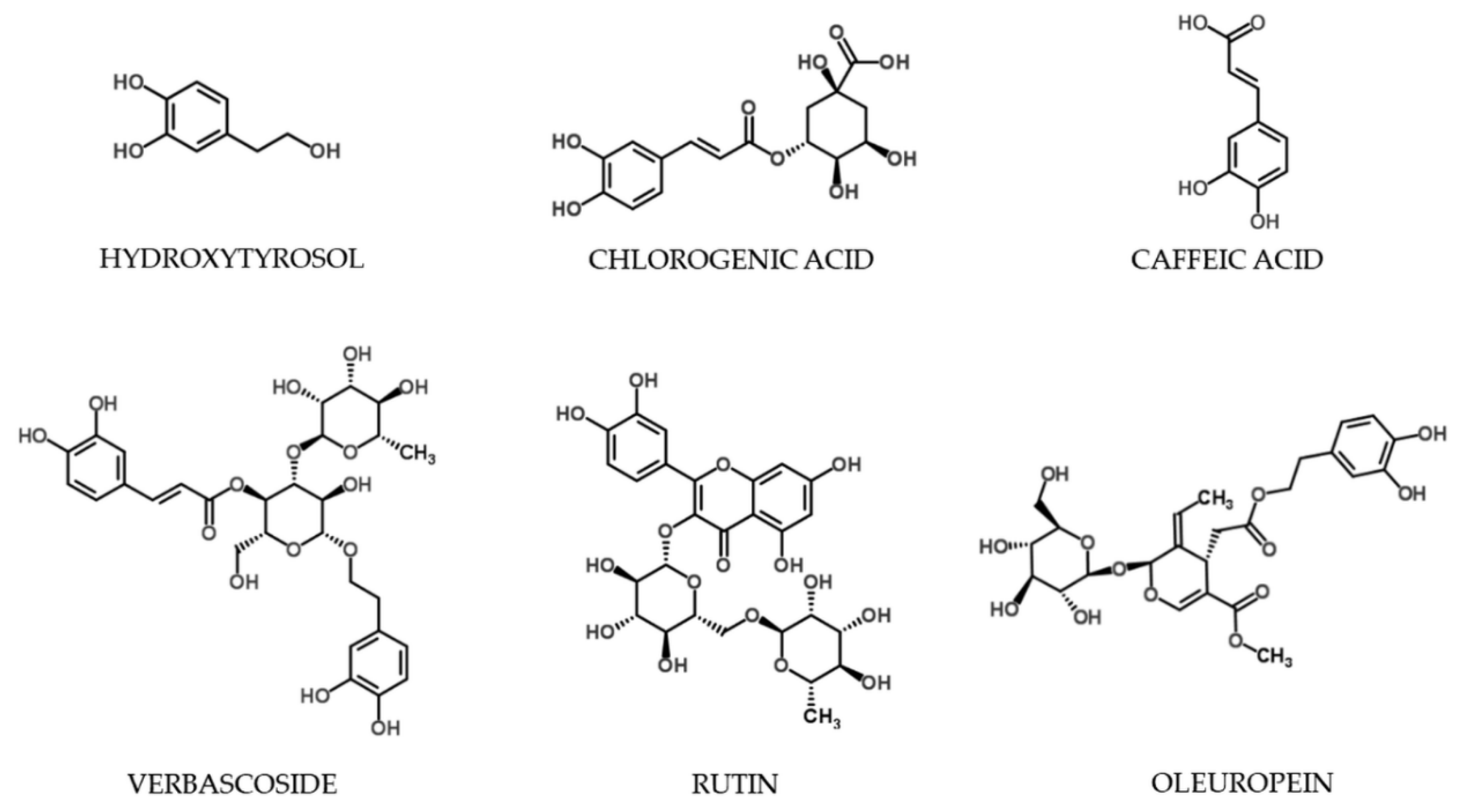

| Extraction Technique | Extraction Parameters | OL | HT | CA | CF | VB | RT | Total Sum | ||
|---|---|---|---|---|---|---|---|---|---|---|
| MAE | Time (min) | Temperature (°C) | Mass (g) | |||||||
| 2 | 45 | 1.5 | 74.11 ± 0.00 | 1.10 ± 0.42 | 0.44 ± 0.00 | 0.26 ± 0.00 | 0.50 ± 0.00 | 2.57 ± 0.00 | 78.98 ± 0.42 | |
| 2 | 45 | 3.0 | 69.51 ± 0.43 | 0.83 ± 0.00 | 0.45 ± 0.01 | 0.28 ± 0.00 | 0.35 ± 0.01 | 2.23 ± 0.01 | 73.65 ± 0.43 | |
| 2 | 80 | 1.5 | 83.41 ± 0.06 | 0.86 ± 0.00 | 0.42 ± 0.00 | 0.24 ± 0.01 | 0.55 ± 0.01 | 2.70 ± 0.02 | 88.19 ± 0.09 | |
| 2 | 80 | 3.0 | 87.75 ± 0.11 | 0.95 ± 0.03 | 0.46 ± 0.01 | 0.32 ± 0.01 | 0.41 ± 0.00 | 2.30 ± 0.01 | 92.18 ± 0.11 | |
| 8.5 | 45 | 1.5 | 52.14 ± 0.04 | 0.61 ± 0.01 | 0.44 ± 0.00 | 0.20 ± 0.00 | 0.31 ± 0.00 | 8.68 ± 0.06 | 62.38 ± 0.02 | |
| 8.5 | 45 | 3.0 | 65.93 ± 0.10 | 0.80 ± 0.01 | 0.41 ± 0.00 | 0.26 ± 0.01 | 0.33 ± 0.00 | 2.15 ± 0.07 | 69.89 ± 0.20 | |
| 8.5 | 80 | 1.5 | 84.82 ± 0.21 | 0.94 ± 0.00 | 0.45 ± 0.00 | 0.29 ± 0.01 | 0.55 ± 0.00 | 2.43 ± 0.01 | 89.49 ± 0.24 | |
| 8.5 | 80 | 3.0 | 82.44 ± 0.57 | 0.93 ± 0.03 | 0.41 ± 0.02 | 0.29 ± 0.00 | 0.40 ± 0.00 | 2.14 ± 0.27 | 86.61 ± 0.89 | |
| 15 | 45 | 1.5 | 64.03 ± 0.05 | 0.82 ± 0.01 | 0.44 ± 0.01 | 0.24 ± 0.01 | 0.47 ± 0.00 | 2.76 ± 0.01 | 68.75 ± 0.05 | |
| 15 | 45 | 3.0 | 70.88 ± 0.05 | 0.87 ± 0.01 | 0.48 ± 0.03 | 0.33 ± 0.04 | 0.34 ± 0.00 | 2.46 ± 0.00 | 75.36 ± 0.12 | |
| 15 | 80 | 1.5 | 80.66 ± 0.25 | 0.92 ± 0.00 | 0.43 ± 0.03 | 0.26 ± 0.01 | 0.54 ± 0.00 | 2.42 ± 0.03 | 85.23 ± 0.25 | |
| 15 | 80 | 3.0 | 82.01 ± 0.18 | 0.82 ± 0.17 | 0.44 ± 0.00 | 0.30 ± 0.01 | 0.42 ± 0.04 | 2.42 ± 0.00 | 86.41 ± 0.04 | |
| UAE | Time (min) | Amplitude (%) | Mass (g) | |||||||
| 7 | 50 | 1.5 | 79.28 ± 0.26 | 3.10 ± 1.11 | 0.46 ± 0.00 | 0.33 ± 0.00 | 0.50 ± 0.00 | 2.54 ± 0.01 | 86.20 ± 0.86 | |
| 7 | 50 | 3.0 | 76.42 ± 0.05 | 2.11 ± 0.00 | 0.53 ± 0.04 | 0.55 ± 0.04 | 0.39 ± 0.01 | 2.09 ± 0.04 | 82.07 ± 0.06 | |
| 7 | 100 | 1.5 | 67.30 ± 0.28 | 1.96 ± 0.21 | 0.37 ± 0.01 | 0.27 ± 0.01 | 0.49 ± 0.01 | 2.17 ± 0.00 | 72.54 ± 0.08 | |
| 7 | 100 | 3.0 | 84.62 ± 0.61 | 1.69 ± 0.04 | 0.59 ± 0.09 | 0.51 ± 0.16 | 0.42 ± 0.00 | 2.06 ± 0.04 | 89.88 ± 0.86 | |
| 14 | 50 | 1.5 | 60.33 ± 0.52 | 1.83 ± 0.06 | 0.35 ± 0.01 | 0.24 ± 0.01 | 0.47 ± 0.00 | 2.04 ± 0.00 | 65.24 ± 0.58 | |
| 14 | 50 | 3.0 | 56.84 ± 0.08 | 1.44 ± 0.01 | 0.39 ± 0.01 | 0.25 ± 0.01 | 0.37 ± 0.01 | 1.81 ± 0.00 | 61.09 ± 0.04 | |
| 14 | 100 | 1.5 | 66.01 ± 0.66 | 2.21 ± 0.02 | 0.42 ± 0.00 | 0.29 ± 0.01 | 0.52 ± 0.00 | 2.37 ± 0.01 | 71.81 ± 0.63 | |
| 14 | 100 | 3.0 | 81.38 ± 0.23 | 2.59 ± 0.02 | 0.53 ± 0.00 | 0.55 ± 0.00 | 0.44 ± 0.01 | 2.22 ± 0.02 | 87.69 ± 0.28 | |
| 21 | 50 | 1.5 | 85.90 ± 0.04 | 2.61 ± 0.01 | 0.50 ± 0.01 | 0.39 ± 0.01 | 0.56 ± 0.01 | 2.54 ± 0.01 | 92.49 ± 0.04 | |
| 21 | 50 | 3.0 | 74.35 ± 0.77 | 2.32 ± 0.04 | 0.48 ± 0.00 | 0.37 ± 0.01 | 0.39 ± 0.00 | 2.00 ± 0.00 | 79.90 ± 0.82 | |
| 21 | 100 | 1.5 | 77.91 ± 0.23 | 2.52 ± 0.02 | 0.48 ± 0.01 | 0.37 ± 0.01 | 0.54 ± 0.01 | 2.43 ± 0.03 | 84.23 ± 0.23 | |
| 21 | 100 | 3.0 | 80.91 ± 0.92 | 1.95 ± 0.00 | 0.54 ± 0.08 | 0.63 ± 0.00 | 0.42 ± 0.00 | 2.11 ± 0.02 | 86.55 ± 1.02 | |
| HPAE | Time (min) | Pressure (MPa) | Mass (g) | |||||||
| 1 | 300 | 1.5 | 69.76 ± 0.42 | 1.58 ± 1.03 | 0.48 ± 0.01 | 0.27 ± 0.00 | 0.47 ± 0.00 | 2.80 ± 0.06 | 75.35 ± 0.55 | |
| 1 | 300 | 3.0 | 67.76 ± 0.49 | 0.82 ± 0.00 | 0.39 ± 0.01 | 0.22 ± 0.01 | 0.50 ± 0.00 | 2.51 ± 0.06 | 72.19 ± 0.44 | |
| 1 | 500 | 1.5 | 72.19 ± 0.18 | 0.85 ± 0.00 | 0.43 ± 0.01 | 0.25 ± 0.01 | 0.51 ± 0.00 | 2.61 ± 0.05 | 76.82 ± 0.14 | |
| 1 | 500 | 3.0 | 72.26 ± 0.33 | 0.85 ± 0.01 | 0.42 ± 0.01 | 0.25 ± 0.01 | 0.52 ± 0.00 | 2.51 ± 0.01 | 76.80 ± 0.32 | |
| 5.5 | 300 | 1.5 | 73.00 ± 0.83 | 0.85 ± 0.00 | 0.45 ± 0.00 | 0.25 ± 0.04 | 0.50 ± 0.00 | 2.60 ± 0.15 | 77.64 ± 1.02 | |
| 5.5 | 300 | 3.0 | 72.74 ± 0.25 | 0.86 ± 0.01 | 0.42 ± 0.03 | 0.25 ± 0.01 | 0.52 ± 0.00 | 2.54 ± 0.02 | 77.32 ± 0.30 | |
| 5.5 | 500 | 1.5 | 66.64 ± 1.20 | 0.79 ± 0.20 | 0.33 ± 0.16 | 0.35 ± 0.07 | 0.35 ± 0.00 | 2.14 ± 0.03 | 70.60 ± 1.51 | |
| 5.5 | 500 | 3.0 | 71.47 ± 0.21 | 0.85 ± 0.23 | 0.22 ± 0.04 | 0.32 ± 0.01 | 0.38 ± 0.00 | 2.27 ± 0.01 | 75.50 ± 0.45 | |
| 10 | 300 | 1.5 | 66.29 ± 0.49 | 0.95 ± 0.01 | 0.43 ± 0.01 | 0.30 ± 0.01 | 0.36 ± 0.00 | 2.26 ± 0.01 | 70.58 ± 0.50 | |
| 10 | 300 | 3.0 | 74.56 ± 1.98 | 1.04 ± 0.00 | 0.34 ± 0.13 | 0.30 ± 0.00 | 0.39 ± 0.01 | 2.25 ± 0.05 | 78.87 ± 2.05 | |
| 10 | 500 | 1.5 | 60.48 ± 0.95 | 0.77 ± 0.19 | 0.39 ± 0.00 | 0.26 ± 0.00 | 0.33 ± 0.01 | 2.04 ± 0.03 | 64.26 ± 0.73 | |
| 10 | 500 | 3.0 | 63.63 ± 0.18 | 0.65 ± 0.01 | 0.38 ± 0.01 | 0.26 ± 0.00 | 0.35 ± 0.00 | 1.93 ± 0.00 | 67.19 ± 0.20 | |
| Extraction Technique | Source of Variation | OL | HT | CA | CF | VB | RT | Total Sum |
|---|---|---|---|---|---|---|---|---|
| MAE | Time (min) | p < 0.01 * | p = 0.26 ns | p = 0.05 * | p = 0.03 * | p < 0.01 * | p < 0.01 * | p < 0.01 * |
| 2 | 78.69 ± 0.08 c | 0.93 ± 0.05 a | 0.44 ± 0.00 ab | 0.27 ± 0.00 ab | 0.45 ± 0.00 b | 2.45 ± 0.03 a | 83.25 ± 0.12 c | |
| 8.5 | 71.33 ± 0.08 a | 0.82 ± 0.05 a | 0.43 ± 0.00 a | 0.26 ± 0.00 a | 0.40 ± 0.00 a | 3.85 ± 0.03 b | 77.09 ± 0.12 a | |
| 15 | 74.40 ± 0.08 b | 0.86 ± 0.05 a | 0.45 ± 0.00 c | 0.28 ± 0.00 c | 0.44 ± 0.00 b | 2.51 ± 0.03 a | 78.94 ± 0.12 b | |
| Temperature (°C) | p < 0.01 * | p = 0.25 ns | p = 0.21 ns | p < 0.01 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | |
| 45 | 66.10 ± 0.07 a | 66.10 ± 0.04 a | 0.44 ± 0.00 a | 0.26 ± 0.00 a | 0.38 ± 0.00 a | 3.47 ± 0.02 b | 71.50 ± 0.10 a | |
| 80 | 83.51 ± 0.07 b | 83.51 ± 0.04 a | 0.44 ± 0.00 a | 0.28 ± 0.00 b | 0.48 ± 0.00 b | 2.40 ± 0.02 a | 88.02 ± 0.10 b | |
| Mass (g) | p < 0.01 * | p = 0.89 ns | p = 0.53 ns | p < 0.01 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | |
| 1.5 | 73.19 ± 0.07 a | 0.88 ± 0.04 a | 0.44 ± 0.00 a | 0.25 ± 0.00 a | 0.49 ± 0.00 b | 3.59 ± 0.02 b | 78.83 ± 0.10 a | |
| 3.0 | 76.42 ± 0.07 b | 0.87 ± 0.04 a | 0.44 ± 0.00 a | 0.30 ± 0.00 b | 0.37 ± 0.00 a | 2.28 ± 0.02 a | 80.68 ± 0.10 b | |
| UAE | Time (min) | p < 0.01 * | p = 0.16 ns | p < 0.01 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | p < 0.01 * |
| 7 | 76.90 ± 0.17 b | 2.21 ± 0.12 a | 0.48 ± 0.01 b | 0.41 ± 0.02 b | 0.45 ± 0.00 a | 2.21 ± 0.01 b | 82.67 ± 0.21 b | |
| 14 | 66.14 ± 0.17 a | 2.01 ± 0.12 a | 0.42 ± 0.01 a | 0.33 ± 0.02 a | 0.45 ± 0.00 a | 2.11 ± 0.01 a | 71.46 ± 0.21 a | |
| 21 | 79.77 ± 0.17 c | 2.35 ± 0.12 a | 0.50 ± 0.01 b | 0.44 ± 0.02 b | 0.47 ± 0.00 b | 2.27 ± 0.01 c | 85.79 ± 0.21 c | |
| Amplitude (%) | p < 0.01 * | p = 0.55 ns | p = 0.03 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | |
| 50 | 72.18 ± 0.14 a | 2.23 ± 0.09 a | 0.45 ± 0.01 a | 0.35 ± 0.01 a | 0.45 ± 0.00 a | 2.17 ± 0.01 a | 77.83 ± 0.17 a | |
| 100 | 76.35 ± 0.14 b | 2.15 ± 0.09 a | 0.48 ± 0.01 b | 0.43 ± 0.01 b | 0.47 ± 0.00 b | 2.22 ± 0.01 b | 82.12 ± 0.17 b | |
| Mass (g) | p < 0.01 * | p = 0.02 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | |
| 1.5 | 72.79 ± 0.14 a | 2.37 ± 0.09 b | 0.43 ± 0.01 a | 0.31 ± 0.01 a | 0.51 ± 0.00 b | 2.35 ± 0.01 b | 78.75 ± 0.17 a | |
| 3.0 | 75.75 ± 0.14 b | 2.02 ± 0.09 a | 0.51 ± 0.01 b | 0.47 ± 0.01 b | 0.40 ± 0.00 a | 2.05 ± 0.01 a | 81.20 ± 0.17 b | |
| HPAE | Time (min) | p < 0.01 * | p = 0.37 ns | p = 0.16 ns | p = 0.04 * | p < 0.01 * | p < 0.01 * | p < 0.01 * |
| 1 | 68.91 ± 0.29 b | 1.01 ± 0.11 a | 0.35 ± 0.02 a | 0.29 ± 0.01 c | 0.42 ± 0.00 a | 2.43 ± 0.02 b | 73.41 ± 0.31 b | |
| 5.5 | 71.32 ± 0.29 c | 0.92 ± 0.11 a | 0.40 ± 0.02 a | 0.27 ± 0.01 ab | 0.44 ± 0.00 b | 2.40 ± 0.02 b | 75.76 ± 0.31 c | |
| 10 | 67.46 ± 0.29 a | 0.78 ± 0.11 a | 0.41 ± 0.02 a | 0.25 ± 0.01 a | 0.42 ± 0.00 a | 2.27 ± 0.02 a | 71.60 ± 0.31 a | |
| Pressure (MPa) | p < 0.01 * | p = 0.37 ns | p = 0.04 * | p = 0.19 ns | p < 0.01 * | p = 0.01 * | p < 0.01 * | |
| 300 | 68.06 ± 0.23 a | 0.96 ± 0.09 a | 0.42 ± 0.02 b | 0.28 ± 0.01 a | 0.42 ± 0.00 a | 2.41 ± 0.02 b | 72.54 ± 0.25 a | |
| 500 | 70.40 ± 0.23 b | 0.84 ± 0.09 a | 0.36 ± 0.02 a | 0.26 ± 0.01 a | 0.44 ± 0.00 b | 2.33 ± 0.02 a | 74.64 ± 0.25 b | |
| Mass (g) | p < 0.01 * | p = 0.34 ns | p = 0.01 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | p < 0.01 * | |
| 1.5 | 71.28 ± 0.23 b | 0.97 ± 0.09 a | 0.43 ± 0.02 b | 0.25 ± 0.01 a | 0.50 ± 0.00 b | 2.59 ± 0.02 b | 76.02 ± 0.25 b | |
| 3.0 | 67.18 ± 0.23 a | 0.84 ± 0.09 a | 0.35 ± 0.02 a | 0.30 ± 0.01 b | 0.36 ± 0.00 a | 2.15 ± 0.02 a | 71.16 ± 0.25 a | |
| 300 | 68.06 ± 0.23 a | 0.96 ± 0.09 a | 0.42 ± 0.02 b | 0.28 ± 0.01 a | 0.42 ± 0.00 a | 2.41 ± 0.02 b | 72.54 ± 0.25 a |
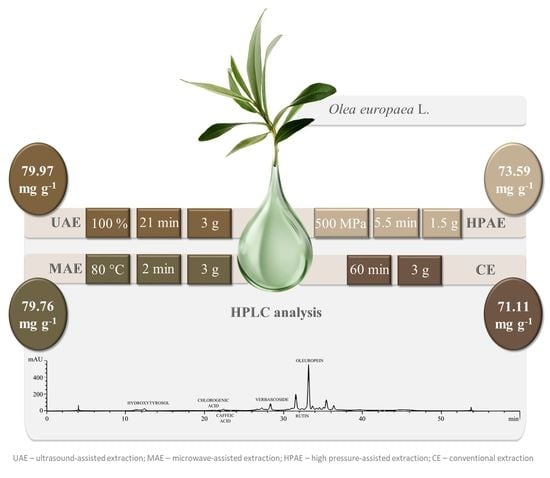
| Extraction Technique | OL | HT | CA | CF | VB | RT | Total Sum |
|---|---|---|---|---|---|---|---|
| MAE | 74.81 ± 2.12 | 0.87 ± 0.03 | 0.44 ± 0.00 | 0.27 ± 0.01 | 0.43 ± 0.02 | 2.94 ± 0.36 | 79.76 ± 1.92 |
| UAE | 74.27 ± 1.90 | 2.19 ± 0.10 | 0.47 ± 0.02 | 0.39 ± 0.03 | 0.46 ± 0.01 | 2.20 ± 0.05 | 79.97 ± 2.01 |
| HPAE | 69.23 ± 0.87 | 0.90 ± 0.07 | 0.39 ± 0.02 | 0.27 ± 0.01 | 0.43 ± 0.02 | 2.37 ± 0.05 | 73.59 ± 0.93 |
| CE | 67.05 ± 1.21 | 0.89 ± 0.07 | 0.41 ± 0.01 | 0.27 ± 0.01 | 0.47 ± 0.02 | 2.01 ± 0.06 | 71.11 ± 1.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrinčić, A.; Repajić, M.; Garofulić, I.E.; Tuđen, L.; Dragović-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes 2020, 8, 1008. https://doi.org/10.3390/pr8091008
Dobrinčić A, Repajić M, Garofulić IE, Tuđen L, Dragović-Uzelac V, Levaj B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes. 2020; 8(9):1008. https://doi.org/10.3390/pr8091008
Chicago/Turabian StyleDobrinčić, Ana, Maja Repajić, Ivona Elez Garofulić, Lucija Tuđen, Verica Dragović-Uzelac, and Branka Levaj. 2020. "Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols" Processes 8, no. 9: 1008. https://doi.org/10.3390/pr8091008
APA StyleDobrinčić, A., Repajić, M., Garofulić, I. E., Tuđen, L., Dragović-Uzelac, V., & Levaj, B. (2020). Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes, 8(9), 1008. https://doi.org/10.3390/pr8091008