Whole-Cell Biotransformation of 1,12-Dodecanedioic Acid from Coconut Milk Factory Wastewater by Recombinant CYP52A17SS Expressing Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganism and Cultivation Conditions
2.3. Biotransformation of CCW-Based Media to DDA and Its pH and Temperature Optimization
2.3.1. Role of the Recombinant CYP52A17SS and CCW-Based Media
2.3.2. Optimal Temperature
2.3.3. Optimal Initial pH of the Medium
2.4. Product Extraction
2.5. Product Analysis
2.6. Statistical Analysis
3. Results and Discussion
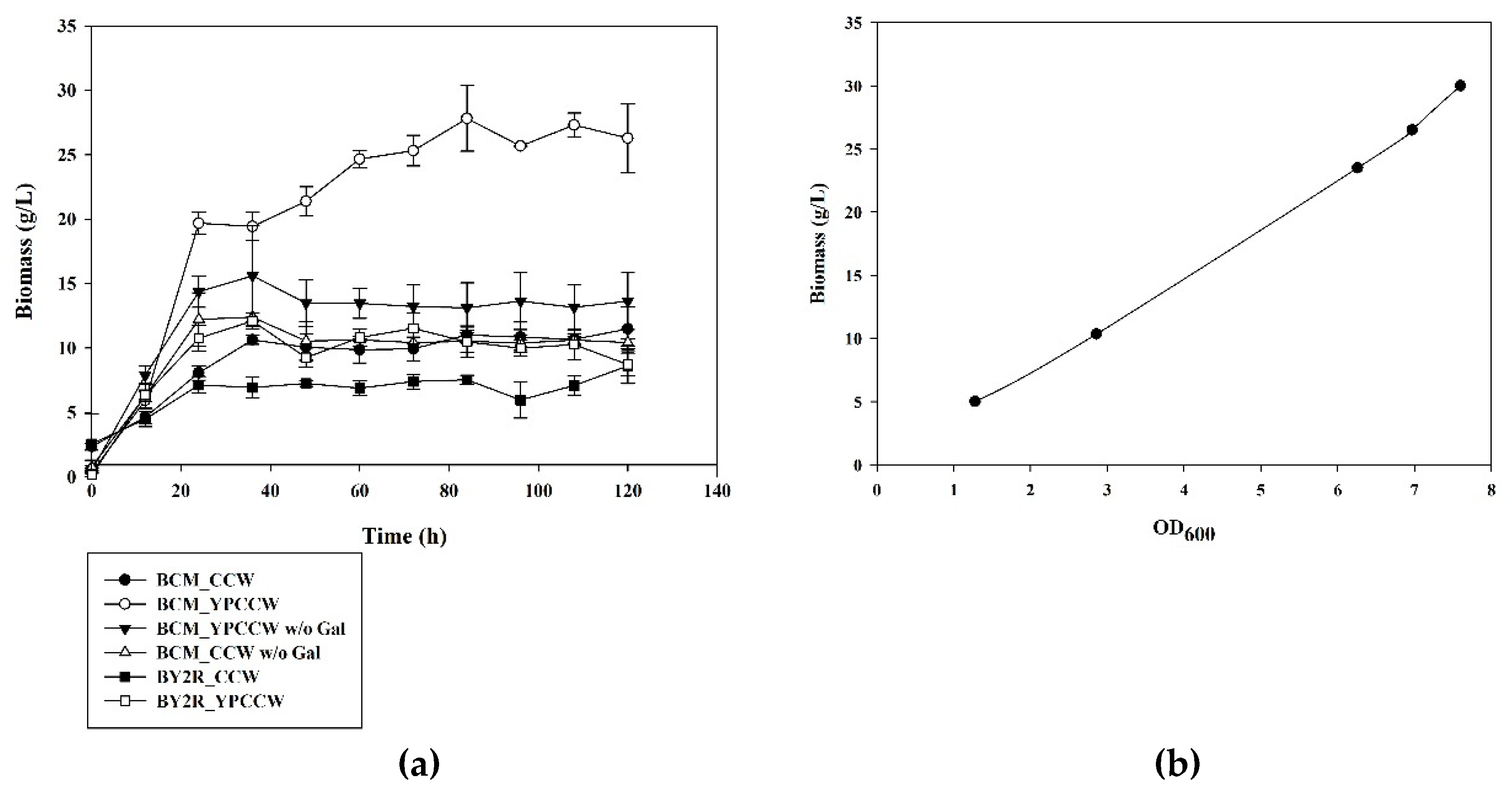
3.1. Cell Growth of S. cerevisiae BCM in CCW and YPCCW with and without Galactose Induction of CYP52A17 Expression
3.2. Production of DDA and HDDA from YPCCW (with Galactose) Using S. cerevisiae BCM
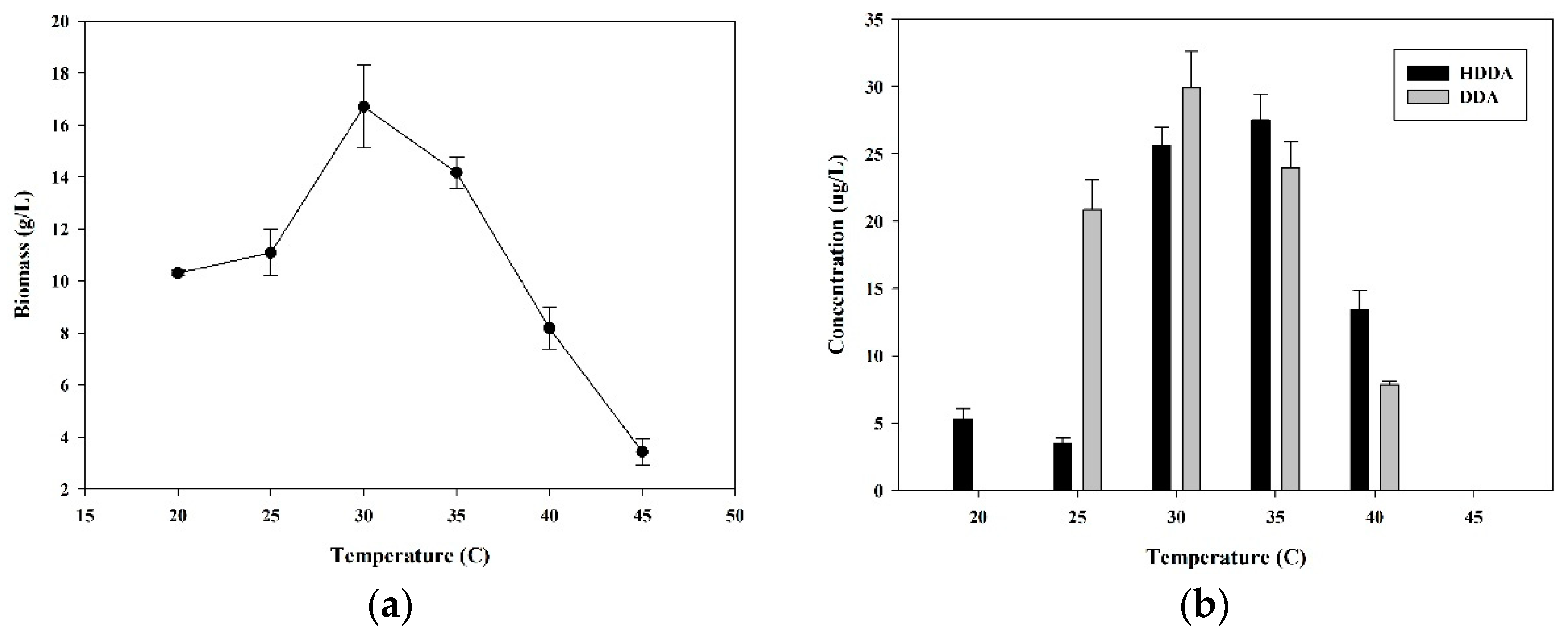
3.3. Effect of Temperature on HDDA and DDA Production
3.4. Effect of pH on DDA Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakpong, P.; Wootthikanokkhan, S. High free fatty acid coconut oil as a potential feedstock for biodiesel production in Thailand. Renew. Energy 2010, 35, 1682–1687. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Lucena, I.L.; Saboya, R.M.; Rodrigues, M.L.; Torres, A.E.; Fernandes, F.A.; Cavalcante, C.L., Jr.; Parente, E.J., Jr. Biodiesel production from waste coconut oil by esterification with ethanol: The effect of water removal by adsorption. Renew. Energy 2010, 35, 2581–2584. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Watsanit, K.; Adachi, S. Carbohydrate content and composition of product from subcritical water treatment of coconut meal. Ind. Eng. Chem. 2012, 18, 225–229. [Google Scholar] [CrossRef]
- Sulaiman, S.; Aziz, A.R.A.; Aroua, M.K. Reactive extraction of solid coconut waste to produce biodiesel. J. Taiwan Inst. Chem. Eng. 2013, 44, 233–238. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kuramochi, H.; Xu, K.Q. Variable oil properties and biomethane production of grease trap waste derived from different resources. Int. Biodeterior. Biodegrad. 2017, 119, 273–281. [Google Scholar] [CrossRef]
- Lertsriwong, S.; Comwien, J.; Chulalaksananukul, W.; Glinwong, C. Isolation and identification of anaerobic bacteria from coconut wastewater factory for ethanol, butanol and 2,3 butanediol production. Int. Biodeterior. Biodegrad. 2017, 119, 461–466. [Google Scholar] [CrossRef]
- Buathong, P.; Boonvitthya, N.; Truan, G.; Chulalaksananukul, W. Biotransformation of lauric acid into 1, 12-dodecanedioic acid using CYP52A17 expressed in Saccharomyces cerevisiae and its application in refining coconut factory wastewater. Int. Biodeterior. Biodegrad. 2019, 139, 70–77. [Google Scholar] [CrossRef]
- Naksagul, N.; Antrakarnapa, K.; Silapanintakul, S. Biogas production from treatment of coconut milk wastewater using anaerobic process. Thai Environ. Eng. J. 2006, 20, 1–10. [Google Scholar]
- Garuda, E. Notification of the Ministry of Science Technology and Environment. No.4 B.E. 2539, 1996. Issued under the Enhancement and Conservation of the National Environmental Quality Act B.E. 2535 (1992). Specifies that Pollution Sources that the above Standards Are to Be Applied Are Factories Group II and III Issued under the Factory Act B.E. 2535 (1992) and Every Kind of Industrial Estates. Royal Government Gazette, 13 February 1996; Part 13 D. Volume 113. [Google Scholar]
- Nur, M.A.; Irawan, M.A. Utilization of coconut milk skim effluent (cmse) as medium growth for Spirulina platensis. Procedia Environ. Sci. 2015, 23, 72–77. [Google Scholar]
- Karnasuta, S.; Punsuvon, V.; Nokkaew, R. Biodiesel production from waste coconut oil in coconut milk manufacturing. Walailak J. Sci. Technol. 2014, 12, 291–298. [Google Scholar]
- Dayrit, F.M. The properties of lauric acid and their significance in coconut oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Seo, E.J.; Yeon, Y.J.; Seo, J.H.; Lee, J.H.; Boñgol, J.P.; Oh, Y.; Park, J.M.; Lim, S.M.; Lee, C.G.; Park, J.B. Enzyme/whole-cell biotransformation of plant oils, yeast derived oils, and microalgae fatty acid methyl esters into n-nonanoic acid, 9-hydroxynonanoic acid, and 1,9-nonanedioic acid. Bioresour. Technol. 2018, 251, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Peng, Y.; Zhang, Y.; Chen, W.; Lin, Y.; Wang, Q. Designing and creating a synthetic omega oxidation pathway in Saccharomyces cerevisiae enables production of medium-chain α,ω-dicarboxylic acids. Front. Microbiol. 2017, 8, 2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eschenfeldt, W.H.; Zhang, Y.; Samaha, H.; Stols, L.; Eirich, L.D.; Wilson, C.R.; Donnelly, M.I. Transformation of fatty acids catalyzed by cytochrome P450 monooxygenase enzymes of Candida tropicalis. Appl. Environ. Microbiol. 2003, 69, 5992–5999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eirich, L.D.; Craft, D.L.; Steinberg, L.; Asif, A.; Eschenfeldt, W.H.; Stols, L.; Donnelly, M.I.; Wilson, C.R. Cloning and characterization of three fatty alcohol oxidase genes from Candida tropicalis strain ATCC 20336. Appl. Environ. Microbiol. 2004, 70, 4872–4879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Ness, J.E.; Xie, W.; Zhang, X.; Minshull, J.; Gross, R.A. Biosynthesis of monomers for plastics from renewable oils. J. Am. Chem. Soc. 2010, 132, 15451–15455. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, H.; Liu, M.; Jiao, P. Engineering the acetyl-CoA transportation system of Candida tropicalis enhances the production of dicarboxylic acid. Biotechnol. J. 2006, 1, 68–74. [Google Scholar] [CrossRef]
- Park, H.; Park, G.; Jeon, W.; Ahn, J.O.; Yang, Y.H.; Choi, K.Y. Whole-cell biocatalysis using cytochrome P450 monooxygenases for biotransformation of sustainable bioresources (fatty acids, fatty alkanes, and aromatic amino acids). Biotechnol. Adv. 2020, 40, 107504. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, S.M.; Lee, J.; Park, J.B. Adding value to plant oils and fatty acids: Biological transformation of fatty acids into ω-hydroxycarboxylic, α,ω-dicarboxylic, and ω-aminocarboxylic acids. J. Biotechnol. 2015, 216, 158–166. [Google Scholar] [CrossRef]
- Van Roermund, C.W.T.; Waterham, H.R.; Ijlst, L.; Wanders, R.J.A. Fatty acid metabolism in Saccharomyces cerevisiae. Cell. Mol. Life Sci. 2003, 60, 1838–1851. [Google Scholar] [CrossRef]
- Durairaj, P.; Malla, S.; Nadarajan, S.P.; Lee, P.G.; Jung, E.; Park, H.H.; Kim, B.G.; Yun, H. Fungal cytochrome P450 monooxygenases of Fusarium oxysporum for the synthesis of ω-hydroxy fatty acids in engineered Saccharomyces cerevisiae. Microb. Cell Factories 2015, 14, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truan, G.; Cullin, C.; Reisdorf, P.; Urban, P.; Pompon, D. Enhanced in vivo monooxygenase activities of mammalian P450s in engineered yeast cells producing high levels of NADPH-P450 reductase and human cytochrome b5. Gene 1993, 125, 49–55. [Google Scholar] [CrossRef]
- De Carvalho, C.C. Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnol. Adv. 2011, 29, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hägerdal, B.; Karhumaa, K.; Larsson, C.U.; Gorwa-Grauslund, M.; Görgens, J.; Van Zyl, W.H. Role of cultivation media in the development of yeast strains for large scale industrial use. Microb. Cell Factories 2005, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Han, C.; Lee, H.W.; Park, G.; Jeon, W.; Ahn, J.; Lee, H. Development of a promising microbial platform for the production of dicarboxylic acids from biorenewable resources. Biotechnol. Biofuels 2018, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Akmalina, R.; Purwadi, R.; Sitompul, J. Bioconversion Studies of Methyl Laurate to Dodecanedioic Acid using a Wild-type of Candida tropicalis. In Proceedings of the MATEC Web of Conferences, Semarang Indonesia, 15–16 November 2017; EDP Sciences: Ulis, France, 2018; Volume 156, p. 01001. [Google Scholar]
- Bestawy, E.; Ahmed, A.H.; Amer, R.; Kashmeri, R.A. Decontamination of domestic wastewater using suspended individual and mixed bacteria in batch system. J. Bioremediat. Biodegrad. 2014, 5, 1. [Google Scholar] [CrossRef]
- Scheps, D.; Malca, S.H.; Richter, S.; Marisch, K.; Nestl, B.M.; Hauer, B. Production of ω-hydroxy dodecanoic acid based on an engineered CYP153A fusion construct. Microb. Biotechnol. 2013, 6, 694–707. [Google Scholar]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. 2017, 10, 52–61. [Google Scholar]
- Qi, W.; Zhang, W.T.; Lu, F.P. Effect of temperature, NaCl and ferulic acid concentration on bioconversion of ferulic acid to 4-vinylguaiacol and 4-ethylguaiacol by halotolerant yeasts Candida versatilis. In Proceedings of the International Conference on Applied Biotechnology, Tianjin, China, 25–27 November 2016; Springer: Singapore, 2016; pp. 289–297. [Google Scholar]
- Green, K.D.; Turner, M.K.; Woodley, J.M. Candida cloacae oxidation of long-chain fatty acids to dioic acids. Enzym. Microb. Technol. 2000, 27, 205–211. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Xie, L. Intracellular pH and metabolic activity of long-chain dicarboxylic acid-producing yeast Candida tropicalis. J. Biosci. Bioeng. 2003, 96, 349–353. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Fang, X.; Cao, Z.A. Optimal pH control strategy for high-level production of long-chain α, ω-dicarboxylic acid by Candida tropicalis. Enzym. Microb. Technol. 2004, 34, 73–77. [Google Scholar] [CrossRef]
- Huf, S.; Krügener, S.; Hirth, T.; Rupp, S.; Zibek, S. Biotechnological synthesis of long-chain dicarboxylic acids as building blocks for polymers. Eur. J. Lipid Sci. Technol. 2011, 113, 548–561. [Google Scholar] [CrossRef]
- Cao, W.; Li, H.; Luo, J.; Yin, J.; Wan, Y. High-level productivity of α, ω-dodecanedioic acid with a newly isolated Candida viswanathii strain. J. Ind. Microbiol. Biotechnol. 2017, 44, 1191–1202. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Value |
|---|---|
| pH | 5.5 ± 0.1 |
| TS (mg/L) | 5836 ± 248 |
| TSS (mg/L) | 5270 ± 197 |
| Total Kjeldahl nitrogen (mg/L) | 83.6 ± 4.5 |
| VS (mg/L) | 5172 ± 258 |
| FOG (mg/L) | 1994 ± 165 |
| BOD (mg/L) | 3516 ± 100 |
| COD (mg/L) | 7982 ± 361 |
| Sulfate (mg/L) | 1 ± 0.12 |
| Total phosphorus (mg/L) | 1.31 ± 0.1 |
| Copper (mg/L) | 0.06 ± 0.0004 |
| Calcium (mg/L) | 3.24 ± 0.2 |
| Magnesium (mg/L) | 19.3 ± 1.6 |
| Reducing sugar (%) | 0.02 ± 0.004 |
| Free fatty acids (%) | 0.21 ± 0.017 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buathong, P.; Boonvitthya, N.; Truan, G.; Chulalaksananukul, W. Whole-Cell Biotransformation of 1,12-Dodecanedioic Acid from Coconut Milk Factory Wastewater by Recombinant CYP52A17SS Expressing Saccharomyces cerevisiae. Processes 2020, 8, 969. https://doi.org/10.3390/pr8080969
Buathong P, Boonvitthya N, Truan G, Chulalaksananukul W. Whole-Cell Biotransformation of 1,12-Dodecanedioic Acid from Coconut Milk Factory Wastewater by Recombinant CYP52A17SS Expressing Saccharomyces cerevisiae. Processes. 2020; 8(8):969. https://doi.org/10.3390/pr8080969
Chicago/Turabian StyleBuathong, Phawadee, Nassapat Boonvitthya, Gilles Truan, and Warawut Chulalaksananukul. 2020. "Whole-Cell Biotransformation of 1,12-Dodecanedioic Acid from Coconut Milk Factory Wastewater by Recombinant CYP52A17SS Expressing Saccharomyces cerevisiae" Processes 8, no. 8: 969. https://doi.org/10.3390/pr8080969
APA StyleBuathong, P., Boonvitthya, N., Truan, G., & Chulalaksananukul, W. (2020). Whole-Cell Biotransformation of 1,12-Dodecanedioic Acid from Coconut Milk Factory Wastewater by Recombinant CYP52A17SS Expressing Saccharomyces cerevisiae. Processes, 8(8), 969. https://doi.org/10.3390/pr8080969