Supercritical Antisolvent Process for Pharmaceutical Applications: A Review
Abstract
:1. Introduction
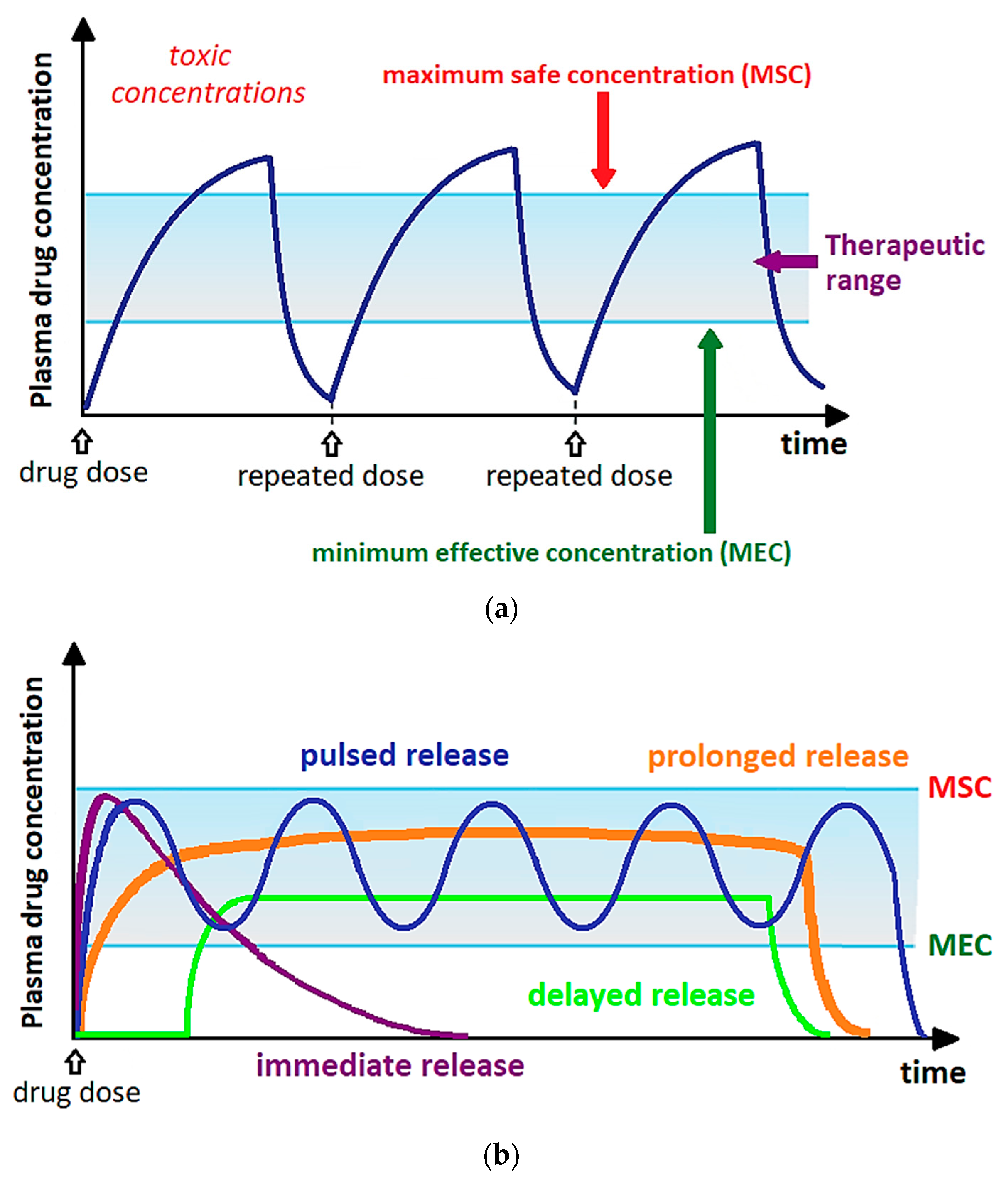
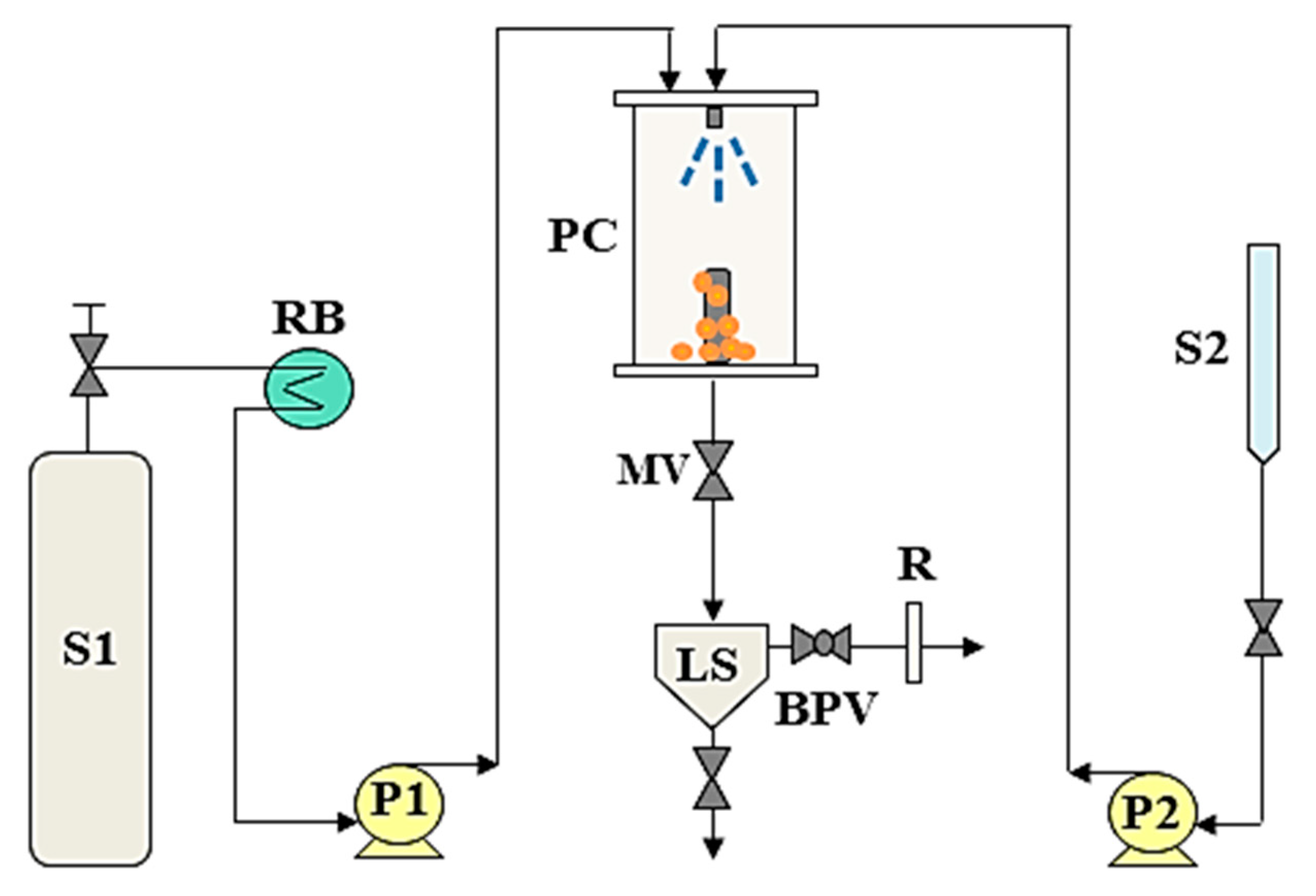
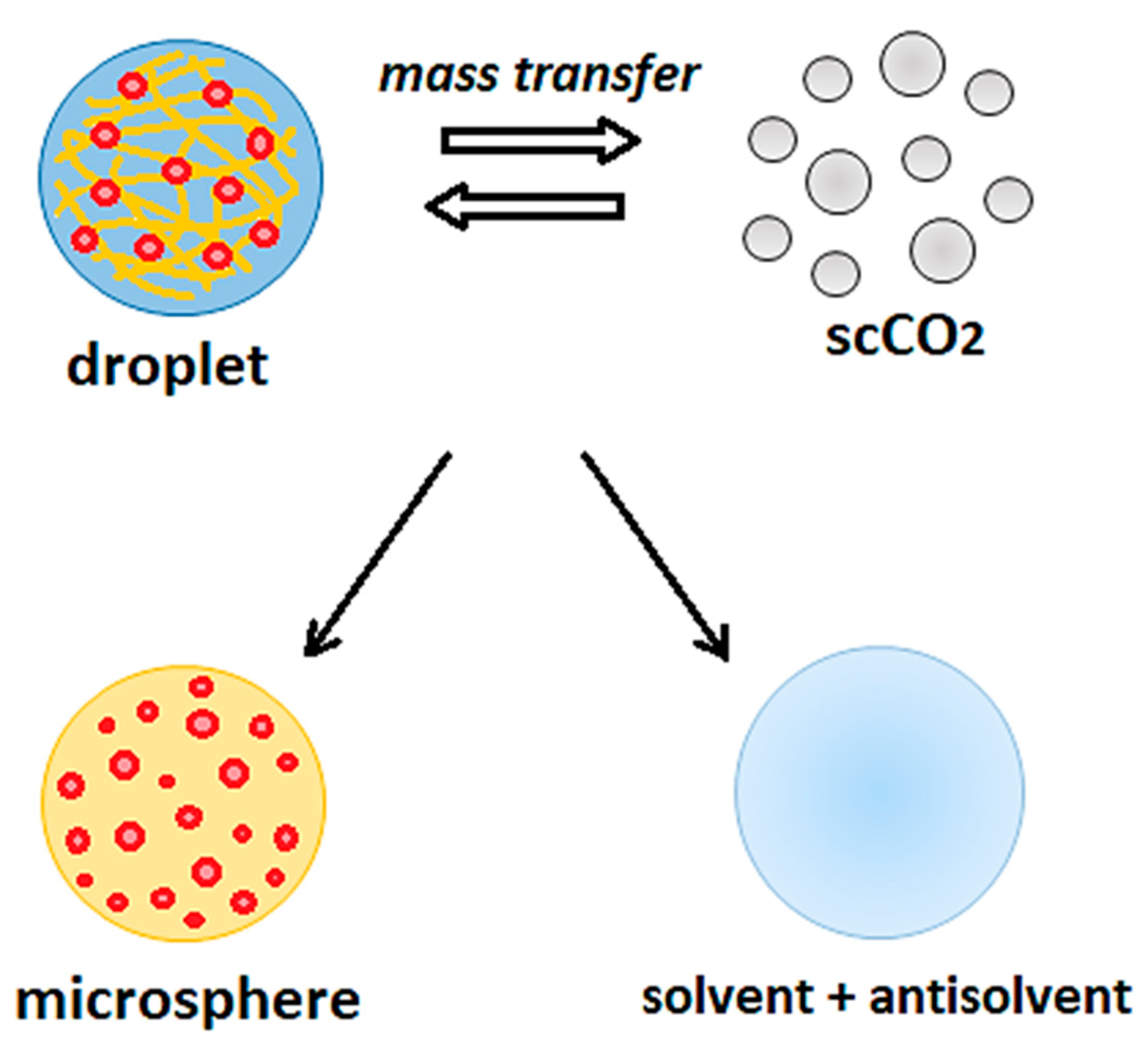
2. SAS Process: Fundamentals and Test Procedure
3. SAS Micronization of Active Compounds
4. SAS Coprecipitation of Active Compounds with Polymeric Carriers
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montes, A.; Wehner, L.; Pereyra, C.; De La Ossa, E.M. Precipitation of submicron particles of rutin using supercritical antisolvent process. J. Supercrit. Fluids 2016, 118, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Tang, M.; Chen, Y.-P. Recrystallization and micronization of sulfathiazole by applying the supercritical antisolvent technology. Chem. Eng. J. 2010, 165, 358–364. [Google Scholar] [CrossRef]
- Zhao, X.; Zu, Y.; Jiang, R.; Wang, Y.; Li, Y.; Li, Q.; Zhao, D.; Zu, B.; Zhang, B.; Sun, Z. Preparation and physicochemical properties of 10-hydroxycamptothecin (HCPT) nanoparticles by supercritical antisolvent (SAS) process. Int. J. Mol. Sci. 2011, 12, 2678–2691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zu, Y.; Li, Q.; Wang, M.; Zu, B.; Zhang, X.; Jiang, R.; Zu, C. Preparation and characterization of camptothecin powder micronized by a supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2010, 51, 412–419. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, L.; Zu, Y.; Li, C.; Liu, S.; Yang, L.; Zhao, X.; Zu, B. Micronization of Ginkgo biloba extract using supercritical antisolvent process. Powder Technol. 2011, 209, 73–80. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Q.; Zhao, X.; Wang, D.; Li, W.; Sui, X.; Zhang, Y.; Jiang, S.; Wang, Q.; Gu, C. Preparation and characterization of vitexin powder micronized by a supercritical antisolvent (SAS) process. Powder Technol. 2012, 228, 47–55. [Google Scholar] [CrossRef]
- Ha, E.-S.; Park, H.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Baek, I.-h.; Kim, M.-S. Pure Trans-Resveratrol Nanoparticles Prepared by A Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats. Antioxidants 2020, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Prosapio, V.; Reverchon, E.; De Marco, I. Formation of PVP/nimesulide microspheres by supercritical antisolvent coprecipitation. J. Supercrit. Fluids 2016, 118, 19–26. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Incorporation of liposoluble vitamins within PVP microparticles using supercritical antisolvent precipitation. J. CO2 Util. 2017, 19, 230–237. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Wang, J.-X.; Zhang, Z.-B.; Le, Y.; Shen, Z.-G.; Chen, J.-F. Micronization of atorvastatin calcium by antisolvent precipitation process. Int. J. Pharm. 2009, 374, 106–113. [Google Scholar] [CrossRef]
- Zahran, F.; Cabañas, A.; Cheda, J.A.R.; Renuncio, J.A.R.; Pando, C. Dissolution rate enhancement of the anti-inflammatory drug diflunisal by coprecipitation with a biocompatible polymer using carbon dioxide as a supercritical fluid antisolvent. J. Supercrit. Fluids 2014, 88, 56–65. [Google Scholar] [CrossRef]
- Wu, K.; Li, J.; Wang, W.; Winstead, D.A. Formation and characterization of solid dispersions of piroxicam and polyvinylpyrrolidone using spray drying and precipitation with compressed antisolvent. J. Pharm. Sci. 2009, 98, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.L.; Lu, T.; Leeke, G.; Prosapio, V.; McConville, C.; Ingram, A. Single-step coprecipitation and coating to prepare curcumin formulations by supercritical fluid technology. J. Supercrit. Fluids 2020, 159, 104758. [Google Scholar] [CrossRef]
- Stebbins, N.D.; Ouimet, M.A.; Uhrich, K.E. Antibiotic-containing polymers for localized, sustained drug delivery. Adv. Drug Del. Rev. 2014, 78, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gürsel, İ.; Korkusuz, F.; Türesin, F.; Alaeddinoǧlu, N.G.; Hasırcı, V. In vivo application of biodegradable controlled antibiotic release systems for the treatment of implant-related osteomyelitis. Biomaterials 2000, 22, 73–80. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, W.; Qian, L.; Zhang, X.; Zeng, P.; Pan, J. In vitro and in vivo studies on mucoadhesive microspheres of amoxicillin. J. Control. Release 2005, 102, 135–144. [Google Scholar] [CrossRef]
- Franco, P.; Reverchon, E.; De Marco, I. Production of zein/antibiotic microparticles by supercritical antisolvent coprecipitation. J. Supercrit. Fluids 2019, 145, 31–38. [Google Scholar] [CrossRef]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID development: Evolution of diclofenac products using pharmaceutical technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Bah, M. NSAIDs in the treatment of postoperative pain. Curr. Pain Headache Rep. 2016, 20, 62. [Google Scholar] [CrossRef]
- Maniar, K.H.; Jones, I.A.; Gopalakrishna, R.; Vangsness, C.T., Jr. Lowering side effects of NSAID usage in osteoarthritis: Recent attempts at minimizing dosage. Expert Opin. Pharmacother. 2018, 19, 93–102. [Google Scholar] [CrossRef]
- Da Fonseca Machado, A.P.; Rezende, C.A.; Rodrigues, R.A.; Barbero, G.F.; e Rosa, P.d.T.V.; Martínez, J. Encapsulation of anthocyanin-rich extract from blackberry residues by spray-drying, freeze-drying and supercritical antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Lee, C.-W.; Kim, S.-J.; Youn, Y.-S.; Widjojokusumo, E.; Lee, Y.-H.; Kim, J.; Lee, Y.-W.; Tjandrawinata, R.R. Preparation of bitter taste masked cetirizine dihydrochloride/β-cyclodextrin inclusion complex by supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2010, 55, 348–357. [Google Scholar] [CrossRef]
- Won, D.-H.; Kim, M.-S.; Lee, S.; Park, J.-S.; Hwang, S.-J. Improved physicochemical characteristics of felodipine solid dispersion particles by supercritical anti-solvent precipitation process. Int. J. Pharm. 2005, 301, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Seo, H.J.; Hong, S.-h.; Ha, E.-S.; Lee, S.; Kim, J.-S.; Baek, I.-h.; Kim, M.-S.; Hwang, S.-J. Characterization and therapeutic efficacy evaluation of glimepiride and L-arginine co-amorphous formulation prepared by supercritical antisolvent process: Influence of molar ratio and preparation methods. Int. J. Pharm. 2020, 581, 119232. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, H.J.; Cho, W.; Cha, K.-H.; Kang, Y.-S.; Hwang, S.-J. Preparation and pharmaceutical characterization of amorphous cefdinir using spray-drying and SAS-process. Int. J. Pharm. 2010, 396, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.D.; Debenedetti, P.G.; Radosz, M.; Schmidt, H.W. Supercritical Antisolvent Process for Substituted Para-Linked Aromatic Polyamides: Phase Equilibrium and Morphology Study. Macromolecules 1993, 26, 6207–6210. [Google Scholar] [CrossRef]
- Yeo, S.D.; Debenedetti, P.G.; Radosz, M.; Giesa, R.; Schmidt, H.W. Supercritical Antisolvent Process for a Series of Substituted Para-Linked Aromatic Polyamides. Macromolecules 1995, 28, 1316–1317. [Google Scholar] [CrossRef]
- Bertucco, A.; Pallado, P.; Benedetti, L. Formation of biocompatible polymer microspheres for controlled drug delivery by a supercritical antisolvent technique. Process Technol. Proc. 1996, 12, 217–222. [Google Scholar]
- Reverchon, E.; Della Porta, G.; Di Trolio, A.; Pace, S. Supercritical Antisolvent Precipitation of Nanoparticles of Superconductor Precursors. Ind. Eng. Chem. Res. 1998, 37, 952–958. [Google Scholar] [CrossRef]
- Reverchon, E.; Celano, C.; Della Porta, G.; Di Trolio, A.; Pace, S. Supercritical antisolvent precipitation: A new technique for preparing submicronic yttrium powders to improve YBCO superconductor. J. Mater. Res. 1998, 13, 284–289. [Google Scholar] [CrossRef]
- Chen, L.-F.; Xu, P.-Y.; Fu, C.-P.; Kankala, R.K.; Chen, A.-Z.; Wang, S.-B. Fabrication of Supercritical Antisolvent (SAS) Process-Assisted Fisetin-Encapsulated Poly (Vinyl Pyrrolidone)(PVP) Nanocomposites for Improved Anticancer Therapy. Nanomaterials 2020, 10, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, T.M.; Bandi, N.; Shulz, R.; Roberts, C.B.; Kompella, U.B. Preparation of budesonide and budesonide-PLA microparticles using supercritical fluid precipitation technology. AAPS PharmSciTech 2002, 3, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Yoon, T.J.; Kwon, D.E.; Yu, K.; Lee, Y.-W. Coprecipitation of hydrochlorothiazide/PVP for the dissolution rate improvement by precipitation with compressed fluid antisolvent process. J. Supercrit. Fluids 2017, 126, 37–46. [Google Scholar] [CrossRef]
- Ha, E.-S.; Choo, G.-H.; Baek, I.-h.; Kim, J.-S.; Cho, W.; Jung, Y.S.; Jin, S.-E.; Hwang, S.-J.; Kim, M.-S. Dissolution and bioavailability of lercanidipine–hydroxypropylmethyl cellulose nanoparticles with surfactant. Int. J. Biol. Macromol. 2015, 72, 218–222. [Google Scholar] [CrossRef]
- Majerik, V.; Charbit, G.; Badens, E.; Horváth, G.; Szokonya, L.; Bosc, N.; Teillaud, E. Bioavailability enhancement of an active substance by supercritical antisolvent precipitation. J. Supercrit. Fluids 2007, 40, 101–110. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Yang, J.; Pfeffer, R.; Dave, R.; Michniak, B. The application of a supercritical antisolvent process for sustained drug delivery. Powder Technol. 2006, 164, 94–102. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.; Kim, J.; Park, H.; Woo, J.; Lee, B.; Hwang, S.J. Controlled delivery of a hydrophilic drug from a biodegradable microsphere system by supercritical anti-solvent precipitation technique. J. Microencapsul. 2006, 23, 741–749. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Ge, Y.; Zhong, Z.; Wang, Z.; Wu, T.; Zhao, X.; Zu, Y. Solubility, Antioxidation, and Oral Bioavailability Improvement of Mangiferin Microparticles Prepared Using the Supercritical Antisolvent Method. Pharmaceutics 2020, 12, 90. [Google Scholar] [CrossRef] [Green Version]
- Ha, E.S.; Kim, J.S.; Baek, I.H.; Yoo, J.W.; Jung, Y.; Moon, H.R.; Kim, M.S. Development of megestrol acetate solid dispersion nanoparticles for enhanced oral delivery by using a supercritical antisolvent process. Drug Des. Dev. Ther. 2015, 9, 4269–4277. [Google Scholar]
- Sacchetin, P.S.C.; Setti, R.F.; e Rosa, P.D.T.V.; Moraes, Â.M. Properties of PLA/PCL particles as vehicles for oral delivery of the androgen hormone 17α-methyltestosterone. Mater. Sci. Eng. C 2016, 58, 870–881. [Google Scholar] [CrossRef]
- Jung, I.-I.; Haam, S.; Lim, G.; Ryu, J.-H. Preparation of peptide-loaded polymer microparticles using supercritical carbon dioxide. Biotechnol. Bioprocess Eng. 2012, 17, 185–194. [Google Scholar] [CrossRef]
- Lee, S.; Nam, K.; Kim, M.S.; Jun, S.W.; Park, J.-S.; Woo, J.S.; Hwang, S.-J. Preparation and characterization of solid dispersions of itraconazole by using aerosol solvent extraction system for improvement in drug solubility and bioavailability. Arch. Pharm. Res. 2005, 28, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.R.C.; Roy, C.; Vega-González, A.; Duarte, C.M.; Subra-Paternault, P. Preparation of acetazolamide composite microparticles by supercritical anti-solvent techniques. Int. J. Pharm. 2007, 332, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, P.; de Marco, I. Eudragit: A Novel Carrier for Controlled Drug Delivery in Supercritical Antisolvent Coprecipitation. Polymers 2020, 12, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, F.; Martín, A.; Mattea, F.; Cocero, M.J. Precipitation of lutein and co-precipitation of lutein and poly-lactic acid with the supercritical anti-solvent process. Chem. Eng. Process. 2008, 47, 1594–1602. [Google Scholar] [CrossRef]
- Franco, P.; Reverchon, E.; De Marco, I. Zein/diclofenac sodium coprecipitation at micrometric and nanometric range by supercritical antisolvent processing. J. CO2 Util. 2018, 27, 366–373. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Huang, Y.; Wang, H.; Jiang, Y. Incorporation of 10-hydroxycamptothecin nanocrystals into zein microspheres. Chem. Eng. Sci. 2016, 155, 405–414. [Google Scholar] [CrossRef]
- Zhong, Q.; Jin, M.; Davidson, P.M.; Zivanovic, S. Sustained release of lysozyme from zein microcapsules produced by a supercritical anti-solvent process. Food Chem. 2009, 115, 697–700. [Google Scholar] [CrossRef]
- Hu, D.; Lin, C.; Liu, L.; Li, S.; Zhao, Y. Preparation, characterization, and in vitro release investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluids. J. Food Eng. 2012, 109, 545–552. [Google Scholar] [CrossRef]
- Abuzar, S.M.; Hyun, S.-M.; Kim, J.-H.; Park, H.J.; Kim, M.-S.; Park, J.-S.; Hwang, S.-J. Enhancing the solubility and bioavailability of poorly water-soluble drugs using supercritical antisolvent (SAS) process. Int. J. Pharm. 2018, 538, 1–13. [Google Scholar] [CrossRef]
- Kalani, M.; Yunus, R. Application of supercritical antisolvent method in drug encapsulation: A review. Int. J. Nanomed. 2011, 6, 1429. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Coloma, A.; Martín, L.; Mainar, A.; Urieta, J.; Fraga, B.M.; Rodríguez-Vallejo, V.; Díaz, C.E. Supercritical extraction and supercritical antisolvent fractionation of natural products from plant material: Comparative results on Persea indica. Phytochem. Rev. 2012, 11, 433–446. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical antisolvent precipitation of micro-and nano-particles. J. Supercrit. Fluids 1999, 15, 1–21. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieth, M.; Siegel, M.G.; Higgs, R.E.; Watson, I.A.; Robertson, D.H.; Savin, K.A.; Durst, G.L.; Hipskind, P.A. Characteristic physical properties and structural fragments of marketed oral drugs. J. Med. Chem. 2004, 47, 224–232. [Google Scholar] [CrossRef]
- Rodriguez-Aller, M.; Guillarme, D.; Veuthey, J.-L.; Gurny, R. Strategies for formulating and delivering poorly water-soluble drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 342–351. [Google Scholar] [CrossRef]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. J. CO2 Util. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef]
- Tenorio, A.; Gordillo, M.D.; Pereyra, C.; Martinez de la Ossa, E.J. Controlled submicro particle formation of ampicillin by supercritical antisolvent precipitation. J. Supercrit. Fluids 2007, 40, 308–316. [Google Scholar] [CrossRef]
- Kalogiannis, C.G.; Pavlidou, E.; Panayiotou, C.G. Production of amoxicillin microparticles by supercritical antisolvent precipitation. Ind. Eng. Chem. Res. 2005, 44, 9339–9346. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Falivene, M. Process parameters and morphology in amoxicillin micro and submicro particles generation by supercritical antisolvent precipitation. J. Supercrit. Fluids 2000, 17, 239–248. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical antisolvent precipitation of cephalosporins. Powder Technol. 2006, 164, 139–146. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I.; Della Porta, G. Rifampicin microparticles production by supercritical antisolvent precipitation. Int. J. Pharm. 2002, 243, 83–91. [Google Scholar] [CrossRef]
- Cardoso, M.T.; Monteiro, G.; Cardoso, J.; Prazeres, T.; Figueiredo, J.; Martinho, J.; Cabral, J.; Palavra, A. Supercritical antisolvent micronization of minocycline hydrochloride. J. Supercrit. Fluids 2008, 44, 238–244. [Google Scholar] [CrossRef]
- Cardoso, M.T.; Geraldes, V.; Cabral, J.; Palavra, A. Characterization of minocycline powder micronized by a supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2008, 46, 71–76. [Google Scholar] [CrossRef]
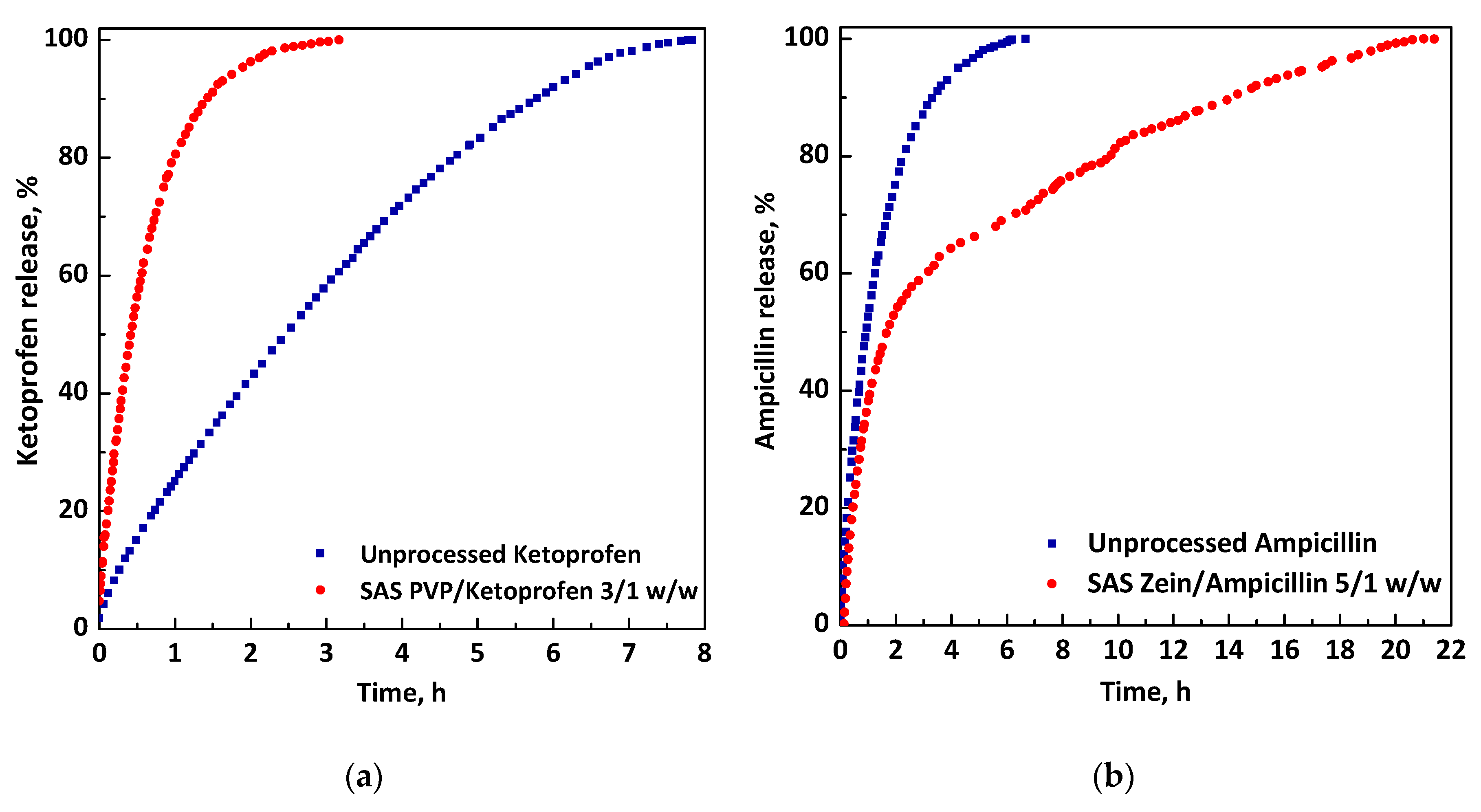
- Franco, P.; Reverchon, E.; De Marco, I. PVP/ketoprofen coprecipitation using supercritical antisolvent process. Powder Technol. 2018, 340, 1–7. [Google Scholar] [CrossRef]
- Montes, A.; Wehner, L.; Pereyra, C.; Martínez de la Ossa, E.J. Mangiferin nanoparticles precipitation by supercritical antisolvent process. J. Supercrit. Fluids 2016, 112, 44–50. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Zahran, F.; Martín, D.; Cabañas, A.; Pando, C. Preparation of 5-fluorouracil microparticles and 5-fluorouracil/poly(l-lactide) composites by a supercritical CO2 antisolvent process. J. Supercrit. Fluids 2019, 143, 64–71. [Google Scholar] [CrossRef]
- Widjojokusumo, E.; Veriansyah, B.; Tjandrawinata, R.R. Supercritical anti-solvent (SAS) micronization of Manilkara kauki bioactive fraction (DLBS2347). J. CO2 Util. 2013, 3-4, 30–36. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Zu, Y.; Jiang, R.; Zhao, D. Recrystallization and micronization of taxol using the supercritical antisolvent (SAS) process. Ind. Eng. Chem. Res. 2012, 51, 9591–9597. [Google Scholar] [CrossRef]
- Park, J.; Cho, W.; Cha, K.-H.; Ahn, J.; Han, K.; Hwang, S.-J. Solubilization of the poorly water soluble drug, telmisartan, using supercritical anti-solvent (SAS) process. Int. J. Pharm. 2013, 441, 50–55. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Pallado, P. Supercritical antisolvent precipitation of salbutamol microparticles. Powder Technol. 2001, 114, 17–22. [Google Scholar] [CrossRef]
- Kim, M.-S.; Jin, S.-J.; Kim, J.-S.; Park, H.J.; Song, H.-S.; Neubert, R.H.; Hwang, S.-J. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2008, 69, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.L.; Johnston, K.P.; Williams, R.O., III. Solution-based particle formation of pharmaceutical powders by supercritical or compressed fluid CO2 and cryogenic spray-freezing technologies. Drug Dev. Ind. Pharm. 2001, 27, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-P.; Tang, M.; Chen, Y.-P. Micronization of sulfamethoxazole using the supercritical anti-solvent process. J. Mater. Sci. 2008, 43, 2328–2335. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Widjojokusumo, E.; Soewandhi, S.; Wikarsa, S.; Tjandrawinata, R.R. Simultaneous cocrystallization and micronization of paracetamol-dipicolinic acid cocrystal by supercritical antisolvent (SAS). Int. J. Pharm. Pharm. Sci. 2016, 8, 89–98. [Google Scholar]
- Kim, M.-S.; Lee, S.; Park, J.-S.; Woo, J.-S.; Hwang, S.-J. Micronization of cilostazol using supercritical antisolvent (SAS) process: Effect of process parameters. Powder Technol. 2007, 177, 64–70. [Google Scholar] [CrossRef]
- Kudryashova, E.; Sukhoverkov, K.; Deygen, I.; Vorobei, A.; Pokrovskiy, O.; Parenago, O.; Presnov, D.; Egorov, A. Moxifloxacin Micronization via Supercritical Antisolvent Precipitation. Russ. J. Phys. Chem. B 2017, 11, 1153–1162. [Google Scholar] [CrossRef]
- Visentin, A.; Rodríguez-Rojo, S.; Navarrete, A.; Maestri, D.; Cocero, M.J. Precipitation and encapsulation of rosemary antioxidants by supercritical antisolvent process. J. Food Eng. 2012, 109, 9–15. [Google Scholar] [CrossRef]
- De Marco, I.; Prosapio, V.; Cice, F.; Reverchon, E. Use of solvent mixtures in supercritical antisolvent process to modify precipitates morphology: Cellulose acetate microparticles. J. Supercrit. Fluids 2013, 83, 153–160. [Google Scholar] [CrossRef]
- Matos, R.L.; Lu, T.; Prosapio, V.; McConville, C.; Leeke, G.; Ingram, A. Coprecipitation of curcumin/PVP with enhanced dissolution properties by the supercritical antisolvent process. J. CO2 Util. 2019, 30, 48–62. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Coprecipitation of polyvinylpyrrolidone/β-carotene by supercritical antisolvent processing. Ind. Eng. Chem. Res. 2015, 54, 11568–11575. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E.; De Marco, I. Dependence of SAS particle morphologies on the ternary phase equilibria. J. Supercrit. Fluids 2017, 130, 273–281. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E.; De Marco, I. PVP microparticles precipitation from acetone-ethanol mixtures using SAS process: Effect of phase behavior. J. Supercrit. Fluids 2019, 143, 321–329. [Google Scholar] [CrossRef]
- Martín, A.n.; Scholle, K.; Mattea, F.; Meterc, D.; Cocero, M.a.J. Production of polymorphs of ibuprofen sodium by supercritical antisolvent (SAS) precipitation. Cryst. Growth Des. 2009, 9, 2504–2511. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Production of antibiotic micro-and nano-particles by supercritical antisolvent precipitation. Powder Technol. 1999, 106, 23–29. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Gupta, R.B. Production of griseofulvin nanoparticles using supercritical CO2 antisolvent with enhanced mass transfer. Int. J. Pharm. 2001, 228, 19–31. [Google Scholar] [CrossRef]
- Bagratashvili, V.; Egorov, A.; Krotova, L.; Mironov, A.; Panchenko, V.Y.; Parenago, O.; Popov, V.; Revelsky, I.; Timashev, P.; Tsypina, S. Supercritical fluid micronization of risperidone pharmaceutical substance. Russ. J. Phys. Chem. B 2012, 6, 804–812. [Google Scholar] [CrossRef]
- Su, C.S.; Lo, W.S.; Lien, L.H. Micronization of fluticasone propionate using supercritical antisolvent (SAS) process. Chem. Eng. Technol. 2011, 34, 535–541. [Google Scholar] [CrossRef]
- Quintana, S.E.; Hernández, D.M.; Villanueva-Bermejo, D.; García-Risco, M.R.; Fornari, T. Fractionation and precipitation of licorice (Glycyrrhiza glabra L.) phytochemicals by supercritical antisolvent (SAS) technique. LWT 2020, 109315. [Google Scholar] [CrossRef]
- Miguel, F.; Martin, A.; Gamse, T.; Cocero, M.J. Supercritical anti solvent precipitation of lycopene: Effect of the operating parameters. J. Supercrit. Fluids 2006, 36, 225–235. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Masmoudi, Y.; Djerafi, R.; Casas, L.; Mantell, C.; de La Ossa, E.M.; Badens, E. Particle design applied to quercetin using supercritical anti-solvent techniques. J. Supercrit. Fluids 2015, 105, 119–127. [Google Scholar] [CrossRef]
- Montes, A.; Pereyra, C.; de la Ossa, E.M. Screening design of experiment applied to the supercritical antisolvent precipitation of quercetin. J. Supercrit. Fluids 2015, 104, 10–18. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Reverchon, E. PVP/corticosteroid microspheres produced by supercritical antisolvent coprecipitation. Chem. Eng. J. 2016, 292, 264–275. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Scognamiglio, M.; Reverchon, E. Folic acid–PVP nanostructured composite microparticles by supercritical antisolvent precipitation. Chem. Eng. J. 2015, 277, 286–294. [Google Scholar] [CrossRef]
- Franceschi, E.; De Cesaro, A.M.; Feiten, M.; Ferreira, S.R.S.; Dariva, C.; Kunita, M.H.; Rubira, A.F.; Muniz, E.C.; Corazza, M.L.; Oliveira, J.V. Precipitation of β-carotene and PHBV and co-precipitation from SEDS technique using supercritical CO2. J. Supercrit. Fluids 2008, 47, 259–269. [Google Scholar] [CrossRef]
- Priamo, W.L.; De Cezaro, A.M.; Ferreira, S.R.S.; Oliveira, J.V. Precipitation and encapsulation of β-carotene in PHBV using carbon dioxide as anti-solvent. J. Supercrit. Fluids 2010, 54, 103–109. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; Capanoglu, E.; De Marco, I. PVP/flavonoid coprecipitation by supercritical antisolvent process. Chem. Eng. Process. 2019, 146, 107689. [Google Scholar] [CrossRef]
- Montes, A.; Kin, N.; Gordillo, M.D.; Pereyra, C.; Martínez de la Ossa, E.J. Polymer–naproxen precipitation by supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2014, 89, 58–67. [Google Scholar] [CrossRef]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; De los Santos, D.M.; Martínez de la Ossa, E.J. Ibuprofen–polymer precipitation using supercritical CO2 at low temperature. J. Supercrit. Fluids 2014, 94, 91–101. [Google Scholar] [CrossRef]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; Martínez de la Ossa, E.J. Polymer and ampicillin co-precipitation by supercritical antisolvent process. J. Supercrit. Fluids 2012, 63, 92–98. [Google Scholar] [CrossRef]
- Montes, A.; Nunes, A.; Gordillo, M.; Pereyra, C.; Duarte, C.M.; Martinez de la Ossa, E.J. Amoxicillin and ethyl cellulose precipitation by two supercritical antisolvent processes. Chem. Eng. Technol. 2013, 36, 665–672. [Google Scholar] [CrossRef]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; Martínez de la Ossa, E.J. Co-precipitation of amoxicillin and ethyl cellulose microparticles by supercritical antisolvent process. J. Supercrit. Fluids 2011, 60, 75–80. [Google Scholar] [CrossRef]
- Uzun, İ.N.; Sipahigil, O.; Dinçer, S. Coprecipitation of Cefuroxime Axetil–PVP composite microparticles by batch supercritical antisolvent process. J. Supercrit. Fluids 2011, 55, 1059–1069. [Google Scholar] [CrossRef]
- De Marco, I.; Franco, P. Production of Eudragit/ampicillin Microparticles by Supercritical Antisolvent Coprecipitation. Chem. Eng. Trans. 2020, 79, 229–234. [Google Scholar]
- Djerafi, R.; Swanepoel, A.; Crampon, C.; Kalombo, L.; Labuschagne, P.; Badens, E.; Masmoudi, Y. Supercritical antisolvent co-precipitation of rifampicin and ethyl cellulose. Eur. J. Pharm. Sci. 2017, 102, 161–171. [Google Scholar] [CrossRef]
- Barrett, A.M.; Dehghani, F.; Foster, N.R. Increasing the Dissolution Rate of Itraconazole Processed by Gas Antisolvent Techniques using Polyethylene Glycol as a Carrier. Pharm. Res. 2007, 25, 1274–1289. [Google Scholar] [CrossRef]
- Jin, H.Y.; Xia, F.; Zhao, Y.P. Preparation of hydroxypropyl methyl cellulose phthalate nanoparticles with mixed solvent using supercritical antisolvent process and its application in co-precipitation of insulin. Adv. Powder Technol. 2012, 23, 157–163. [Google Scholar] [CrossRef]
- Elvassore, N.; Bertucco, A.; Caliceti, P. Production of protein-loaded polymeric microcapsules by compressed CO2 in a mixed solvent. Ind. Eng. Chem. Res. 2001, 40, 795–800. [Google Scholar] [CrossRef]
- Kalantarian, P.; Haririan, I.; Najafabadi, A.R.; Shokrgozar, M.A.; Vatanara, A. Entrapment of 5-fluorouracil into PLGA matrices using supercritical antisolvent processes. J. Pharm. Pharmacol. 2011, 63, 500–506. [Google Scholar] [CrossRef]
- Ha, E.-S.; Kim, J.-S.; Baek, I.-h.; Hwang, S.-J.; Kim, M.-S. Enhancement of dissolution and bioavailability of ezetimibe by amorphous solid dispersion nanoparticles fabricated using supercritical antisolvent process. J. Pharm. Investig. 2015, 45, 641–649. [Google Scholar] [CrossRef]
- Argemí, A.; Vega, A.; Subra-Paternault, P.; Saurina, J. Characterization of azacytidine/poly(l-lactic) acid particles prepared by supercritical antisolvent precipitation. J. Pharm. Biomed. Anal. 2009, 50, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Z.; Pu, X.M.; Kang, Y.Q.; Liao, L.; Yao, Y.D.; Yin, G.F. Preparation of 5--Fluorouracil--Poly (l--lactide) Microparticles Using Solution--Enhanced Dispersion by Supercritical CO2. Macromol. Rapid Commun. 2006, 27, 1254–1259. [Google Scholar] [CrossRef]
- Alias, D.; Yunus, R.; Chong, G.H.; Che Abdullah, C.A. Single step encapsulation process of tamoxifen in biodegradable polymer using supercritical anti-solvent (SAS) process. Powder Technol. 2017, 309, 89–94. [Google Scholar] [CrossRef]
- Huang, Y.; Zu, Y.; Zhao, X.; Wu, M.; Feng, Z.; Deng, Y.; Zu, C.; Wang, L. Preparation of inclusion complex of apigenin-hydroxypropyl-β-cyclodextrin by using supercritical antisolvent process for dissolution and bioavailability enhancement. Int. J. Pharm. 2016, 511, 921–930. [Google Scholar] [CrossRef]
- Lestari, S.D.; Machmudah, S.; Winardi, S.; Kanda, H.; Goto, M. Particle micronization of Curcuma mangga rhizomes ethanolic extract/biopolymer PVP using supercritical antisolvent process. J. Supercrit. Fluids 2019, 146, 226–239. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Xu, P.-Y.; Chen, B.-Q.; Fu, C.-P.; Kankala, R.K.; Chen, A.-Z.; Wang, S.-B. Supercritical Antisolvent Process-assisted Fabrication of Chrysin-polyvinylpyrrolidone Sub-microparticles for Improved Anticancer Efficiency. J. Supercrit. Fluids 2020, 162, 104847. [Google Scholar] [CrossRef]
- Machmudah, S.; Winardi, S.; Wahyudiono; Kanda, H.; Goto, M. Formation of Fine Particles from Curcumin/PVP by the Supercritical Antisolvent Process with a Coaxial Nozzle. ACS Omega 2020, 5, 6705–6714. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, G.; Wu, J.; Jiang, Y. Co-precipitation of 10-hydroxycamptothecin and poly (l-lactic acid) by supercritical CO2 anti-solvent process using dichloromethane/ethanol co-solvent. J. Supercrit. Fluids 2013, 74, 137–144. [Google Scholar] [CrossRef]
- Patomchaiviwat, V.; Paeratakul, O.; Kulvanich, P. Formation of inhalable rifampicin–poly (l-lactide) microparticles by supercritical anti-solvent process. AAPS PharmSciTech 2008, 9, 1119–1129. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, V.M.H.; Balcão, V.M.; Vila, M.M.D.C.; Oliveira Júnior, J.M.; Aranha, N.; Chaud, M.V.; Gremião, M.P.D. Zidovudine-Poly(l-Lactic Acid) Solid Dispersions with Improved Intestinal Permeability Prepared by Supercritical Antisolvent Process. J. Pharm. Sci. 2015, 104, 1691–1700. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Liu, G.; Zhao, Z.; Wei, D.; Pang, J.; Jiang, Y. Design of gefitinib-loaded poly (l-lactic acid) microspheres via a supercritical anti-solvent process for dry powder inhalation. Int. J. Pharm. 2017, 532, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Montes, A.; Wehner, L.; Pereyra, C.; Martínez de la Ossa, E.J. Generation of microparticles of ellagic acid by supercritical antisolvent process. J. Supercrit. Fluids 2016, 116, 101–110. [Google Scholar] [CrossRef]
- Chhouk, K.; Kanda, H.; Kawasaki, S.-I.; Goto, M. Micronization of curcumin with biodegradable polymer by supercritical anti-solvent using micro swirl mixer. Front. Chem. Sci. Eng. 2018, 12, 184–193. [Google Scholar] [CrossRef]
- Guha, R.; Vinjamur, M.; Mukhopadhyay, M. Demonstration of mechanisms for coprecipitation and encapsulation by supercritical antisolvent process. Ind. Eng. Chem. Res. 2011, 50, 1079–1088. [Google Scholar] [CrossRef]
- Kalogiannis, C.G.; Michailof, C.M.; Panayiotou, C.G. Microencapsulation of amoxicillin in poly (l-lactic acid) by supercritical antisolvent precipitation. Ind. Eng. Chem. Res. 2006, 45, 8738–8743. [Google Scholar] [CrossRef]
- Li, W.; Liu, G.; Li, L.; Wu, J.; LÜ, Y.; Jiang, Y. Effect of Process Parameters on Co-precipitation of Paclitaxel and Poly(l-lactic Acid) by Supercritical Antisolvent Process. Chin. J. Chem. Eng. 2012, 20, 803–813. [Google Scholar] [CrossRef]
- Liu, G.; Hu, M.; Zhao, Z.; Lin, Q.; Wei, D.; Jiang, Y. Enhancing the stability of astaxanthin by encapsulation in poly (l-lactic acid) microspheres using a supercritical anti-solvent process. Particuology 2019, 44, 54–62. [Google Scholar] [CrossRef]
- Nerome, H.; Machmudah, S.; Wahyudiono; Fukuzato, R.; Higashiura, T.; Youn, Y.S.; Lee, Y.W.; Goto, M. Nanoparticle formation of lycopene/β-cyclodextrin inclusion complex using supercritical antisolvent precipitation. J. Supercrit. Fluids 2013, 83, 97–103. [Google Scholar] [CrossRef]
- Martín, A.; Mattea, F.; Gutiérrez, L.; Miguel, F.; Cocero, M.J. Co-precipitation of carotenoids and bio-polymers with the supercritical anti-solvent process. J. Supercrit. Fluids 2007, 41, 138–147. [Google Scholar] [CrossRef]
- Lang, Z.M.; Hong, H.L.; Han, L.M.; Zhu, N.; Suo, Q.L. Preparation of emodin-polyethylene glycol composite microparticles using a supercritical antisolvent process. Chem. Eng. Technol. 2012, 35, 362–368. [Google Scholar] [CrossRef]
- Machado, F.R.S.; Trevisol, T.C.; Boschetto, D.L.; Burkert, J.F.M.; Ferreira, S.R.S.; Oliveira, J.V.; Burkert, C.A.V. Technological process for cell disruption, extraction and encapsulation of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2016, 218, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boschetto, D.L.; Aranha, E.M.; de Souza, A.A.U.; Souza, S.M.A.G.U.; Ferreira, S.R.S.; Priamo, W.L.; Oliveira, J.V. Encapsulation of bixin in PHBV using SEDS technique and in vitro release evaluation. Ind Crops Prod. 2014, 60, 22–29. [Google Scholar] [CrossRef]
- Boschetto, D.L.; Dalmolin, I.; de Cesaro, A.M.; Rigo, A.A.; Ferreira, S.R.S.; Meireles, M.A.A.; Batista, E.A.C.; Vladimir Oliveira, J. Phase behavior and process parameters effect on grape seed extract encapsulation by SEDS technique. Ind. Crops Prod. 2013, 50, 352–360. [Google Scholar] [CrossRef]
- Andrade, K.S.; Aguiar, G.P.S.; Rebelatto, E.A.; Lanza, M.; Oliveira, J.V.; Ferreira, S.R. Encapsulation of pink pepper extract by SEDS technique: Phase behavior data and process parameters. J. Supercrit. Fluids 2020, 161, 104822. [Google Scholar] [CrossRef]
- Franceschi, E.; De Cezaro, A.; Ferreira, S.R.S.; Kunita, M.H.; Muniz, E.C.; Rubira, A.F.; Oliveira, J.V. Co-precipitation of beta-carotene and bio-polymer using supercritical carbon dioxide as antisolvent. Open Chem. Eng. J. 2010, 4, 11–20. [Google Scholar] [CrossRef]
- Lee, S.Y.; Abdullah, L.C.; Rahman, R.A.; Abas, F.; Chong, G.H. Role of polymers as crystal growth inhibitors in coprecipitation via solution-enhanced dispersion by supercritical fluids (SEDS) to improve andrographolide dissolution from standardized Andrographis paniculata extract. J. Drug Deliv. Sci. Technol. 2019, 50, 145–154. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, F.; Guo, Z.; Zhao, Y. Preparation and characterization of resveratrol/hydroxypropyl--β--cyclodextrin inclusion complex using supercritical antisolvent technology. J. Food Process Eng. 2012, 35, 677–686. [Google Scholar] [CrossRef]
- Yan, T.; Ji, M.; Sun, Y.; Yan, T.; Zhao, J.; Zhang, H.; Wang, Z. Preparation and characterization of baicalein/hydroxypropyl-β-cyclodextrin inclusion complex for enhancement of solubility, antioxidant activity and antibacterial activity using supercritical antisolvent technology. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 285–295. [Google Scholar] [CrossRef]
- Sun, J.; Hong, H.; Zhu, N.; Han, L.; Suo, Q. Response surface methodology to optimize the preparation of tosufloxacin tosylate/hydroxypropyl-β-cyclodextrin inclusion complex by supercritical antisolvent process. J. Mol. Struct. 2019, 1198, 126939. [Google Scholar] [CrossRef]
- Jun, S.W.; Kim, M.-S.; Kim, J.-S.; Park, H.J.; Lee, S.; Woo, J.-S.; Hwang, S.-J. Preparation and characterization of simvastatin/hydroxypropyl-β-cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2007, 66, 413–421. [Google Scholar] [CrossRef]
- Zhong, Q.; Jin, M. Nanoscalar structures of spray-dried zein microcapsules and in vitro release kinetics of the encapsulated lysozyme as affected by formulations. J. Agric. Food Chem. 2009, 57, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Condori, S.; Saldaña, M.D.; Temelli, F. Microencapsulation of flax oil with zein using spray and freeze drying. LWT-Food Sci. Technol. 2011, 44, 1880–1887. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y. Eugenol nanoemulsion stabilized with zein and sodium caseinate by self-assembly. J. Agric. Food Chem. 2017, 65, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.S.; Dehghani, F.; Foster, N. Micronisation and microencapsulation of pharmaceuticals using a carbon dioxide antisolvent. Powder Technol. 2002, 126, 134–149. [Google Scholar] [CrossRef]
- Kikic, I.; Vecchione, F. Supercritical impregnation of polymers. Curr. Opin. Solid State Mater. Sci. 2003, 7, 399–405. [Google Scholar] [CrossRef]
- Lian, Z.; Epstein, S.A.; Blenk, C.W.; Shine, A.D. Carbon dioxide-induced melting point depression of biodegradable semicrystalline polymers. J. Supercrit. Fluids 2006, 39, 107–117. [Google Scholar] [CrossRef]
- Liu, G.; Wei, D.; Wang, H.; Hu, Y.; Jiang, Y. Self-assembly of zein microspheres with controllable particle size and narrow distribution using a novel built-in ultrasonic dialysis process. Chem. Eng. J. 2016, 284, 1094–1105. [Google Scholar] [CrossRef]
- Rosa, M.T.M.; Alvarez, V.H.; Albarelli, J.Q.; Santos, D.T.; Meireles, M.A.A.; Saldaña, M.D. Supercritical Anti-solvent Process as an Alternative Technology for Vitamin Complex Encapsulation Using Zein as Wall Material: Technical-economic Evaluation. J. Supercrit. Fluids 2019. [Google Scholar] [CrossRef]
- He, W.; Suo, Q.; Hong, H.; Shan, A.; Li, C.; Huang, Y.; Li, Y.; Zhu, M. Production of natural carotene-dispersed polymer microparticles by SEDS-PA co-precipitation. J. Mater. Sci. 2007, 42, 3495–3501. [Google Scholar] [CrossRef]
- Mezzomo, N.; Paz, E.d.; Maraschin, M.; Martín, Á.; Cocero, M.J.; Ferreira, S.R.S. Supercritical anti-solvent precipitation of carotenoid fraction from pink shrimp residue: Effect of operational conditions on encapsulation efficiency. J. Supercrit. Fluids 2012, 66, 342–349. [Google Scholar] [CrossRef]
- Fraile, M.; Buratto, R.; Gómez, B.; Martín, Á.; Cocero, M.J. Enhanced Delivery of Quercetin by Encapsulation in Poloxamers by Supercritical Antisolvent Process. Ind. Eng. Chem. Res. 2014, 53, 4318–4327. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Pereyra, C.; Martínez de la Ossa, E.J. Generation of quercetin/cellulose acetate phthalate systems for delivery by supercritical antisolvent process. Eur. J. Pharm. Sci. 2017, 100, 79–86. [Google Scholar] [CrossRef] [PubMed]
- García-Casas, I.; Montes, A.; Pereyra, C.; Martínez De La Ossa, E.J. Co-precipitation of mangiferin with cellulose acetate phthalate by supercritical antisolvent process. J. CO2 Util. 2017, 22, 197–207. [Google Scholar] [CrossRef]
- Ober, C.A.; Kalombo, L.; Swai, H.; Gupta, R.B. Preparation of rifampicin/lactose microparticle composites by a supercritical antisolvent-drug excipient mixing technique for inhalation delivery. Powder Technol. 2013, 236, 132–138. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Lu, P.-M.; Huang, C.-Y.; Wu, J.-J. Encapsulation of lycopene with lecithin and α-tocopherol by supercritical antisolvent process for stability enhancement. J. Supercrit. Fluids 2017, 130, 246–252. [Google Scholar] [CrossRef]
- Elizondo, E.; Sala, S.; Imbuluzqueta, E.; González, D.; Blanco-Prieto, M.J.; Gamazo, C.; Ventosa, N.; Veciana, J. High loading of gentamicin in bioadhesive PVM/MA nanostructured microparticles using compressed carbon-dioxide. Pharm. Res. 2011, 28, 309–321. [Google Scholar] [CrossRef]
- Junior, O.V.; Cardoso, F.A.R.; Giufrida, W.M.; de Souza, M.F.; Cardozo-Filho, L. Production and computational fluid dynamics-based modeling of PMMA nanoparticles impregnated with ivermectin by a supercritical antisolvent process. J. CO2 Util. 2020, 35, 47–58. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Cabañas, A.; Cheda, J.A.; Türk, M.; Pando, C. Cocrystallization of the anticancer drug 5-fluorouracil and coformers urea, thiourea or pyrazinamide using supercritical CO2 as an antisolvent (SAS) and as a solvent (CSS). J. Supercrit. Fluids 2020, 104813. [Google Scholar] [CrossRef]
- Saad, S.; Ahmad, I.; Kawish, S.M.; Khan, U.A.; Ahmad, F.J.; Ali, A.; Jain, G.K. Improved cardioprotective effects of hesperidin solid lipid nanoparticles prepared by supercritical antisolvent technology. Colloids Surf. B Biointerfaces 2020, 187, 110628. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Velaga, S.P.; Matos, H.A.; de Azevedo, E.G. Formation of indomethacin–saccharin cocrystals using supercritical fluid technology. Eur. J. Pharm. Sci. 2009, 38, 9–17. [Google Scholar] [CrossRef]
| Active Compound | Solvent | P [MPa] | T [°C] | C [mg/mL] | Morphology | Applications | Reference |
|---|---|---|---|---|---|---|---|
| Risperidone | CHF | 5–20 | 40 | 25–100 | C (size: 10–200 μm) | antipsychotic to treat bipolar and obsessive-compulsive disorders, schizophrenia | [87] |
| Sulfathiazole | AC, EtOH | 10–14 | 35, 45, 55 | 3–13 | C+MP (m.s.: 2.1–16.9 µm) | antimicrobial drug | [2] |
| Paracetamol | MeOH | 10 | 40 | Not reported | C (size: 4.2 μm) | analgesic and antipyretic to treat fever, headaches, and others | [75] |
| Cefonicid, Cefuroxime, Cefoperazone | DMSO | 9–18 | 40–60 | 10–90 | SMP, MP or BL of Cefonicid and Cefuroxime SMP or MP of Cefoperazone (size: 0.1–50 μm) | cephalosporins used before-surgery, to treat pneumonia, skin infections, urinary tract and post-operative infections | [61] |
| Ampicillin | NMP, EtOH, DMSO | 8–15 | 40 | 20 | SMP, MP, cSMP, irregular MP (m.s.: 0.26–1.2 µm) | antibiotic to treat respiratory, gastrointestinal and urinary tract infections | [58] |
| Amoxicillin | DMSO, DMSO/EtOH, DMSO/MeOH | 10–25 | 40 | 0.005–0.02 | SMPs, MPs (size: 0.2–1.6 µm) | antibiotic to treat infections of the skin, urinary and respiratory tracts | [59] |
| Amoxicillin | NMP, DMSO | 15 | 40 | 20–100 | SMP, MP (m.s.: 0.25–1.2 µm) | [60] | |
| Ampicillin, Amoxicillin | DMSO | 9 | 40 | 20 | SMP (m.s.: 0.23 µm for Ampicillin, 0.26 µm for Amoxicillin) | antibiotics | [17] |
| Griseofulvin, Ampicillin, Amoxicillin, Tetracycline | NMP, DMSO, EtOH, DCM | 10–18 | 40 | 20–120 | C of Griseofulvin AGG of Ampicillin Film of Amoxicillin cSMP of Tetracycline (m.s.: 0.2–0.6 µm) | Antibiotics for various infections | [85] |
| Griseofulvin | THF, DCM | 9.7 | 35 | 5 | C, needles, irregular NP-SMP (m.s. 0.13–0.51µm) | Antibiotic and antifungal drug | [86] |
| Cefdinir | MeOH | 12 | 45 | 20 | NP (m.s.: 0.15 μm) | antibiotic to treat infections of skin, eyes and respiratory tract | [25] |
| Rifampicin | DMSO, NMP, MeOH, EtAc, DCM | 9–14.5 | 40 | 5–70 | C, SMP, MP (m.s.: 0.4–5.0 µm) | antibiotic to treat tuberculosis, meningitis, biliary tract infections | [62] |
| Minocycline hydrochloride | ethanol | 7.5–13 | 35–50 | 1–20 | cSMP/AGG (m.s.: 0.2–0.3 µm) | antibiotic to treat infections of skin, urinary and respiratory tracts | [63,64] |
| Moxifloxacin | DMSO, DMFA, MeOH, acetic acid | 15 | 40 | 1–50 | C (m.s.: 0.3–8.2 µm) | antibiotic to treat tuberculosis | [77] |
| Sulfamethoxazole | AC | 10–12 | 35 | 88 | C (m.s.: 42–5 µm) | antibiotic to treat urinary tract infections, otitis, shigellosis, interstitial pneumonia | [74] |
| Theophylline | DMSO | 9 | 40 | 20 | C | bronchodilator to treat asthma | [44] |
| Salbutamol | DMSO, MeOH, EtOH/H2O | 9.5–15 | 40 | 3–10 | BL, cMP, rods (length: 1–3 µm; diameter: 0.2–0.4 µm) | drug to treat bronchial asthma | [71] |
| Fluticasone propionate | DCM | 6.5–11 | 40–60 | 5–17 | C (m.s.: 3.7–9.1 μm) | corticosteroid to prevent asthma symptoms | [88] |
| Dexamethasone, prednisolone, budesonide | EtOH | 9–15 | 40 | 20 | C | corticosteroids to treat ocular and pulmonary diseases, hepatitis, ulcers | [93] |
| Budesonide | DMC | 7.9–13.9 | 35–45 | 0.002–0.01 | AGG, MP (m.s. 1.4–2.0 μm) | corticosteroid to treat asthma, nasal polyps, bronchiectasis, pulmonary disease | [32] |
| Cilostazol | DCM, acetic acid | 8–15 | 40–60 | 50–150 | AGG (size: 1.0–4.5 μm) | vasodilator drug to treat vascular claudication | [76] |
| Telmisartan | EtOH/DCM | 12 | 45 | 25 | Irregular cSMP (m.s.: 740 µm) | drug for hypertension treatment | [70] |
| HCT | DMF, NMP, DMSO, THF, AC, 2-butanone | 15 | 42 | 20 | AGG, irregular particles | drug to treat hypertension | [33] |
| Atorvastatin | MeOH | 10–18 | 40–60 | 25–150 | NP, SMP (m.s.: 0.15–0.86 μm) | drug to treat hyper-cholesterolemia | [72] |
| Piroxicam | DCM | 9.7 | 25 | 0.05 | C | NSAID to treat arthritis, osteoarthritis, spondylitis | [12] |
| Diclofenac sodium | DMSO | 9 | 40 | 20 | NP (m.s.: 0.14 µm) | NSAID to treat arthritis, osteoarthritis, spondylitis | [46] |
| Diflunisal | AC, AC/DCM | 14–15 | 35–40 | 0.02–0.04 | C | NSAID for tuberculosis treatment | [11] |
| Ibuprofen sodium | EtOH | 8–12 | 35–50 | 0.0002–0.0004 | C | NSAID to treat fever, pains, various inflammations | [84] |
| Licorice | EtOH | 15–20 | 40 | 10–14 | AGG | thrombin inhibitor with antiulcer, antimicrobial, antidiabetic, hepatoprotective and anticancer activities | [89] |
| 5-fluorouracil | DMSO, DMSO/DCM | 15–18 | 40–50 | 0.1–0.2 | AGG, cSMP (m.s.: 0.22–0.67 µm) | anticancer drug | [67] |
| DLBS2347 | DMSO, EtOH, MeOH, EtAc, AC, DCM | 8–20 | 40–60 | Not reported | Film, C, NP-AGG | anticancer drug | [68] |
| Camptothecin | DMSO | 10–25 | 35–68 | 1–5 | cNP, SMP (m.s.: 0.4–0.9 µm) | anticancer drug | [4] |
| HCPT | DMSO | 10–25 | 35–68 | 0.5–5 | NP (m.s.: 0.18 µm) | anticancer drug | [3] |
| Taxol | EtOH | 10–25 | 35–68 | 2.5–10 | NP, MP (m.s.: 0.2–1.9 µm) | anticancer drug | [69] |
| GBE | EtOH | 10–40 | 35–80 | 1–5 | NP (m.s.: 0.1–0.2 µm) | antioxidant, antifungal and antitumor drug to treat diabetes cardiovascular diseases, cerebral insufficiency, dementia | [5] |
| Curcumin | EtOH, AC, AC/EtOH | 9 | 40 | 2–10 | AGG | polyphenol with antioxidant and anticancer properties | [80] |
| Mangiferin | DMSO, AC, NMP, DMSO/AC, DMSO/EtOH, NMP/AC, NMP/EtOH | 8–15 | 40–50 | 8–14 | NP, SMP, cSMP (size: 0.22–1.44 µm) | polyphenol with antioxidant, analgesic, anti-allergic, anticancer properties to treat diabetes, aging, periodontitis, neurodegenerative disease | [66] |
| Mangiferin | NMP | 10–20 | 35–59 | 5–59 | cSMP, SMP, MP (m.s. 0.56–1.04 µm) | antioxidant | [38] |
| Curcumin | AC/EtOH | 9–12 | 40 | 20 | Needles | polyphenol | [13] |
| Resveratrol | MeOH, EtOH, MeOH/DMC, EtOH/DMC | 15 | 40 | Not reported | NP, SMP (m.s.: 0.15–0.50 μm) | phenol to treat diabetes, cancer, cardiovascular and neurological disease | [7] |
| Folic acid | DMSO | 15 | 40 | 20 | AGG | vitamin B9 to prevent neural tube defects in infants, vascular diseases and megaloblastic anemia | [94] |
| Lycopene | DCM | 7–15 | 35–45 | 0.13–0.5 | C (m.s.: 10–80 μm) | carotenoid with antioxidant and anticancer properties | [90] |
| Lutein | EtAc | 6.5–9 | 35–45 | 0.5–0.9 | needles, cNP | carotenoid | [45] |
| β-carotene | AC/EtOH | 8.5 | 40 | 4–8 | C (m.s. 4–247 µm) | carotenoid with antioxidant and anticancer properties, also used to treat cardiovascular diseases and osteoporosis | [81] |
| β-carotene | DCM | 8–12 | 40 | 32–61 | C | carotenoid | [95] |
| β-carotene | DCM | 8–20 | 40 | 4–8 | C | carotenoid | [96] |
| Rosemary extracts | EtOH | 8–12 | 25–50 | Not reported | AGG, cSMP (size: 0.2–1 µm) | antioxidants with antimicrobial, anti-inflammatory and anticancer activities | [78] |
| Quercetin, Rutin | DMSO | 9, 13 | 40 | 20 | C | flavonoids with antioxidant and anticancer properties, also used to prevent cardiovascular disease | [97] |
| Quercetin | EtAc | 10 | 35 | 1.4 | needles (0.63 ± 0.06 µm) | flavonoid | [91] |
| Quercetin | EtOH | 8–25 | 35–65 | 2–11 | C, AGG, needles | flavonoid | [92] |
| Rutin | DMSO, DMSO/EtOH, DMSO/AC | 8–20 | 40–60 | 20–85 | cSMP, SMP, MP (m.s.: 0.3–1.9 μm) | flavonoid | [1] |
| Fisetin | EtOH/DCM | 10 | 45 | 1 | rods | flavonoid with antioxidant, neuroprotective, anticancer effects | [31] |
| Vitexin | DMSO | 15–30 | 40–70 | 1–2.5 | Irregular NP (m.s.: 0.13 µm) | flavonoid to prevent heart disease | [6] |
| Polymeric Carrier | Active Compound | Solvent | P [MPa] | T [°C] | Ctot [mg/mL] | Morphology | Reference |
|---|---|---|---|---|---|---|---|
| PVP | Cefuroxime axetil | MeOH | 7–20 | 35–50 | 50–150 | cMP, MP (m.s. 1.88–3.97 µm) | [103] |
| Ezetimibe | EtOH | 15 | 40 | 25 | NP (m.s. 0.21–0.23 µm) | [110] | |
| Dexamethasone, prednisolone, budesonide | EtOH | 9–15 | 40 | 10–30 | Dexamethasone MP (m.s. 1.82–2.51 µm), prednisolone MP (m.s. 1.96–3.03 µm), budesonide MP (m.s. 3.06–3.58 µm) | [93] | |
| Telmisartan | EtOH/DCM | 12 | 45 | 25 | SMP, MP (m.s. 0.38–0.60 µm) | [70] | |
| HCT | DMSO, DMSO/AC | 8.6–19 | 30–40 | 10–30 | NP (0.05–0.21 µm) | [33] | |
| Oxeglitazar | EtOH/CHF | 8 | 35 | 30 | C | [35] | |
| Nimesulide | DMSO | 9–15 | 35–45 | 20–35 | AGG or MP (m.s. 1.67–4.04 µm) | [8] | |
| Piroxicam | DCM | 9.7 | 25 | 0.05 | MP (0.1–5.0 µm) | [12] | |
| Ketoprofen | DMSO | 9–15 | 40 | 10–50 | MP (m.s. 2.41–3.81 μm) | [65] | |
| Diflunisal | AC/DCM | 12–14 | 35 | 18–36 | cNP, cMP (size: 0.4–8.1 µm) | [11] | |
| Folic Acid | DMSO | 9–15 | 35–40 | 20–40 | NP 0.05–0.20, SMP, MP (m.s. 0.30–3.80 µm) | [94] | |
| α-tocopherol, menadione | DMSO | 9–15 | 35–50 | 20–60 | α-tocopherol MP (m.s. 1.80–4.08 µm), menadione MP (m.s. 2.64–5.09 µm) | [9] | |
| Quercetin, rutin | DMSO | 9 | 40–50 | 20–40 | Quercetin MP (m.s. 0.47–9.52 μm), rutin MP (m.s. 0.84–8.17 μm) | [97] | |
| Fisetin | EtOH/DCM | 10 | 45 | Not reported | NP, SMP (0.08–0.72 μm) | [31] | |
| Chrysin | AC/EtOH | 12 | 40 | 1–3 | SMP (m.s. 0.27–0.96 μm) | [116] | |
| β-carotene | AC/EtOH | 8.5–10 | 40 | 5–7 | NPs (m.s. 0.25– µm), MP (m.s. 0.81–2.43 µm) | [81] | |
| Anthocyanins | EtOH | 10 | 40 | Not reported | C, AGG | [21] | |
| Curcuma | AC/EtOH | 15–21 | 35–45 | 2–10 | NP (m.s.0.11–0.21 µm) | [115] | |
| Curcumin | AC/EtOH | 8–12 | 40–60 | 1 | cSMP, SMP (size <1 µm) | [117] | |
| Curcumin | AC/EtOH | 10–20 | 30–50 | 1–10 | NP, SMP (m.s. 0.03–0.34 µm) | [123] | |
| Curcumin | EtOH, AC/EtOH | 2–12 | 35–50 | 5–20 | AGG, NP or SMP (m.s. 0.05–0.33 µm) | [80] | |
| PVP and MCC, starch or lactose | Curcumin | AC/EtOH | 9–12 | 40 | 20 | irregular particles/C of MCC (size: 175 µm), starch (size: 15 µm) or lactose (size <5 µm) coated with PVP/curcumin particles | [13] |
| PLA | Budesonide | DCM | 8.6 | 40 | 14 | MP (1.26 µm) | [32] |
| Cholesterol | DCM | 9 | 45 | 10, 46 | MP (1.70 µm), C (8.0 µm) | [124] | |
| Insulin | DMSO/ DCM | 8.5–13 | 20–38 | 1 | MP (0.50–2.0 µm) | [108] | |
| Lutein | EtAc | 10 | 17 | 21.8–22.2 | cMP (m.s. 1.0–10.0 µm) | [45] | |
| PLA/PCL | 17α-MT | DCM | 8 | 40 | 0.01 | MP (23.0–54.0 µm) | [40] |
| PLLA | Ibuprofen | DCM | 12–20 | 40–50 | 5–10 | MP (0.93–1.97 µm) | [99] |
| Naproxen | DCM | 10–20 | 40–50 | 5–8 | MP (0.56–1.43 µm) | [98] | |
| Amoxicillin | DMSO/ DCM | 10–20 | 29–50 | 2–9 | MP | [125] | |
| Rifampicin | DCM | 14–21 | 33–50 | 10–30 | MP (m.s. 3.26–30.53 µm) | [119] | |
| Azacytidine | DMSO/ DCM | 11 | 40 | 19 | C+MP (2.0 µm) | [111] | |
| Leuprolide acetate | DCM/ MeOH | 13 | 35 | 11–12 | MP | [41] | |
| Zidovudine | EtOH/ DCM | 8.5–13.5 | 45 | 0.2 | Filaments | [120] | |
| 5-fluorouracil | EtOH/ DCM | 12 | 33 | 4 | MP (0.98 µm) | [112] | |
| 5-fluorouracil | DMSO/DCM | 12–25 | 35–50 | 0.1–0.2 | AGG | [67] | |
| HCPT | EtOH/DCM | 7.5–12 | 30–40 | 1–9 | MP (0.57–1.37 µm) | [118] | |
| Paclitaxel | DCM, DCM/ DMSO, DCM/EtOH | 8–14 | 30–45 | 7–14 | MP (0.83–1.43 µm) | [126] | |
| Tamoxifen citrate | DCM | 13 | 38 | 13.5 | MP | [113] | |
| Astaxanthin | DCM/AC | 8–12 | 30–42 | 5–12 | Irregular MP (size: 0.6–2.0 µm) | [127] | |
| Gefitinib | EtOH/ DCM | 9–12 | 33–48 | 6–13 | MP (size: 1.1–3.8 µm) | [121] | |
| zein | Lutein | AC/ DMSO | 10 | 32–45 | 10–20 | SMP (m.s. 0.20–0.36 µm) | [49] |
| riboflavin, δ-tocopherol, β-carotenein | EtOH | 16 | 40 | 22–270 | EMP, MP (m.s. 8–18 µm) | [148] | |
| HCPT | DMSO, DMSO/EtOH | 8–14 | 35–45 | 6–21 | Rods, C+NP | [47] | |
| Lysozyme | EtOH/H2O | 10 | 40 | 0.05 | Collapsed MP/EMP with internal porosity (size up to 50 µm | [48] | |
| Diclofenac sodium | DMSO | 9 | 40 | 30–50 | SMP, MP (m.s. 0.42–1.3 µm) | [46] | |
| Amoxicillin, Ampicillin | DMSO | 9 | 40–50 | 50 | SMP, MP (m.s.: 0.65–12.0 µm for amoxicillin, 0.36–19 µm for ampicillin) | [17] | |
| β-CD | Lycopene | DMF | 10–14 | 40–50 | 1 | NP (m.s. 0.04–0.12 µm) | [128] |
| Cetirizine dihydrochloride | DMSO | 15 | 35 | 10–24 | SMP-MP (0.29–4.16 µm) | [22] | |
| H-β-CD | Apigenin | DMF | 10–25 | 35–65 | 67–402 | needles + cSMP (m.s. 0.4 µm) | [114] |
| Simvastatin | DCM/EtOH | 12 | 40 | Not reported | AGG | [140] | |
| Tosufloxacin tosylate | DCM/DMF | 8–16 | 35–55 | Not reported | C | [139] | |
| Resveratrol | EtOH | 12 | 40 | 0.03 | AGG/cNP | [137] | |
| Baicalein | AC/EtOH | 8–14 | 35–50 | Not reported | C, AGG (m.s. 0.3–1.0 µm) | [138] | |
| Eudragit RS100, Eudragit RL100 | Acetazolamide | AC | 5.7–9 | 27–40 | 15–24 | Needles + cMP, AGG | [43] |
| Eudragit L100 | Ellagic acid | NMP | 15 | 40 | 20 | MP+C | [122] |
| Ibuprofen | AC | 12–20 | 40, 50 | 5–10 | NP (0.08–0.21 µm), SMP (51 µm) | [99] | |
| Naproxen | EtOH | 10–20 | 40–50 | 5–8 | NP (0.08–0.15 µm) SMP (0.31 µm) | [98] | |
| Eudragit L100–55 | Ampicillin | DMSO | 9–10 | 40 | 20–40 | cMP, MP (m.s.: 1.5–2.5 μm | [104] |
| Diclofenac sodium, theophylline | DMSO | 9–15 | 40 | 20–50 | C, EMP, cMP, MP (m.s.: 1.5–2.9 µm for diclofenac, 1.6–6.8 µm for theophylline) | [44] | |
| PLGA | 5-Fluoracil | Acetone, DCM/ methanol | 11 | 36, 36–45 | 6, 6–30 | Film, cMP | [109] |
| Hydrocortisone | AC, MeOH/ DCM | 9 | 33 | 20 | C | [36] | |
| PLGA, PLGA/PLLA | Bupivacaine HCl | EtOH/ DCM | 8 | 40 | Not reported | MP + Fibers (5.56–7.07 µm), MP (4.39–10.9 µm) | [37] |
| PEG | β-carotene, Lutein | DCM | 8–10 | 15 | 13–17 | MP and C for β-carotene, AGG and irregular cMP for lutein | [129] |
| Carotene | DCM | 16 | 35–50 | 6 | C+cMP (m.s. 1–10 µm) | [149] | |
| Emodin | DCM/ MeOH | 8–20 | 35–50 | 1 | C (m.s. 3–20µm) | [130] | |
| Itraconazole | AC | 19 | 40 | 2 | C+ MP (m.s. 3 µm) | [106] | |
| Oxeglitazar | CHF | 8 | 35 | 30 | C | [35] | |
| mPEG/PLLA | Leuprolide acetate | DCM/ MeOH | 13 | 35, 15 | 11–12 | MP (m.s. 2.86–5.63 µm) | [41] |
| Ethyl cellulose | Amoxicillin | DMSO/ DCM | 10–25 | 35–65 | 10 | cMP (m.s. 0.23–2.66 µm) | [102] |
| Ampicillin | DMSO/ DCM | 10–25 | 35–55 | 11 | cMP (m.s. 1.0–3.0 µm) | [100] | |
| Amoxicillin | DMSO/ DCM | 10–15 | 35–50 | 23–24 | AGG, MP | [101] | |
| Quercetin | EtAc | 10 | 35 | 1.4 | cNP, cSMP (m.s. 0.18–0.34 µm) | [91] | |
| Rifampicin | EtAc, EtAc/ DMSO | 10 | 35–60 | 13–15 | NP+C NP (m.s. 0.19–0.23 µm) | [105] | |
| PMC | Insulin | DMSO/AC | 12 | 32 | 2, 4–7 | NP (0.14 µm), SMP (m.s. 0.27–0.34 µm) | [107] |
| Itraconazole | EtOH/ DCM | 8–15 | 45–60 | Not reported | C+NP/cSMP (size: 0.1–0.5 µm) | [42] | |
| Lercanidipine | EtOH/ DCM | 15 | 40 | 50 | NP, SMP (m.s. 0.22–0.44 µm) | [34] | |
| Megestrol acetate | EtOH/ DCM | 15 | 40 | 52 | NP, SMP (m.s. 0.14–0.50 µm) | [39] | |
| Telmisartan | EtOH/ DCM | 12 | 45 | 25 | SMP (m.s. 0.45–0.50 µm) | [70] | |
| HPMC/poloxamers/HCO-60 | Felodipine | EtOH/ DCM | 10 | 45 | Not reported | cNP/cSMP (m.s. 0.20–0.25 µm) | [23] |
| Poloxamers | Oxeglitazar | DMC, EtOH/CHF | 8 | 35 | 23 | C | [35] |
| Rosemary extracts | EtOH | 10 | 50 | Not reported | cSMP | [78] | |
| Astaxanthin | AC | 8–12 | 35–40 | 6 | cMP | [150] | |
| Quercetin | AC | 10 | 40 | 0.03–0.2 | cMP (1 µm) | [151] | |
| PHBV | β-carotene | DCM | 8 | 40 | 21–48 | Irregular cMP, EMP, AGG | [135] |
| β-carotene | DCM | 8–20 | 40 | 32–61 | Not reported | [96] | |
| Astaxanthin | DCM | 8–10 | 35 | 5–10 | SMP (m.s. 0.22–0.40 µm) | [131] | |
| Bixin | DCM | 8–10 | 35–40 | 1.4–20.4 | SMP (m.s. 0.20–0.55 µm) | [132] | |
| Grape seed extract | DCM | 8–12 | 35–45 | 27–40 | SMP (m.s. 0.62–0.72 µm) | [133] | |
| Pink pepper extract | DCM | 8–12.5 | 35–55 | 30 | cSMP, MP (m.s. 0.39–25.4 µm) | [134] | |
| CAP | Quercetin | AC | 9 | 40 | 20 | NP+C | [152] |
| Mangiferin | AC/DMSO | 18 | 50 | 8–26 | cSMP (m.s. 0.25–0.41 µm) | [153] | |
| HPC | Ezetimibe | EtOH | 12–18 | 40–50 | 10–25, 50–100 | NP (m.s. 0.15–0.24 µm) SMP, MP (m.s. 0.33–0.91 µm) | [110] |
| sulfamethoxazole | AC | 10 | 35 | 88 | AGG | [74] | |
| Lactose | Rifampicin | MeOH | 12.4 | 40 | 1–5 | cMP < 8 µm | [154] |
| Lecithin/α-tocopherol | Lycopene | DMF | 8–12 | 35 | 20–30 | C | [155] |
| PVM/MA | Gentamicin | AC | 10 | 25 | 140 | NP, MP (m.s. 0.05–0.93 µm) | [156] |
| PMMA | ivermectin | AC | 9–11 | 40–60 | 33 | cNP, NP (m.s. 0.05–0.17 µm) | [157] |
| Urea, thiourea or PZA | 5-fluorouracil | MeOH | 7–15 | 40 | 4–8 | C | [158] |
| L-arginine | glimepiride | DCM/EtOH | 12 | 60 | 7 | C (m.s. 5–79 µm) | [24] |
| Stearic acid | hesperidin | DMSO | 8–20 | 35–45 | 75 | NP-SMP (m.s. 0.15–0.39 µm) | [159] |
| Saccharin | Indomethacin | EtOH, MeOH, EtAc | 8.4–9 | 50 | Not reported | C | [160] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, P.; De Marco, I. Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes 2020, 8, 938. https://doi.org/10.3390/pr8080938
Franco P, De Marco I. Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes. 2020; 8(8):938. https://doi.org/10.3390/pr8080938
Chicago/Turabian StyleFranco, Paola, and Iolanda De Marco. 2020. "Supercritical Antisolvent Process for Pharmaceutical Applications: A Review" Processes 8, no. 8: 938. https://doi.org/10.3390/pr8080938
APA StyleFranco, P., & De Marco, I. (2020). Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes, 8(8), 938. https://doi.org/10.3390/pr8080938






