Abstract
Wendita calysina (commonly known as burrito) is an indigenous Paraguayan medical plat, whose essential oil (EO) is characterized by some pharmaceutical properties. In particular, the main component is D-carvone, with anticancer action and antimicrobial properties against various microorganisms. In this work, supercritical CO2 (SC-CO2) was used for the extraction of volatile compounds from this plant, selecting different operative conditions to optimize the extract yield and purity. Pressure was varied from 80 to 250 bar, and two CO2 flow rates (0.8 kg/h and 1.2 kg/h) were tested. The highest EO percentage in the extract was obtained operating at 100 bar and 40 °C, using ground Wendita calysina leaves of 250 µm. CO2 flow rate did not influence the extraction yield, indicating that an internal mass transfer resistance governs this process. The largely prevailing compound identified in the extract was D-carvone, with a mean percentage up to 90% w/w.
1. Introduction
The adoption of a correct diet together with regular physical exercise are recognized as useful for the prevention of some human diseases [1,2,3]. For instance, the daily consumption of foods rich in antioxidants can reduce free radical degradation of cells and tissues in an organism [2,4]. Herbal infusions are a possible sources of these bioactive molecules [5,6]; green tea is one of the major examples, widely used particularly in oriental regions due to its medical effects in the prevention and treatment of various diseases, such as cancer, cardiovascular disease, diabetes and obesity [7].
Infusions of Wendita calysina (Geraniaceae) leaves, a Paraguayan herbaceous plant, generally known as burrito, are also recognized as healthy for humans, thanks to the well-known antimicrobial, anti-inflammatory, antioxidant and anti-rheumatic properties [8,9]. Marzocco et al. [9] demonstrated that Wendita calysina water-soluble extract can be used in the therapy of acute inflammation. Mesa et al. [10] reported an ultrasonic extraction procedure from Wendita calysina leaves using a water/ethanol mixture (40/60% v/v), followed by spray-drying. These authors determined the antiplatelet action of this extract to be used as food supplement and phytomedicine. Wendita calysina is rich in D-carvone, a monoterpenic ketone with antimicrobial, anticonvulsant, and antioxidant properties [11]. In particular, it shows antimicrobial properties against various microorganisms, like Listeria monocytogenes [12], Escherichia coli, Salmonella typhimurium, Photobacterium leiognathi [13], Fusarium subglutinans, Fusarium cerealis, Fusarium verticillioides, Fusarium proliferatum, Fusarium oxysporum, Fusarium sporotrichioides, Aspergillus tubingensis, Aspergillus carbonarius, Alternaria alternata and Penicillium sp. [14]. Aydin et al. [15] demonstrated that carvone has also anticancer potential in vitro, which improves the general healthy Wendita calysina plant effects [8,9,10].
Maceration is the most frequently used traditional technique to produce extracts from a vegetable matter [16]; an organic solvent and, in some cases, water, affine with the compounds of interest, is used at a specific ratio with the starting vegetable matter. This procedure can last from some hours to some days, and the final extract is recovered after solvent evaporation. However, maceration is not a selective extraction technique and the obtained extracts have to be generally post-processed to remove the organic solvent residues and/or impurities [16].
Supercritical fluid extraction (SFE) is a green alternative technique to extract high-value compounds from vegetable matter, avoiding the use of toxic organic solvents that pollute the final product and the environment [17,18,19]. Supercritical fluids and, in particular, supercritical CO2 (SC-CO2), are characterized by favorable physico-chemical properties (i.e., gas-like diffusivity, liquid-like density, and near to zero surface tension) and reduced cost [20]. Numerous vegetal materials were processed by SFE [21,22]. Parisotto et al. [23] found that the antitumor activity of Cordia verbenacea extracts obtained by SFE was superior in the reduction of tumor cells viability and proliferation with respect to the extract obtained by classical organic solvent extraction with ethanol. Radojković et al. [24] performed SFE process on Morus leaves working at 300 bar and 40 °C. They obtained a linoleic and linolenic acid-rich extract to be used as dietary supplement and food product. Souza et al. [25] determined the effects of temperature and pressure on the extraction yield and chemical composition of extracts from Aloysia gratissima leaves obtained by SFE. Operative temperature was varied from 40 to 60 °C; whereas pressure was from 150 to 200 bar. The chemical analysis showed the presence of guaiol, pinocamphone, caryophyllene oxide and spathulenol as the major chemical components of this plant extract that contributed to a significant reduction of carrageenan-induced paw edema and myeloperoxidase activity.
SFE technology can also allow a fractional separation of the extracts, to obtain high purity compounds [19]. This result is possible when process conditions are opportunely selected. Operative pressure and temperature influence SC-CO2 solvent power through CO2 density; therefore, depending on the solubility and on the position inside the vegetable matter of the compounds of interest, these parameters should be selected, together with CO2 flow rate and vegetable matter particle size, with the aim of minimizing the co-extraction of undesired compounds [26]. Another possibility is to perform the fractional extraction, changing the SC-CO2 solvent power during processing. In particular, depending on the molecular weight (and on the solubility) of the various components, a reduced SC-CO2 solvent power can be selected in a first step to extract essential oil compounds (low molecular weight compounds); then, in a second step, operative pressure is increased, to extract the other higher molecular weight compounds [26].
Taking into account these considerations, to our knowledge, this work performed SFE of Wendita calysina leaves for the first time, to obtain a high purity essential oil with enhanced healthy properties for pharmaceutical applications. Systematic gas chromatography–mass spectrometry (GC-MS) analyses were carried out on the extracts, to optimize the parameters related to SFE processing and on the starting vegetable matter, to produce a high added-value, solvent free product.
2. Materials and Methods
2.1. Plant Material
Dried Wendita calysina Martius leaves (moisture content lower than 10% w/w), produced by Coprosa Ltd.a, Paraguay (Cooperativa multiactiva de Produccìon Servicios publicos, Consumo, Ahorro y Credito, San Andres), were supplied by Commercio Alternativo s.r.l. (Ferrara, Italy). Before processing, Wendita calysina leaves were ground using a cooled milling apparatus (Waring Commercial Blender); mean particle size was determined using mechanical sieving.
2.2. Supercritical Fluid Extraction
Supercritical CO2 extraction was performed in a lab-scale apparatus equipped with a 400 cm3 internal volume extractor. CO2 (purity 99%) was purchased from Morlando Group s.r.l. (Sant’Antimo, NA, Italy). An amount of 100 g of the vegetable matter (mean particle size of 250 µm) was mixed with 85 g of 3-mm glass beads to avoid caking and channeling phenomena in the fixed bed of particles inside the extractor. Extract fractions were recovered using two separation vessels in series, with an internal volume of 200 cm3. Cooling of the first separator was achieved using a thermostated bath (Julabo, mod. F38-EH, Seelbach, Germany). The second separator allowed the continuous discharge of the product using a valve located at the bottom of the vessel. A membrane high-pressure pump (Lewa, mod. LDB1 M210S, Mazzo di Rho, Italy) pumped liquid CO2 at the desired flow rate. CO2 was heated up to the extraction temperature in a thermostated oven, and its flow rate was monitored by a calibrated rotameter (ASA, mod. N.5-2500, Serval 115022, Sesto San Giovanni, Italy). Temperatures along the extraction apparatus were measured by thermocouples and regulated using PID controllers (mod. 305, Watlow, Corsico (MI), Italy); whereas pressure was measured by test gauges (mod. MP1, OMET, Lecco, Italy). A schematic representation of an SFE plant can be found in [27]. Each experiment was performed in duplicate.
2.3. GC–MS Analyses of Compounds from Wendita Calysina Extracts
Wendita calysina extracts obtained by SFE were characterized by gas chromatography–mass spectrometry (GC-MS). The apparatus was a Varian (mod. Saturn 2100T, San Fernando, CA, USA); separation was achieved using a fused-silica capillary column (mod. DB-5, J&W, Folsom, CA, USA) with a 30-m length, 0.25-mm internal diameter and 0.25-µm film thickness. GC conditions were oven temperature at 40 °C for 5 min; then, programmed heating from 40 to 250 °C at 2 °C/min. After 60 min at 250 °C, temperature was increased to 270 °C (speed 2 °C/min) and, finally, from 270 to 290 °C, at 1 °C/min. Helium was used as the carrier gas (1 mL/min). Samples were run in dichloromethane using a dilution factor of 0.0008% w/w. All the components were identified according to the retention time and molecular weight. The same procedure was adopted for the identification of waxes.
3. Results
The aim of this work was the extraction of a high purity volatile oil from Wendita calysina leaves by SFE. Since this was the first time this technology was applied to Wendita calysina, a preliminary series of experiments was performed using different CO2 flow rates, to determine the kind of mass transfer resistance governing this process. CO2 flow rate was varied from 0.8 to 1.2 kg/h; whereas the plant mean particle size was fixed at 250 µm, obtained using the procedure described in Materials and Methods. SFE was performed at 250 bar and 40 °C (ρCO2 = 0.880 g/cm3); the first separator was set at −14 °C and the second separator was operated at 25 °C and 15 bar. The selection of these values of temperature in the two separators was based on previous works [17,19,28]; the aim was to induce waxes (i.e., paraffins) precipitation in the first separator due to the solubility reduction of these components in SC-CO2 at low temperature, and the extract recovery in the second separator at conditions near to the ambient ones to avoid thermal stress on the liquid extract.
The extraction yield, measured as the ratio between the weight of the extract collected at regular time intervals and the starting vegetable matter loaded in the extractor, was similar using the two CO2 flow rates. This information indicates that an internal mass transfer resistance governs this process [20]: i.e., the compounds of interest are located inside the vegetable matter, therefore, the effect of CO2 flow rate is negligible. Once the kind of extraction mechanism was ascertained, all the following experiments were performed using a CO2 flow rate of 0.8 kg/h and Wendita calysina leaves particle size of 250 µm.
The second part of the work was focused on the obtainment of a higher essential oil (EO) purity. Operative pressure was varied from 80 to 250 bar and temperature was fixed at 40 °C, to avoid thermal stresses on the vegetable material, and with the aim of determining the effect of SC-CO2 solvent power. In all experiments, separators’ conditions were the same as the preliminary experiments.
SFE was carried out in two steps (fractional extraction): the first one was performed at 80 bar and 40 °C (ρCO2 = 0.281 g/cm3), the second one at 150 bar and 40 °C (ρCO2 = 0.780 g/cm3). Extraction was stopped when a negligible increment of the extract was obtained. A final total yield of 0.763% w/w and an EO yield of 0.717% w/w were measured. However, working at 80 bar and 40 °C, due to the reduced SC-CO2 density, a too low EO extraction yield was obtained (≈0.03% w/w); therefore, these operative conditions were discarded. Moreover, observing the last 100 min of processing, the distance between the total extract yield curve and the EO yield curve tended to enlarge due to the extraction of non-terpenic compounds: i.e., higher molecular weight compounds were co-extracted that do not belong to Wendita calysina essential oil. Waxes extraction yield was 1.91% w/w, measured in the first separator at the end of the supercritical processing.
A further SFE experiment was performed at 250 bar e 40 °C, to determine the effect of a larger SC-CO2 density on the total and EO yield. However, as expected, increasing SC-CO2 density and, thus, its solvent power, the final total yield (0.794% w/w) was larger than the one measured working at 150 bar and 40 °C (0.763% w/w); but, also the difference between the final total yield and the EO yield (0.636% w/w) was still larger, due to the co-extraction of other undesired families of compounds (see Figure 1). Therefore, a lower EO purity characterized this extract. In Figure 1, this trend is clearly visible, even if the extraction kinetics were faster (420 min). Waxes collected in the first separator had a yield of 2.02% w/w.
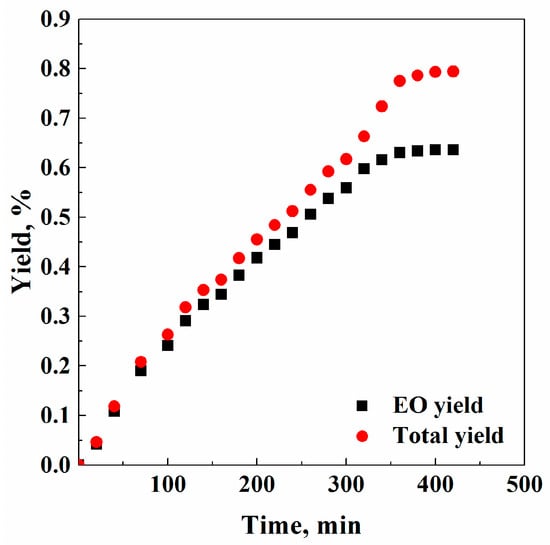
Figure 1.
Representation of supercritical fluid extraction (SFE) total yield and essential oil (EO) yield versus time: 250 bar and 40 °C.
Taking into account these results, another series of experiments was performed at intermediate SC-CO2 solvent power: i.e., 100 bar and 40 °C (ρCO2 = 0.623 g/cm3), to balance a fast extraction and a high EO purity in the extract. Figure 2 reports that a very similar final total yield (0.697% w/w) and EO yield (0.693% w/w) were obtained: i.e., the two curves practically overlap. This result confirms that adopting the opportune SFE operative conditions, it is possible to selectively extract the compounds of interest. Waxes yield was 1.56% w/w in this case.
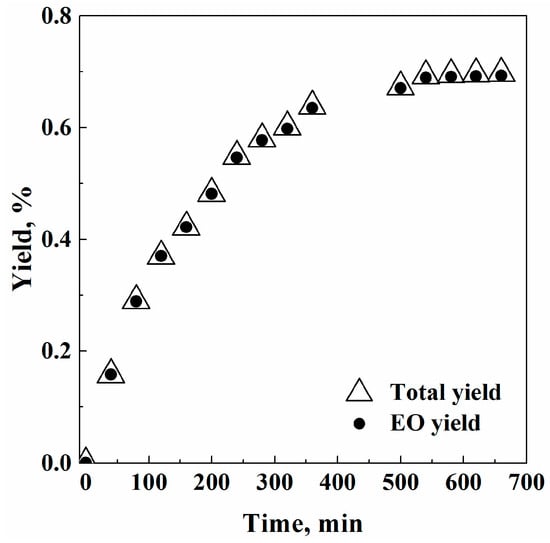
Figure 2.
Representation of SFE total yield and EO yield versus time: 100 bar and 40 °C.
Summarizing these results, SFE confirmed its flexibility in extracting the compounds of interest changing SC-CO2 solvent power. This peculiarity, coupled with fractional separation, favored the obtainment of a high purity EO in the extract, without the use of toxic organic solvents.
Extracts collected in the first and in the second separator were systematically characterized by GC-MS, as described in Materials and Methods. The extracts composition was in all cases similar and mainly formed by monoterpenes and sesquiterpenes (at the second separator): identification of compounds related to SFE performed at 100 bar and 40 °C is reported in Table 1. In particular, D-carvone, a monoterpene responsible for the characteristic odor of caraway, was the major compound forming the Wendita calysina essential oil, as demonstrated by the GC-MS trace in Figure 3. Its mean amount in the extract represented up to 90% of the EO composition by supercritical processing.

Table 1.
Gas chromatography–mass spectrometry (GC-MS) analysis on SFE extracts obtained at 100 bar and 40 °C.
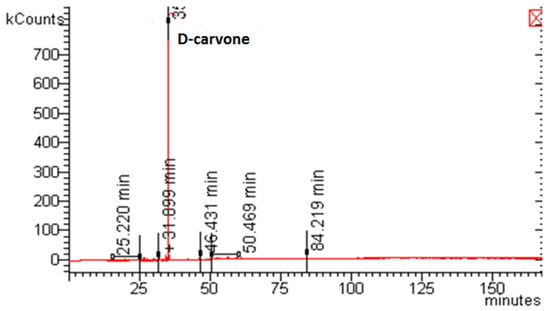
Figure 3.
Example of a GC-MS trace related to the extract collected in the II separator.
Waxes collected in the first separator were white, odorless and mainly formed by Nonacosane (C29) 1.227%, Triacontane (C30) 12.616%, traces of Hentriacontane (C31) and Dotriacontane (C32) 86.150%, as shown in Figure 4. These results demonstrate the efficiency of SFE fractional separation since, in this separator, only waxes (paraffinic compounds) were precipitated; other peaks, related to other compounds were not detected. Moreover, waxes extraction yield increased from 1.56% w/w (@100 bar) to 2.02% w/w (@250 bar) when a larger SC-CO2 solvent power was tested.
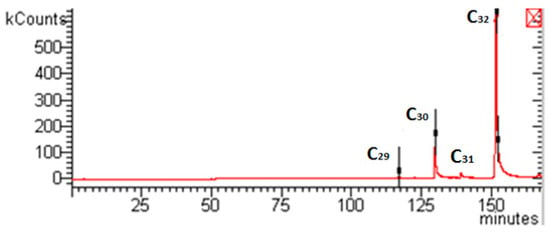
Figure 4.
Example of a GC-MS trace related to waxes collected in the I separator at −14 °C and 100 bar.
4. Conclusions
Extracts produced by SFE can have a larger added value, since the co-extraction of undesired families of compounds can be minimized. A selective extraction is possible by obtaining a high purity, solvent-free extract. Operating at 100 bar and 40 °C, SFE applied to Wendita calysina leaves was successful in obtaining an extract with a content of D-carvone up to 90% w/w. This result was possible thanks to the addition of a fractional separation scheme that allowed waxes precipitation in the first separator, and pure extract collection in the second one. This product can be used in the pharmaceutical field thanks to its antimicrobial, anticancer, anti-inflammatory, antioxidant and anti-rheumatic properties.
A subsequent study will be focused on the testing of pharmaceutical properties of Wendita calysina leaves extract, in order to determine its efficacy and the doses required.
Author Contributions
Conceptualization, L.B. and E.R.; methodology, E.R.; formal analysis, L.B.; investigation, L.B.; resources, E.R.; writing—original draft preparation, L.B.; writing—review and editing, L.B. and E.R.; supervision, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sur, P.; Chandhuri, T.; Vedasiromoni, J.R.; Gomes, A.; Ganguly, D.K. Antiinflammatory and antioxidant property of saponins of tea [Camellia sinensis (L) O. Kuntze] root extract. Phytother. Res. 2001, 15, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Wongkham, S.; Laupattarakasaem, P.; Pienthaweechai, K.; Areejitranusorn, P.; Wongkham, C.; Techanitiswad, T. Antimicrobial activity of Streblus asper leaf extract. Phytother. Res. 2001, 15, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.Z.; Tan, H.W.; Zhang, H.; Su, X.L.; Wei, W.Z. Bulk acoustic wave bacterial growth sensor applied to analysis of antimicrobial properties of tea. Biotechnol. Prog. 1998, 14, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Hakamata, H.; Takahashi, K.; Kotani, A.; Kusu, F. Determination of quercetin in human plasma after ingestion of commercial canned green tea by semi-micro HPLC with electrochemical detection. Biomed. Chromatogr. 2004, 18, 662–666. [Google Scholar] [CrossRef]
- Marongiu, B.; Porcedda, S.; Piras, A.; Rosa, A.; Deiana, M.; Dessi, M.A. Antioxidant activity of supercritical extract of Melissa officinalis subsp officinalis and Melissa officinalis subsp inodora. Phytother. Res. 2004, 18, 789–792. [Google Scholar] [CrossRef]
- Wu, S.J.; Ng, L.T.; Lin, C.C. Antioxidant activities of some common ingredients of traditional Chinese medicine, Angelica sinensis, Lycium barbarum and Poria cocos. Phytother. Res. 2004, 18, 1008–1012. [Google Scholar] [CrossRef]
- Arab, H.; Maroofian, A.; Golestani, S.; Shafaee, H.; Sohrabi, K.; Forouzanfar, A. Review of the therapeutic effects of Camellia sinensis (green tea) on oral and periodontal health. J. Med. Plants Res. 2011, 5, 5465–5469. [Google Scholar]
- Piccinelli, A.L.; De Simone, F.; Passi, S.; Rastrelli, L. Phenolic Constituents and antioxidant activity of Wendita calysina leaves (Burrito), a folk paraguayan tea. J. Agric. Food Chem. 2004, 52, 5863–5868. [Google Scholar] [CrossRef]
- Marzocco, S.; Piccinelli, A.L.; Rastrelli, L.; Mazzon, E.; Cuzzocrea, S.; Autore, G. Inhibition of inducible nitric oxide synthase in vitro and in vivo by a water-soluble extract of Wendita calysina leaves. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007, 375, 349–358. [Google Scholar] [CrossRef]
- Mesa, M.G.; Piccinelli, A.L.; Antonia, M.; Valiente, A.; Pinto, A.; Fazio, A.; Rastrelli, L. Inhibition of human platelet aggregation in vitro by standardized extract of Wendtia calycina. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2011, 21, 884–888. [Google Scholar] [CrossRef][Green Version]
- Bicas, J.L.; Neri-Numa, I.A.; Ruiz, A.L.; De Carvalho, J.E.; Pastore, G.M. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem. Toxicol. 2011, 49, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A 2012, 29, 415–422. [Google Scholar]
- Aydın, E.; Türkez, H.; Keleş, M.S. Potential anticancer activity of carvone in N2a neuroblastoma cell line. Toxicol. Ind. Health 2015, 31, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Alizadehdakhel, A.; Golzary, A. An environmental friendly process for extraction of active constituents from herbal plants. Environ. Energy Econ. Res. 2020, 4, 69–81. [Google Scholar]
- Baldino, L.; Reverchon, E.; Della Porta, G. An optimized process for SC-CO2 extraction of antimalarial compounds from Artemisia annua L. J. Supercrit. Fluids 2017, 128, 89–93. [Google Scholar] [CrossRef]
- Baldino, L.; Della Porta, G.; Sesti Osseo, L.; Reverchon, E.; Adami, R. Concentrated oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J. Supercrit. Fluids 2018, 133, 65–69. [Google Scholar] [CrossRef]
- Baldino, L.; Adami, R.; Reverchon, E. Concentration of Ruta graveolens active compounds using SC-CO2 extraction coupled with fractional separation. J. Supercrit. Fluids 2018, 131, 82–86. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Challenges in the production of pharmaceutical and food related compounds by SC-CO2 processing of vegetable matter. J. Supercrit. Fluids 2018, 134, 269–273. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Parisotto, E.B.; Michielin, E.M.Z.; Biscaro, F.; Ferreira, S.R.S.; Wilhelm Filho, D.; Pedrosa, R.C. The antitumor activity of extracts from Cordia verbenacea D.C. obtained by supercritical fluid extraction. J. Supercrit. Fluids 2012, 61, 101–107. [Google Scholar] [CrossRef]
- Radojković, M.; Zeković, Z.; Mašković, P.; Vidović, S.; Mandić, A.; Mišanb, A.; Ðurović, S. Biological activities and chemical composition of Morus leaves extracts obtained by maceration and supercritical fluid extraction. J. Supercrit. Fluids 2016, 117, 50–58. [Google Scholar] [CrossRef]
- Souza, M.A.; Guzatti, J.G.G.; Martello, R.H.; Schindler, M.S.Z.; Calisto, J.F.F.; Morgan, L.V.; Aguiar, G.P.S.; Locateli, G.; Scapinello, J.; Müller, L.G.; et al. Supercritical CO2 extraction of Aloysia gratissima leaves and evaluation of anti-inflammatory activity. J. Supercrit. Fluids 2020, 159, 104753. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Extraction of rotenoids from Derris elliptica using supercritical CO2. J. Chem. Technol. Biotechnol. 2018, 93, 3656–3660. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Artemisia annua organic solvent extract, processed by supercritical CO2. J. Chem. Technol. Biotechnol. 2018, 93, 3171–3175. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).