Compound Identification and In Vitro Cytotoxicity of the Supercritical Carbon Dioxide Extract of Papaya Freeze-Dried Leaf Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Supercritical Fluid Extraction (SFE)
2.3. Gas Chromatography–Mass Spectrometry
2.4. Sample Preparation for UHPLC–QToF-MS Analysis
2.5. UHPLC–QToF-MS Analysis
2.5.1. Calibration Curves, Linearity Ranges, Limits of Detection (LOD), and Limits of Quantification (LOQ)
2.5.2. Quantification of dl-α-Tocopherol and Phytosterols by LC–QToF-MS
2.6. Sample Preparation for the Cell Viability Assay
2.7. Cell Culture Conditions
Cell Viability Assay
2.8. Statistical Analysis
3. Results
3.1. Supercritical Carbon Dioxide Extraction
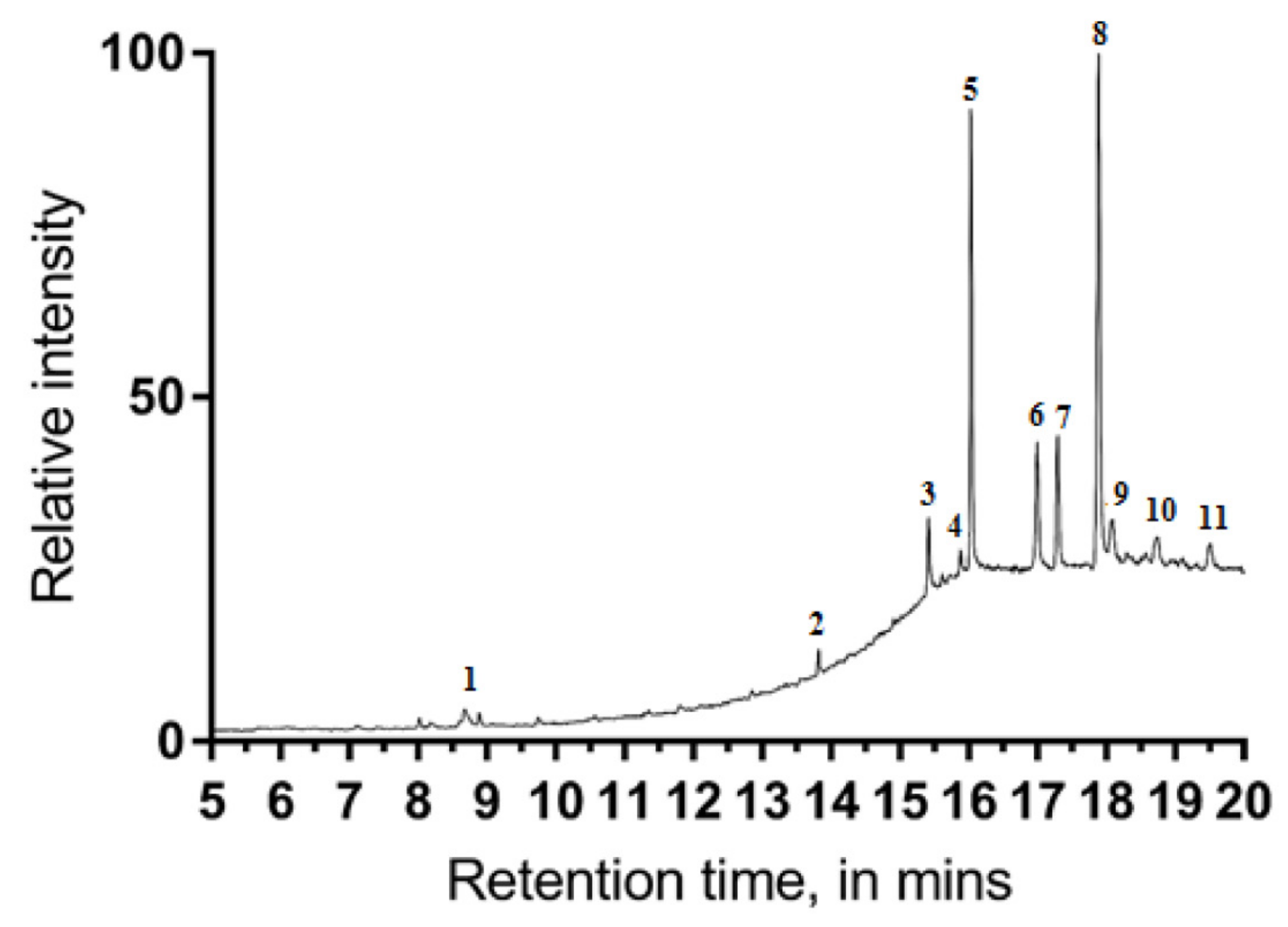
3.2. Chemical Analysis of scCO2 Extract with GC–MS
3.3. Compound Identification by Comparison with Authentic Standards
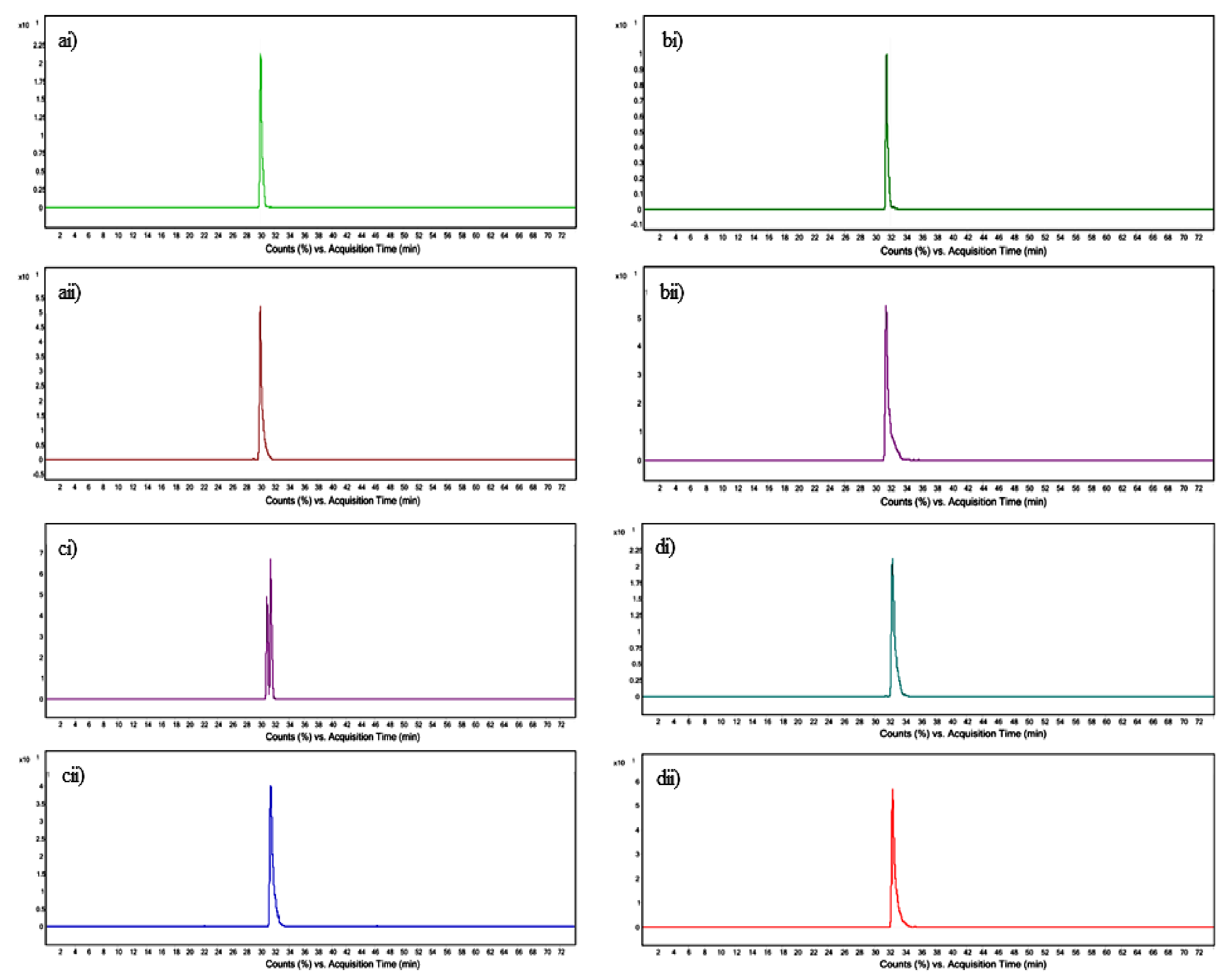
3.4. Quantification of Tocopherol and Phytosterols by UPLC–QToF-MS
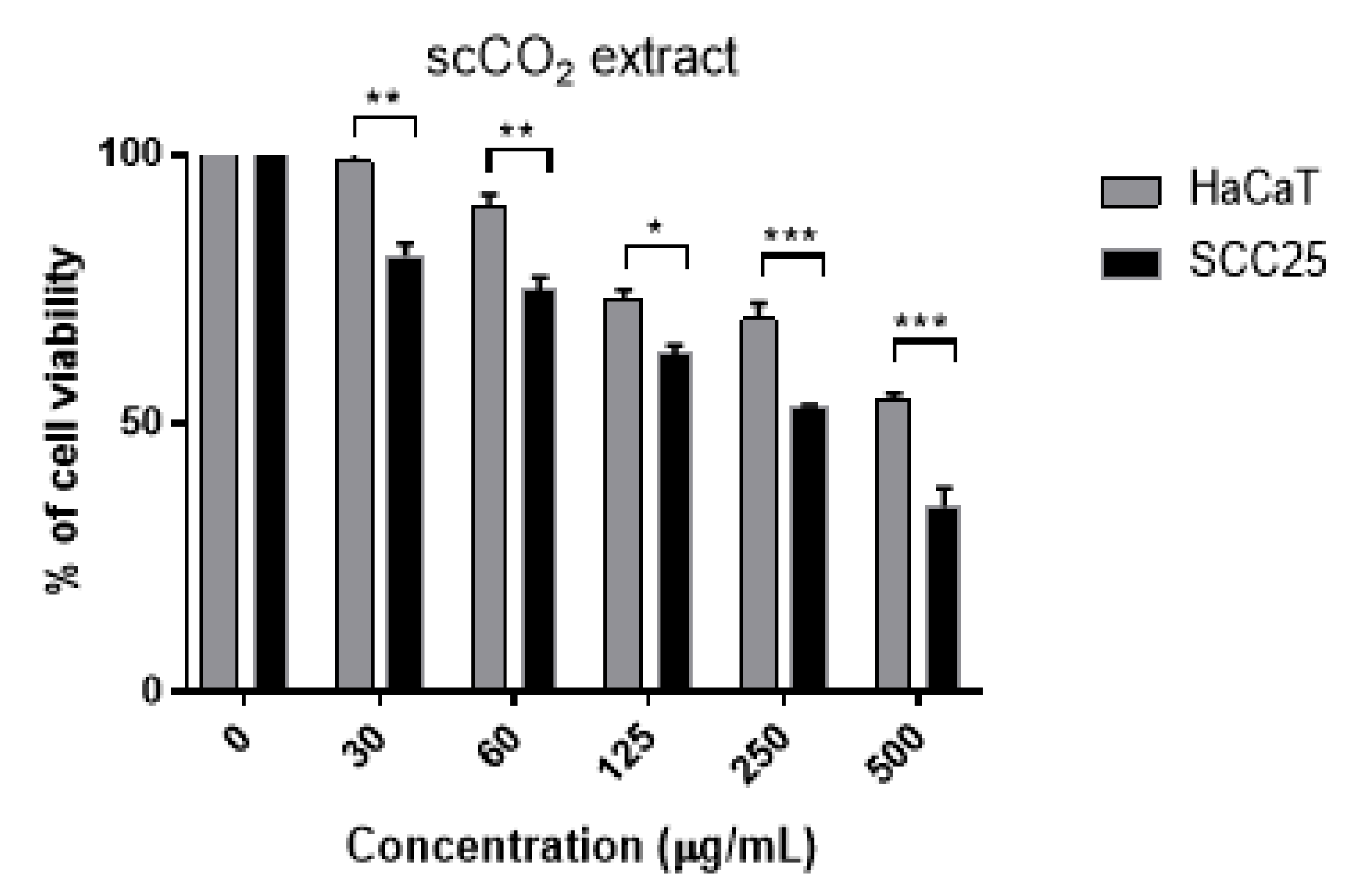
3.5. Cytotoxicity of scCO2 Extract and Identified Compounds
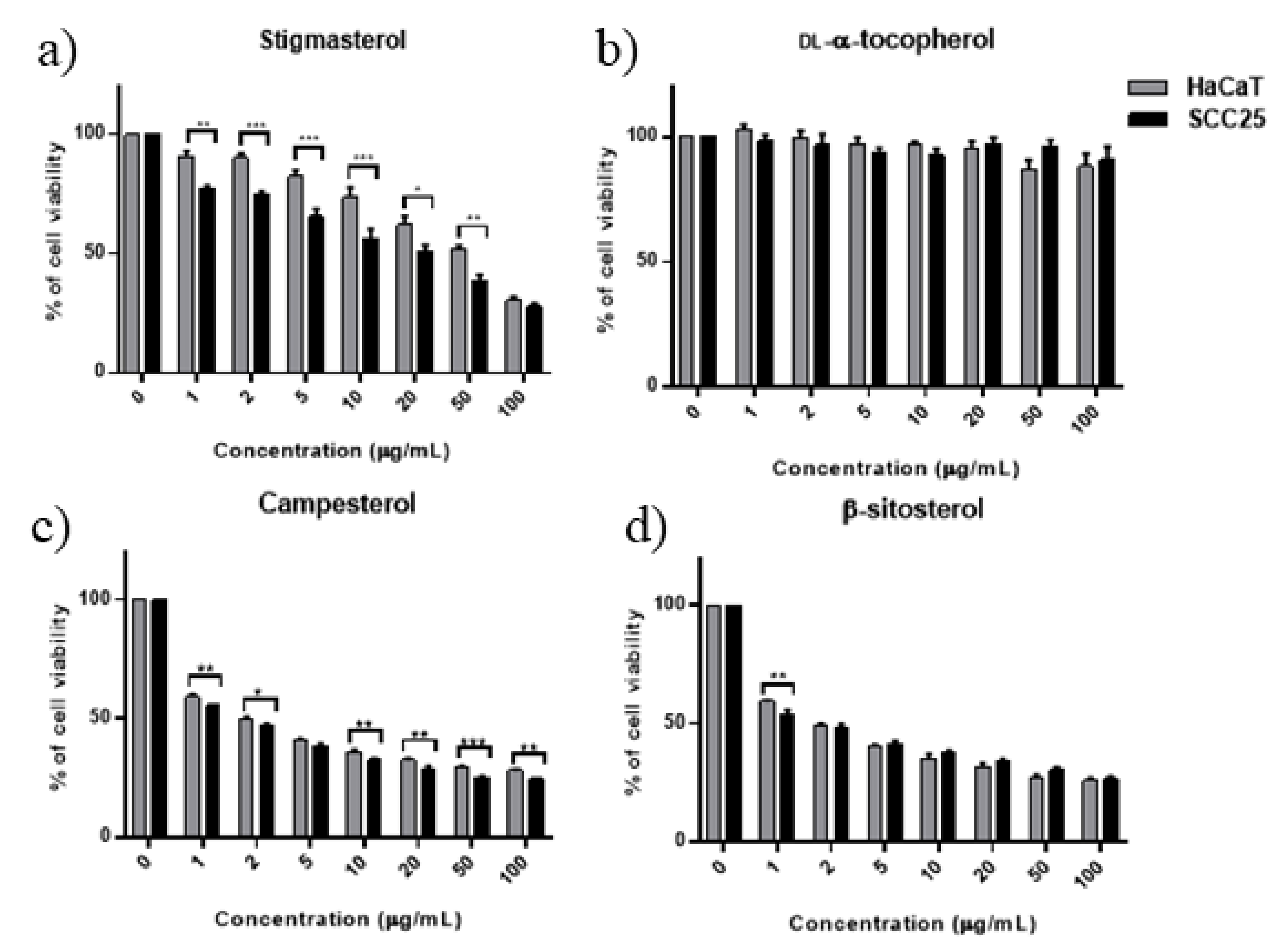
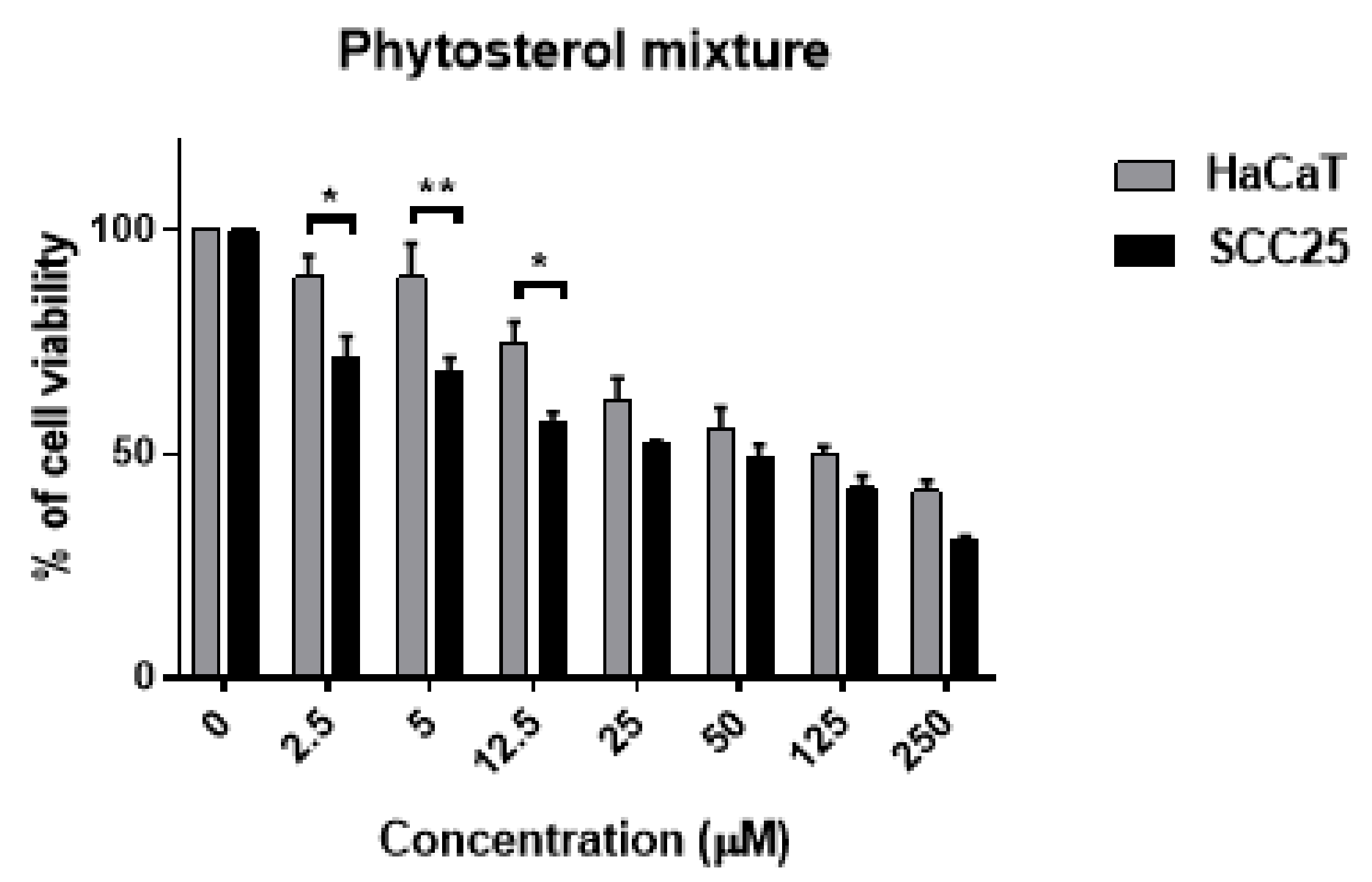
3.6. Effect of Combined Phytosterols against SCC25 and HaCaT Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Antunes Carvalho, F.; Renner, S.S. A dated phylogeny of the papaya family (Caricaceae) reveals the crop’s closest relatives and the family’s biogeographic history. Mol. Phylogenet. Evol. 2012, 65, 46–53. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.J.; Williams, D.J. Papaya as a Medicinal Plant. In Genetics and Genomics of Papaya; Ming, R., Moore, P.H., Eds.; Springer: New York, NY, USA, 2014; pp. 391–407. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Shaw, P.N.; Parat, M.-O.; Hewavitharana, A.K. Anticancer activity of Carica papaya: A review. Mol. Nutr. Food Res. 2013, 57, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Fazal, H.; Ayaz, M.; Abbasi, B.H.; Mohammad, I.; Fazal, L. Dengue fever treatment with Carica papaya leaves extracts. Asian Pac. J. Trop. Biomed. 2011, 1, 330–333. [Google Scholar] [CrossRef]
- Gunjan, M.; Karna, L.; Dayalan, K.; Sasigaran, P. A review and search of phytomedicine used by traditional people in Malaysia (Ipoh, Perak). Int. J. Phytoterapy Res. 2012, 2, 26–41. [Google Scholar]
- Kapoor, S.; Saraf, S. Topical herbal therapies an alternative and complementary choice to combat acne. Res. J. Med. Plant 2011, 5, 650–669. [Google Scholar] [CrossRef]
- Lim, T.K. Carica papaya, Edible Medicinal and Non-Medicinal Plants: Volume 1, Fruits; Springer: Dordrecht, The Netherlands, 2012; pp. 693–717. [Google Scholar] [CrossRef]
- Pandey, S.; Cabot, P.J.; Shaw, P.N.; Hewavitharana, A.K. Anti-inflammatory and immunomodulatory properties of Carica papaya. J. Immunotoxicol. 2016, 13, 590–602. [Google Scholar] [CrossRef]
- Vij, T.; Prashar, Y. A review on medicinal properties of Carica papaya Linn. Asian Pac. J. Trop. Dis. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Nguyen, T.; Parat, M.-O.; Hodson, M.; Pan, J.; Shaw, P.; Hewavitharana, A. Chemical Characterization and in vitro Cytotoxicity on Squamous Cell Carcinoma Cells of Carica Papaya Leaf Extracts. Toxins 2016, 8, 7. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Parat, M.-O.; Shaw, P.N.; Hewavitharana, A.K.; Hodson, M.P. Traditional Aboriginal Preparation Alters the Chemical Profile of Carica papaya Leaves and Impacts on Cytotoxicity towards Human Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0147956. [Google Scholar] [CrossRef]
- Gogna, N.; Hamid, N.; Dorai, K. Metabolomic profiling of the phytomedicinal constituents of Carica papaya L. leaves and seeds by 1H NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. Anal. 2015, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lieb, V.M.; Esquivel, P.; Cubero Castillo, E.; Carle, R.; Steingass, C.B. GC–MS profiling, descriptive sensory analysis, and consumer acceptance of Costa Rican papaya (Carica papaya L.) fruit purees. Food Chem. 2018, 248, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Melariri, P.; Campbell, W.; Etusim, P.; Smith, P. Antiplasmodial Properties and Bioassay-Guided Fractionation of Ethyl Acetate Extracts from Carica papaya Leaves. J. Parasitol. Res. 2011, 2011, 104954. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Nguyen, T.T.T.; Pandey, S.; Thurecht, K.J.; Falconer, J.R. Factorial design-assisted supercritical carbon-dioxide extraction of cytotoxic active principles from Carica papaya leaf juice. Sci. Rep. 2019, 9, 1716. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Walpole, C.; Cabot, P.J.; Shaw, P.N.; Batra, J.; Hewavitharana, A.K. Selective anti-proliferative activities of Carica papaya leaf juice extracts against prostate cancer. Biomed. Pharmacother. 2017, 89, 515–523. [Google Scholar] [CrossRef]
- Otsuki, N.; Dang, N.H.; Kumagai, E.; Kondo, A.; Iwata, S.; Morimoto, C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J. Ethnopharmacol. 2010, 127, 760–767. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Hartmann, M.-A. 5 Sterol metabolism and functions in higher plants. In Lipid Metabolism and Membrane Biogenesis; Daum, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 183–211. [Google Scholar] [CrossRef]
- Ali, H.; Dixit, S.; Ali, D.; Alqahtani, S.M.; Alkahtani, S.; Alarifi, S. Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des. Dev. Ther. 2015, 9, 2793–2800. [Google Scholar] [CrossRef]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Molnár, J.; Forgo, P.; Vasas, A.; Kele, Z.; Hohmann, J. Antiproliferative Constituents of the Roots of Conyza canadensis. Planta Med. 2011, 77, 1183–1188. [Google Scholar] [CrossRef]
- van Vuuren, S.; Viljoen, A. Plant-Based Antimicrobial Studies—Methods and Approaches to Study the Interaction between Natural Products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Wang, X.N.; Han, X.; Xu, L.N.; Yin, L.H.; Xu, Y.W.; Qi, Y.; Peng, J.Y. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine 2008, 15, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
| Peak | Retention Time (Min) | Peak Area | a Peak Area (%) | Tentative Compound Identification |
|---|---|---|---|---|
| 1 | 8.673 | 863,427 | 1.71 | Linolenic acid |
| 2 | 13.820 | 542,784 | 1.07 | Squalene |
| 3 | 15.417 | 2,380,812 | 4.71 | γ-Tocopherol |
| 4 | 15.884 | 624,002 | 1.23 | β-Sitosterol acetate |
| 5 | 16.034 | 12,690,750 | 25.15 | dl-α-Tocopherol |
| 6 | 17.000 | 4,980,249 | 9.87 | Campesterol |
| 7 | 17.293 | 4,474,003 | 8.86 | Stigmasterol |
| 8 | 17.89 | 20,663,841 | 40.96 | β-Sitosterol |
| 9 | 18.08 | 1,819,771 | 3.60 | α-Amyrin |
| 10 | 18.74 | 1,403,996 | 2.78 | Brassicasterin |
| 11 | 19.51 | 1,289,165 | 2.53 | 3-Oxocholest-4-en-27-yl acetate |
| Standard | Mass of Standard [M + H − H2O]+ m/z | Retention Time of Standard (min) | Product ion Standard m/z | Mass of Feature [M + H − H2O]+ m/z | Retention Time of Feature (min) | Product ion of Feature m/z | Molecular Formula |
|---|---|---|---|---|---|---|---|
| DL-α-Tocopherol | 431.3823 | 30.049 | 165.120 | 431.3884 | 30.043 | 165.139 | C29H50O2 |
| Campesterol | 383.3683 | 31.429 | 161.180 | 383.3662 | 31.464 | 161.187 | C28H48O |
| Stigmasterol | 395.3669 | 31.457 | 255.187 | 395.3668 | 31.497 | 255.220 | C29H48O |
| β-Sitosterol | 397.1347 | 32.407 | 161.185 | 397.1361 | 32.409 | 161.188 | C29H50O |
| Compound | Calibration Curve | R2 | Linear Range (µg/mL) | LODs (µg/mL) | LOQs (µg/mL) |
|---|---|---|---|---|---|
| DL-α-Tocopherol | y = 2668.2x + 4301.4 | 0.9992 | 50–1.56 | 0.19 | 0.78 |
| Stigmasterol | y = 956.46x + 2623.6 | 0.9979 | 50–3.12 | 0.39 | 1.56 |
| Campesterol | y = 1008.1x + 1213.6 | 0.9987 | 50–1.56 | 0.39 | 1.56 |
| β-Sitosterol | y = 1718.1x + 1316.6 | 0.9991 | 50–1.56 | 0.39 | 1.56 |
| Sample | Content (µg/100 mg ± SEM of Original Leaf) n = 3 Independent Extractions | |||
|---|---|---|---|---|
| DL-α-Tocopherol | Stigmasterol | Campesterol | β-Sitosterol | |
| scCO2 extract | 1.07 ± 0.36 | 0.33 ± 0.10 | 0.55 ± 0.08 | 1.91 ± 0.45 |
| Cell Lines | IC50 (95% Confidence Interval) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DL-α-Tocopherol | Stigmasterol | Campesterol | β-Sitosterol | Equimolar Sterol * Mixture | scCO2 Extract | ||||
| µg/mL | µM | µg/mL | µM | µg/mL | µM | µM | |||
| SCC25 | ND | 14.35 (11.8–18.2) | 34.8 | 0.63 (0.51–0.74) | 1.57 | 0.41 (0.2–0.56) | 0.98 | 5.98 (3.9–8.1) | 88.07 (67.9–111) |
| HaCaT | ND | 23.41 (19.1–28.7) | 57.7 | 0.63 (0.53–0.73) | 1.57 | 0.78 (0.65–0.91) | 1.88 | 15.60 (11.2–21.6) | 120.60 (95.7–152.3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaw, K.-Y.; Shaw, P.N.; Parat, M.-O.; Pandey, S.; Falconer, J.R. Compound Identification and In Vitro Cytotoxicity of the Supercritical Carbon Dioxide Extract of Papaya Freeze-Dried Leaf Juice. Processes 2020, 8, 610. https://doi.org/10.3390/pr8050610
Khaw K-Y, Shaw PN, Parat M-O, Pandey S, Falconer JR. Compound Identification and In Vitro Cytotoxicity of the Supercritical Carbon Dioxide Extract of Papaya Freeze-Dried Leaf Juice. Processes. 2020; 8(5):610. https://doi.org/10.3390/pr8050610
Chicago/Turabian StyleKhaw, Kooi-Yeong, Paul Nicholas Shaw, Marie-Odile Parat, Saurabh Pandey, and James Robert Falconer. 2020. "Compound Identification and In Vitro Cytotoxicity of the Supercritical Carbon Dioxide Extract of Papaya Freeze-Dried Leaf Juice" Processes 8, no. 5: 610. https://doi.org/10.3390/pr8050610
APA StyleKhaw, K.-Y., Shaw, P. N., Parat, M.-O., Pandey, S., & Falconer, J. R. (2020). Compound Identification and In Vitro Cytotoxicity of the Supercritical Carbon Dioxide Extract of Papaya Freeze-Dried Leaf Juice. Processes, 8(5), 610. https://doi.org/10.3390/pr8050610





