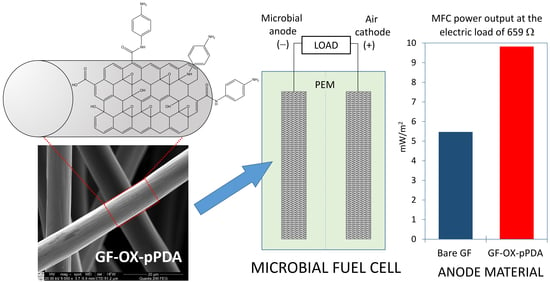
Enhanced Performance of Microbial Fuel Cells with Anodes from Ethylenediamine and Phenylenediamine Modified Graphite Felt
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Conditions
2.2. Electrodes
- Bare graphite felt (GF) as a control;
- GF modified with ethylenediamine (EDA);
- GF modified with p-phenylenediamine (pPDA).
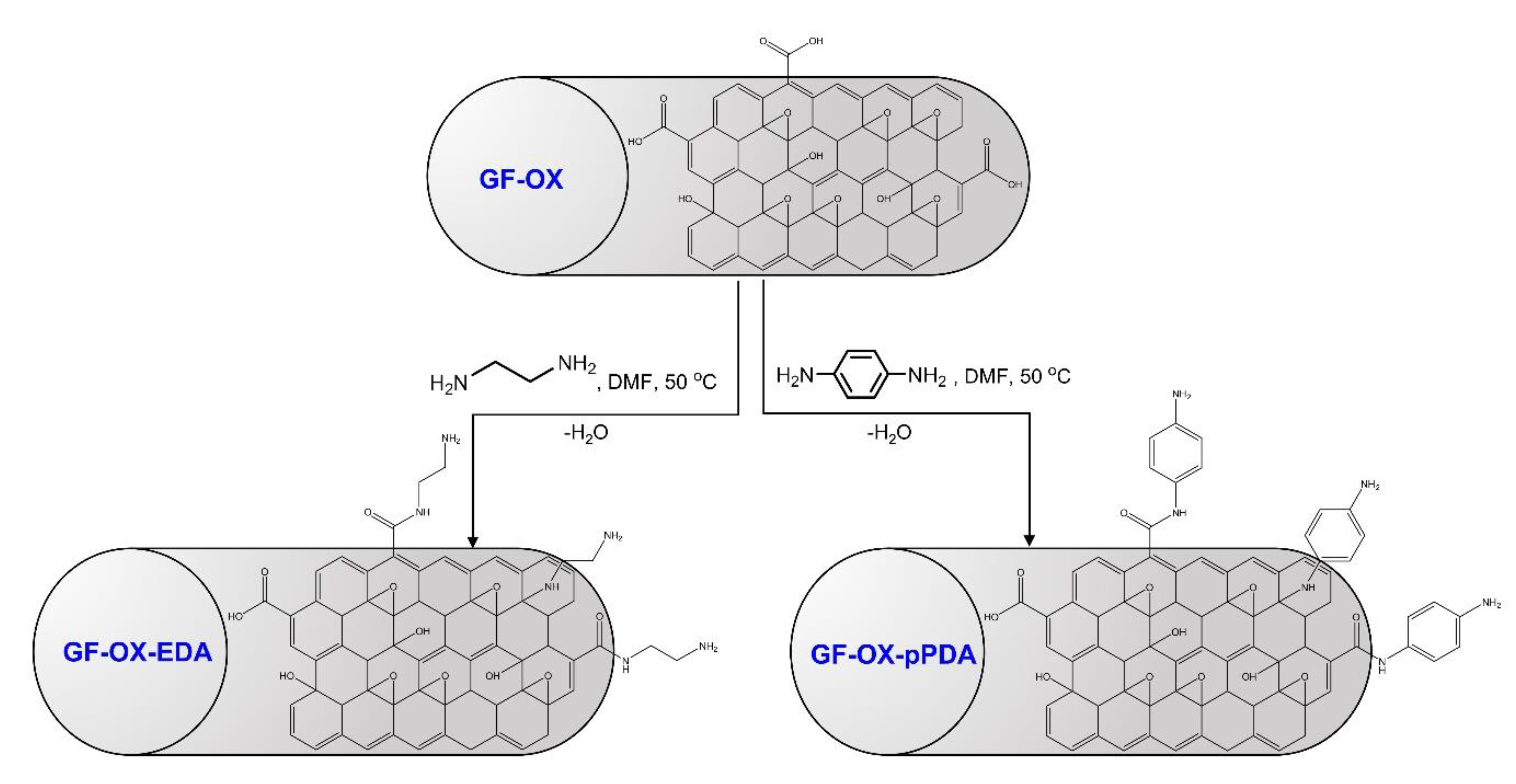
2.3. Anode Material Treatment
2.3.1. Electrochemical Oxidation of GF
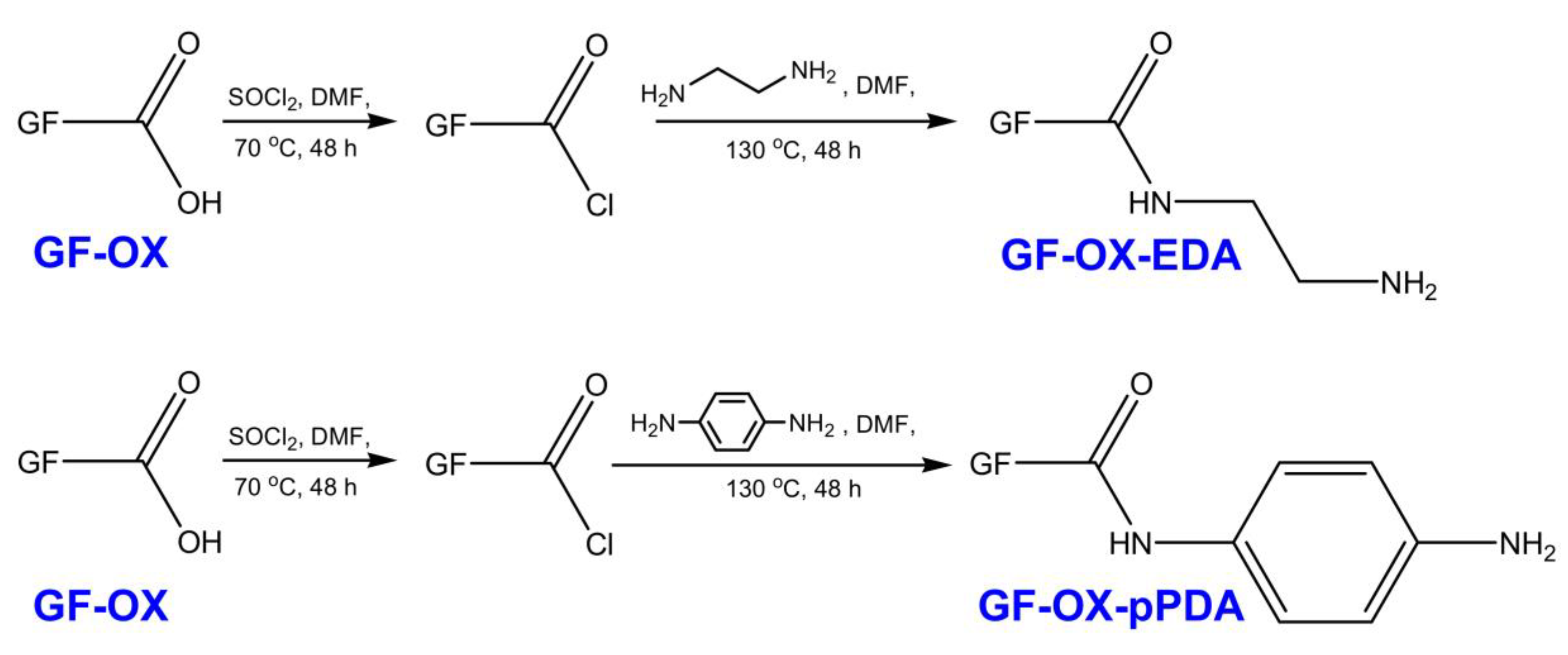
2.3.2. Treatment with Ethylenediamine (Ethane-1,2-Diamine or EDA)
2.3.3. Treatment with p-Phenylenediamine (Benzene-1,4-Diamine or pPDA)
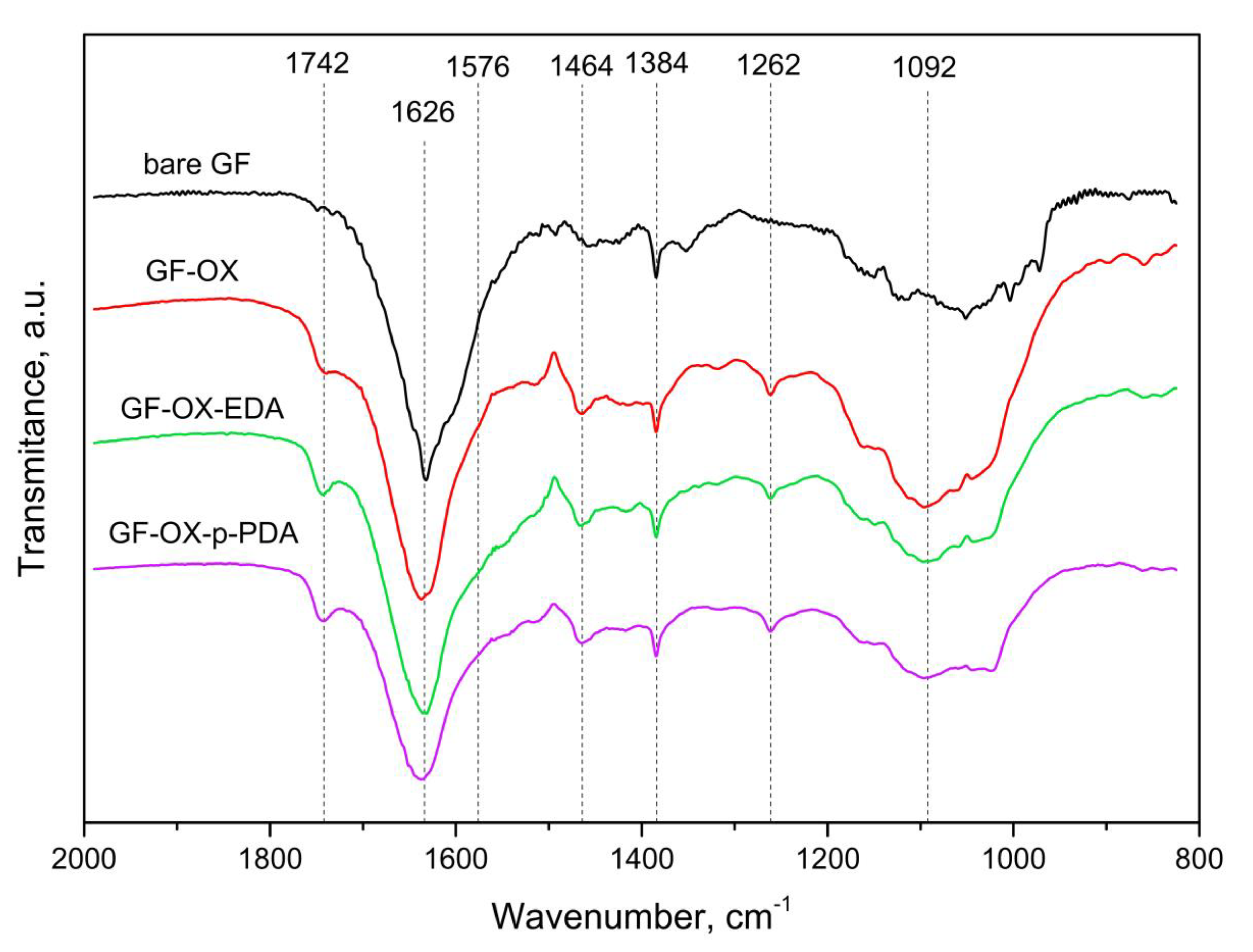
2.4. Characterization of Electrode Materials
2.5. Cell Cultures and Media
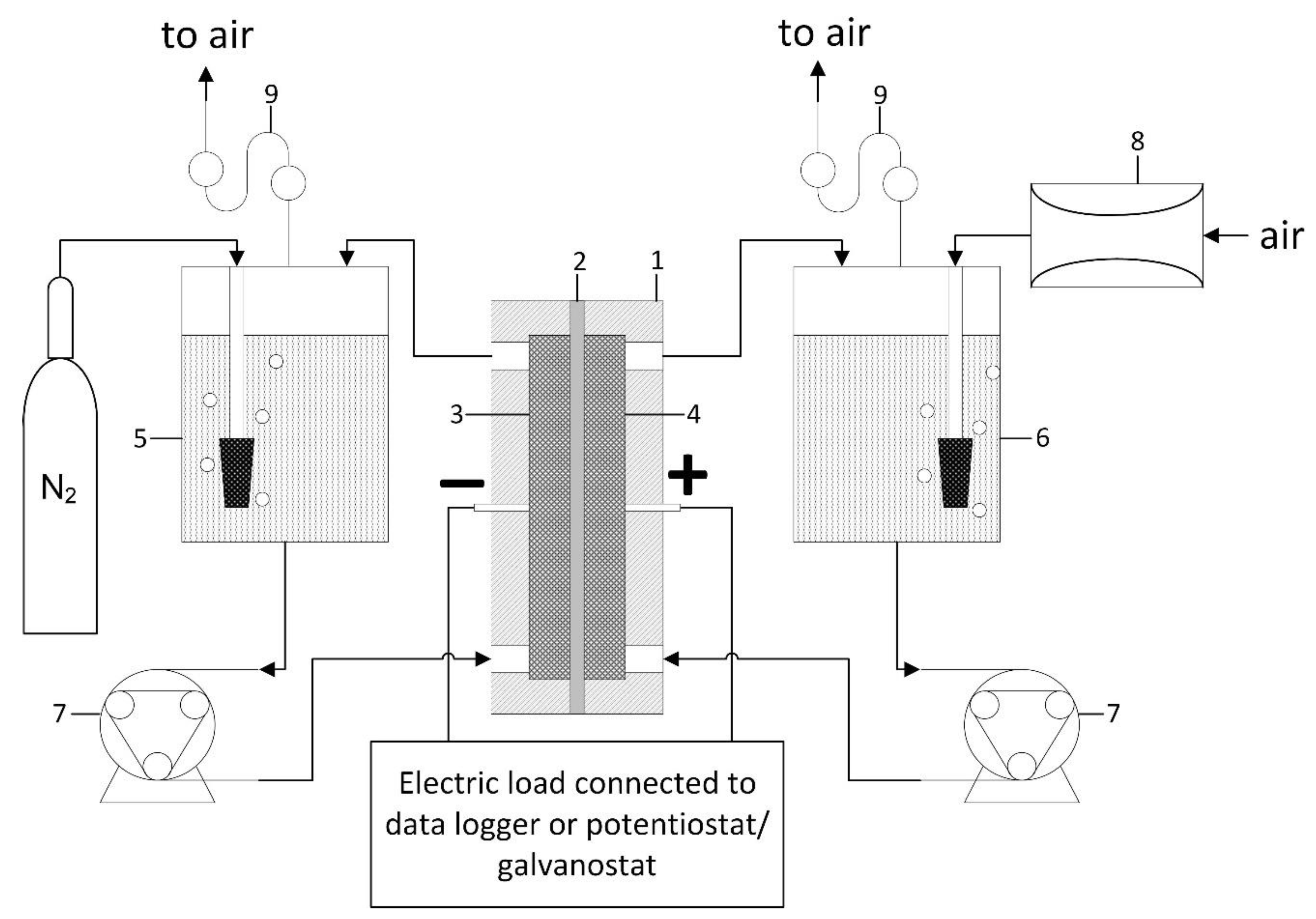
2.6. MFC Design and Operation
3. Results and Discussion
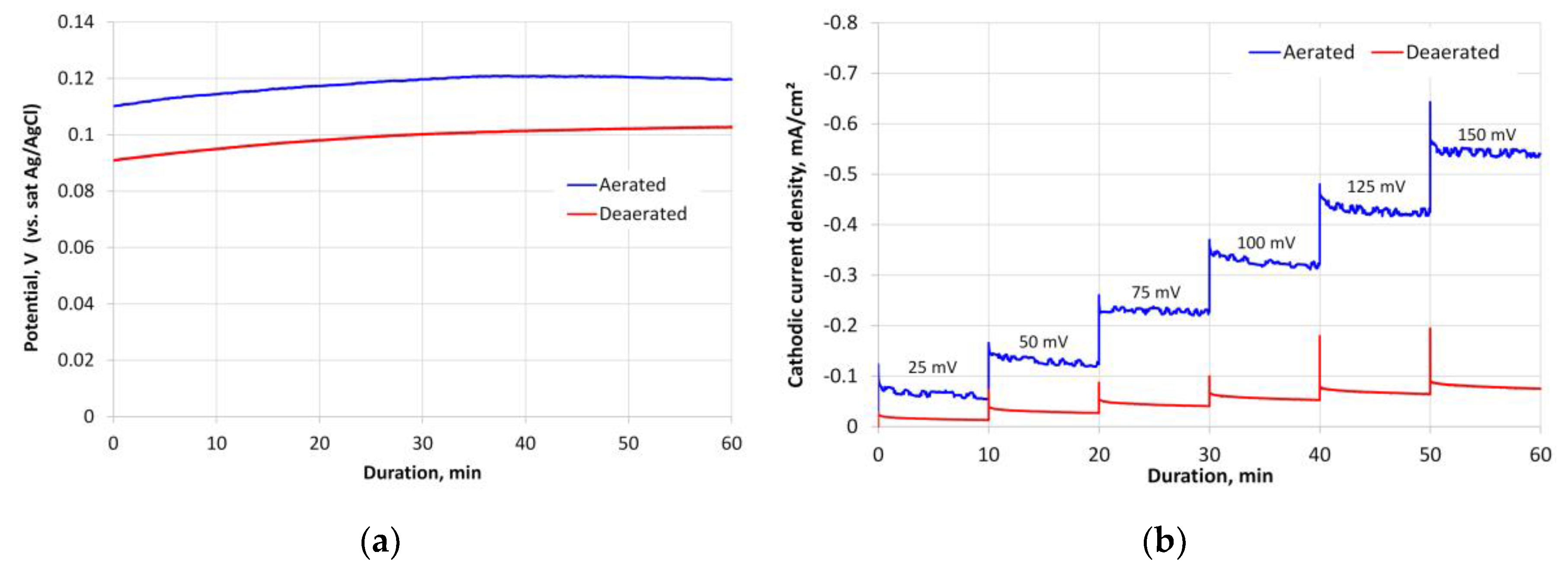
3.1. Anode Material Treatment
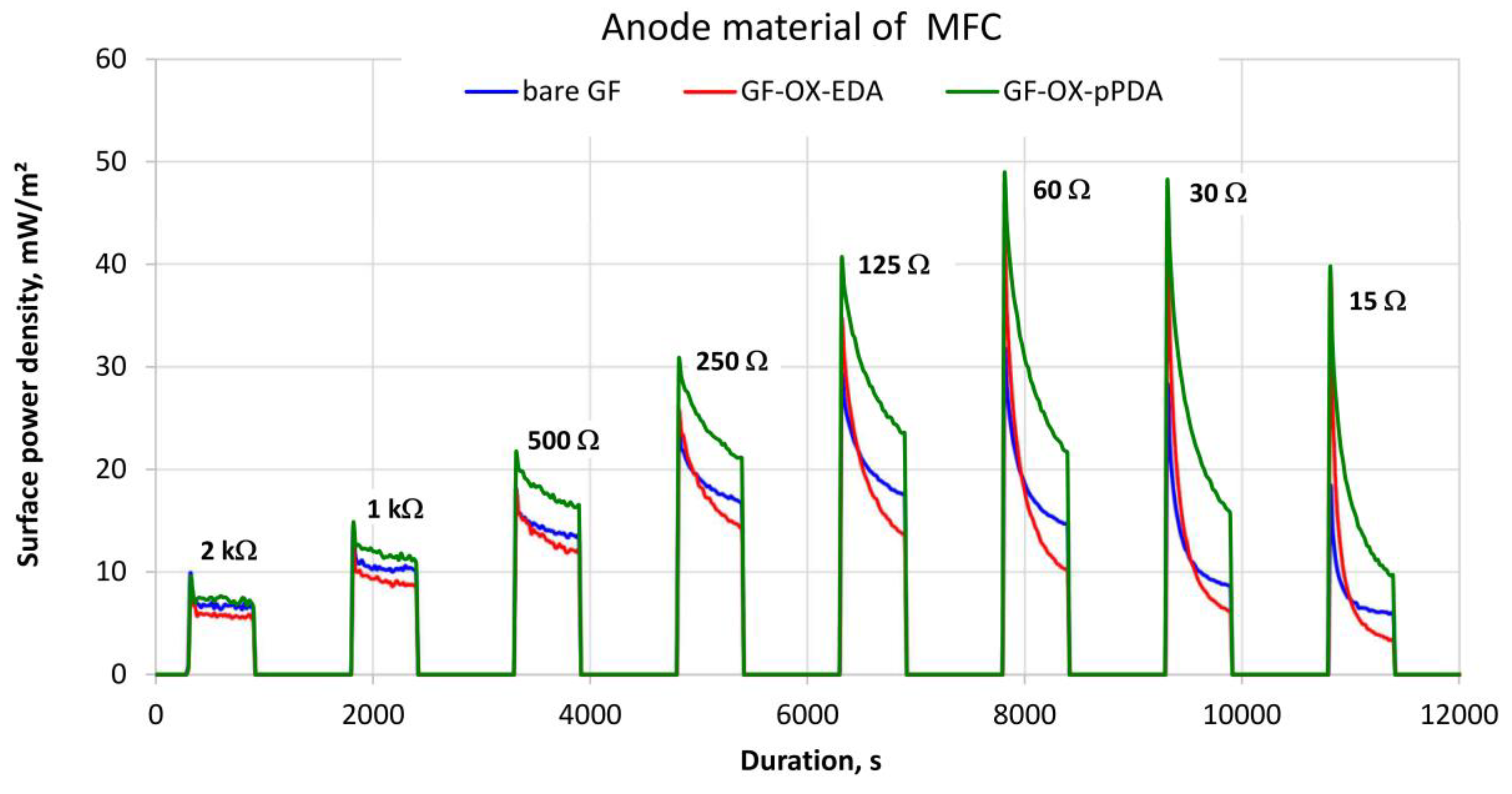
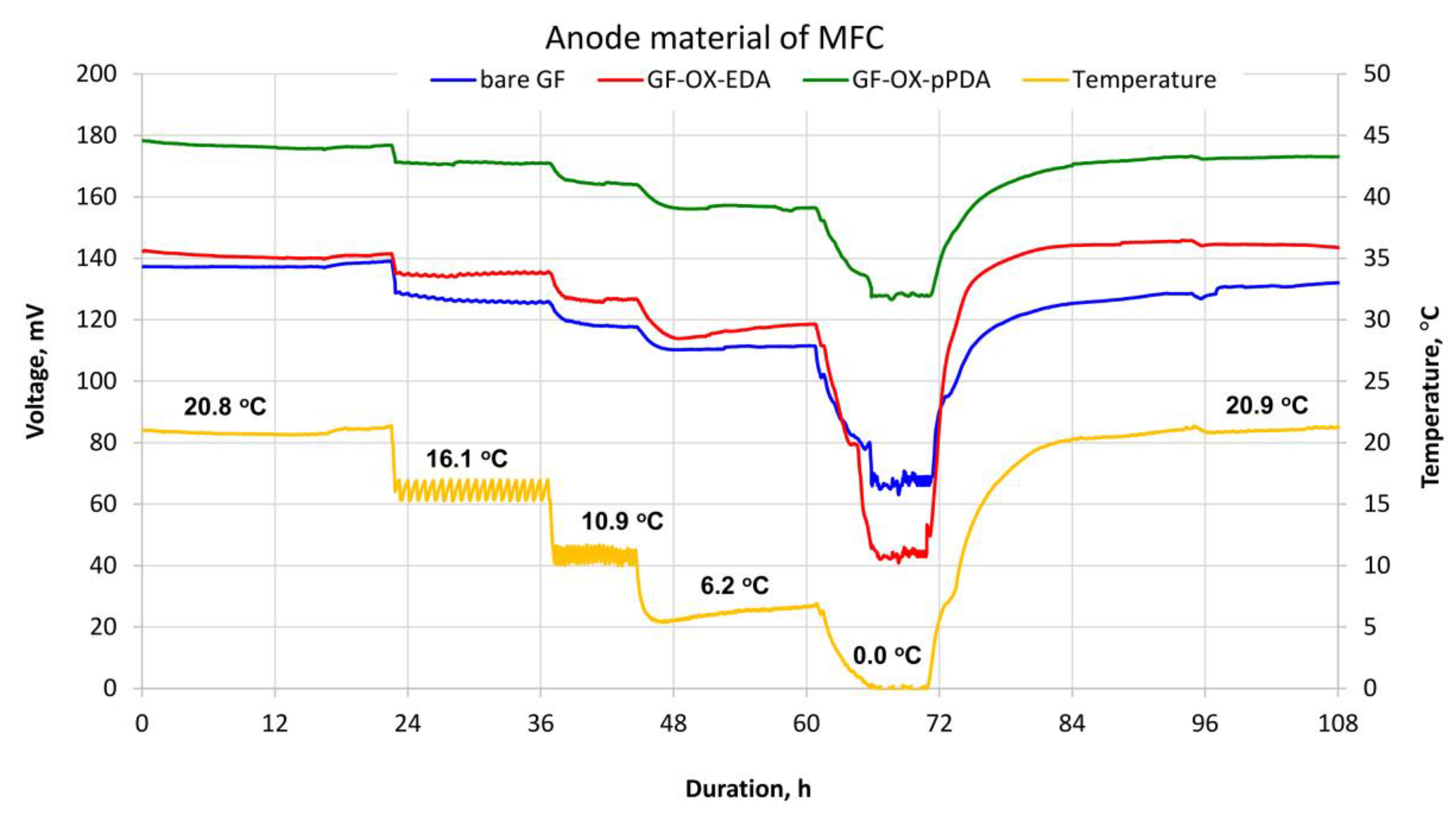
3.2. MFC Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, W.W.; Yu, H.Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef] [Green Version]
- Deb, D.; Patel, R.; Balas, V.E. A Review of Control-Oriented Bioelectrochemical Mathematical Models of Microbial Fuel Cells. Processes 2020, 8, 583. [Google Scholar] [CrossRef]
- Wang, H.; Park, J.-D.; Ren, Z.J. Practical energy harvesting for microbial fuel cells: A review. Environ. Sci. Technol. 2015, 49, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Kumar, G.; Bakonyi, P.; Xu, K.; Zhao, Y. Microbial electrolysis cell platform for simultaneous waste biorefinery and clean electrofuels generation: Current situation, challenges and future perspectives. Prog. Energy Combust. Sci. 2017, 63, 119–145. [Google Scholar] [CrossRef]
- Raslavičius, L.; Striūgas, N.; Felneris, M. New insights into algae factories of the future. Renew. Sustain. Energ. Rev. 2018, 81, 643–654. [Google Scholar] [CrossRef]
- Bajracharya, S.; Srikanth, S.; Mohanakrishna, G.; Zacharia, R.; Strik, D.; Pant, D. Biotransformation of carbon dioxide in bioelectrochemical systems: State of the art and future prospects. J. Power Sources 2017, 356, 256–273. [Google Scholar] [CrossRef]
- Greenman, J.; Ieropoulos, I.A. Allometric scaling of microbial fuel cells and stacks: The lifeform case for scale-up. J. Power Sources 2017, 356, 365–370. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, B.; Tian, C.; Liu, Y.; Shi, C.; Cheng, M.; Feng, C. Enhanced microbial reduction of vanadium (V) in groundwater with bioelectricity from microbial fuel cells. J. Power Sources 2015, 287, 43–49. [Google Scholar] [CrossRef]
- Grattieri, M.; Suvira, M.; Hasan, K.; Minteer, S.D. Halotolerant extremophile bacteria from the Great Salt Lake for recycling pollutants in microbial fuel cells. J. Power Sources 2017, 356, 310–318. [Google Scholar] [CrossRef]
- Tommasi, T.; Salvador, G.P.; Quaglio, M. New insights in Microbial Fuel Cells: Novel solid phase anolyte. Sci. Rep. 2016, 6, 29091. [Google Scholar] [CrossRef] [Green Version]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; An, J.; Li, J.; Zhou, L.; Li, N.; Wang, X. Polydopamine as a new modification material to accelerate startup and promote anode performance in microbial fuel cells. J. Power Sources 2017, 343, 477–482. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Deng, W.; Tan, Y.; Xie, Q. Macroporous graphitic carbon foam decorated with polydopamine as a high-performance anode for microbial fuel cell. J. Power Sources 2017, 363, 27–33. [Google Scholar] [CrossRef]
- Hindatu, Y.; Annuara, M.S.M.; Gumel, A.M. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sust. Energ. Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Losic, D.; Saint, C.P.; Pant, D. Applications of graphene in microbial fuel cells: The gap between promise and reality. Renew. Sustain. Energ. Rev. 2017, 72, 1389–1403. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, Y.; Chen, S.; Yang, F.; Zheng, S.; Hou, H. Carbon nanofibers modified graphite felt for high performance anode in high substrate concentration microbial fuel cells. Sci. World J. 2014, 130185. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef]
- Li, C.; Cheng, S. Functional group surface modifications for enhancing the formation and performance of exoelectrogenic biofilms on the anode of a bioelectrochemical system. Crit. Rev. Biotechnol. 2019, 39, 1015–1030. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, Z.; Song, H.; Stoll, Z.A.; Xu, P. Melamine modified carbon felts anode with enhanced electrogenesis capacity toward microbial fuel cells. J. Energy Chem. 2017, 26, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.A.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–4496. [Google Scholar] [CrossRef]
- Zhu, N.; Chen, X.; Zhang, T.; Wu, P.; Li, P.; Wu, J. Improved performance of membrane free single-chamber air-cathode microbial fuel cells with nitric acid and ethylenediamine surface modified activated carbon fiber felt anodes. Bioresour. Technol. 2011, 102, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Bajracharya, S.; Gildemyn, S.; Patil, S.A.; Alvarez-Gallego, Y.; Pant, D.; Rabaey, K.; Dominguez-Benettona, X. A critical revisit of the key parameters used to describe microbial electrochemical systems. Electrochim. Acta 2014, 140, 191–208. [Google Scholar] [CrossRef]
- Wu, X.; Zou, L.; Huang, Y.; Qiao, Y.; Long, Z.; Liu, H.; Li, C.M. Shewanella putrefaciens CN32 outer membrane cytochromes MtrC and UndA reduce electron shuttles to produce electricity in microbial fuel cells. Enzyme Microb. Technol. 2018, 115, 23–28. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Harnisch, F.; Kuhlicke, U.; Neu, T.R.; Schröder, U. Electron transfer and biofilm formation of Shewanella putrefaciens as function of anode potential. Bioelectrochemistry 2013, 93, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Dutta, R.K. A facile method of synthesizing ammonia modified graphene oxide for efficient removal of uranyl ions from aqueous medium. RSC Adv. 2015, 5, 77192. [Google Scholar] [CrossRef]
- Bagge, D.; Hjelm, M.; Johansen, C.; Huber, I.; Gram, L. Shewanella putrefaciens adhesion and biofilm formation on food processing surfaces. Appl. Environ. Microbiol. 2001, 67, 2319–2325. [Google Scholar] [CrossRef] [Green Version]
- Price, P.B.; Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl. Acad. Sci. USA 2004, 101, 4631–4636. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.P.; Xie, J.; Cheng, Y.; Zhang, Z.; Zhao, Y.; Qian, Y.F. Response of Shewanella putrefaciens to low temperature regulated by membrane fluidity and fatty acid metabolism. LWT Food Sci. Technol. 2020, 117, 108638. [Google Scholar] [CrossRef]
- Gao, X.; Liu, W.; Mei, J.; Xie, J. Quantitative Analysis of Cold Stress Inducing Lipidomic Changes in Shewanella putrefaciens Using UHPLC-ESI-MS/MS. Molecules 2019, 24, 4609. [Google Scholar] [CrossRef] [Green Version]



| Sample | Concentration of Element, at. % | ||
|---|---|---|---|
| C | O | N | |
| Bare GF | 92.9 | 1.0 | 6.1 |
| GF-OX | 78.0 | 18.8 | 3.2 |
| GF-OX-EDA | 77.8 | 9.0 | 13.2 |
| GF-OX-pPDA | 77.9 | 9.8 | 12.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griškonis, E.; Ilginis, A.; Jonuškienė, I.; Raslavičius, L.; Jonynas, R.; Kantminienė, K. Enhanced Performance of Microbial Fuel Cells with Anodes from Ethylenediamine and Phenylenediamine Modified Graphite Felt. Processes 2020, 8, 939. https://doi.org/10.3390/pr8080939
Griškonis E, Ilginis A, Jonuškienė I, Raslavičius L, Jonynas R, Kantminienė K. Enhanced Performance of Microbial Fuel Cells with Anodes from Ethylenediamine and Phenylenediamine Modified Graphite Felt. Processes. 2020; 8(8):939. https://doi.org/10.3390/pr8080939
Chicago/Turabian StyleGriškonis, Egidijus, Arminas Ilginis, Ilona Jonuškienė, Laurencas Raslavičius, Rolandas Jonynas, and Kristina Kantminienė. 2020. "Enhanced Performance of Microbial Fuel Cells with Anodes from Ethylenediamine and Phenylenediamine Modified Graphite Felt" Processes 8, no. 8: 939. https://doi.org/10.3390/pr8080939
APA StyleGriškonis, E., Ilginis, A., Jonuškienė, I., Raslavičius, L., Jonynas, R., & Kantminienė, K. (2020). Enhanced Performance of Microbial Fuel Cells with Anodes from Ethylenediamine and Phenylenediamine Modified Graphite Felt. Processes, 8(8), 939. https://doi.org/10.3390/pr8080939