Electrical Resistivity of Carbonaceous Bed Material at High Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Materials
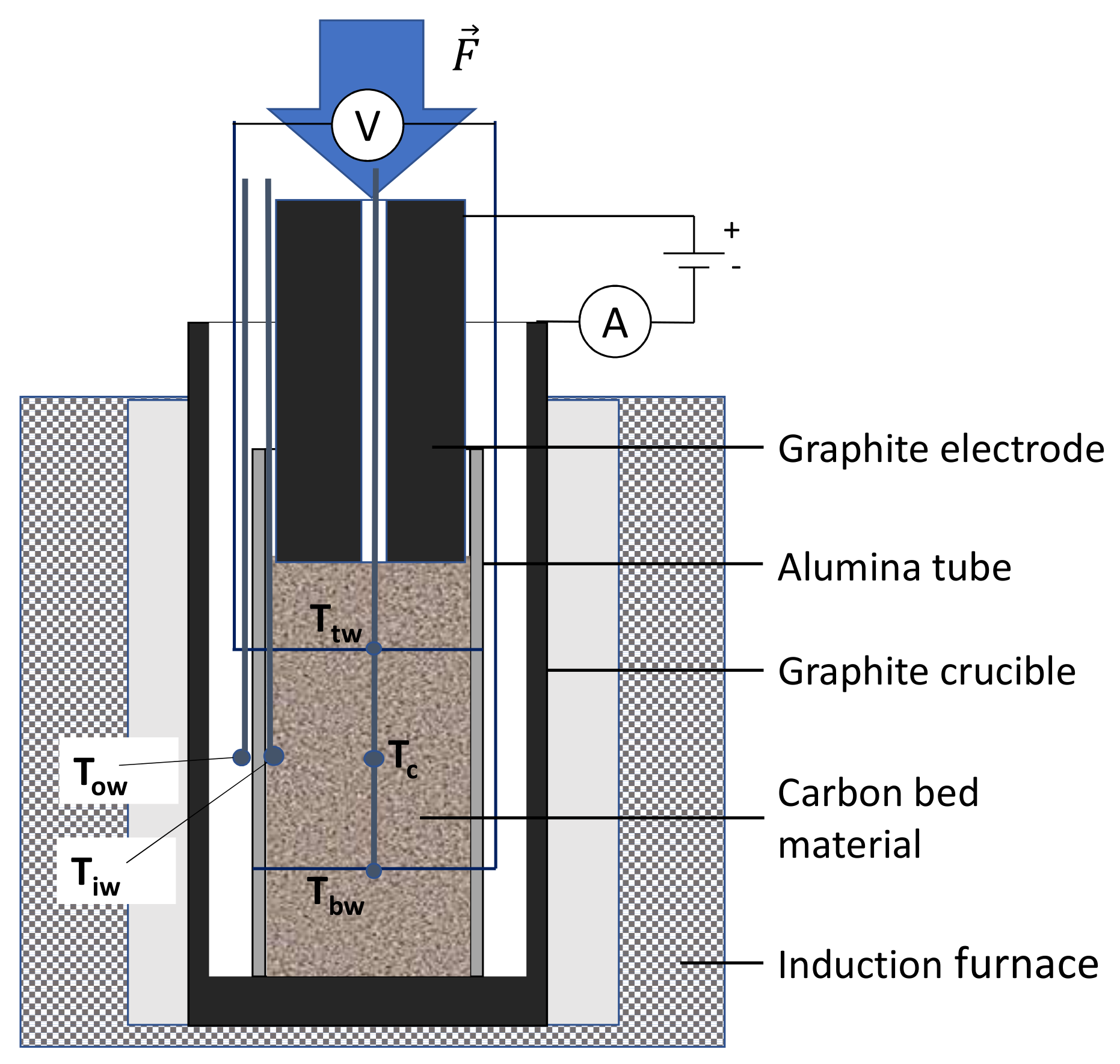
2.2. Resistivity at High-Temperature
2.3. Resistivity Under Load
2.4. Solid Characterization
- Proximate analysis
- Elemental analysis
- Scanning electron microscopy
- Compression strength
- Mechanical durability
3. Results
3.1. Feed Material Composition
3.2. Product Yield
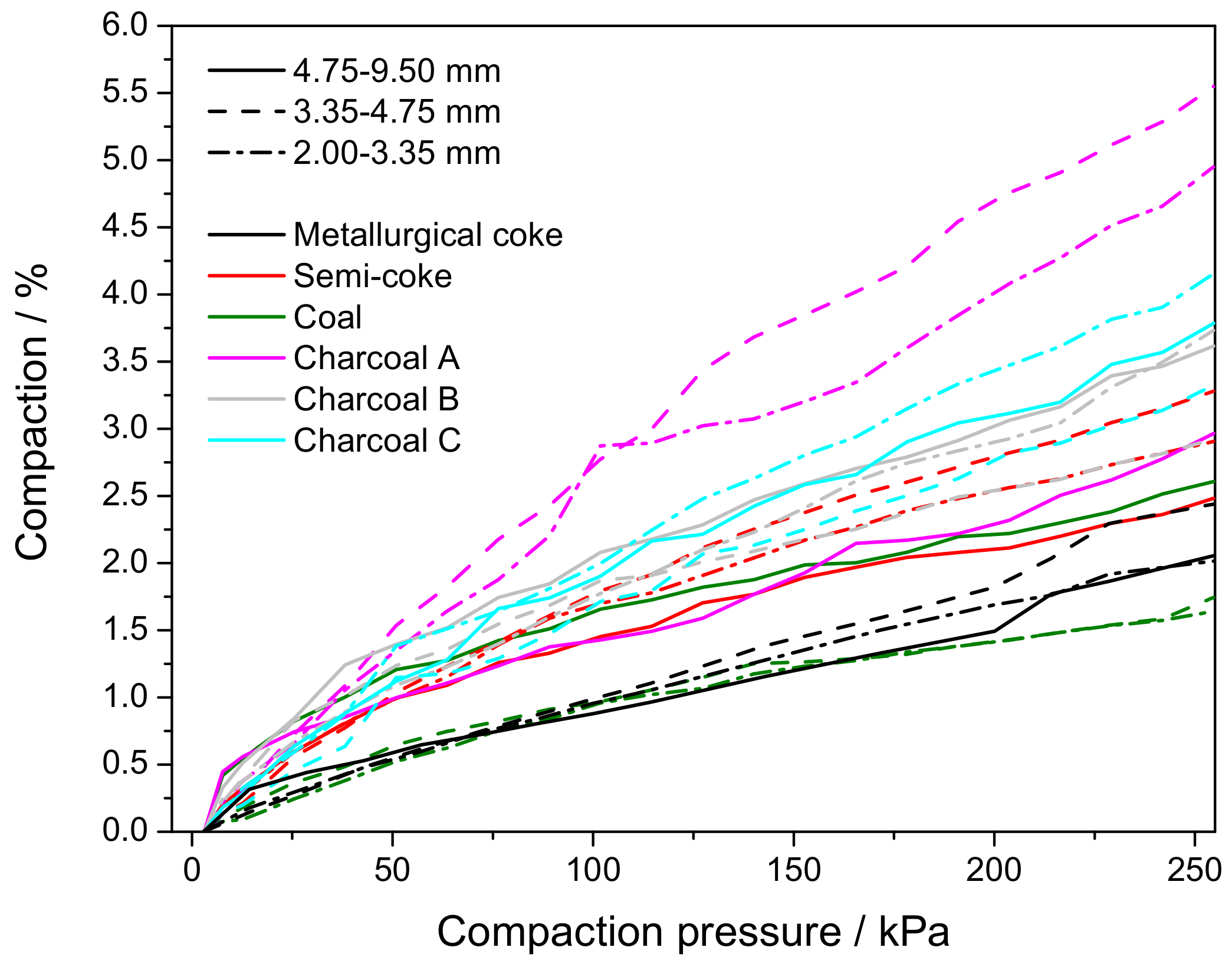
3.3. Mechanical Properties
3.4. Electrical Resistivity at Elevated Temperature
3.5. Electrical Resistivity under Load
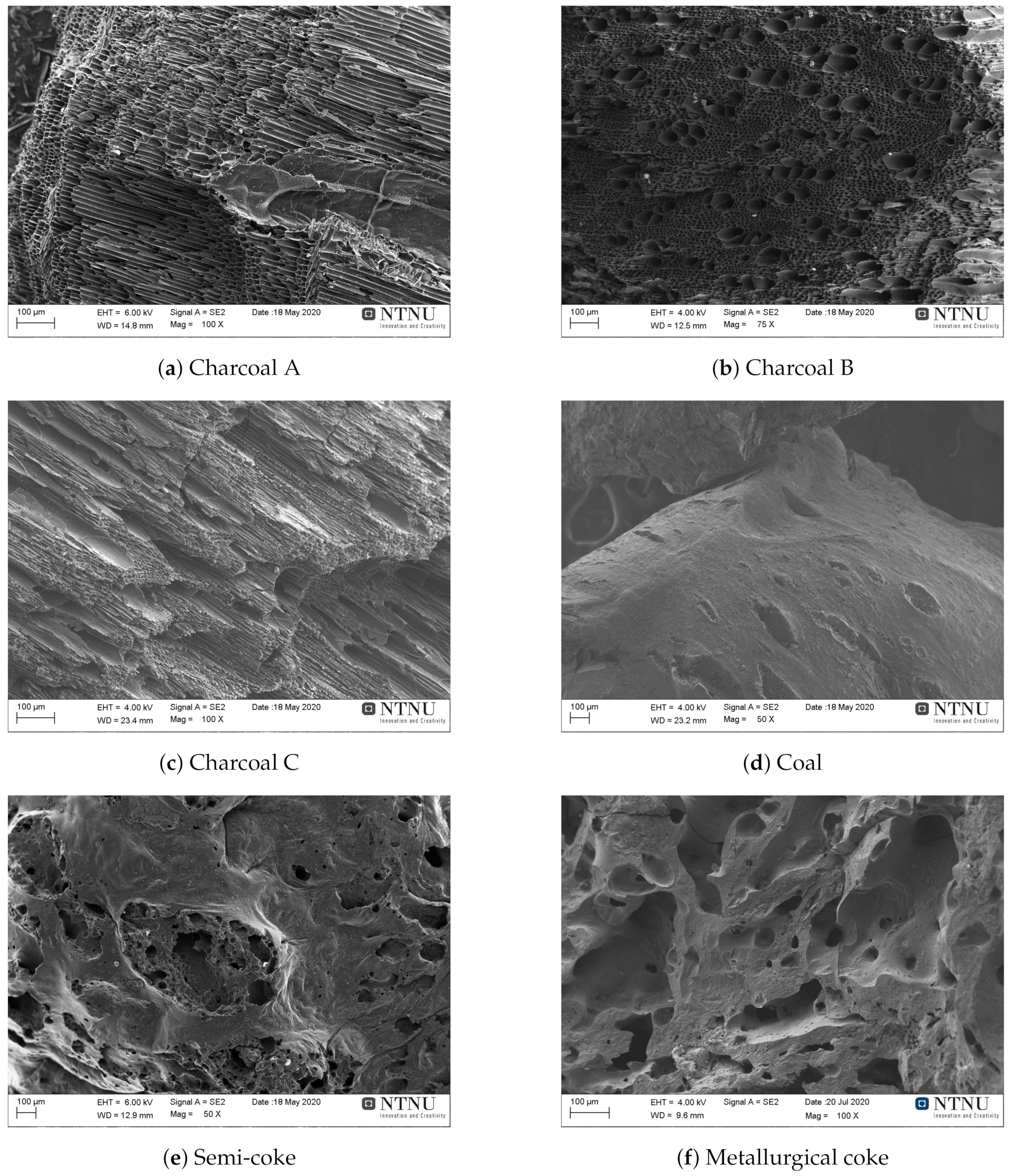
4. Surface Morphology
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Cross-sectional area |
| ar | as received |
| db | dry basis |
| EAF | Electric arc furnace |
| I | Current |
| IF | Induction furnace |
| l | length |
| NTNU | Norwegian University of Science and Technology |
| SAF | Submerged arc furnace |
| TS | Thermal shrinkage |
| T | Temperature at the bottom Mo-wire |
| T | Temperature in the center of the bulk |
| T | Temperature at the inner wall of the Al-tube |
| T | Temperature at the outer wall of the Al-tube |
| T | Temperature at the top Mo-wire |
| V | Voltage |
Appendix A. Composition of Heat Treated Material
| Particle Size | Solid Yield | Ash | Fixed Carbon | Volatile Matter | C | H | N | O | S |
|---|---|---|---|---|---|---|---|---|---|
| /mm | /wt.%, db | /wt.%, db | |||||||
| Metallurgical coke | |||||||||
| <2.0 mm | 94.0 ± 0.3 | 11.4 | 88.5 | 0.1 ± 0.02 | 88.5 | <0.1 | <0.5 | <0.1 | 0.5 |
| 2.0 ≤ d ≤ 4.75 | 94.0 ± 0.3 | 11.4 | 88.5 | 0.1 ± 0.02 | 88.4 | <0.1 | <0.5 | <0.1 | 0.5 |
| 4.75 ≤ d ≤ 9.5 | 93.8 ± 0.3 | 11.9 | 88.0 | 0.1 ± 0.02 | 88.1 | <0.1 | <0.5 | <0.1 | 0.5 |
| Semi-coke | |||||||||
| <2.0 mm | 91.4 ± 0.3 | 2.3 | 97.6 | 0.1 ± 0.02 | 97.9 | <0.1 | <0.5 | <0.1 | 0.3 |
| 2.0 ≤ d ≤ 4.75 | 90.1 ± 0.3 | 2.4 | 97.5 | 0.1 ± 0.02 | 97.3 | <0.1 | <0.5 | <0.1 | 0.3 |
| 4.75 ≤ d ≤ 9.5 | 89.9 ± 0.3 | 2.3 | 97.6 | 0.1 ± 0.03 | 97.1 | <0.1 | <0.5 | <0.1 | 0.3 |
| Coal | |||||||||
| <2.0 mm | 61.9 ± 0.3 | 1.7 ± 0.3 | 98.0 | 0.3 ± 0.04 | 99.2 | <0.1 | <0.5 | <0.1 | 0.4 |
| 2.0 ≤ d ≤ 4.75 | 62.8 ± 0.3 | 1.8 ± 0.2 | 97.9 | 0.3 ± 0.03 | 98.3 | <0.1 | <0.5 | <0.1 | 0.3 |
| 4.75 ≤ d ≤ 9.5 | 62.9 ± 0.3 | 1.7 ± 0.3 | 98.0 | 0.3 ± 0.06 | 98.0 | <0.1 | <0.5 | <0.1 | 0.3 |
| Charcoal A | |||||||||
| <2.0 mm | 82.5 ± 0.3 | 2.0 ± 0.3 | 97.7 | 0.3 ± 0.03 | 97.9 | <0.1 | <0.5 | <0.1 | - |
| 2.0 ≤ d ≤ 4.75 | 80.4 ± 0.3 | 2.5 ± 0.2 | 97.2 | 0.3 ± 0.04 | 97.5 | <0.1 | <0.5 | <0.1 | - |
| 4.75 ≤ d ≤ 9.5 | 81.0 ± 0.3 | 3.0 ± 0.3 | 96.7 | 0.3 ± 0.02 | 97.1 | <0.1 | <0.5 | <0.1 | - |
| Charcoal B | |||||||||
| <2.0 mm | 79.7 ± 0.3 | 1.4 ± 0.2 | 98.4 | 0.2 ± 0.05 | 98.5 | <0.1 | <0.5 | <0.1 | - |
| 2.0 ≤ d ≤ 4.75 | 81.5 ± 0.3 | 1.5 ± 0.1 | 98.3 | 0.2 ± 0.04 | 98.4 | <0.1 | <0.5 | <0.1 | - |
| 4.75 ≤ d ≤ 9.5 | 78.9 ± 0.3 | 1.8 ± 0.3 | 97.9 | 0.3 ± 0.05 | 98.1 | <0.1 | <0.5 | <0.1 | - |
| Charcoal C | |||||||||
| <2.0 mm | 83.3 ± 1.3 | 1.9 ± 0.1 | 97.9 | 0.2 ± 0.03 | 98.0 | <0.1 | <0.5 | <0.1 | - |
| 2.0 ≤ d ≤ 4.75 | 84.0 ± 0.9 | 1.7 ± 0.2 | 98.1 | 0.2 ± 0.05 | 98.1 | <0.1 | <0.5 | <0.1 | - |
| 4.75 ≤ d ≤ 9.5 | 81.4 ± 1.5 | 2.1 ± 0.3 | 97.7 | 0.2 ± 0.05 | 97.9 | <0.1 | <0.5 | <0.1 | - |
Appendix B. Electrical Resistivity at Low Heat Treatment Temperature

Appendix C. Residence Time


Appendix D. Electrical Resistivity under Load
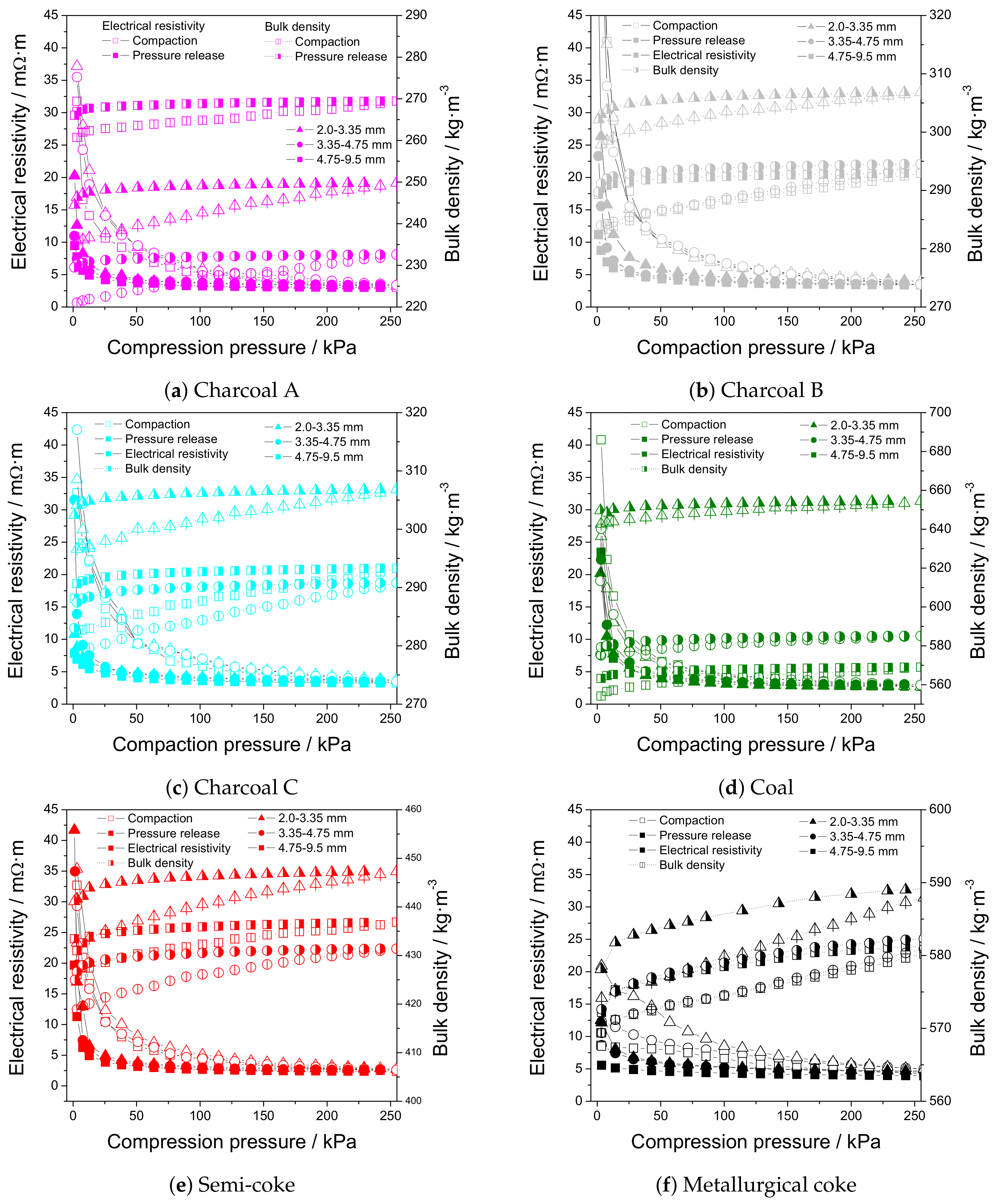
| IF75 | 4-Probe Setup | |||||
|---|---|---|---|---|---|---|
| Electrical Resistivity | Bulk Density | Electrical Resistivity | Bulk Density | |||
| /m·m | /kg·m | /m·m | /kg·m | |||
| Hot * | After Cooling | Compaction | Pressure Release | |||
| Metallurgical coke | ||||||
| Fine | 7.32 | 6.50 | 920 | 11.97 | 8.84 | 550 |
| Small fraction | 7.87 | 6.47 | 616 | 13.88 | 8.37 | 560 |
| Large fraction | 6.03 | 6.38 | 600 | 10.24 | 6.72 | 560 |
| Semi-coke | ||||||
| Fine | 8.68 | 10.77 | 620 | 20.28 | 11.54 | 660 |
| Small fraction | 9.41 | 9.02 | 420 | 19.42 | 7.44 | 430 |
| Large fraction | 11.05 | 9.48 | 390 | 20.94 | 6.25 | 430 |
| Coal | ||||||
| Fine | 3.48 | 5.98 | 590 | 36.03 | 12.43 | 750 |
| Small fraction | 6.01 | 9.01 | 520 | 19.02 | 12.24 | 580 |
| Large fraction | 5.00 | 8.37 | 480 | 22.33 | 8.84 | 560 |
| Charcoal A | ||||||
| Fine | 7.84 | 12.94 | 240 | 41.72 | 18.70 | 350 |
| Small fraction | 9.73 | 11.66 | 230 | 24.27 | 6.12 | 270 |
| Large fraction | 11.71 | 12.24 | 230 | 16.61 | 5.66 | 270 |
| Charcoal B | ||||||
| Fine | 7.64 | 19.00 | 385 | 43.08 | 17.10 | 400 |
| Small fraction | 11.23 | 12.13 | 265 | 34.14 | 9.15 | 290 |
| Large fraction | 11.39 | 13.08 | 255 | 40.99 | 6.97 | 290 |
| Charcoal C | ||||||
| Fine | 8.22 | 16.75 | 360 | 45.76 | 11.62 | 410 |
| Small fraction | 10.97 | 11.80 | 280 | 30.99 | 9.13 | 290 |
| Large fraction | 12.48 | 10.49 | 260 | 24.83 | 6.13 | 290 |
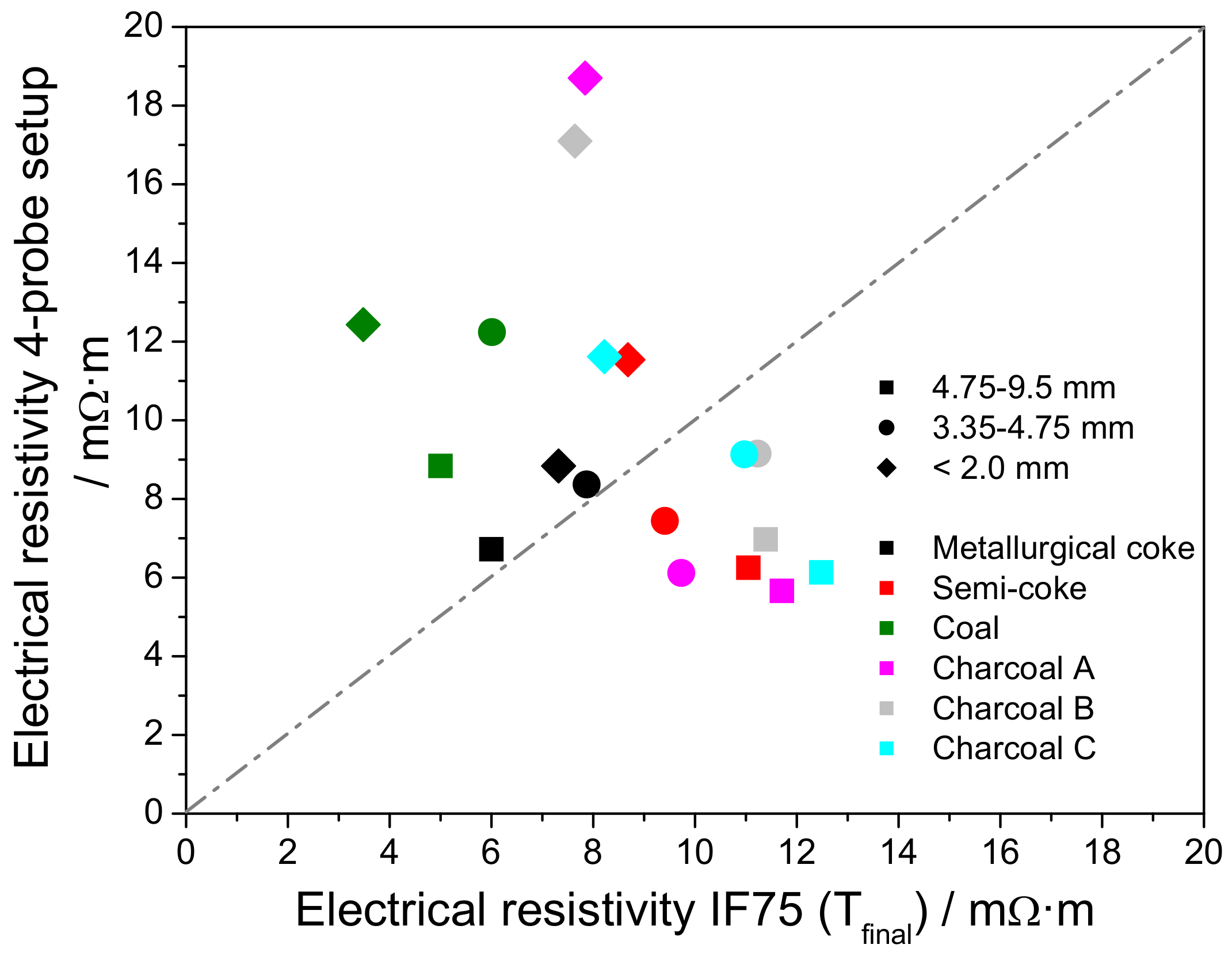

Appendix E. Estimation of the Burden Load on the Carbon Bed
References
- Mochidzuki, K.; Soutric, F.; Takokoro, K.; Antal, M.J.; Toth, M.; Zelei, B.; Varhegyi, G. Electrical and Physical Properties of Carbonized Charcoals. Ind. Eng. Chem. Res. 2003, 42, 5140–5151. [Google Scholar] [CrossRef]
- Hoffmann, V.; Rodriguez Correa, C.; Sautter, D.; Maringolo, E.; Kruse, A. Study of the electrical conductivity of biobased carbonaceous powder materials under moderate pressure. GCB Bioenergy 2019, 11, 230–248. [Google Scholar] [CrossRef] [Green Version]
- Antal, M.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Vorob’ev, V.; Golunov, A.; Ignat’ev, A. Carbon Reductants for the Production of Manganese Ferroalloys. Russ. Metall. 2009, 8, 752–755. [Google Scholar] [CrossRef]
- Griessachenr, T.; Antrekowitsch, J.; Steinlechner, S. Charcoal from agricultural residues as alternative reducing agent in metal recycling. Biomass Bioenergy 2012, 39, 139–146. [Google Scholar] [CrossRef]
- Adrados, A.; De Marco, I.; López-Urionabarrenchea, A.; Solar, J.; Caballero, B.; Gastelu, N. Biomass Pyrolysis Solids as Reducing Agents: Comparison with Commercial Reducing Agents. Materials 2016, 9, 3. [Google Scholar] [CrossRef]
- Monsen, B.; Tangstad, M.; Midtgaard, H. Use of charcoal in silicomanganese production. In Proceedings of the International Ferro-Alloys Congress X, Cape Town, South Africa, 1–4 February 2004; pp. 392–404. [Google Scholar]
- Surup, G.; Nielsen, H.; Großarth, M.; Deike, R.; Van den Bulcke, J.; Kibleur, P.; Müller, M.; Ziegner, M.; Yazhenskikh, E.; Beloshapkin, S.; et al. Effect of operating conditions and feedstock composition on the properties of manganese oxide or quartz charcoal pellets for the use in ferroalloy industries. Energy 2020, 193, 116736. [Google Scholar] [CrossRef]
- Surup, G.; Hunt, A.; Attard, T.; Budarin, V.L.; Forsberg, F.; Arshadi, M.; Abdelsayed, V.; Shekhawat, D.; Trubetskaya, A. The effect of wood composition and supercritical CO2 extraction on charcoal production in ferroalloy industries. Energy 2020, 193, 116696. [Google Scholar] [CrossRef]
- World Steel Association. Steel’s Contribution to a Low Carbon Future and Climate Resilient Societies—World Steel Position Paper; World Steel Association: Brussels, Belgium, 2017. [Google Scholar]
- Olsen, S.; Monsen, B.; Lindstad, T. Emissions from the Production of Manganese and Chromium Alloys in Norway. In Proceedings of the 56th Electric Furnace Conference, New Orleans, LA, USA, 15–18 November 1998; pp. 1–7. [Google Scholar]
- Lindstad, T.; Olsen, S.; Tranell, G.; Færden, T.; Lubetsky, J. Greenhouse gas emissions from ferroalloy production. In Proceedings of the International Ferro-Alloys Congress XI, New Delhi, India, 18–21 February 2007; pp. 457–466. [Google Scholar]
- Kero, I.; Eidem, P.; Ma, Y.; Indresand, H.; Aarhaug, T.; Grådahl, S. Airborne Emissions from Mn Ferroalloy Production. JOM 2019, 71, 349–365. [Google Scholar] [CrossRef] [Green Version]
- Westfall, L.; Davourie, J.; Ali, M.; McGough, D. Cradle-to-gate life cycle assessment of global manganese alloy production. Int. J. Life Cycle Assess. 2016, 21, 1573–1579. [Google Scholar] [CrossRef] [Green Version]
- Mathieson, J.; Rogers, H.; Somerville, M.; Jahanshahi, S.; Ridgeway, P. Potential for the use of biomass in the iron and steel industry. In Proceedings of the Chemeca 2011, Engineering a Better World, Sydney, Australia, 18–21 September 2011; pp. 1–12. [Google Scholar]
- Surup, G.; Nielsen, H.; Heidelmann, M.; Trubetskaya, A. Characterization and reactivity of charcoal from high temperature pyrolysis (800–1600 ∘C). Fuel 2019, 235, 1544–1554. [Google Scholar] [CrossRef]
- Lindstad, T.; Monsen, B.; Osen, K. How the ferroalloys industry can meet greenhouse gas regulations. In Proceedings of the International Ferro-Alloys Congress XII, Helsinki, Finland, 6–9 June 2010; pp. 63–70. [Google Scholar]
- Eidem, P.; Tangstad, M.; Bakken, J.; Ishak, R. Influence of coke particle size on the electrical resistivity of coke beds. In Proceedings of the International Ferro-Alloys Congress XII, Helsinki, Finland, 6–9 June 2010; pp. 349–358. [Google Scholar]
- Hussein, A.; Larachi, F.; Ziegler, D.; Alamdari, H. Effects of heat treatment and acid washing on properties and reactivity of charcoal. Biomass Bioenergy 2016, 90, 101–113. [Google Scholar] [CrossRef]
- Surup, G.; Foppe, M.; Schubert, D.; Deike, R.; Heidelmann, M.; Timko, M.T.; Trubetskaya, A. The effect of feedstock origin and temperature on the structure and reactivity of char from pyrolysis at 1300–2800 ∘C. Fuel 2019, 235, 306–316. [Google Scholar] [CrossRef]
- Eidem, P. Electrical Resistivity of Coke Beds. Ph.D. Thesis, NTNU, Trondheim, Norway, 2008. [Google Scholar]
- Surup, G. Renewable Reducing Agents for the Use in Ferroalloy Industries. Ph.D. Thesis, University of Agder, Kristiansand, Norway, 2019. [Google Scholar]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The potential of using biomass-based reducing agents in the blast furnace: A review of thermochemical conversion technologies and assessments related to sustainability. Renew. Sustain. Energy Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of biomass in integrated steelmaking—Status quo, future needs and comparison to other low-CO2 steel production technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef] [Green Version]
- Dijs, H.; Smith, D.J. Factors affecting the resistivity and reactivity of carbonaceous reducing agents for the electric-smelting industry. J. S. Afr. Inst. Min. Metall. 1980, 80, 286–296. [Google Scholar]
- Adinaveen, T.; Vijaya, J.J.; Kennedy, L. Comparative Study of Electrical Conductivity on Activated Carbons Prepared from Various Cellulose Materials. Arab. J. Sci. Eng. 2014, 41, 55–65. [Google Scholar] [CrossRef]
- Espinola, A.; Miguel, P.; Salles, M.; Pinto, A. Electrical properties of carbons - resistance of powder materials. Carbon 1986, 24, 337–341. [Google Scholar] [CrossRef]
- Sánchez-González, J.; Macías-García, A.; Alexandre-Franco, M.; Gómez-Serrano, V. Electrical conductivity of carbon blacks under compression. Carbon 2005, 43, 741–747. [Google Scholar] [CrossRef]
- Tsybul’kin, G. On the Calculation of the Electrical Resistivity of Conducting Loose Materials. Elektrometallurgiya 2010, 2011, 594–598. [Google Scholar] [CrossRef]
- Eidem, P.; Tangstad, M.; Bakken, J. Measurement of material resistivity and contact resistance of metallurgical coke. In Proceedings of the International Ferro-Alloys Congress XI, New Delhi, India, 18–21 February 2007; pp. 561–571. [Google Scholar]
- Buryak, V.; Vasil’chenko, G.; Chirka, T.; Konstantinov, S. Specific electrical resistance of carbon materials. Refract. Ind. Ceram. 2013, 54, 215–219. [Google Scholar] [CrossRef]
- Tangstad, M.; Beukes, J.; Steenkamp, J.; Ringdalen, E. 14—Coal-based reducing agents in ferroalloys and silicon production. In New Trends in Coal Conversion Combustion, Gasification, Emissions, and Coking; Woodhead Publishing: Cambridge, UK, 2019; pp. 405–438. [Google Scholar]
- Monsen, B.; Tangstad, M.; Solheim, I.; Syvertsen, I.; Ishak, R.; Midtgaard, H. Charcoal for manganese alloy production. In Proceedings of the International Ferro-Alloys Congress XI, New Delhi, India, 18–21 February 2007; pp. 297–310. [Google Scholar]
- Surup, G.; Vehus, T.; Eidem, P.; Trubetskaya, A.; Nielsen, H. Characterization of renewable reductants and charcoal-based pellets for the use in ferroalloy industries. Energy 2019, 167, 337–345. [Google Scholar] [CrossRef]
- Antal, M.; Mok, W.; Varhegyi, G.; Szekely, T. Review of methods for improving the yield of charcoal from biomass. Energy Fuels 1990, 4, 221–225. [Google Scholar] [CrossRef]
- Olsson, J.; Jaglid, U.; Pettersson, J.; Hald, P. Alkali metal emission during pyrolysis of biomass. Energy Fuels 1997, 11, 779–784. [Google Scholar] [CrossRef]
- Riaza, J.; Mason, P.; Jones, J.; Gibbins, J.; Chalmers, H. High temperature volatile yield and nitrogen partitioning during pyrolysis of coal and biomass fuels. Fuel 2019, 248, 215–220. [Google Scholar] [CrossRef]
- Efika, E.; Onwudili, J.; Williams, P. Products from the high temperature pyrolysis of RDF at slow and rapid heating rates. J. Anal. Appl. Pyrolysis 2015, 112, 14–22. [Google Scholar] [CrossRef]
- Wakchaure, G.; Mani, I. Thermal and Storage Characteristics of Biomass Briquettes with Organic Binders. J. Agric. Eng. Res. 2011, 48, 43–53. [Google Scholar]
- Wang, L.; Barta-Rajnai, E.; Hu, K.; Higashi, C.; Skreiberg, Ø.; Grønli, M.; Czégény, Z.; Jakab, E.; Myrvågnes, V.; Várhegyi, G.; et al. Biomass Charcoal Properties Changes during Storage. Energy Procedia 2017, 105, 830–835. [Google Scholar] [CrossRef]
- Koursaris, A.; See, J. The resistivity of mixtures of Mamatwan manganese ore and reducing agents. J. S. Afr. Inst. Min. Metall. 1980, 80, 229–238. [Google Scholar]
- Mrozowski, S. Semiconductivity and Diamagnetism of Polycrystalline Graphite and Condensed Ring Systems. Phys. Rev. 1952, 85, 609–620. [Google Scholar] [CrossRef]
- Golden, T.; Jenkins, R.; Otake, Y.; Scaroni, A. Oxygen Complexes on Carbon Surfaces. In Proceedings of the Workshop on The Electrochemistry of Carbon, Cleveland, OH, USA, 17–19 August 1983; Volume 84, pp. 61–78. [Google Scholar]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Sümmchen, L.; Roy, C. Electrical conductivity of thermal carbon blacks Influence of surface chemistry. Carbon 2001, 39, 1147–1158. [Google Scholar] [CrossRef]
- JoséSánchez-González, J.; Stoeckli, F.; Centeno, T. The role of the electric conductivity of carbons in the electrochemical capacitor performance. J. Electroanal. Chem. 2011, 657, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Razd’yakonova, G. The electrical conductivity of carbon-black filled vulcanisates. Int. Polym. Sci. Tech. 2013, 40, 11–15. [Google Scholar] [CrossRef]
- Panshin, A.; De Zeeuw, C. Textbook of Wood Technology: Structure, Identification, Properties, and uses of the Commercial Woods of the United States and Canada; Number Bd. 1 in McGraw-Hill Series in Forest Resources; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Trubetskaya, A.; Jensen, P.; Jensen, A.; Steibel, M.; Spliethoff, H.; Glarborg, P. Influence of fast pyrolysis conditions on yield and structural transformation of biomass chars. Fuel Process. Technol. 2015, 140, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Díez, M.; Alvarez, R.; Barriocanal, C. Coal for metallurgical coke production: Predictions of coke quality and future requirements for cokemaking. Int. J. Coal. Geol. 2002, 50, 389–412. [Google Scholar] [CrossRef]
- Babu, S.; Santella, M.L.; Feng, Z.; Riemer, B.; Cohron, J. Empirical model of effects of pressure and temperature on electrical contact resistance of metals. Sci. Technol. Weld. Join. 2001, 6, 126–132. [Google Scholar] [CrossRef]
- Rani, A.; Nam, S.; Oh, K.; Park, M. Electrical Conductivity of Chemically Reduced Graphene Powders under Compression. Carbon Lett. 2010, 11, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Mao, H. Solid Carbon at High Pressure: Electrical Resistivity and Phase Transition. Phys. Chem. Miner. 1994, 21, 1–5. [Google Scholar]
- Xiao, G.; Xiao, R.; Jin, B.; Zuo, W.; Liu, J.; Grace, J. Study on Electrical Resistivity of Rice Straw Charcoal. J. Biobased Mater. Bioenergy 2010, 4, 426–429. [Google Scholar] [CrossRef]
- Emerson, D.; Schmidt, P. Pyrolusitic supergene manganese oxides: Inductive properties, EM conductivity and magnetic susceptibility. Preview 2018, 197, 42–50. [Google Scholar]
- Montiano, M.; Díaz-Faes, E.; Barriocanal, E.; Alvarez, R. Influence of biomass on metallurgical coke quality. Fuel 2014, 116, 175–182. [Google Scholar] [CrossRef] [Green Version]
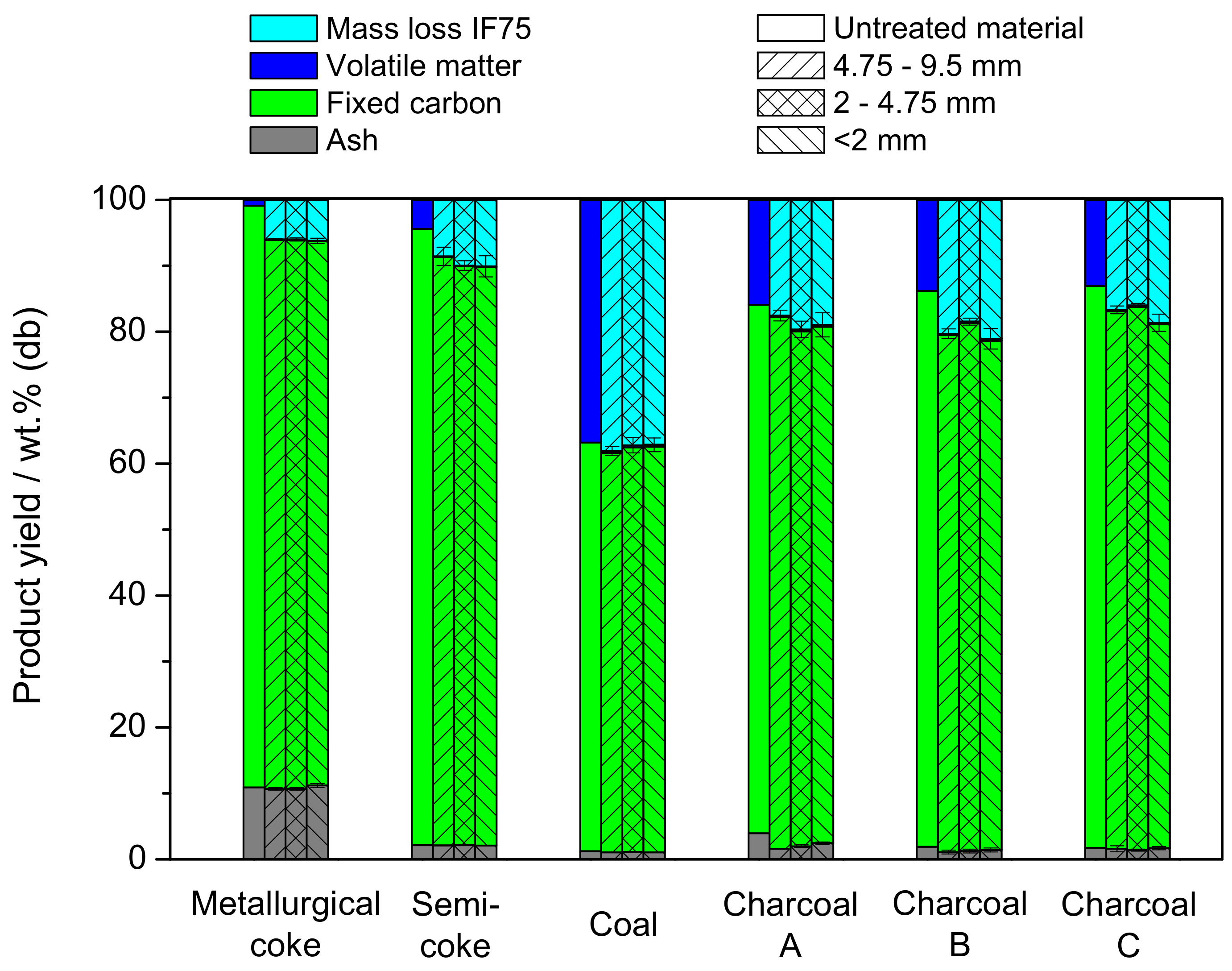

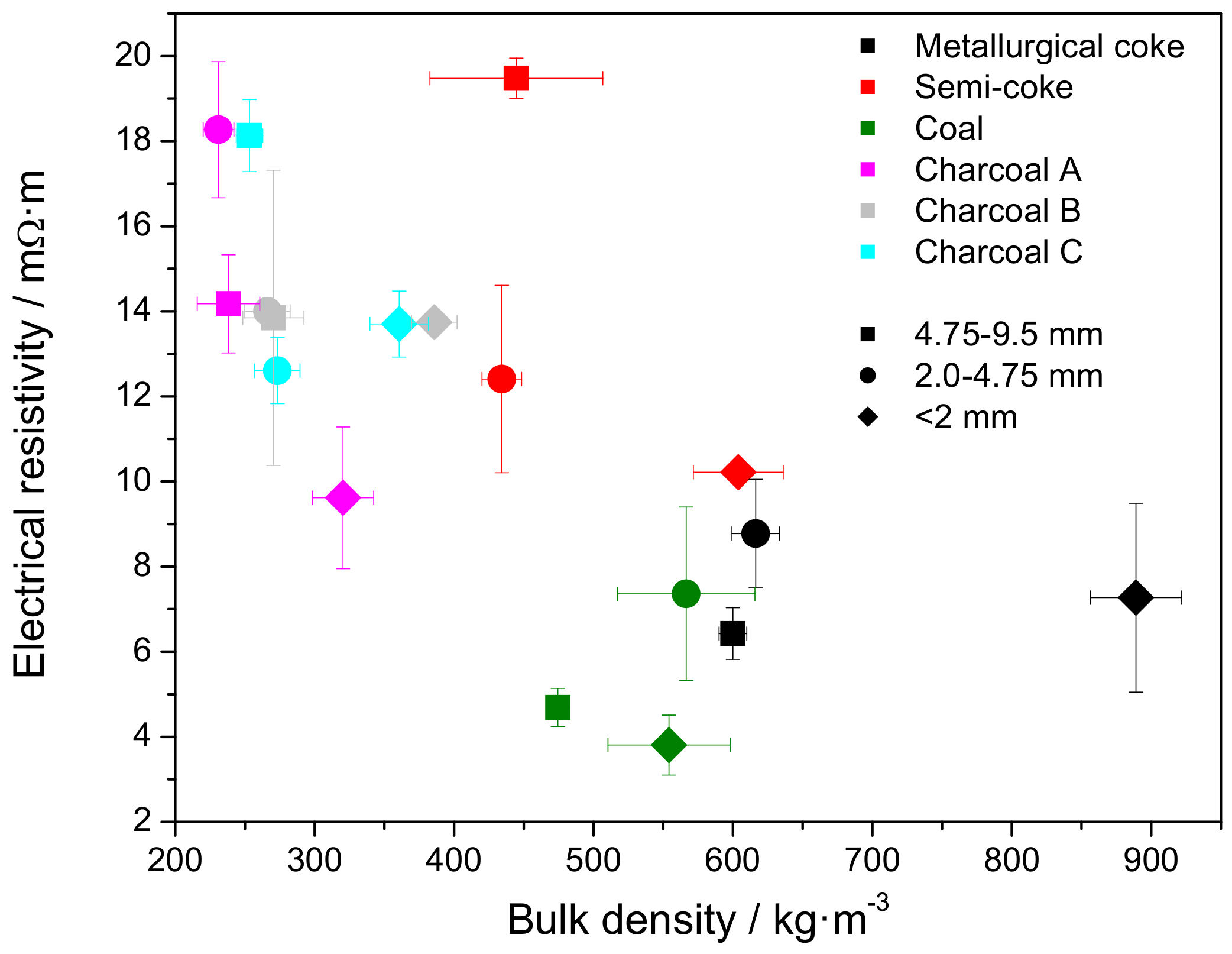
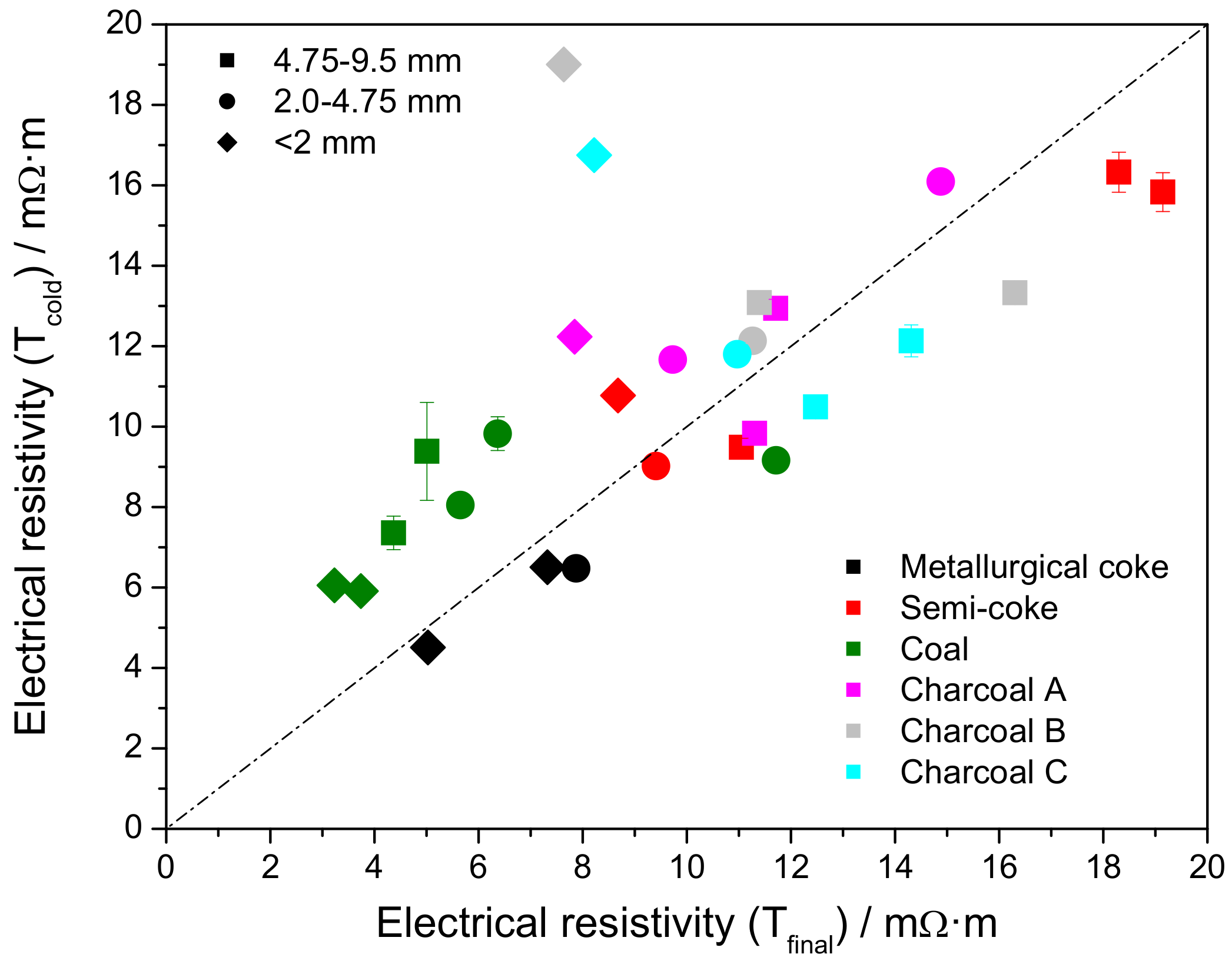
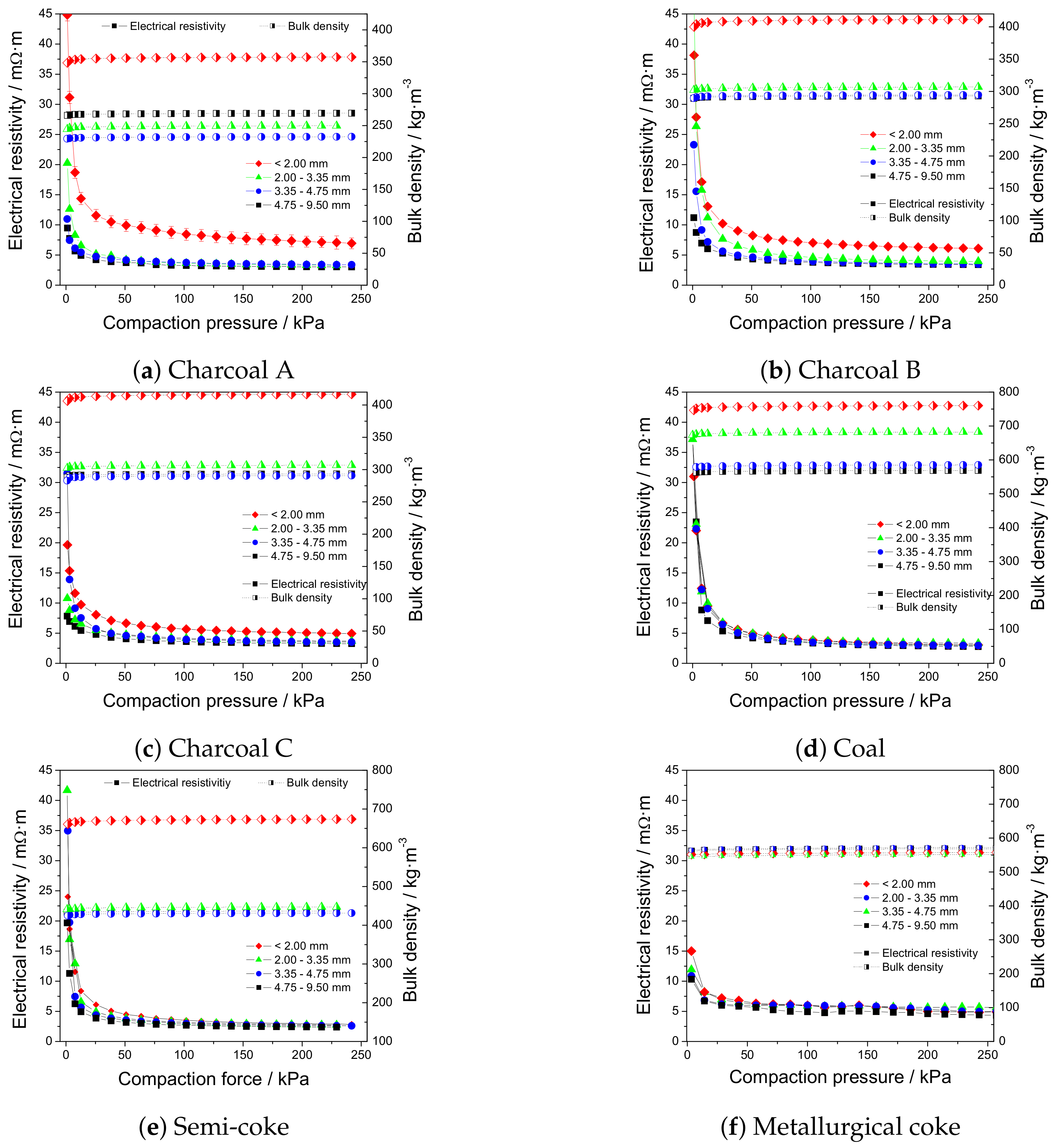
| Feedstock | Charcoal A | Charcoal B | Charcoal C | Coal | Semi-Coke | Metallurgical Coke |
|---|---|---|---|---|---|---|
| Proximate analysis (wt.%) | ||||||
| Moisture (ar) | 27.5 | 12.1 | 5.02 | 12.2 | 8.8 | <0.1 |
| Volatile matter (db) | 15.9 | 13.8 | 13.1 | 38.8 | 5.4 | 0.9 |
| Fixed carbon (db) | 82.8 | 84.8 | 85.2 | 59.3 | 88.6 | 87.9 |
| Ash (db) | 3.97 | 1.89 | 1.75 | 1.25 | 5.87 | 10.90 |
| Ultimate analysis (wt.%, dry basis) | ||||||
| C | 85.5 | 83.5 | 88.1 | 78.3 | 88.5 | 87.7 |
| H | 1.95 | 2.28 | 2.51 | 4.72 | 1.24 | 0.21 |
| N | <0.50 | <0.50 | <0.50 | 1.61 | 1.44 | 1.75 |
| O | 7.40 | 8.68 | 7.14 | 13.77 | 2.64 | <0.10 |
| S | <0.01 | <0.01 | <0.01 | 0.35 | 0.31 | 0.57 |
| TS/% | Mechanical Durability/% | Compression Strength/N | |||
|---|---|---|---|---|---|
| Sample | Heat-Treated | Raw | Heat-Treated | Raw | Heat-Treated |
| Charcoal A | 17.6 ± 1.2 | 93.6 | 95.6 ± 1.3 | 20–60 | 40–80 |
| Charcoal B | 16.6 ± 2.4 | 93.5 | 96.1 ± 0.4 | 30–60 | 40–80 |
| Charcoal C | 19.4 ± 3.1 | 91.8 | 96.0 ± 0.1 | 30–60 | 40–80 |
| Coal | 4.5 ± 0.6 | 99.4 | 94.3 ± 1.1 | 50 | 70 |
| Semi-coke | 7.6 ± 0.6 | 99.8 | 98.4 ± 0.6 | 80 | 100 |
| Metallurgical coke | 1.9 ± 0.1 | 99.8 | 99.6 ± 0.1 | 100 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surup, G.R.; Pedersen, T.A.; Chaldien, A.; Beukes, J.P.; Tangstad, M. Electrical Resistivity of Carbonaceous Bed Material at High Temperature. Processes 2020, 8, 933. https://doi.org/10.3390/pr8080933
Surup GR, Pedersen TA, Chaldien A, Beukes JP, Tangstad M. Electrical Resistivity of Carbonaceous Bed Material at High Temperature. Processes. 2020; 8(8):933. https://doi.org/10.3390/pr8080933
Chicago/Turabian StyleSurup, Gerrit Ralf, Tommy Andre Pedersen, Annah Chaldien, Johan Paul Beukes, and Merete Tangstad. 2020. "Electrical Resistivity of Carbonaceous Bed Material at High Temperature" Processes 8, no. 8: 933. https://doi.org/10.3390/pr8080933
APA StyleSurup, G. R., Pedersen, T. A., Chaldien, A., Beukes, J. P., & Tangstad, M. (2020). Electrical Resistivity of Carbonaceous Bed Material at High Temperature. Processes, 8(8), 933. https://doi.org/10.3390/pr8080933





