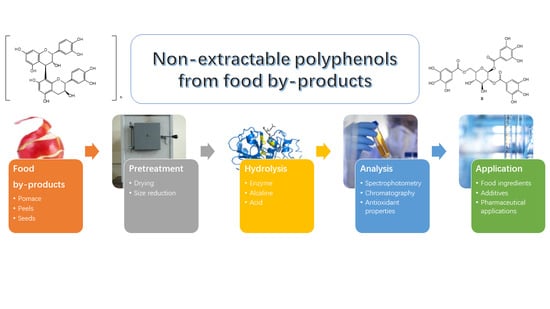
Non-Extractable Polyphenols from Food By-Products: Current Knowledge on Recovery, Characterisation, and Potential Applications
Abstract
:1. Introduction
2. NEPs Recovery from Food By-Products
2.1. Food By-Products
2.2. Non-Extractable Polyphenols
2.3. NEPs Recovery Process
3. Steps for the Extraction of NEPs from Food By-Products
3.1. By-Products Pre-Treatments
3.2. Isolation of Extractable Polyphenols (EPPs)
3.3. Extraction of NEPs
3.3.1. Acid Hydrolysis
3.3.2. Alkaline Hydrolysis
3.3.3. Enzymatic Hydrolysis
3.4. Innovative NEPs Extraction and Purification
3.4.1. Ultrasound-Assisted Extraction (UAE)
3.4.2. Microwave-Assisted Extraction (MAE)
3.4.3. Supercritical Fluid Extraction (SFE)
3.4.4. Solid Phase Extraction (SPE)
4. NEPs Characterisation Methods
4.1. Spectrophotometry
4.2. Liquid Chromatography and Mass Spectrometry
4.3. Antioxidant Activity of NEPs
5. Potential Applications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable Polyphenols in Plant Foods: Nature, Isolation, and Analysis; Elsevier Academic Press: Cambridge, MA, USA, 2014; ISBN 9780123979346. [Google Scholar]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res. Int. 2018, 111, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High Contents of Nonextractable Polyphenols in Fruits Suggest That Polyphenol Contents of Plant Foods Have Been Underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Concept and Health-Related Properties of Nonextractable Polyphenols: The Missing Dietary Polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef]
- Saura-Calixto, F.J.P.-J. Contents. In Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; CPI Group: Croydon, UK, 2018; pp. P007–P015. ISBN 9781788011006. [Google Scholar]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Nunes, C.; Castro, A.; Ferreira, P.; Coimbra, M.A. Influence of grape pomace extract incorporation on chitosan films properties. Carbohydr. Polym. 2014, 113, 490–499. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic profile and antioxidant activity of olive mill wastewater from two Sicilian olive cultivars: Cerasuola and Nocellara etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- da Silva, L.M.R.; de Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; de Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Mauro, M.D.; Tomasello, B.; Giardina, R.C.; Dattilo, S.; Mazzei, V.; Sinatra, F.; Caruso, M.; D’Antona, N.; Renis, M. Sugars and minerals enriched fraction from olive mill wastewater for promising cosmeceutical application: Characterization, in vitro and in vivo studies. Food Funct. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Zhang, X.; Chen, H.; Xia, S.; Jia, C.; Zhong, F. Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Sep. Purif. Technol. 2010, 73, 371–376. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- Arranz, S.; Saura Calixto, F. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Zhang, R.; Khan, S.A.; Chi, J.; Wei, Z.; Zhang, Y.; Deng, Y.; Liu, L.; Zhang, M. Different effects of extrusion on the phenolic profiles and antioxidant activity in milled fractions of brown rice. LWT 2018, 88, 64–70. [Google Scholar] [CrossRef]
- Ti, H.; Li, Q.; Zhang, R.; Zhang, M.; Deng, Y.; Wei, Z.; Chi, J.; Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in southern China. Food Chem. 2014, 159, 166–174. [Google Scholar] [CrossRef]
- Speroni, C.S.; Stiebe, J.; Guerra, D.R.; Beutinger Bender, A.B.; Ballus, C.A.; dos Santos, D.R.; Dal Pont Morisso, F.; da Silva, L.P.; Emanuelli, T. Micronization and granulometric fractionation improve polyphenol content and antioxidant capacity of olive pomace. Ind. Crops Prod. 2019, 137, 347–355. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Raes, K.; Vanhoutte, H.; Coelus, S.; Smagghe, G.; Van Camp, J. Liquid chromatography–mass spectrometry coupled with multivariate analysis for the characterization and discrimination of extractable and nonextractable polyphenols and glucosinolates from red cabbage and Brussels sprout waste streams. J. Chromatogr. A 2015, 1402, 60–70. [Google Scholar] [CrossRef]
- Ferrentino, G.; Asaduzzaman, M.; Scampicchio, M.M. Current technologies and new insights for the recovery of high valuable compounds from fruits by-products. Crit. Rev. Food Sci. Nutr. 2016, 58, 1–19. [Google Scholar] [CrossRef]
- Stenmarck, Å.; Jensen, C.; Quested, T.; Moates, G.; Cseh, B.; Juul, S.; Parry, A.; Politano, A.; Redlingshofer, B.; Scherhaufer, S.; et al. FUSIONS—Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016; ISBN 9789188319012. [Google Scholar]
- Pimentel-Moral, S.; de la Luz Cádiz-Gurrea, M.; Rodríguez-Pérez, C.; Segura-Carretero, A. Recent advances in extraction technologies of phytochemicals applied for the revaluation of agri-food by-products. In Functional and Preservative Properties of Phytochemicals; Elsevier: Cambridge, MA, USA, 2020; pp. 209–239. ISBN 9780128185933. [Google Scholar]
- Ran, X.; Zhang, M.; Wang, Y.; Adhikari, B. Novel technologies applied for recovery and value addition of high value compounds from plant byproducts: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Food Sci. Nutr. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of agro-industrial by-products, effluents and waste: Concept, opportunities and the case of olive mill wastewaters. J. Chem. Technol. Biotechnol. 2009, 84, 895–900. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Vella, F.M.; Cautela, D.; Laratta, B. Characterization of Polyphenolic Compounds in Cantaloupe Melon By-Products. Foods 2019, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Bilen, F.D.; Gonzales, G.B.; Grootaert, C.; Van de Wiele, T.; Van Camp, J. Bioaccessibility of Polyphenols from Plant-Processing Byproducts of Black Carrot (Daucus carota L.). J. Agric. Food Chem. 2016, 64, 2450–2458. [Google Scholar] [CrossRef]
- Peričin, D.; Krimer, V.; Trivić, S.; Radulović, L. The distribution of phenolic acids in pumpkin’s hull-less seed, skin, oil cake meal, dehulled kernel and hull. Food Chem. 2009, 113, 450–456. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Process optimisation for recovery of carotenoids from tomato waste. Food Chem. 2011, 129, 747–752. [Google Scholar] [CrossRef]
- Vargas, E.F.; Jablonski, A.; Flôres, S.H.; Rios, A.D. Waste from peach (Prunus persica) processing used for optimisation of carotenoids ethanolic extraction. Int. J. Food Sci. Technol. 2017, 52, 757–762. [Google Scholar] [CrossRef]
- Beveridge, T.H.J.; Girard, B.; Kopp, T.; Drover, J.C.G. Yield and Composition of Grape Seed Oils Extracted by Supercritical Carbon Dioxide and Petroleum Ether: Varietal Effects. J. Agric. Food Chem. 2005, 53, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Kittiphoom, S.; Sutasinee, S. Mango seed kernel oil and its physicochemical properties. Int. Food Res. J. 2013, 20, 1145–1149. [Google Scholar]
- Vági, E.; Simándi, B.; Vásárhelyiné, K.P.; Daood, H.; Kéry, Á.; Doleschall, F.; Nagy, B. Supercritical carbon dioxide extraction of carotenoids, tocopherols and sitosterols from industrial tomato by-products. J. Supercrit. Fluids 2007, 40, 218–226. [Google Scholar] [CrossRef]
- Tanaka, M.; Takamizu, A.; Hoshino, M.; Sasaki, M.; Goto, M. Extraction of dietary fiber from Citrus junos peel with subcritical water. Food Bioprod. Process. 2012, 90, 180–186. [Google Scholar] [CrossRef]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Serna-Saldivar, S.O.; Welti-Chanes, J. Dietary Fiber Concentrates from Fruit and Vegetable By-products: Processing, Modification, and Application as Functional Ingredients. Food Bioprocess Technol. 2018, 11, 1439–1463. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef]
- Haslam, E.; Cai, Y. Plant polyphenols (vegetable tannins): Gallic acid metabolism. Nat. Prod. Rep. 1994, 11, 41. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, M.D.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.G.; Centonze, G.; Maggiore, R.; D’antona, N. Polyphenolic fraction from olive mill wastewater: Scale-up and in vitro studies for ophthalmic nutraceutical applications. Antioxidants 2019, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.; Yan, H.; Han, C.; Chen, X.; Wang, W.; Xie, C.; Qu, J.; Gong, Z.; Shi, X. Acid and alkaline hydrolysis extraction of non-extractabke polyphenols in blueberries optimisation by response surface methodology. Czech J. Food Sci. 2014, 32, 218–225. [Google Scholar] [CrossRef] [Green Version]
- de Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier–El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef] [Green Version]
- Arapitsas, P. Hydrolyzable tannin analysis in food. Food Chem. 2012, 135, 1708–1717. [Google Scholar] [CrossRef]
- dos Santos Grasel, F.; Ferrão, M.F.; Wolf, C.R. Ultraviolet spectroscopy and chemometrics for the identification of vegetable tannins. Ind. Crops Prod. 2016, 91, 279–285. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G. Non-covalent interaction between procyanidins and apple cell wall material. Biochim. Biophys. Acta Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Akram, S.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction: An alternative and green technology for non-extractable polyphenols. Anal. Bioanal. Chem. 2017, 409, 3645–3655. [Google Scholar] [CrossRef]
- Cheng, A.; Chen, X.; Wang, W.; Gong, Z.; Liu, L. Contents of extractable and non-extractable polyphenols in the leaves of blueberry. Czech J. Food Sci. 2013, 31, 275–282. [Google Scholar] [CrossRef]
- Esparza-Martínez, F.J.; Miranda-López, R.; Mata-Sánchez, S.M.; Guzmán-Maldonado, S.H. Extractable and Non-Extractable Phenolics and Antioxidant Capacity of Mandarin Waste Dried at Different Temperatures. Plant Foods Hum. Nutr. 2016, 71, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Cruz, D.; Peréz-Ramírez, I.F.; Pérez-Jiménez, J.; Nava, G.M.; Reynoso-Camacho, R. Comparison of the bioactive potential of Roselle (Hibiscus sabdariffa L.) calyx and its by-product: Phenolic characterization by UPLC-QTOF MS and their anti-obesity effect in vivo. Food Res. Int. 2019, 126, 108589. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. Waste Manag. 2016, 48, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Hellström, J.K.; Mattila, P.H. HPLC Determination of Extractable and Unextractable Proanthocyanidins in Plant Materials. J. Agric. Food Chem. 2008, 56, 7617–7624. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.H.; Cazarin, C.B.B.; Quatrin, A.; Minuzzi, N.M.; Costa, E.L.; Morari, J.; Velloso, L.A.; Leal, R.F.; Rodrigues, E.; Bochi, V.C.; et al. Grape peel powder promotes intestinal barrier homeostasis in acute TNBS-colitis: A major role for dietary fiber and fiber-bound polyphenols. Food Res. Int. 2019, 123, 425–439. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire Djantou, E.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic Profiles and Antioxidant Activity of Black Rice Bran of Different Commercially Available Varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels. Sep. Purif. Technol. 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Landbo, A.-K.; Meyer, A.S. Enzyme-Assisted Extraction of Antioxidative Phenols from Black Currant Juice Press Residues (Ribes nigrum). J. Agric. Food Chem. 2001, 49, 3169–3177. [Google Scholar] [CrossRef]
- Saxena, S.N.; Sharma, Y.K.; Rathore, S.S.; Singh, K.K.; Barnwal, P.; Saxena, R.; Upadhyaya, P.; Anwer, M.M. Effect of cryogenic grinding on volatile oil, oleoresin content and anti-oxidant properties of coriander (Coriandrum sativum L.) genotypes. J. Food Sci. Technol. 2015, 52, 568–573. [Google Scholar] [CrossRef]
- An, X.; Xu, Y.; Jiang, L.; Huan, C.; Yu, Z. Effects of postharvest temperature on apoptosis-related enzyme activity and gene expression in peach fruits (Prunus persica L. cv. Xiahui 8). Sci. Hortic. 2019, 245, 178–184. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, I.F.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Comprehensive Characterization of Extractable and Nonextractable Phenolic Compounds by High-Performance Liquid Chromatography–Electrospray Ionization–Quadrupole Time-of-Flight of a Grape/Pomegranate Pomace Dietary Supplement. J. Agric. Food Chem. 2018, 66, 661–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Scientia Horticulturae; Academic Press: Cambridge, MA, USA, 1999; Volume 213, pp. 152–178. [Google Scholar]
- Liu, L.; Guo, J.; Zhang, R.; Wei, Z.; Deng, Y.; Guo, J.; Zhang, M. Effect of degree of milling on phenolic profiles and cellular antioxidant activity of whole brown rice. Food Chem. 2015, 185, 318–325. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Hartzfeld, P.W.; Forkner, R.; Hunter, M.D.; Hagerman, A.E. Determination of Hydrolyzable Tannins (Gallotannins and Ellagitannins) after Reaction with Potassium Iodate. J. Agric. Food Chem. 2002, 50, 1785–1790. [Google Scholar] [CrossRef]
- Tow, W.W.; Premier, R.; Jing, H.; Ajlouni, S. Antioxidant and Antiproliferation Effects of Extractable and Nonextractable Polyphenols Isolated from Apple Waste Using Different Extraction Methods. J. Food Sci. 2011, 76, T163–T172. [Google Scholar] [CrossRef]
- Agostini, F.; Bertussi, R.A.; Agostini, G.; Atti dos Santos, A.C.; Rossato, M.; Vanderlinde, R. Supercritical Extraction from Vinification Residues: Fatty Acids, α -Tocopherol, and Phenolic Compounds in the Oil Seeds from Different Varieties of Grape. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, I.A.; Mirghani, M.E.S.; Yusof, F.; Al-khatib, M. Effects of Time, Temperature, and Solvent Ratio on the Extraction of Non-Extractable Polyphenols with Anticancer Activity of Barhi Date Palm Kernels Extracts Using Response Surface Methodology. Preprints 2019. [Google Scholar] [CrossRef] [Green Version]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Naczk, M.; Shahidi, F. The effect of methanol-ammonia-water treatment on the content of phenolic acids of canola. Food Chem. 1989, 31, 159–164. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Release of Bound Procyanidins from Cranberry Pomace by Alkaline Hydrolysis. J. Agric. Food Chem. 2010, 58, 7572–7579. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B.; Smagghe, G.; Raes, K.; Van Camp, J. Combined Alkaline Hydrolysis and Ultrasound-Assisted Extraction for the Release of Nonextractable Phenolics from Cauliflower (Brassica oleracea var. botrytis) Waste. J. Agric. Food Chem. 2014, 62, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Tsao, R.; Yang, R.; Cui, S. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Ajila, C.M.; Prasada Rao, U.J.S. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- Fernández, K.; Vega, M.; Aspé, E. An enzymatic extraction of proanthocyanidins from País grape seeds and skins. Food Chem. 2015, 168, 7–13. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.S.; Rathod, V.K. Immobilization of pectinase onto chitosan magnetic nanoparticles by macromolecular cross-linker. Carbohydr. Polym. 2017, 157, 677–685. [Google Scholar] [CrossRef]
- Long, J.; Fu, Y.; Zu, Y.; Li, J.; Wang, W.; Gu, C.; Luo, M. Ultrasound-assisted extraction of flaxseed oil using immobilized enzymes. Bioresour. Technol. 2011, 102, 9991–9996. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Koubaa, M.; Barba, F.J.; Grimi, N.; Mhemdi, H.; Koubaa, W.; Boussetta, N.; Vorobiev, E. Recovery of colorants from red prickly pear peels and pulps enhanced by pulsed electric field and ultrasound. Innov. Food Sci. Emerg. Technol. 2016, 37, 336–344. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pereira, A.L.D.; Barbero, G.F.; Martínez, J. Recovery of anthocyanins from residues of Rubus fruticosus, Vaccinium myrtillus and Eugenia brasiliensis by ultrasound assisted extraction, pressurized liquid extraction and their combination. Food Chem. 2017, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Herrero, M.; Prodanov, M.; Ibáñez, E.; Cifuentes, A. Characterization of grape seed procyanidins by comprehensive two-dimensional hydrophilic interaction × reversed phase liquid chromatography coupled to diode array detection and tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4627–4638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Enhancement of the total phenolic compounds and antioxidant activity of aqueous Citrus limon L. pomace extract using microwave pretreatment on the dry powder. J. Food Process. Preserv. 2017, 41, e13152. [Google Scholar] [CrossRef] [Green Version]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef] [Green Version]
- Chiremba, C.; Rooney, L.W.; Beta, T. Microwave-Assisted Extraction of Bound Phenolic Acids in Bran and Flour Fractions from Sorghum and Maize Cultivars Varying in Hardness. J. Agric. Food Chem. 2012, 60, 4735–4742. [Google Scholar] [CrossRef] [Green Version]
- Hayat, K.; Zhang, X.; Farooq, U.; Abbas, S.; Xia, S.; Jia, C.; Zhong, F.; Zhang, J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010, 123, 423–429. [Google Scholar] [CrossRef]
- Haman, N.; Morozova, K.; Tonon, G.; Scampicchio, M.; Ferrentino, G. Antimicrobial Effect of Picea abies Extracts on E. coli Growth. Molecules 2019, 24, 4053. [Google Scholar] [CrossRef] [Green Version]
- Hennion, M.-C. Solid-phase extraction: Method development, sorbents, and coupling with liquid chromatography. J. Chromatogr. A 1999, 856, 3–54. [Google Scholar] [CrossRef]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef]
- Kostić, D.; Velicković, J.; Mitić, S.; Mitić, M.; Randelović, S. Phenolic Content, and Antioxidant and Antimicrobial Activities of Crataegus Oxyacantha L (Rosaceae) Fruit Extract from Southeast Serbia. Trop. J. Pharm. Res. 2012, 11, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Wroistad, R.E. Color and Pigment Analyses in Fruit Products; Agricultural Experiment Station: Corvallis, OR, USA, 1993; pp. 4–20. [Google Scholar]
- Karger, B.L. HPLC: Early and Recent Perspectives. J. Chem. Educ. 1997, 74, 45. [Google Scholar] [CrossRef]
- Hümmer, W.; Schreier, P. Analysis of proanthocyanidins. Mol. Nutr. Food Res. 2008, 52, 1381–1398. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Proximate and Polyphenolic Characterization of Cranberry Pomace. J. Agric. Food Chem. 2010, 58, 4030–4036. [Google Scholar] [CrossRef]
- Koupai-Abyazani, M.R.; McCallum, J.; Bohm, B.A. Identification of the constituent flavanoid units in sainfoin proanthocyanidins by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1992, 594, 117–123. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Suwannachot, J. Non-extractable polyphenols and in vitro bile acid-binding capacity of dried persimmon (Diospyros kaki) fruit. Food Chem. 2019, 293, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ames, J.M. Food Analysis by HPLC; CRC Press: Boca Raton, FL, USA, 1993; Volume 46, ISBN 9781439830857. [Google Scholar]
- Kongwong, P.; Morozova, K.; Ferrentino, G.; Poonlarp, P.; Scampicchio, M. Rapid Determination of the Antioxidant Capacity of Lettuce by an E-Tongue Based on Flow Injection Coulometry. Electroanalysis 2018, 30, 230–237. [Google Scholar] [CrossRef]
- Longo, E.; Morozova, K.; Loizzo, M.R.; Tundis, R.; Savini, S.; Foligni, R.; Mozzon, M.; Martin-Vertedor, D.; Scampicchio, M.; Boselli, E. High resolution mass approach to characterize refrigerated black truffles stored under different storage atmospheres. Food Res. Int. 2017, 102, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Quintanilla-López, J.E.; Gómez-Cordovés, C.; Bartolomé, B.; Lebrón-Aguilar, R. MALDI-TOF MS analysis of plant proanthocyanidins. J. Pharm. Biomed. Anal. 2010, 51, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2012, 1. [Google Scholar] [CrossRef] [Green Version]
- Calixto, S. Antioxidant Ingredient with Low Calorie Content, Method for Obtaining Same and Use Thereof. United States Patent Application No. US20140348925A1, 30 April 2019. [Google Scholar]
- Calixto, S.; Perze-Jimenez, J. Method for Producing a Concentrate of Polymer Proanthocyanidins with a Concentration of More than 40% by Means of Enzymaztic Treatments and without Using Organic Solvents. United States Patent Application No. US2018303792A1, 25 October 2018. [Google Scholar]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Belviso, S.; Giordano, M.; Ghirardello, D.; Torri, L.; Piochi, M.; Zeppa, G. Yogurt Enrichment with Grape Pomace: Effect of Grape Cultivar on Physicochemical, Microbiological and Sensory Properties. J. Food Qual. 2016, 39, 77–89. [Google Scholar] [CrossRef]
- Frumento, D.; do Espirito Santo, A.P.; Aliakbarian, B.; Casazza, A.A.; Gallo, M.; Converti, A.; Perego, P. Development of lactobacillus acidophilus fermented milk fortified with vitis vinifera marc flour. Food Technol. Biotechnol. 2013, 51, 370–375. [Google Scholar]
- Sánchez-Alonso, I.; Solas, M.T.; Borderías, A.J. Physical Study of Minced Fish Muscle with a White-Grape By-Product Added as an Ingredient. J. Food Sci. 2007, 72, E94–E101. [Google Scholar] [CrossRef]
- Ribeiro, B.; Cardoso, C.; Silva, H.A.; Serrano, C.; Ramos, C.; Santos, P.C.; Mendes, R. Effect of grape dietary fibre on the storage stability of innovative functional seafood products made from farmed meagre (Argyrosomus regius). Int. J. Food Sci. Technol. 2013, 48, 10–21. [Google Scholar] [CrossRef]
- Yadav, S.; Malik, A.; Pathera, A.; Islam, R.U.; Sharma, D. Development of dietary fibre enriched chicken sausages by incorporating corn bran, dried apple pomace and dried tomato pomace. Nutr. Food Sci. 2016, 46, 16–29. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Process optimization for antioxidant enriched sorghum flour and apple pomace based extrudates using liquid CO2 assisted extrusion. LWT 2017, 86, 544–554. [Google Scholar] [CrossRef]
- Struck, S.; Straube, D.; Zahn, S.; Rohm, H. Interaction of wheat macromolecules and berry pomace in model dough: Rheology and microstructure. J. Food Eng. 2018, 223, 109–115. [Google Scholar] [CrossRef]
- Rohm, H.; Brennan, C.; Turner, C.; Günther, E.; Campbell, G.; Hernando, I.; Struck, S.; Kontogiorgos, V. Adding Value to Fruit Processing Waste: Innovative Ways to Incorporate Fibers from Berry Pomace in Baked and Extruded Cereal-based Foods—A SUSFOOD Project. Foods 2015, 4, 690. [Google Scholar] [CrossRef] [Green Version]
- Acun, S.; Gül, H. Effects of grape pomace and grape seed flours on cookie quality. Qual. Assur. Saf. Crop. Foods 2014, 6, 81–88. [Google Scholar] [CrossRef]
- Aksoylu, Z.; Çağindi, Ö.; Köse, E. Effects of Blueberry, Grape Seed Powder and Poppy Seed Incorporation on Physicochemical and Sensory Properties of Biscuit. J. Food Qual. 2015, 38, 164–174. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Zawirska-Wojtasiak, R.; Górecka, D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J. Sci. Food Agric. 2013, 93, 389–395. [Google Scholar] [CrossRef]
- Rodríguez-González, S.; Pérez-Ramírez, I.F.; Amaya-Cruz, D.M.; Gallegos-Corona, M.A.; Ramos-Gomez, M.; Mora, O.; Reynoso-Camacho, R. Polyphenol-rich peach (Prunus persica L.) by-product exerts a greater beneficial effect than dietary fiber-rich by-product on insulin resistance and hepatic steatosis in obese rats. J. Funct. Foods 2018, 45, 58–66. [Google Scholar] [CrossRef]
- Bowser, S.M.; Moore, W.T.; McMillan, R.P.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Hulver, M.W.; Neilson, A.P. High-molecular-weight cocoa procyanidins possess enhanced insulin-enhancing and insulin mimetic activities in human primary skeletal muscle cells compared to smaller procyanidins. J. Nutr. Biochem. 2017, 39, 48–58. [Google Scholar] [CrossRef]
- Sánchez-Tena, S.; Lizárraga, D.; Miranda, A.; Vinardell, M.P.; García-García, F.; Dopazo, J.; Torres, J.L.; Saura-Calixto, F.; Capellà, G.; Cascante, M. Grape antioxidant dietary fiber inhibits intestinal polyposis in Apc Min/+ mice: Relation to cell cycle and immune response. Carcinogenesis 2013, 34, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Lizarraga, D.; Vinardell, M.P.; Noé, V.; van Delft, J.H.; Alcarraz-Vizán, G.; van Breda, S.G.; Staal, Y.; Günther, U.L.; Reed, M.A.; Ciudad, C.J.; et al. A Lyophilized Red Grape Pomace Containing Proanthocyanidin-Rich Dietary Fiber Induces Genetic and Metabolic Alterations in Colon Mucosa of Female C57BL/6J Mice. J. Nutr. 2011, 141, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.P.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Saura-Calixto, F. Effects of grape antioxidant dietary fiber in cardiovascular disease risk factors. Nutrition 2008, 24, 646–653. [Google Scholar] [CrossRef] [PubMed]
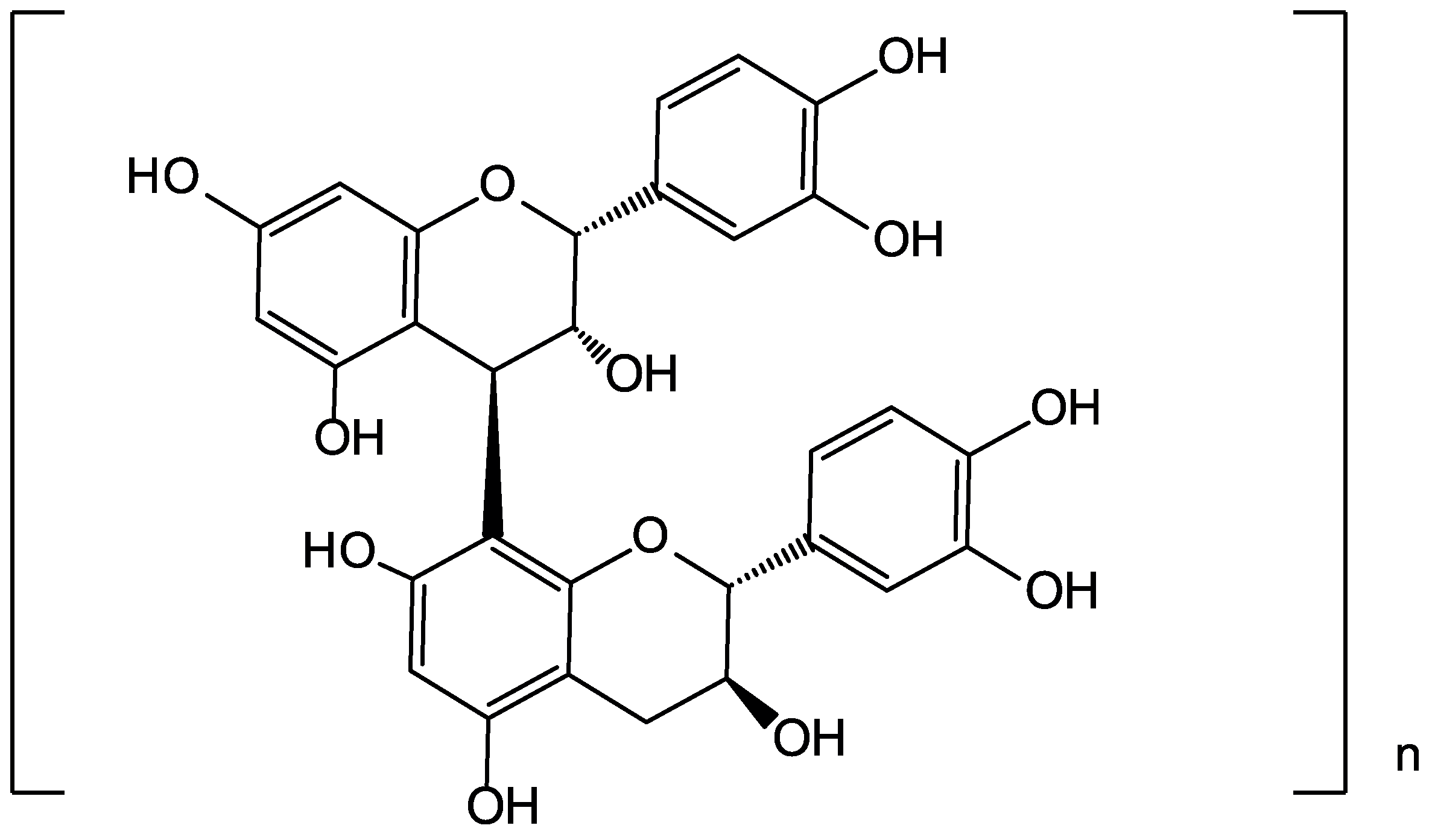
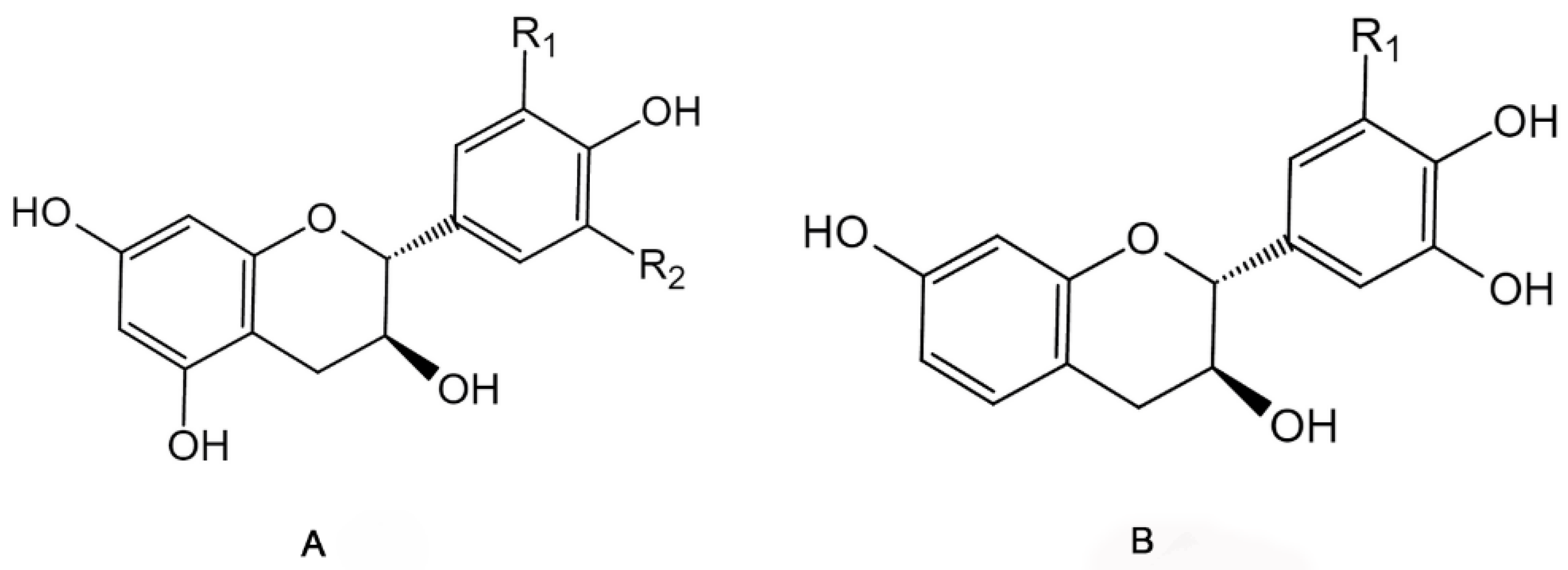
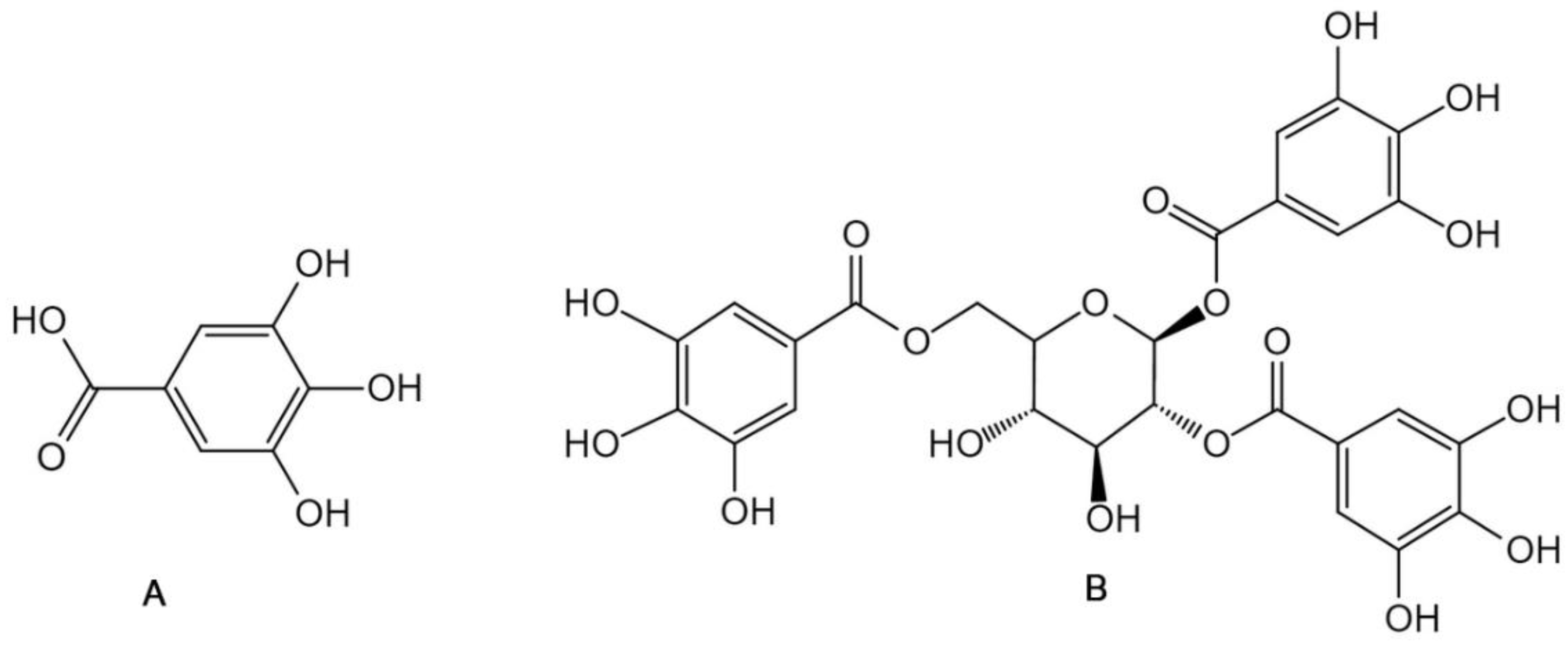

| Categories | Bioactive Compounds | Food By-Products Sources | Concentration Range | Extraction Method | Reference |
|---|---|---|---|---|---|
| Phenolic compounds | Polyphenols | Apple pomace | 12.38–13.76 mg/g GAE | Aqueous methanol extraction with acid hydrolysis | [15] |
| Polyphenols | Cantaloupe melons peel | 25.58 mg/g GAE 1 | 95% ethanol extraction | [30] | |
| Hesperidin | Citrus peel | Up to 673 mg/g DW 2 | Aqueous ethanol extraction | [31] | |
| Phenolic acid | Wild carrot pomace | 569.2 ± 55.9 mg/g DW | Aqueous methanol extraction | [32] | |
| Phenolic acid | Pumpkin oil cake meal | Up to 89.52 mg/kg DW | Aqueous methanol extraction | [33] | |
| Lipids | Carotenoids | Tomato waste | 36.5 ± 1.1 mg/kg dry waste | Hexane–ethyl acetate (50:50) extraction | [34] |
| Peach pulp residue | 256.1 mg/kg dry residues | Absolute ethanol extraction | [35] | ||
| β-sitosterol | Grape seed | Up to 11.2 g/kg dry seeds | Supercritical CO2 extraction | [36] | |
| Lipids | Mango kernel | Up to 84.6 mg/g dry kernel | Hexane extraction | [37] | |
| Vitamins | Tocopherols | Tomato pomace | Up to 195.99 mg/100 g dry pomace | Supercritical CO2 extraction | [38] |
| Dietary fiber - | Dietary fiber - | Citrus peel | 780 mg/g | Subcritical water extraction | [39] |
| Apple peel | 170–920 mg/g | Blanching or scalding | [40] |
| Hydrolysis Methods | Food By-Product | Pretreatment | Protocol | Reference |
|---|---|---|---|---|
| Acid Hydrolysis | Apple, mango, papaya, pineapple, etc., left over | Freeze-dried. | 1.5 M HCl in 85% ethanol, extracted under refrigeration for 13 h in the dark. | [12] |
| Apple pomace | Liquid nitrogen dried and milled. Defatted. | Sequence extraction with acetone/water/acetic acid and acetone/8 M urea/acetic acid. Facilitated by microwave and purified by SPE 3 (C18 Sep-Pak cartridges). | [66] | |
| Apple waste | Freeze-dried. Extracted with 80% ethanol and 0.18 M HCl. | 200 mg sample (DW) extracted with 10 mL butanol/HCl/FeCl3 (97.5/2.5 v/v, 0.7 g) at 100 °C for 60 min. | [72] | |
| Blueberry leaves | Oven dried. Extracted with different concentration of methanol/water solvent. | 200 mg sample (DW) extracted with 10 mL butanol/HCl/FeCl3 (97.5/2.5 v/v, 0.7 g) at 100 °C for 60 min. 100 mg sample (DW) extracted with 10 mL methanol/H2SO4 (90:10 °C) at 85 °C for 20 h. | [54] | |
| Blueberry pomace | Freeze-dried. Extracted with acidic ethanol/water (50:50), pH adjusted to 2 by HCl. | Extracted with acidified water at pH = 2.0. Facilitated by PLE 4 (10.0 MPa, 80 °C for 30 min), UAE 5 (37 KHz, 580 W, for 90 min) and two methods combined. | [89] | |
| Date palm kernels | Extracted for 2.37 h at 43.23 °C in 75.39% methanol/ethanol concentration and 54.57 mL/g of solvent/sample ratio. | Acid hydrolysis with butanol/HCl (97.5:2.5, v/v) and heated at 100 °C. Continuously stirred for three hours. | [74] | |
| Fruit peels (apple, kiwi, banana, melon) | Freeze-dried. Extracted with acidic methanol/water (50:50), pH adjusted to 2 by HCl. Washed by acetone/water (70:30 v/v). | Acid hydrolysis with methanol and sulphuric acid for 20 h at 85 °C. Then subjected to SPE 5 treatment (Oasis HLB cartridges). Acid hydrolysis with butanol/HCl/FeCl3 at 100 °C for 1 h. | [3] | |
| Grape peels | Freeze-dried. Extracted with acidic methanol/water (50:50), pH adjusted to 2 by HCl. Washed by acetone/water (70:30 v/v). | Acid hydrolysis with HCl/butanol/FeCl3 (5:95 v/v containing 0.7 g/L FeCl3) solution in a boiling water bath for three hours. Acid hydrolysis with methanol/H2SO4 (90:10 v/v) at 85 °C under slight shaking for 20 h. Purified with a SPE-C18 cartridge. | [59] | |
| Grape pomace | Freeze-dried. Extracted with acidic methanol/water (50:50), pH adjusted to 2 by HCl. Washed by acetone/water (70:30 v/v). | Acid hydrolysis with methanol/H2SO4 (10:1 v/v) at 85 °C under slight shaking for 20 h. Acid hydrolysis with HCl/butanol (97.5:2.5 v/v with 0.1% FeCl3) solution at 100 °C for one hour. | [67] | |
| Grape seed | Air dried. Extracted with 60% methanol. | Acid hydrolysis with HCl/butanol (5:95 v/v containing 2% ferric reagent). Performed with liquid–liquid extraction. | [73] | |
| Mandarin waste | Oven dried. Extracted with acidic methanol/water (50:50), pH adjusted to 2 by HCl. Washed by acetone/water (70:30 v/v). | Acid hydrolysis with methanol/H2SO4 (90:10 v/v). | [55] | |
| Olive pomace | Freeze-dried. Defatted. Extracted with acidic methanol/water (50:50), pH adjusted to 2 by HCl. Washed by acetone/water (70:30 v/v). Lipid removed by hexanes. | Acid hydrolysis with methanol/H2S O4 for 20 h at 85 °C. Acid hydrolysis with butanol/HCl (95:5 v/v, containing FeCl3) at 100 °C for 1 h. | [20] | |
| Roselle by-products | Forced circulation dried. Extracted with acidic methanol/water (50:50), pH adjusted to 2 by HCl. Washed by acetone/water (70:30 v/v). | Acid hydrolysis with butanol/HCl (95:5 v/v containing 2% w/v NH4F e(SO4)2·12 H2O in 2 M HCl) at 100 °C for 1 h. | [56] | |
| Alkaline hydrolysis | Black rice bran | Air dried. Extracted with chilled acidified methanol (95% methanol:1 M HCl 85:15 v/v). Lipid removed with hexanes. | Alkaline hydrolysis with 2 M NaOH at room temperature for 1 h. | [61] |
| Brown rice bran | Extracted with 80% chilled acetone. Lipid removed by hexanes. | Alkaline hydrolysis with 2 M NaOH at room temperature for 1 h with continuous shaking under nitrogen gas. | [18] | |
| Cauliflower waste | Freeze-dried. Extracted with pure methanol. | Alkaline hydrolysis with 2 M NaOH for 15 min at 60 °C in a screw-capped test tube previously flushed with nitrogen. The extraction was facilitated with ultrasound (37 KHz, 180 W). The NEP went through SPE (C18 SPE cartridge) prior to analysis. | [78] | |
| Citrus peels | Sun dried. Extracted with 80% methanol. | Alkaline hydrolysis (4 M NaOH) at ambient temperature for 1 h. Facilitated with microwave (for heating) and ultrasound. | [14] | |
| Chestnut peel | Freeze-dried. | Optimise the alkaline hydrolysis condition with different concentrations of Na2S O3 and NaOH (from 1% to 8%) for a different period (from 30 to 960 min) at 85 °C. | [57] | |
| Cranberry pomace | Lipid removed by hexane. | Optimise the alkaline hydrolysis condition with NaOH (2 M, 4 M, and 6 M) with water shaking bath (200 rpm) at different times (from 5 min to 24 h) under 25, 40, or 60 °C. NEPs isolated with SPE (Sephadex LH-20). | [77] | |
| Mango peels | Extracted with 80% chilled acetone. Air dried. | Alkaline hydrolysis with 1 M NaOH (containing 0.5% NaBH4) under nitrogen atmosphere. | [81] | |
| Red cabbage and Brussels sprouts waste | Freeze-dried. Extracted with methanol. | Optimise the alkaline hydrolysis condition with NaOH with different temperatures and incubation time. Extraction facilitated with ultrasound. Purified with SPE (C18-cartridge). | [21] | |
| Rice bran | Air dried. Extracted with chilled acidified methanol (95% methanol:1 M HCl 85:15 v/v). Lipid removed with hexanes. | Alkaline hydrolysis with 2 M NaOH at ambient temperature for 1 h under nitrogen condition. | [19] | |
| Sorghum bran | Air dried. | Alkaline hydrolysis with 2 M NaOH facilitated by microwave oven (1400 W, 45 s). | [93] | |
| Sequential hydrolysis or acid and alkaline hydrolysis comparison study | Apple pomace | Freeze-dried. Extracted with methanol/water (80:20 v/v with 1% formic acid). | Multistep sequential extraction with acid, alkaline and combined hydrolysis (2 M NaOH and 2 M HCl). | [15] |
| Wheat bran | Freeze-dried. Extracted with acidic methanol/water (50:50), pH adjusted to 2 by HCl. Washed by acetone/water (70:30 v/v). | Acid (methanol/H2S O4 90:10 v/v at 85 °C for 20 h) and alkaline hydrolysis (2 M NaOH at room temperature for 4 h). | [17] | |
| Wheat bran | Defatted with hexane. Extracted with 80% methanol. | Acid (6 M HCl at 95 °C for 20 h) and alkaline (2 M NaOH at room temperature for 4 h) hydrolysis. | [79] | |
| Enzyme Hydrolysis | Black tea leftover | Washed thoroughly and dried under ambient condition. | Enzyme hydrolysis (Kemzyme, Alcalase, Pectinex, etc.). Applied ultrasound for degas. Facilitated by SFE 6. | [53] |
| Citrus peels | Liquid nitrogen dried and milled. | Enzyme hydrolysis with Cellulase MX, Cellulase CL, and Kleerase AFP. Purified by SPE (C18 Sep-Pak cartridge). | [62] | |
| Grape pomace | Oven-dried. | Enzyme hydrolysis with pectinase, cellulase, tannase, etc. | [16] | |
| Grape skin and seeds | Freeze-dried. | Enzyme hydrolysis with pectinase, cellulase, and tannase. | [82] | |
| Juice pomace | Freeze-dried. | Enzyme hydrolysis with Pectinases Macer 8 FJ, 8R, and Novozym 89 acid protease. | [63] | |
| White grape pomace | Oven-dried. | Enzyme hydrolysis with Pectinex 3X L, Pectinex Ultra SPL, Termamyl, Fungamyl, Pentopan, 500B G, and Celluclast. | [97] |
| Food By-Product | Featured Extraction/Purification Method | Characterisation (Spectrophotometry or Chromatography) | Antioxidant Capacity | Main Compounds | Reference |
|---|---|---|---|---|---|
| Apple, mango, papaya, pineapple etc., left over | Acid hydrolysis | TAC 7, TPC 8 | - | Cherry left over has the highest TPC (12,696.03 mg GAE 9/100 g DW 10), followed by cashew apple (6588.41 mg GAE/100g DW) and pineapple (2787.09 mg GAE/100g DW). | [12] |
| Apple pomace | Chemical hydrolysis | TPC, UHPLC 11-MS 12(Q- Exactive Orbitrap MS, ESI 13, 80–1200m/z), ORAC 14 | Highest antioxidant capacity achieved by alkaline hydrolysis (478.66 μmol
TE 15/g DW). | Sequence extraction with both acid and alkaline hydrolysis achieved the highest TPC (12.38–13.76 mg GAE/g DW). Quercetin-3-O-galactoside is the main compound identified by MS. | [15] |
| Apple pomace | Sequential extraction facilitated by microwave | UHPLC-DAD 16-ESI-MS (RP 17, Ultra ion trap MS, ESI, 100–1000 m/z) | - | Flavan-3-ols are the major class of polyphenols (2.88 g/kg of dry apple pomace), with an average DP 18 of 4.7. Main compounds after alkaline hydrolysis of apple procyanidins are 3,4-dihydroxybenzoic acid (0.67 M/kg) and catechol (0.15 M/kg). | [66] |
| Apple waste | Acid hydrolysis | TAC 19, TPC, DPPH 20, ABTS 21 | NEPs has a significantly higher rate of radical scavenging capacity than EEPs. | The NEPs content ranges from 18.40 to 23.48 mg anthocyanidins equivalent/100g DW, which occupies 64.07% of TPC in apple waste. | [72] |
| Black rice bran | Alkaline hydrolysis | TPC, TAC, ORAC | The antioxidant ability ranges from 47.91 to 79.48 μM of TE/g DW. | The NEPs content ranges from 221.2 to 382.7 mg GAE/100 g DW. | [61] |
| Black tea leftover | Enzyme hydrolysis, facilitated with SFE 22 | TPC, UHPLC-DAD- MS (RP, Triple quadrupole MS, ESI, 100–1200 m/z), DPPH, FRAP 23, ABTS | Enzymatically hydrolysed sample has liberated a greater amount of ABTS radical cations comparative to Trolox (1,156.56 ± 46.88 μM TE/g). | The optimised condition achieved 521.44 mg GAE/g of TPC during supercritical fluid based extraction. p-coumaric acid (208.33 μg/mL) is the major phenolic acid in the black tea left over. | [53] |
| Blueberry leaves | Acid hydrolysis | TPC, TAC, TPAC | - | The NEPs occupies 2.81% to 3.73% of TPC. The non-extractable proanthocyanidins content ranges from 10.06 to 11.69 mg/g with different extract conditions. | [54] |
| Blueberry pomace | Acid hydrolysis, facilitated by ultrasound | TPC, HPLC-MS (Q-TOF-MS, ESI, 100–800 m/z) | - | UAE 24 + PLE 25 is the most efficient method to extract polyphenols (8.54 mg GAE/g DW). Main compound after UAE is cyanidin-3-O-galactoside (0.32 mg/g DW). | [89] |
| Brown rice bran | Alkaline hydrolysis | TPC, HPLC-PDA (RP), FRAP. ORAC | The antioxidant ability of NEPs ranges from 207 to 267 mg of TE/100 g DW, which is significantly lower than EPPs (range from 452 to 589 mg TE/100 g DW). | The TPC after alkaline hydrolysis reaches 276 mg GAE/100 g DW. Ferulic acid (1,617 μg/g DW) and p-coumaric acid (394 μg/g DW) are the main compounds in NEPs after hydrolysis. | [18] |
| Cauliflower waste | Alkaline hydrolysis, facilitated with ultrasound | TPC, HPLC-ESI-MS (HDMS 26-TOF 27, ESI, 100-1500 Da) (qualify), HPLC-DAD (RP) (quantify) | - | Alkaline and UAE achieves the highest TPC extraction (7.3 ± 0.17 mg GAE/g DW). Kaempferol-3-O-diglucoside-7-O-glucoside is the most abundant flavonoid present in the NEP fraction (2.4 ± 0.1 mg/g DW). | [78] |
| Chestnut peel | Alkaline hydrolysis with Na2SO3 and NaOH | TPC, HPLC-DAD (RP) | - | The highest TPC achieved by extracting with 1% NaOH for 4 h (4,112.1 μg/g DW). The ellagic acid is the main compound after hydrolysis (3,542.6 μg/g DW). | [57] |
| Citrus peels | Acid and alkaline hydrolysis in a sequence | HPLC-PDA 28 (RP), DPPH | The antioxidant capacity of the extract increased with microwave power after alkaline hydrolysis (the maximise scavenging activity reaches 26.30%). | Microwave treatment of citrus peels cleaves and liberates phenolic compounds (maximum TPC is 3,583.5 μg/g DW). The ferulic acid is the main compound after hydrolysis (2,162.6 μg/g DW). | [14] |
| Citrus peels | Enzyme hydrolysis | TPC, FRAP, HPLC-PDA (RP) | Grapefruit has the highest total antioxidant activity (1.719 ± 0.075 mM FeSO4/100 g fresh peel). | The grapefruit peels contain the highest amount of TPC after enzyme hydrolysis, which vary from 90 to 162 mg GAE/100 g fresh peel. | [62] |
| Cranberry pomace | Alkaline hydrolysis | HPLC-DAD (NP 29), HPLC-MS (Q-ion trap MS, ESI), MALDI 30-TOF-MS (TOF-MS, MALDI) | - | Alkaline hydrolysis resulted in a 30% increase in total procyanidins compared to conventional extraction (1,685 mg/100 g DW and 1,292 mg/100 g DW, respectively). | [77] |
| Date palm kernels | Acid hydrolysis | TPC, TPAC 31, DPPH | The antioxidant capacity (IC50) ranges between 58.12 ± 3.32 and 70.5 ± 9.66 µg/mL. | Maximum extract yield of NEPs (14.2%) achieved at 85 °C extracted for 3 h with 1:20 solid to liquid ratio (g/mL). | [74] |
| Fruit peels (apple, kiwi, banana, melon) | Acid hydrolysis | TPC, HPLC-DAD (RP) | - | NEPs contribute from 7% (mango) to 82% (banana) of TPC. The highest concentration is 9.62 mg/g (banana) among fruit peels. Hydroxycinnamic acids are detected as main compounds in the hydrolysable part of polyphenols in melon, orange, and pear. | [3] |
| Grape peels | Acid hydrolysis | HPLC-PDA-MS/MS (RP, Q-TOF analyser, ESI,
100–1000m/z) | - | The major phenolic compounds found in the NEPs are procyanidin, syringic acid, p-coumaric acid, and hydroxybenzoic acid derivatives. | [59] |
| Grape pomace | Enzyme hydrolysis | TPC, HPLC-DAD (RP), ABTS | The highest antioxidant capacity is 5.58 mM TE/100 g DW. | With the optimised condition (188 U/g DW of cellulase and 198 U/g DW of tannase at 45 °C), the TPC content reaches 0.81 g GAE/100 g DW. Gallic acid is the main compound after hydrolysis (0.16 g/100 g DW). | [16] |
| Grape pomace | Acid hydrolysis | TPC, HPLC-ESI-MS (Q-TOF, ESI, 100-1200 Da &TOF, MALDI) | The antioxidant ability is 440 and 324 µM TE/g DW by FRAP and ABTS, respectively. | The NEPs occupy 14.4% of dry weight in grape skin while the EPPs occupy 3.5%. Dihydroxybenzoic acid is the main compound determined after hydrolysis (74.3 mg/100 g DW). | [67] |
| Grape seed | Acid hydrolysis, facilitated by SFE | TPC | - | The TPC ranges from 1.23 to 2.37 mg GAE/100 g DW. | [73] |
| Grape skin and seeds | Enzyme hydrolysis | TPC, HPLC-DAD (RP) | - | The three enzymes used individually are able to increase the total phenol release of grape seed by 1.26, 1.32, and 1.34 times, respectively. | [82] |
| Juice pomace | Enzyme hydrolysis | TPC | - | The maximum TPC yield achieved when adding 10% Macer8 FJ and Grindamyl pectinase enzyme (383 mg GAE/L DW). | [63] |
| Mandarin waste | Acid hydrolysis | HPLC-DAD (RF), ABTS, ORAC, DPPH, FRAP | The antioxidant ability ranges from 333.43 to 351.55 (μM TE/g DW). | The highest TPC achieved when the sample was dried at 120 °C (74.56 mg GAE/g DW). Non-extractable hesperidin occupies 35.7% of phenolics in fresh mandarin waste. | [55] |
| Mango peels | Acid hydrolysis | HPLC-DAD (RP) | - | The bound phenolic content in mango peel dietary fibre ranges from 8.12 to 29.52 mg/g, while the bound flavonoids content ranges from 0.101 to 0.392 mg/g. Gallic acid is the major phenolic acid in both raw and ripe mango peel (6.29 and 16.60 mg/g DW, respectively). | [81] |
| Olive pomace | Acid hydrolysis | TPC, HPLC-PDA(RP) | - | More NEPs released with granulometric fractionised and micronised samples (maximum 13.2 g GAE/100 g DW). | [20] |
| Red cabbage and Brussels sprouts waste | Alkaline hydrolysis, facilitated by ultrasound | TPC, HPLC-MS (HDMS-TOF, ESI, 100-1500 Da) | - | The maximised condition to extract NEPs from red cabbage waste achieved when extracting with 4 M NaOH at 80 °C for 40 min (7.8 mg GAE/g DW). | [21] |
| Rice bran | Alkaline hydrolysis | TPC, HPLC-PDA (RP), FRAP, ABTS, ORAC | The IC50 values of the bound fraction ranged from 78.7 to 153.6 mg TE/100 g DW with the percentage contribution to the total antioxidant ability ranging from 10.5% to 21.1% by ORAC assay. | The bound phenolic content ranges from 91.1 to 126.8 mg GAE/100 g with the percentage contribution to the total ranging from 10.8% to 14.5%. Ferulic acid is the main component after hydrolysis (1.24 mg/g DW). | [19] |
| Roselle by-products | Acid hydrolysis | TPC, UHPLC-MS (Q-TOF, ESI, 50–1800 Da) | - | The hydrolysable polyphenols and proanthocyanidins content of roselle calyx by-products are 6.18 mg GAE/g and 6.67 mg proanthocyanidins equivalent/g. The NEPs content occupied 71.2% of the TPC. | [56] |
| Sorghum bran | Alkaline hydrolysis | HPLC-PDA (RP), HPLC-MS (Q-TOF-MS,100–1500 m/z) | - | The ferulic acid and p-coumaric acid are the main compounds (1,189 and 179 μg/g DW) in maize bran. | [93] |
| Wheat bran | Acid and alkaline hydrolysis | HPLC-MS -DAD (RP, ESI, 100–1000 m/z) | - | Caffeic, ferulic, and cinnamic acids are the main hydroxycinnamic acids in bran (43% of total hydrolysable polyphenols). | [17] |
| Wheat bran | Acid and alkaline hydrolysis | TPC, HPLC-DAD (RP) | - | The TPC content of wheat bran ranges from 654 to 2,326 μg GAE/g. Ferulic acid is the predominant phenolic acid after acid hydrolysis. | [79] |
| White grape pomace | Enzyme hydrolysis | TPC, HPLC-DAD (RP), ABTS | The highest antioxidant activity is detected in the dried powder extraction for 6 h with 2% Celluclast at 37 °C (7.82 g ascorbic acid equivalent/L). | For wet pomace, the optimised TPC extraction condition reaches at 2 h of incubation with enzyme hydrolysis (1,316 mg GAE/L). For dry pomace, the incubation time poses no effect to the TPC content (2,636 mg GAE/L). | [97] |
| Food By-Products | Final Product | Purpose | Reference |
|---|---|---|---|
| Fruit pulps residual | Functional beverage, juice, milkshake, etc. | As antioxidant ingredients with low calorie content (<20 kcal/100 g) | [113] |
| Fruit peels | Polymer proanthocyanidins in different food varieties | Food supplements, functional ingredient, pharmaceutical or cosmetic products | [114] |
| Black rice bran | Different food varieties | As food colourants | [61] |
| Grape skin | Yogurt products | Increase the TPC without sensory and storage changes | [8] |
| Dried grape pomace | Yogurt products | Accelerate the milk fermentation, increase antioxidant ability | [117] |
| Grape dietary fiber | Meagre sausage | Increase nutritional value | [119] |
| Grape pomace powder | Minced fish muscle | Increase water and oil retention capacity | [118] |
| Corn bran, dried apple, and tomato pomace | Chicken sausage | As microbiological preservative | [120] |
| Apple pomace | Sorghum and maize flour blends | Increase the content of bioactive compounds | [121] |
| Berry pomace | Wheat flour dough | Improve microstructure and texture | [122,123] |
| Grape or blueberry pomace | Cookies | Increase TPC | [124] |
| White grape pomace | Wheat biscuits | Increase texture and total dietary fibre | [125] |
| Blueberry and grape seed powder | Biscuits | Increase antioxidant ability, increase TPC | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Morozova, K.; Scampicchio, M.; Ferrentino, G. Non-Extractable Polyphenols from Food By-Products: Current Knowledge on Recovery, Characterisation, and Potential Applications. Processes 2020, 8, 925. https://doi.org/10.3390/pr8080925
Ding Y, Morozova K, Scampicchio M, Ferrentino G. Non-Extractable Polyphenols from Food By-Products: Current Knowledge on Recovery, Characterisation, and Potential Applications. Processes. 2020; 8(8):925. https://doi.org/10.3390/pr8080925
Chicago/Turabian StyleDing, Yubin, Ksenia Morozova, Matteo Scampicchio, and Giovanna Ferrentino. 2020. "Non-Extractable Polyphenols from Food By-Products: Current Knowledge on Recovery, Characterisation, and Potential Applications" Processes 8, no. 8: 925. https://doi.org/10.3390/pr8080925
APA StyleDing, Y., Morozova, K., Scampicchio, M., & Ferrentino, G. (2020). Non-Extractable Polyphenols from Food By-Products: Current Knowledge on Recovery, Characterisation, and Potential Applications. Processes, 8(8), 925. https://doi.org/10.3390/pr8080925