Design, Fundamental Principles of Fabrication and Applications of Microreactors
Abstract
:1. Introduction
2. Construction of a Microreactor
2.1. Materials Used in Microreactor Construction
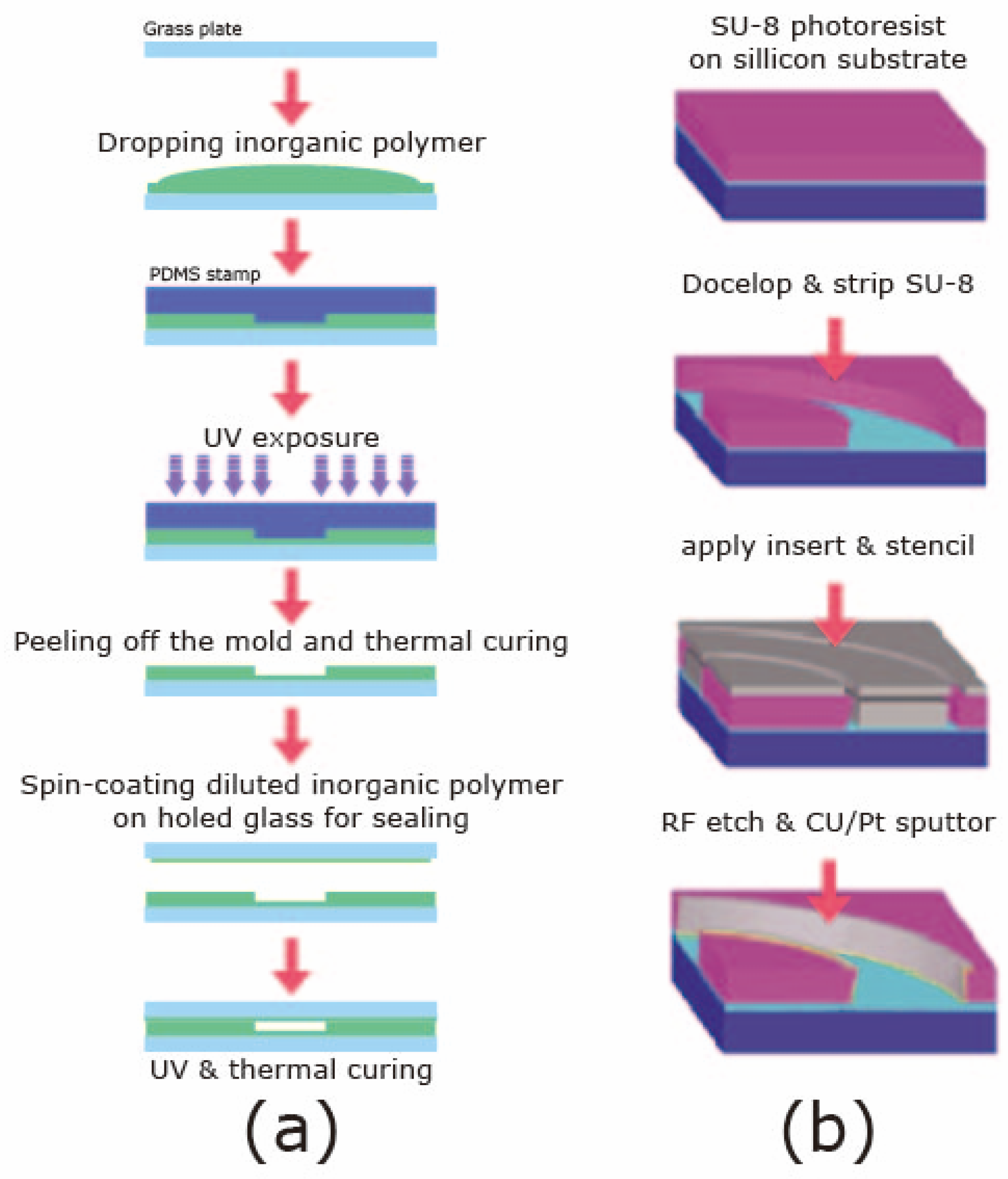
2.2. Manufacturing Methods of Microreactors
2.2.1. Etching Methods for Microreactor Construction
2.2.2. Micromachining Method for Microconstruction
2.2.3. Lithography, Electroplating, and Molding or Lithography, Galvanoforming, and Abforming (LIGA) Methods
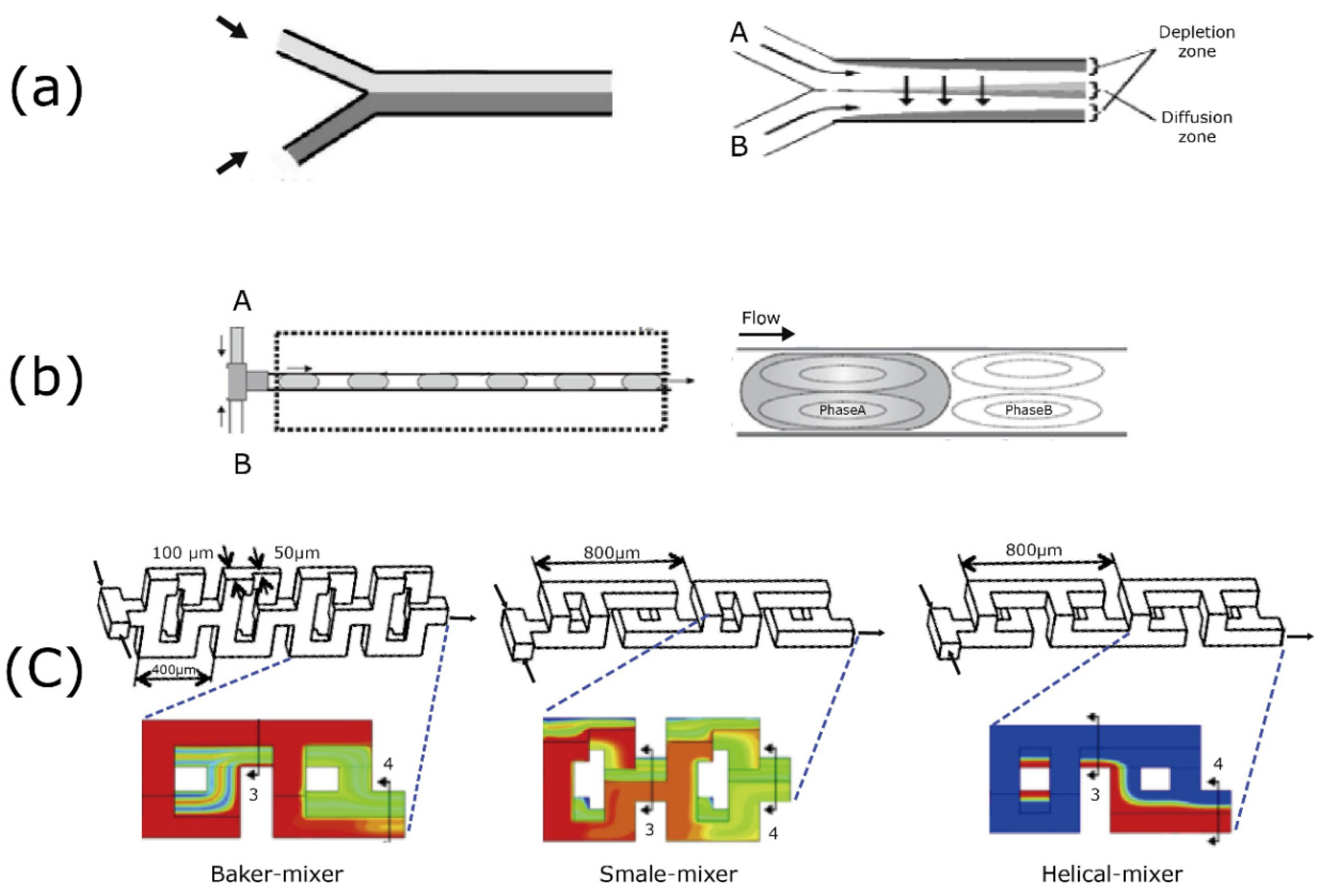
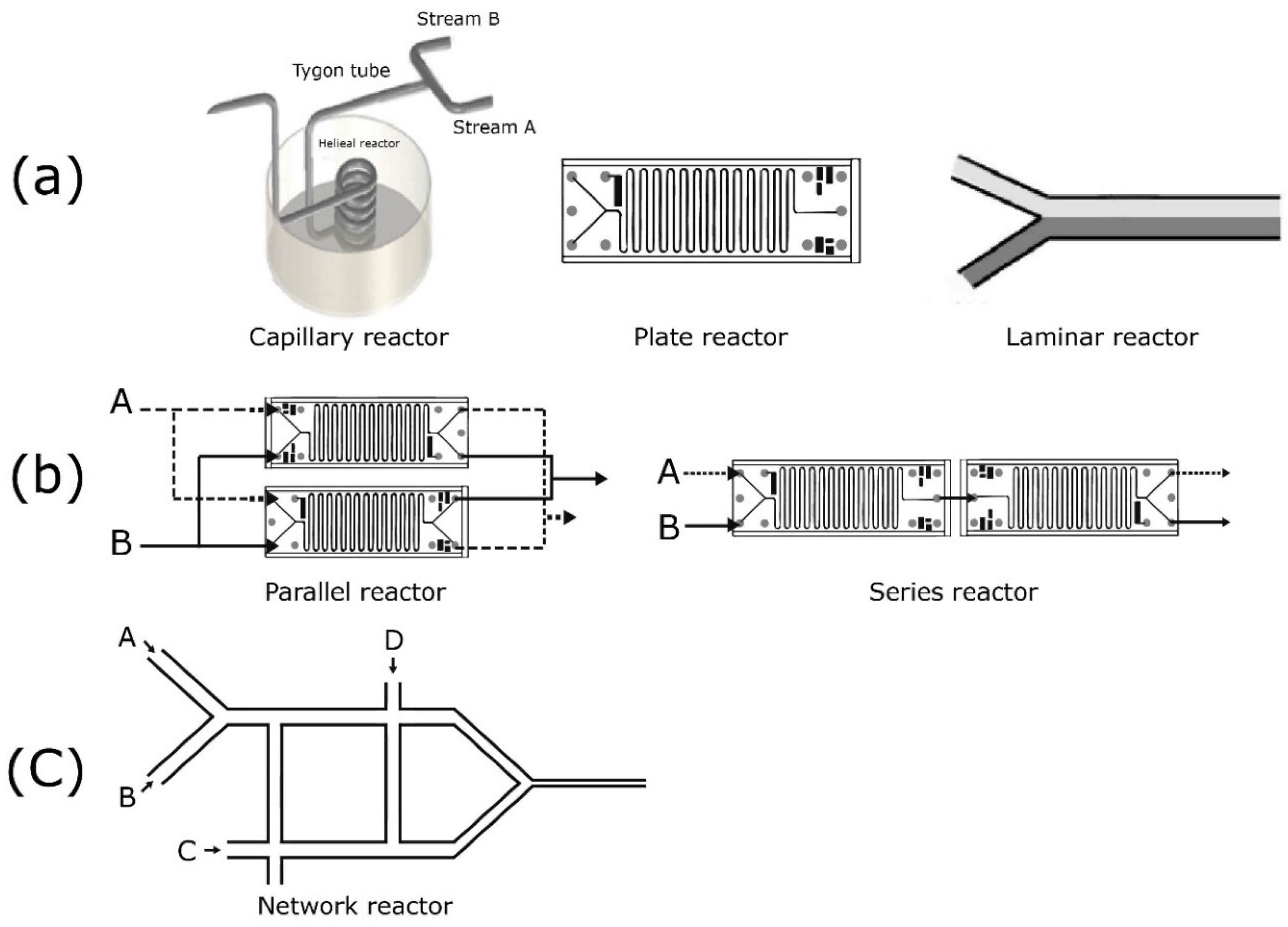
3. Schematic Fundamentals and Approaches in the Microreactor’s Design
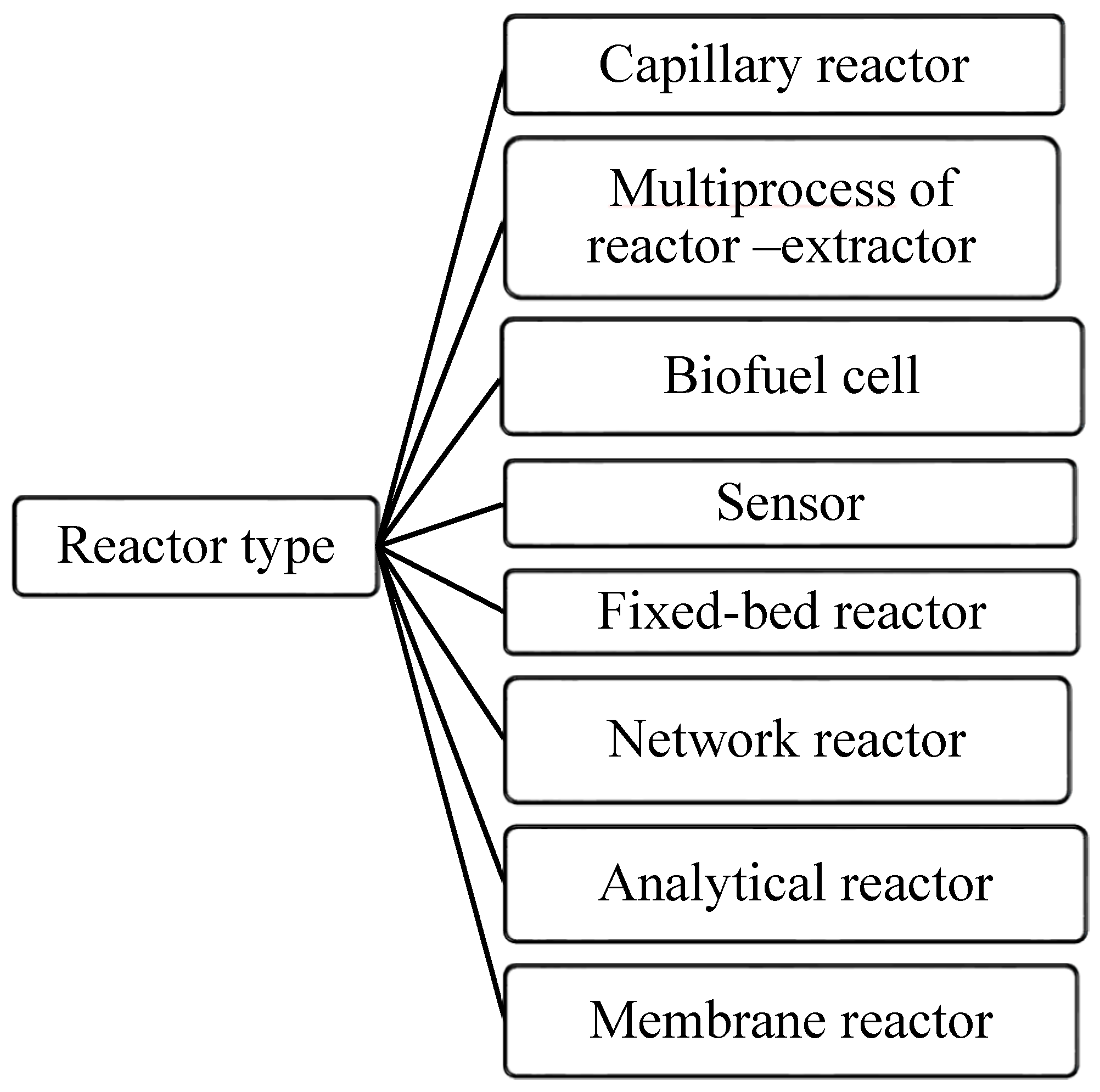
4. Innovative Solicitations of Microreactor
4.1. Synthesis of Chemical and (Bio)Polymers
4.2. Microreactors for Biological and Pharmaceutical Applications
4.3. Nanoparticle Synthesis Using Microreactors
4.3.1. Microfluidic Synthesis Schemes of Nanoparticles
Continuous Flow Microreactors
Gas–Liquid Segmented Microfluidic Microreactors
4.4. Numerical Models of Microreactors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoshida, J.I.; Takahashi, Y.; Nagaki, A. Flash chemistry: Flow chemistry that cannot be done in batch. Chem. Commun. 2013, 49, 9896–9904. [Google Scholar] [CrossRef] [Green Version]
- Baraldi, P.T.; Hessel, V. Micro reactor and flow chemistry for industrial applications in drug discovery and development. Green Process. Synth. 2012, 1, 149–167. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer based micro-reactors. Rev. Mol. Biotechnol. 2001, 82, 89–99. [Google Scholar] [CrossRef]
- Bothe, D.; Stemich, C.; Warnecke, H.J. Fluid mixing in a T-shaped micro-mixer. Chem. Eng. Sci. 2006, 61, 2950–2958. [Google Scholar] [CrossRef]
- Haswell, S.J.; Middleton, R.J.; O’Sullivan, B.; Skelton, V.; Watts, P.; Styring, P. The application of micro reactors to synthetic chemistry. Chem. Commun. 2001, 43, 391–398. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Fletcher, P.D.; Haswell, S.J.; Pombo-Villar, E.; Warrington, B.H.; Watts, P.; Wong, S.Y.; Zhang, X. Micro reactors: Principles and applications in organic synthesis. Tetrahedron 2002, 58, 4735–4757. [Google Scholar] [CrossRef] [Green Version]
- Pennemann, H.; Kolb, G. Review: Microstructured reactors as efficient tool for the operation of selective oxidation reactions. Catal. Today 2016, 278, 3–21. [Google Scholar] [CrossRef]
- Plouffe, P.; Roberge, D.M.; Macchi, A. Liquid–liquid flow regimes and mass transfer in various micro-reactors. Chem. Eng. J. 2016, 300, 9–19. [Google Scholar] [CrossRef]
- Rizkin, B.A.; Popovic, F.G.; Hartman, R.L. Review article: Spectroscopic microreactors for heterogeneous catalysis. J. Vac. Sci. Technol. A 2019, 37, 050801. [Google Scholar] [CrossRef] [Green Version]
- Gavriilidis, A.; Angeli, P.; Cao, E.; Yeong, K.K.; Wan, Y.S.S. Technology and applications of microengineered reactors. Chem. Eng. Res. Design 2002, 80, 3–30. [Google Scholar] [CrossRef]
- Haswell, S.J.; Watts, P. Green chemistry: Synthesis in micro reactors. Green Chem. 2003, 5, 240–249. [Google Scholar] [CrossRef]
- Kundu, A.; Ahn, J.E.; Park, S.S.; Shul, Y.G.; Han, H.S. Process intensification by micro-channel reactor for steam reforming of methanol. Chem. Eng. J. 2008, 135, 113–119. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.; Luo, L.; Bellettre, J.; Yue, J. Preparation of Pt/γ-Al2O3 catalyst coating in microreactors for catalytic methane combustion. Chem. Eng. J. 2020, 380, 122424. [Google Scholar] [CrossRef]
- NÉmethnÉ-SÓVÁGÓ, J.; BENKE, M. Micro-reactors: A new concept for chemical synthesis and technological feasibility. Mater. Sci. Eng. 2014, 39, 89–101. [Google Scholar]
- Plouffe, P.; Bittel, M.; Sieber, J.; Roberge, D.M.; Macchi, A. On the scale-up of micro-reactors for liquid–liquid reactions. Chem. Eng. Sci. 2016, 143, 216–225. [Google Scholar] [CrossRef]
- Wohlgemuth, R.; Plazl, I.; Žnidaršič-Plazl, P.; Gernaey, K.V.; Woodley, J.M. Microscale technology and biocatalytic processes: Opportunities and challenges for synthesis. Trends Biotechnol. 2015, 33, 302–314. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Bau, H.; Gorte, R.J. Fabrication of micro-reactors using tape-casting methods. Catal. Lett. 2001, 77, 173–177. [Google Scholar] [CrossRef]
- Zebda, A.; Innocent, C.; Renaud, L.; Certin, M.; Pichot, F.; Ferrigno, R.; Tingry, S. Enzyme-Based Microfluidic Biofuel Cell to Generate Micropower in Biofuel’s Engineering Process Technology; IntechOpen Limited: London, UK, 2011; p. 564. [Google Scholar]
- Kjeang, E.; Sinton, D.; Harrington, D.A. Strategic enzyme patterning for microfluidic biofuel cells. J. Power Sources 2006, 158, 1–12. [Google Scholar] [CrossRef]
- Kjeang, E.; Djilali, N.; Sinton, D. Microfluidic fuel cells: A review. J. Power Sources 2009, 186, 353–369. [Google Scholar] [CrossRef]
- Love, J.C.; Anderson, J.R.; Whitesides, G.M. Fabrication of three-dimensional microfluidic systems by soft lithography. Mrs Bulletin 2001, 26, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef]
- Turkenburg, D.H.; Fischer, H.R. Diels-Alder based, thermo-reversible cross-linked epoxies for use in self-healing composites. Polymer 2015, 79, 187–194. [Google Scholar] [CrossRef]
- Shukla, A.K.; Raman, R.K.; Scott, K. Advances in mixed-reactant fuel cells. Fuel Cells 2005, 5, 436–447. [Google Scholar] [CrossRef]
- Bullen, R.A.; Arnot, T.C.; Lakeman, J.B.; Walsh, F.C. Biofuel cells and their development. Biosens. Bioelectron. 2006, 21, 2015–2045. [Google Scholar] [CrossRef] [Green Version]
- Volpatti, L.R.; Yetisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef]
- Krishna, K.S.; Li, Y.; Li, S.; Kumar, C.S.S.R. Lab-on-a-chip synthesis of inorganic nanomaterials and quantum dots for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 1470–1495. [Google Scholar] [CrossRef]
- Phillips, T.W.; Lignos, I.G.; Maceiczyk, R.M.; deMello, A.J.; deMello, J.C. Nanocrystal synthesis in microfluidic reactors: Where next? Lab Chip 2014, 14, 3172–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolliffe, H.G.; Gerogiorgis, D.I. Process modelling and simulation for continuous pharmaceutical manufacturing of ibuprofen. Chem. Eng. Res. Design 2015, 97, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, I.; Compagnoni, M. Chemical reaction engineering, process design and scale-up issues at the frontier of synthesis: Flow chemistry. Chem. Eng. J. 2016, 296, 56–70. [Google Scholar] [CrossRef]
- Lo, I.C.; Wu, H.S. Methanol formation from carbon dioxide hydrogenation using Cu/ZnO/Al2O3 catalyst. J. Taiwan Inst. Chem. Eng. 2019, 98, 124–131. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, G.; Zhang, Q.; Liu, B.; Zhang, L. Magnesium modified mesh-type Cu/γ-Al2O3/Al catalysts: Low acid density catalysts for methanol steam reforming. Catal. Lett. 2020. [Google Scholar] [CrossRef]
- Yen, B.K.H.; Stott, N.E.; Jensen, K.F.; Bawendi, M.G. A continuous-flow microcapillary reactor for the preparation of a size series of CdSe nanocrystals. Adv. Mater. 2003, 15, 1858–1862. [Google Scholar] [CrossRef]
- Cherkasov, N.; Al-Rawashdeh, M.m.; Ibhadon, A.O.; Rebrov, E.V. Scale up study of capillary microreactors in solvent-free semihydrogenation of 2-methyl-3-butyn-2-ol. Catal. Today 2016, 273, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, D.E.; Ley, S.V. Engineering chemistry: Integrating batch and flow reactions on a single, automated reactor platform. React. Chem. Eng. 2016, 1, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.F.; Reizman, B.J.; Newman, S.G. Tools for chemical synthesis in microsystems. Lab Chip 2014, 14, 3206–3212. [Google Scholar] [CrossRef] [Green Version]
- Kralj, J.G.; Sahoo, H.R.; Jensen, K.F. Integrated continuous microfluidic liquid–liquid extraction. Lab Chip 2007, 7, 256–263. [Google Scholar] [CrossRef]
- Honda, T.; Miyazaki, M.; Yamaguchi, Y.; Nakamura, H.; Maeda, H. Integrated microreaction system for optical resolution of racemic amino acids. Lab Chip 2007, 7, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Šalić, A.; Tušek, A.; Zelić, B. Application of microreactors in medicine and biomedicine. J. Appl. Biomed. 2012, 10, 137–153. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhang, Y.; Du, L.; Liu, J.; Yao, J. Review of the applications of microreactors. Renew. Sustain. Energy Rev. 2015, 47, 519–539. [Google Scholar] [CrossRef]
- Yue, J. Multiphase flow processing in microreactors combined with heterogeneous catalysis for efficient and sustainable chemical synthesis. Catal. Today 2018, 308, 3–19. [Google Scholar] [CrossRef]
- Urban, S.; Kieninger, J.; Deschner, B.J.; Kraut, M.; Dittmeyer, R.; Urban, G.A.; Weltin, A. Multiparametric, spatially resolved detection of H2O2 and O2 with electrochemical microsensor array in synthesis membrane microreactors. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin/Heidelbrg, Germany, 23–27 June 2019; pp. 1297–1300. [Google Scholar]
- Chucherd, N.; Pavarajarn, V. Reactive extraction of metal ion and simultaneous stripping of nanofibers-supported liquid membrane in a microchannel. In Proceedings of the 2019 Pure and Applied Chemistry International Conference, Flic-en-Flac, Mauritius, 6–10 July 2020. [Google Scholar]
- Jensen, K.F. Flow chemistry—Microreaction technology comes of age. AIChE J. 2017, 63, 858–869. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. An online immobilized α-glucosidase microreactor for enzyme kinetics and inhibition assays. RSC Adv. 2015, 5, 56841–56847. [Google Scholar] [CrossRef]
- Martínez-Cisneros, C.S.; Pedro, S.G.-d.; Puyol, M.; García-García, J.; Alonso-Chamarro, J. Design, fabrication and characterization of microreactors for high temperature syntheses. Chem. Eng. J. 2012, 211-212, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Perumal, J.; Wang, J.; Wang, H.; Sharma, S.; Kim, D.-P. Whole ceramic-like microreactors from inorganic polymers for high temperature or/and high pressure chemical syntheses. Lab Chip 2014, 14, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Shang, M.; Noël, T.; Su, Y.; Hessel, V. Kinetic study of hydrogen peroxide decomposition at high temperatures and concentrations in two capillary microreactors. AIChE J. 2017, 63, 689–697. [Google Scholar] [CrossRef]
- Saksena, P.; Tadigadapa, S.; Yetter, R.A. Design, fabrication and analysis of stagnation flow microreactors used to study hypergolic reactions. Lab Chip 2015, 15, 2248–2257. [Google Scholar] [CrossRef]
- Floyd, T.M.; Losey, M.W.; Firebaugh, S.L.; Jensen, K.F.; Schmidt, M.A. Novel liquid phase microreactors for safe production of hazardous specialty chemicals. In Microreaction technology: Industrial Prospects; Springer: Berlin/Heidelberg, Germany, 2000; pp. 171–180. [Google Scholar]
- Srinivasan, R.; Hsing, I.-M.; Berger, P.E.; Jensen, K.F.; Firebaugh, S.L.; Schmidt, M.A.; Harold, M.P.; Lerou, J.J.; Ryley, J.F. Micromachined reactors for catalytic partial oxidation reactions. AIChE J. 1997, 43, 3059–3069. [Google Scholar] [CrossRef]
- Azouz, A.B.; Murphy, S.; Karazi, S.; Vázquez, M.; Brabazon, D. Fast fabrication process of microfluidic devices based on cyclic olefin copolymer. Mater. Manuf. Processes 2014, 29, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Fouillet, Y.; Parent, C.; Gropplero, G.; Davoust, L.; Achard, J.L.; Revol-Cavalier, F.; Verplanck, N. Stretchable material for microfluidic applications. Proceedings 2017, 1, 501. [Google Scholar] [CrossRef] [Green Version]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Kucuk, I.; Edirisinghe, M. Microfluidic preparation of polymer nanospheres. J. Nanoparticle Res. 2014, 16, 2626. [Google Scholar] [CrossRef] [Green Version]
- Zilio, C.; Sola, L.; Damin, F.; Faggioni, L.; Chiari, M. Universal hydrophilic coating of thermoplastic polymers currently used in microfluidics. Biomed. Microdevices 2014, 16, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.; Shin, W.C.; Besser, R.S. Novel microfabrication approaches for directly patterning PEM fuel cell membranes. J. Power Sources 2003, 123, 172–181. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2013; Volume 2. [Google Scholar]
- Tiggelaar, R.M.; Benito-López, F.; Hermes, D.C.; Rathgen, H.; Egberink, R.J.M.; Mugele, F.G.; Reinhoudt, D.N.; van den Berg, A.; Verboom, W.; Gardeniers, H.J.G.E. Fabrication, mechanical testing and application of high-pressure glass microreactor chips. Chem. Eng. J. 2007, 131, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Leng, C.; Du, G. Polyvinyl alcohol/fulvic acid composite adsorbent gel prepared by microreactor. Earth Environ. Sci. 2019, 300, 052006. [Google Scholar] [CrossRef]
- Fath, V.; Szmais, S.; Lau, P.; Kockmann, N.; Röder, T. Model-based scale-up predictions: From micro to millireactors using inline FT-IR spectroscopy. Org. Process Res. Dev. 2019, 23, 2020–2030. [Google Scholar] [CrossRef]
- Fletcher, P.D.; Haswell, S.J.; Zhang, X. Electrical currents and liquid flow rates in micro-reactors. Lab Chip 2001, 1, 115–121. [Google Scholar] [CrossRef]
- Hessel, V. From microreactor design to microreactor process design. Chem. Eng. Technol. 2005, 28, 243. [Google Scholar] [CrossRef]
- Vican, J.; Gajdeczko, B.F.; Dryer, F.L.; Milius, D.L.; Aksay, I.A.; Yetter, R.A. Development of a microreactor as a thermal source for microelectromechanical systems power generation. Proc. Combust. Inst. 2002, 29, 909–916. [Google Scholar] [CrossRef]
- Sharada, S.; Suryawanshi, P.L.; Kumar, P.R.; Gumfekar, S.P.; Narsaiah, T.B.; Sonawane, S.H. Synthesis of palladium nanoparticles using continuous flow microreactor. Colloids Surf. A 2016, 498, 297–304. [Google Scholar] [CrossRef]
- Sun, L.; Luan, W.; Shan, Y.; Tu, S.-T. One-step synthesis of monodisperse Au–Ag alloy nanoparticles in a microreaction system. Chem. Eng. J. 2012, 189, 451–455. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer microfluidics: Simple, low-cost fabrication process bridging academic lab research to commercialized production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Friend, J.; Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 2010, 4, 026502. [Google Scholar] [CrossRef] [Green Version]
- Eddings, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J. Micromechanics Microengineering 2008, 18, 067001. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Tor, S.B.; Loh, N.H.; Wong, T.N.; Gañán-Calvo, A.M.; Tan, S.H.; Nguyen, N.-T. Acoustofluidic control of bubble size in microfluidic flow-focusing configuration. Lab Chip 2015, 15, 996–999. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.H.; Nguyen, N.T.; Chua, Y.C.; Kang, T.G. Oxygen plasma treatment for reducing hydrophobicity of a sealed polydimethylsiloxane microchannel. Biomicrofluidics 2010, 4, 032204. [Google Scholar] [CrossRef] [Green Version]
- Yoon, T.-H.; Park, S.-H.; Min, K.-I.; Zhang, X.; Haswell, S.; Kim, D. Novel inorganic polymer derived microreactors for organic microchemistry applications. Lab Chip 2008, 8, 1454–1459. [Google Scholar] [CrossRef] [Green Version]
- West, J.; Karamata, B.; Lillis, B.; Gleeson, J.; Alderman, J.; Collins, J.; Lane, B.; Mathewson, A.; Berney, H. Application of magnetohydrodynamic actuation to continuous flow chemistry. Lab Chip 2002, 2, 224–230. [Google Scholar] [CrossRef]
- Mills, P.L.; Nicole, J.F. Multiple automated reactor systems (MARS). 2. effect of microreactor configurations on homogeneous gas-phase and wall-catalyzed reactions for 1,3-butadiene oxidation. Ind. Eng. Chem. Res. 2005, 44, 6453–6465. [Google Scholar] [CrossRef]
- Watts, P.; Haswell, S.J. The application of micro reactors for organic synthesis. Chem. Soc. Rev. 2005, 34, 235–246. [Google Scholar] [CrossRef]
- Tiwari, A.; Rajesh, V.M.; Yadav, S. Biodiesel production in micro-reactors: A review. Energy Sustain. Dev. 2018, 43, 143–161. [Google Scholar] [CrossRef]
- Knitter, R.; Göhring, D.; Risthaus, P.; Haußelt, J. Microfabrication of ceramic microreactors. Microsyst. Technol. 2001, 7, 85–90. [Google Scholar] [CrossRef]
- Acikgoz, C.; Hempenius, M.A.; Huskens, J.; Vancso, G.J. Polymers in conventional and alternative lithography for the fabrication of nanostructures. Eur. Polym. J. 2011, 47, 2033–2052. [Google Scholar] [CrossRef] [Green Version]
- Jena, R.K.; Yue, C.Y. Cyclic olefin copolymer based microfluidic devices for biochip applications: Ultraviolet surface grafting using 2-methacryloyloxyethyl phosphorylcholine. Biomicrofluidics 2012, 6, 012822. [Google Scholar] [CrossRef] [Green Version]
- Konstantinou, D.; Shirazi, A.; Sadri, A.; Young, E.W.K. Combined hot embossing and milling for medium volume production of thermoplastic microfluidic devices. Sens. Actuators B 2016, 234, 209–221. [Google Scholar] [CrossRef]
- Pattekar, A.V.; Kothare, M.V. A microreactor for hydrogen production in micro fuel cell applications. J. Microelectromech. Syst. 2004, 13, 7–18. [Google Scholar] [CrossRef]
- Plouffe, P. Micro-Reactor Design for Fast Liquid Liquid Reactions. Ph.D. Thesis, University of Ottawa, Ottawa, ON, Canada, 2015. [Google Scholar]
- Urban, P.L.; Goodall, D.M.; Bruce, N.C. Enzymatic microreactors in chemical analysis and kinetic studies. Biotechnol. Adv. 2006, 24, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Waterkamp, D.A.; Heiland, M.; Schlüter, M.; Sauvageau, J.C.; Beyersdorff, T.; Thöming, J. Synthesis of ionic liquids in micro-reactors—A process intensification study. Green Chem. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Cheemalapati, S.; Ladanov, M.; Winskas, J.; Pyayt, A. Optimization of dry etching parameters for fabrication of polysilicon waveguides with smooth sidewall using a capacitively coupled plasma reactor. Appl. Opt. 2014, 53, 5745–5749. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, Y.; Kondo, Y.; Sekine, M.; Ishikawa, K.; Hayashi, T.; Takeda, K.; Kondo, H.; Yamazaki, A.; Ito, A.; Matsumoto, H.; et al. Highly Selective Etching of SiO2over Si3N4and Si in Capacitively Coupled Plasma Employing C5HF7Gas. Jpn. J. Appl. Phys. 2013, 52, 016201. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.D.; Beebe, D.J.; Young, E.W.K. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [Green Version]
- Roberge, D. Lonza—Hazardous flow chemistry for streamlined large scale synthesis. Green Process. Synth. 2012, 1, 129. [Google Scholar] [CrossRef]
- Pileni, M.P. Reverse micelles as microreactors. J. Phys. Chem. 1993, 97, 6961–6973. [Google Scholar] [CrossRef]
- Zeng, D.; Pan, M.; Wang, L.; Tang, Y. Fabrication and characteristics of cube-post microreactors for methanol steam reforming. Appl. Energy 2012, 91, 208–213. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Z. Screening of cathepsin B inhibitors in traditional Chinese medicine by capillary electrophoresis with immobilized enzyme microreactor. J. Pharm. Biomed. Anal. 2019, 176, 112811. [Google Scholar] [CrossRef]
- Li, Y.; Yan, L.; Liu, Y.; Qian, K.; Liu, B.; Yang, P.; Liu, B. High-efficiency nano/micro-reactors for protein analysis. RSC Adv. 2015, 5, 1331–1342. [Google Scholar] [CrossRef]
- Muhler, M.; Schlögl, R.; Eder, S.; Ertl, G. Design of a continuous flow microreactor attached to a surface analysis system: First results with an iron oxide based catalyst. Surf. Sci. 1987, 189-190, 69–79. [Google Scholar] [CrossRef]
- Kolb, G. Review: Microstructured reactors for distributed and renewable production of fuels and electrical energy. Chem. Eng. Process. Process Intensif. 2013, 65, 1–44. [Google Scholar] [CrossRef]
- Effenhauser, C.S.; Bruin, G.J.M.; Paulus, A.; Ehrat, M. Integrated capillary electrophoresis on flexible silicone microdevices: Analysis of dna restriction fragments and detection of single DNA molecules on microchips. Anal. Chem. 1997, 69, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Gambhire, S.; Patel, N.; Gambhire, G.; Kale, S. A Review on different micromixers and its micromixing within microchannel. Int. J. Curr. Eng. Technol. 2016, 4, 409–413. [Google Scholar]
- Ober, T.J.; Foresti, D.; Lewis, J.A. Active mixing of complex fluids at the microscale. Proc. Natl. Acad. Sci. USA 2015, 112, 12293–12298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanović, J.; Rebrov, E.V.; Nijhuis, T.A.; Kreutzer, M.T.; Hessel, V.; Schouten, J.C. Liquid–liquid flow in a capillary microreactor: Hydrodynamic flow patterns and extraction performance. Ind. Eng. Chem. Res. 2012, 51, 1015–1026. [Google Scholar] [CrossRef]
- Farshchian, B.; Amirsadeghi, A.; Choi, J.; Park, D.S.; Kim, N.; Park, S. 3D nanomolding and fluid mixing in micromixers with micro-patterned microchannel walls. Nano Converg. 2017, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.; Falke, F.H.; Schouten, J.C.; Nijhuis, T.A. Microreactors with integrated UV/Vis spectroscopic detection for online process analysis under segmented flow. Lab Chip 2013, 13, 4855–4863. [Google Scholar] [CrossRef] [Green Version]
- Gañán-Calvo, A.M.; Gordillo, J.M. Perfectly monodisperse microbubbling by capillary flow focusing. Phys. Rev. Lett. 2001, 87, 274501. [Google Scholar] [CrossRef]
- Machsun, A.L.; Gozan, M.; Nasikin, M.; Setyahadi, S.; Yoo, Y.J. Membrane microreactor in biocatalytic transesterification of triolein for biodiesel production. Biotechnol. Bioprocess Eng. 2010, 15, 911–916. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Wang, J.; Xu, J.-J.; Chen, H.-Y. Energy transfer between CdS quantum dots and Au nanoparticles in photoelectrochemical detection. Chem. Commun. 2011, 47, 10990–10992. [Google Scholar] [CrossRef] [PubMed]
- Karande, R.; Schmid, A.; Buehler, K. Miniaturizing biocatalysis: Enzyme-catalyzed reactions in an aqueous/organic segmented flow capillary microreactor. Adv. Synth. Catal. 2011, 353, 2511–2521. [Google Scholar] [CrossRef]
- Marques, P.; Gonçalves, G.; Cruz, S.; Almeida, N.; Singh, M.; Grácio, J.; Sousa, A. Functionalized graphene nanocomposites. Adv. Nanocomposite Technol. 2011, 11, 247–272. [Google Scholar]
- Vankayala, B.K.; Löb, P.; Hessel, V.; Menges, G.; Hofmann, C.; Metzke, D.; Krtschil, U.; Kost, H.-J. Scale-up of process intensifying falling film microreactors to pilot production scale. Int. J. Chem. React. Eng. 2007, 5, 1542–6580. [Google Scholar] [CrossRef]
- Fu, H.; Dencic, I.; Tibhe, J.; Pedraza, C.S.; Wang, Q.; Noel, T.; Meuldijk, J.; de Croon, M.; Hessel, V.; Weizenmann, N. Threonine aldolase immobilization on different supports for engineering of productive, cost-efficient enzymatic microreactors. Chem. Eng. J. 2012, 207, 564–576. [Google Scholar] [CrossRef]
- Woitalka, A.; Kuhn, S.; Jensen, K.F. Scalability of mass transfer in liquid–liquid flow. Chem. Eng. Sci. 2014, 116, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Doku, G.N.; Verboom, W.; Reinhoudt, D.N.; van den Berg, A. Microbubble beam (MBB), a potential dispersion mechanism for multiphase gas−liquid microreactor systems. Ind. Eng. Chem. Res. 2003, 42, 3721–3730. [Google Scholar] [CrossRef]
- Choban, E.R.; Markoski, L.J.; Wieckowski, A.; Kenis, P.J.A. Microfluidic fuel cell based on laminar flow. J. Power Sources 2004, 128, 54–60. [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Todaro, S.; Migliori, M.; Giordano, G.; Frusteri, F. Interaction effects between CuO-ZnO-ZrO2 methanol phase and zeolite surface affecting stability of hybrid systems during one-step CO2 hydrogenation to DME. Catal. Today 2019, 345, 175–182. [Google Scholar] [CrossRef]
- Cabeza, V.S. High and efficient production of nanomaterials by microfluidic reactor approaches. In Advances in Microfluidics–New Applications in Biology, Energy, and Materials Sciences; InTech Rijeka: Rijeka, Croatia, 2016. [Google Scholar]
- Kashid, M.N.; Harshe, Y.M.; Agar, D.W. Liquid−liquid slug flow in a capillary: An alternative to suspended drop or film contactors. Ind. Eng. Chem. Res. 2007, 46, 8420–8430. [Google Scholar] [CrossRef]
- Kashid, M.N.; Gupta, A.; Renken, A.; Kiwi-Minsker, L. Numbering-up and mass transfer studies of liquid–liquid two-phase microstructured reactors. Chem. Eng. J. 2010, 158, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.M.; Wu, H.S. Interfacial mechanism and kinetics of phase-transfer catalysis. Catal. Rev. 2003, 45, 463–540. [Google Scholar] [CrossRef]
- Ueno, M.; Hisamoto, H.; Kitamori, T.; Kobayashi, S. Phase-transfer alkylation reactions using microreactors. Chem. Commun. 2003, 10, 936–937. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.S.; Wang, C.S. Liquid–solid–liquid phase-transfer catalysis in sequential phosphazene reaction: Kinetic investigation and reactor design. Chem. Eng. Sci. 2003, 58, 3523–3534. [Google Scholar] [CrossRef]
- Aljbour, S.; Yamada, H.; Tagawa, T. Ultrasound-assisted phase transfer catalysis in a capillary microreactor. Chem. Eng. Process. Process Intensif. 2009, 48, 1167–1172. [Google Scholar] [CrossRef]
- Ahmed-Omer, B.; Barrow, D.; Wirth, T. Effect of segmented fluid flow, sonication and phase transfer catalysis on biphasic reactions in capillary microreactors. Chem. Eng. J. 2008, 135, S280–S283. [Google Scholar] [CrossRef]
- Ruijin, W.; Beiqi, L.; Dongdong, S.; Zefei, Z. Investigation on the splitting-merging passive micromixer based on Baker’s transformation. Sensors Actuators B Chem. 2017, 249, 395–404. [Google Scholar] [CrossRef]
- Anika, N.N.; Djenidi, L.; Tardu, S. Roughness effect in an initially laminar channel flow. J. Fluid Mech. 2020, 892. [Google Scholar] [CrossRef]
- Karande, R. Development and Application of Microreactors for Biocatalytic Reactions. Ph.D. Thesis, Technische Universität Dortmund Fakultät Bio- und Chemieingenieurwesen, Dortmund, Germany, 2014. Available online: http://129.217.131.68:8080/bitstream/2003/33133/2/Summary.pdf (accessed on 25 July 2020).
- Fletcher, P.D.I.; Haswell, S.J.; Paunov, V.N. Theoretical considerations of chemical reactions in micro-reactors operating under electroosmotic and electrophoretic control. Analyst 1999, 124, 1273–1282. [Google Scholar] [CrossRef]
- Erickson, D. Towards numerical prototyping of labs-on-chip: Modeling for integrated microfluidic devices. Microfluid. Nanofluidics 2005, 1, 301–318. [Google Scholar] [CrossRef]
- Ferziger, J.H.; Perić, M.; Street, R.L. Computational Methods for Fluid Dynamics; Springer: Berlin/Heidelberg, Germany, 2002; Volume 3. [Google Scholar]
- Liao, Y.S.; Chen, Y.T. Precision fabrication of an arrayed micro metal probe by the laser-LIGA process. J. Micromech. Microeng. 2005, 15, 2433–2440. [Google Scholar] [CrossRef]
- Potic, B.; Kersten, S.R.A.; Ye, M.; van der Hoef, M.A.; Kuipers, J.A.M.; van Swaaij, W.P.M. Fluidization with hot compressed water in micro-reactors. Chem. Eng. Sci. 2005, 60, 5982–5990. [Google Scholar] [CrossRef]
- Schenk, R.; Hessel, V.; Hofmann, C.; Kiss, J.; Löwe, H.; Ziogas, A. Numbering-up of micro devices: A first liquid-flow splitting unit. Chem. Eng. J. 2004, 101, 421–429. [Google Scholar] [CrossRef]
- Jensen, K.F. Microreaction engineering—Is small better? Chem. Eng. Sci. 2001, 56, 293–303. [Google Scholar] [CrossRef]
- Mendorf, M.; Nachtrodt, H.; Mescher, A.; Ghaini, A.; Agar, D.W. Design and control techniques for the numbering-up of capillary microreactors with uniform multiphase flow distribution. Ind. Eng. Chem. Res. 2010, 49, 10908–10916. [Google Scholar] [CrossRef]
- Chován, T.; Guttman, A. Microfabricated devices in biotechnology and biochemical processing. Trends Biotechnol. 2002, 20, 116–122. [Google Scholar] [CrossRef]
- Choi, C.H.; Su, Y.W.; Chang, C.H. Effects of fluid flow on the growth and assembly of ZnO nanocrystals in a continuous flow microreactor. CrystEngComm 2013, 15, 3326–3333. [Google Scholar] [CrossRef]
- Brandner, j.J. Fabrication of microreactors made from metals and ceramics. In Microreactors in Organic Synthesis and Catalysis; Wiley Online Library: Hoboken, NJ, USA, 2008; pp. 1–7. [Google Scholar]
- Wagner, J.; Kirner, T.; Mayer, G.; Albert, J.; Köhler, J.M. Generation of metal nanoparticles in a microchannel reactor. Chem. Eng. J. 2004, 101, 251–260. [Google Scholar] [CrossRef]
- Wang, S.; Su, P.; Yang, Y. Online immobilized enzyme microreactor for the glucose oxidase enzymolysis and enzyme inhibition assay. Anal. Biochem. 2012, 427, 139–143. [Google Scholar] [CrossRef]
- Junkers, T. Precision polymer design in microstructured flow reactors: Improved control and first upscale at once. Macromol. Chem. Phys. 2017, 218, 1600421. [Google Scholar] [CrossRef]
- Cantillo, D.; Kappe, C.O. Halogenation of organic compounds using continuous flow and microreactor technology. React. Chem. Eng. 2017, 2, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Movsisyan, M.; Delbeke, E.; Berton, J.; Battilocchio, C.; Ley, S.; Stevens, C. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef] [PubMed]
- Nettekoven, M.; Püllmann, B.; Martin, R.E.; Wechsler, D. Evaluation of a flow-photochemistry platform for the synthesis of compact modules. Tetrahedron Lett. 2012, 53, 1363–1366. [Google Scholar] [CrossRef]
- Aillet, T.; Loubiere, K.; Dechy-Cabaret, O.; Prat, L. Photochemical synthesis of a “cage” compound in a microreactor: Rigorous comparison with a batch photoreactor. Chem. Eng. Process. Process Intensif. 2013, 64, 38–47. [Google Scholar] [CrossRef] [Green Version]
- El Zanati, E.; Abdallah, H.; Elnahas, G. Micro-reactor for non-catalyzed esterification reaction: Performance and modeling. Int. J. Chem. React. Eng. 2017, 15. [Google Scholar] [CrossRef]
- Inoue, T.; Ohtaki, K.; Murakami, S.; Matsumoto, S. Direct synthesis of hydrogen peroxide based on microreactor technology. Fuel Process. Technol. 2013, 108, 8–11. [Google Scholar] [CrossRef]
- Freakley, S.J.; Piccinini, M.; Edwards, J.K.; Ntainjua, E.N.; Moulijn, J.A.; Hutchings, G.J. Effect of reaction conditions on the direct synthesis of hydrogen peroxide with a AuPd/TiO2 catalyst in a flow reactor. ACS Catal. 2013, 3, 487–501. [Google Scholar] [CrossRef]
- Paunovic, V.; Schouten, J.C.; Nijhuis, T. Direct synthesis of hydrogen peroxide in a wall-coated microchannel reactor over Au–Pd catalyst: A performance study. Catal. Today 2015, 248, 160–168. [Google Scholar] [CrossRef]
- Westermann, T.; Mleczko, L. Heat management in microreactors for fast exothermic organic syntheses first design principles. Org. Process Res. Dev. 2016, 20, 487–494. [Google Scholar] [CrossRef]
- Kulkarni, A.A. Continuous flow nitration in miniaturized devices. Beilstein J. Org. Chem. 2014, 10, 405–424. [Google Scholar] [CrossRef]
- Aida, T.M.; Oshima, M.; Smith Jr, R.L. Controlled conversion of proteins into high-molecular-weight peptides without additives with high-temperature water and fast heating rates. ACS Sustain. Chem. Eng. 2017, 5, 7709–7715. [Google Scholar] [CrossRef]
- Reichart, B.; Tekautz, G.; Kappe, C.O. Continuous flow synthesis of n-alkyl chlorides in a high-temperature microreactor environment. Org. Process Res. Dev. 2013, 17, 152–157. [Google Scholar] [CrossRef]
- Min, K.I.; Lee, H.J.; Kim, D.P. Three-dimensional flash flow microreactor for scale-up production of monodisperse PEG–PLGA nanoparticles. Lab Chip 2014, 14, 3987–3992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.W.; Sinton, D.; Moffitt, M.G. Morphological control via chemical and shear forces in block copolymer self-assembly in the lab-on-chip. ACS Nano 2013, 7, 1424–1436. [Google Scholar] [CrossRef]
- Bains, A.; Cao, Y.; Kly, S.; Wulff, J.E.; Moffitt, M.G. Controlling structure and function of polymeric drug delivery nanoparticles using microfluidics. Mol. Pharm. 2017, 14, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.; Wulff, J.E.; Moffitt, M.G. Microfluidic synthesis of dye-loaded polycaprolactone-block-poly (ethylene oxide) nanoparticles: Insights into flow-directed loading and in vitro release for drug delivery. J. Colloid Interface Sci. 2016, 475, 136–148. [Google Scholar] [CrossRef]
- Corrigan, N.; Rosli, D.; Jones, J.W.J.; Xu, J.; Boyer, C. Oxygen tolerance in living radical polymerization: Investigation of mechanism and implementation in continuous flow polymerization. Macromolecules 2016, 49, 6779–6789. [Google Scholar] [CrossRef]
- Daniloska, V.; Carretero, P.; Tomovska, R.; Asua, J.M. High performance pressure sensitive adhesives by miniemulsion photopolymerization in a continuous tubular reactor. Polymer 2014, 55, 5050–5056. [Google Scholar] [CrossRef]
- Tomovska, R.; de la Cal, J.C.; Asua, J.M. Miniemulsion photo-copolymerization of styrene/butyl acrylate in a continuous tubular reactor. Ind. Eng. Chem. Res. 2014, 53, 7313–7320. [Google Scholar] [CrossRef]
- Qiu, M.; Zha, L.; Song, Y.; Xiang, L.; Su, Y. Numbering-up of capillary microreactors for homogeneous processes and its application in free radical polymerization. React. Chem. Eng. 2019, 4, 351–361. [Google Scholar] [CrossRef]
- Su, Y.; Song, Y.; Xiang, L. Continuous-flow Microreactors for polymer synthesis: Engineering principles and applications. In Accounts on Sustainable Flow Chemistry; Noël, T., Luque, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 147–190. [Google Scholar]
- Yoshida, J.; Nagaki, A.; Iwasaki, T.; Suga, S. Enhancement of chemical selectivity by microreactors. Chem. Eng. Technol. 2005, 28, 259–266. [Google Scholar] [CrossRef]
- Watts, P.; Wiles, C. Micro reactors: A new tool for the synthetic chemist. Org. Biomol. Chem. 2007, 5, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.; Wiles, C. Micro reactors, flow reactors and continuous flow synthesis. J. Chem. Res. 2012, 36, 181–193. [Google Scholar] [CrossRef]
- Watkins, N.N.; Hassan, U.; Damhorst, G.; Ni, H.; Vaid, A.; Rodriguez, W.; Bashir, R. Microfluidic CD4+ and CD8+ T lymphocyte counters for point-of-care HIV diagnostics using whole blood. Sci. Transl. Med. 2013, 5, 214ra170. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Marques, M.P.; Fernandes, P.; Cabral, J.M.; Žnidaršič-Plazl, P.; Plazl, I. Continuous steroid biotransformations in microchannel reactors. New Biotechnol. 2012, 29, 227–234. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M. Thermomyces lanuginosus lipase-catalyzed synthesis of natural flavor esters in a continuous flow microreactor. 3 Biotech 2016, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.I.; Sask, K.N.; Brash, J.L.; Selvaganapathy, P.R. Polyurethane-based microfluidic devices for blood contacting applications. Lab Chip 2012, 12, 960–970. [Google Scholar] [CrossRef]
- Porta, R.; Benaglia, M.; Puglisi, A. Flow chemistry: Recent developments in the synthesis of pharmaceutical products. Org. Process Res. Dev. 2016, 20, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Khodashenas, B.; Zadghaffari, R.; Jafari, S.D. Process intensification approach for the synthesis of metal nanoparticles: A mini review. Orient. J. Chem. 2015, 31, 249–257. [Google Scholar] [CrossRef]
- Su, M. Synthesis of highly monodisperse silica nanoparticles in the microreactor system. Korean J. Chem. Eng. 2017, 34, 484–494. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Wiesbauer, J.; Nidetzky, B. Biotransformations in microstructured reactors: More than flowing with the stream? Trends Biotechnol. 2011, 29, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-X.; He, L.; Qiao, S.Z.; Middelberg, A.P.J. Nanoparticle synthesis in microreactors. Chem. Eng. Sci. 2011, 66, 1463–1479. [Google Scholar] [CrossRef]
- Nightingale, A.M.; deMello, J.C. Segmented flow reactors for nanocrystal synthesis. Adv. Mater. 2013, 25, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Lignos, I.; Protesescu, L.; Stavrakis, S.; Piveteau, L.; Speirs, M.J.; Loi, M.A.; Kovalenko, M.V.; de Mello, A.J. Facile droplet-based microfluidic synthesis of monodisperse IV–VI semiconductor nanocrystals with coupled in-line NIR fluorescence detection. Chem. Mater. 2014, 26, 2975–2982. [Google Scholar] [CrossRef]
- Gutierrez, L.; Gomez, L.; Irusta, S.; Arruebo, M.; Santamaria, J. Comparative study of the synthesis of silica nanoparticles in micromixer–microreactor and batch reactor systems. Chem. Eng. J. 2011, 171, 674–683. [Google Scholar] [CrossRef]
- Ju, J.; Zeng, C.; Zhang, L.; Xu, N. Continuous synthesis of zeolite NaA in a microchannel reactor. Chem. Eng. J. 2006, 116, 115–121. [Google Scholar] [CrossRef]
- Appalakutti, S.; Sonawane, S.; Bhanvase, B.A.; Mittal, V.; Ashokkumar, M. Process intensification of copper chromite (CuCr2O4) nanoparticle production using continuous flow microreactor. Chem. Eng. Process. Process Intensif. 2015, 89, 28–34. [Google Scholar] [CrossRef]
- Azimi, N.; Rahimi, M.; Abdollahi, N. Using magnetically excited nanoparticles for liquid–liquid two-phase mass transfer enhancement in a Y-type micromixer. Chem. Eng. Process. Process Intensif. 2015, 97, 12–22. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wang, A.; Li, X.; Wang, G.; Zhao, L. Synthesis of ZnO nanoparticles from microemulsions in a flow type microreactor. Chem. Eng. J. 2014, 235, 191–197. [Google Scholar] [CrossRef]
- Du, L.; Wang, Y.J.; Lu, Y.C.; Luo, G.S. Process intensification of BaSO4 nanoparticle preparation with agitation of microbubbles. Powder Technol. 2013, 247, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Lesmana, D.; Wu, H.S. Cu/ZnO/Al2O3/Cr2O3/CeO2 catalyst for hydrogen production by oxidative methanol reforming via washcoat catalyst preparation in microchannel reactor. Bull. Chem. React. Eng. Catal. 2017, 12, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Günther, A.; Jensen, K.F. Multiphase microfluidics: From flow characteristics to chemical and materials synthesis. Lab Chip 2006, 6, 1487–1503. [Google Scholar] [CrossRef]
- Aubin, J.; Ferrando, M.; Jiricny, V. Current methods for characterising mixing and flow in microchannels. Chem. Eng. Sci. 2010, 65, 2065–2093. [Google Scholar] [CrossRef]
- Kjeang, E.; Michel, R.; Harrington, D.A.; Sinton, D.; Djilali, N. An alkaline microfluidic fuel cell based on formate and hypochlorite bleach. Electrochim. Acta 2008, 54, 698–705. [Google Scholar] [CrossRef]
- Bamgbopa, M.O.; Almheiri, S.; Sun, H. Prospects of recently developed membraneless cell designs for redox flow batteries. Renew. Sustain. Energy Rev. 2017, 70, 506–518. [Google Scholar] [CrossRef]
- Suss, M.E.; Conforti, K.; Gilson, L.; Buie, C.R.; Bazant, M.Z. Membraneless flow battery leveraging flow-through heterogeneous porous media for improved power density and reduced crossover. RSC Adv. 2016, 6, 100209–100213. [Google Scholar] [CrossRef] [Green Version]
- Hollinger, A.S.; Kenis, P.J.A. Electrohydrodynamic-jet deposition of pt-based fuel cell catalysts. In International Conference on Fuel Cell Science, Engineering and Technology; American Society of Mechanical Engineers: New York, NY, USA, 2016. [Google Scholar]
- Zebda, A.; Renaud, L.; Cretin, M.; Pichot, F.; Innocent, C.; Ferrigno, R.; Tingry, S. A microfluidic glucose biofuel cell to generate micropower from enzymes at ambient temperature. Electrochem. Commun. 2009, 11, 592–595. [Google Scholar] [CrossRef]
- Choban, E.R.; Spendelow, J.S.; Gancs, L.; Wieckowski, A.; Kenis, P.J.A. Membraneless laminar flow-based micro fuel cells operating in alkaline, acidic, and acidic/alkaline media. Electrochimica Acta 2005, 50, 5390–5398. [Google Scholar] [CrossRef]
- Zou, H.; Chen, J.; Fang, Y.; Ding, J.; Peng, W.; Liu, R. A dual-electrolyte based air-breathing regenerative microfluidic fuel cell with 1.76V open-circuit-voltage and 0.74V water-splitting voltage. Nano Energy 2016, 27, 619–626. [Google Scholar] [CrossRef]
- Lalaoui, N.; Means, N.; Walgama, C.; Le Goff, A.; Holzinger, M.; Krishnan, S.; Cosnier, S. Enzymatic versus electrocatalytic oxidation of NADH at carbon-nanotube electrodes modified with glucose dehydrogenases: Application in a bucky-paper-based glucose enzymatic fuel cell. ChemElectroChem 2016, 3, 2058–2062. [Google Scholar] [CrossRef]
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic biofuel cells: 30 years of critical advancements. Biosens. Bioelectron. 2016, 76, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjeang, E.; Brolo, A.G.; Harrington, D.A.; Djilali, N.; Sinton, D. Hydrogen peroxide as an oxidant for microfluidic fuel cells. J. Electrochem. Soc. 2007, 154, B1220. [Google Scholar] [CrossRef]
- Oruc, M.E.; Desai, A.V.; Nuzzo, R.G.; Kenis, P.J.A. Design, fabrication, and characterization of a proposed microchannel water electrolyzer. J. Power Sources 2016, 307, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Terrier, O.; Bourdon, J.-C.; Rosa-Calatrava, M. Method for Improving the Production of Influenza Viruses and Vaccine Seeds. U.S. Patent No. US938,123,8B2, 5 July 2016. [Google Scholar]
- Narvaez Villarrubia, C.W.; Soavi, F.; Santoro, C.; Arbizzani, C.; Serov, A.; Rojas-Carbonell, S.; Gupta, G.; Atanassov, P. Self-feeding paper based biofuel cell/self-powered hybrid μ-supercapacitor integrated system. Biosens. Bioelectron. 2016, 86, 459–465. [Google Scholar] [CrossRef]
- Kim, C.J.; Hur, J.; Meng, D. Self-Pumping Membraneless Fuel Cell. U.S. Patent No. US960,178,9B2, 21 March 2017. [Google Scholar]
- Verma, R.; Lal, S.; Deepa, M.; Janardhanan, V.M.; Sahu, K.C. Sodium percarbonate based, mixed-media fuel cells supported on paper with gold/nickel oxide catalysts. ChemElectroChem 2017, 4, 310–319. [Google Scholar] [CrossRef]
- Li, L.; Fan, W.; Xuan, J.; Leung, M.K.H.; Zheng, K.; She, Y. Design principles of current collectors in microfluidic fuel cell with flow-through porous electrodes. Energy Procedia 2017, 105, 1557–1563. [Google Scholar] [CrossRef]
- Kwok, Y.H.; Tsang, A.C.H.; Wang, Y.; Leung, D.Y.C. Ultra-fine Pt nanoparticles on graphene aerogel as a porous electrode with high stability for microfluidic methanol fuel cell. J. Power Sources 2017, 349, 75–83. [Google Scholar] [CrossRef]
- Ojani, R.; Hamidi, P.; Raoof, J.-B. Efficient nonenzymatic hydrogen peroxide sensor in acidic media based on Prussian blue nanoparticles-modified poly(o-phenylenediamine)/glassy carbon electrode. Chin. Chem. Lett. 2016, 27, 481–486. [Google Scholar] [CrossRef]
- Qian, W.; Wilkinson, D.P.; Shen, J.; Wang, H.; Zhang, J. Architecture for portable direct liquid fuel cells. J. Power Sources 2006, 154, 202–213. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368. [Google Scholar] [CrossRef] [PubMed]
- Lauga, E.; Brenner, M.; Stone, H. Microfluidics: The no-slip boundary condition. In Springer Handbook of Experimental Fluid Mechanics; Tropea, C., Yarin, A.L., Foss, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1219–1240. [Google Scholar]
- Choi, S.D.; Choi, J.H.; Kim, Y.H.; Kim, S.Y.; Dwivedi, P.K.; Sharma, A.; Goel, S.; Kim, G.M. Enzyme immobilization on microelectrode arrays of CNT/Nafion nanocomposites fabricated using hydrogel microstencils. Microelectron. Eng. 2015, 141, 193–197. [Google Scholar] [CrossRef]
- Ramasamy, R.P. Photosynthetic Electrochemical Cells. U.S. Patent No. 1,005,665,9B2, 21 August 2018. [Google Scholar]
- Minteer, S.D.; Martin, R.S.; Moore, C.M. Microfluidic Biofuel Cell. Google Patents No. 7,709,134, 4 May 2010. [Google Scholar]
- Bedekar, A.; Feng, J.; Lim, K.; Krishnamoorthy, S.; Palmore, G.; Sundaram, S. In Proceedings of the Computational Analysis of Microfluidic Biofuel Cells, Austin, TX, USA, 7–12 November 2004.
- Ding, S.-N.; Holzinger, M.; Mousty, C.; Cosnier, S. Laccase electrodes based on the combination of single-walled carbon nanotubes and redox layered double hydroxides: Towards the development of biocathode for biofuel cells. J. Power Sources 2010, 195, 4714–4717. [Google Scholar] [CrossRef]
- Lee, J.Y.; Shin, H.Y.; Kang, S.W.; Park, C.; Kim, S.W. Application of an enzyme-based biofuel cell containing a bioelectrode modified with deoxyribonucleic acid-wrapped single-walled carbon nanotubes to serum. Enzym. Microb. Technol. 2011, 48, 80–84. [Google Scholar] [CrossRef]
- Amatore, C.; Da Mota, N.; Lemmer, C.; Pebay, C.; Sella, C.; Thouin, L. Theory and experiments of transport at channel microband electrodes under laminar flows. 2. electrochemical regimes at double microband assemblies under steady state. Anal. Chem. 2008, 80, 9483–9490. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Materials | Merits | Demerits |
|---|---|---|
| Metal |
|
|
|
| |
| ||
| Glass |
|
|
| ||
| ||
| Polymers |
|
|
|
| |
| ||
| Silicon |
|
|
|
| |
| Ceramic |
|
|
|
|
| Type | Phase | Merits | Demerits | Ref |
|---|---|---|---|---|
| Membrane reactor | S-L, G-L-S | The experimental phases are easily separable | Expensive Enzyme inactiveness | [106] |
| Monolith microreactor | L-S, G-L-S | Ease of pressure control No hindrance in active transportation of molecules | Only applicable to certain phase Immobilization of Catalyst | [107] |
| Segmented flow microreactor | L-L, G-L-S, L-L-G-S | Low pressure drop Higher surface area | Inadequate choice of flow rates | [108] |
| Co-flow microreactor | L-L | Good mass transfer Lower pressure drop | Inadequate choice of flow rates | [109] |
| Falling film microreactor | G-L, L-S, G-L-S | Good surface area in L-G | Poor residence time | [110] |
| Overflowing bed microreactor | L-S, G-L-S | Easy to operate Good enzymatic loading for enzymatic reaction | Unequal flow rate control | [111] |
| Reactor Design | Dimension (mm) | Flow Rate (mL/min) | Nanoparticle | Size (nm) | Refs |
|---|---|---|---|---|---|
| Capillary Reactor | D = 0.26, L= 110–152 | 0.06 | PbS/PbSe | 3–6 | [176] |
| Y-shaped Capillary | D = 0.2, L = 350 | 0.2 | CdSe-ZnS | 2 | [139] |
| Interdigital Micro mixer | D = 1.4, L = 1000 | 0.5 | SiO2 | 173 | [177] |
| Capillary | D = 0.74, L = 1400 | 0.22–0.84 | Zeolite | 279–427 | [178] |
| Y-shaped Microchannel | D = 0.9, L = 2200 | 0.8 | Cu2Cr2O5 | 68–265 | [179] |
| Y-shaped Microchannel | D= 0.9, L = 113 | 3–30 | Fe3O4 | 10–23 | [180] |
| T-shaped Microchannel | - | 7 | Zn/Fe3O4 | 4 | [4] |
| Y-shaped Microchannel with tubes interconnected | D = 0.35, L = 3.5 | 2–6 | ZnO | 17 | [181] |
| Microchannel | D = 0.5, L = 20 | 64 | BaSO4 | 60 | [182] |
| Electrolyte | Nanomaterials | Microchannel Scheme (W/H/L) | Electrodeposition | References |
|---|---|---|---|---|
| Phosphate solvent 0.1 M | Palladium Palladium or Platinum | Co-laminar flow T-shaped2.0 mm/0.072–0.173 mm/ 10.2 mm | Bottom-most wall | [21,187,188,189] |
| H2SO4 Solution 0.3 M | Platinum-black Platinum-black | Co-laminar flow Y-shaped 0.54 or 1.0 mm/0.53 or 1.0 mm/30.2 mm | Side walls | [190,191,192] |
| H2SO4 0.1 M | Platinum | Co-laminar flow F-shaped 0.383 mm/1.0 mm/50.1 mm | Top-/bottom-most walls | [191,193] |
| PBS and NaCl (0.1–0.2 M) | Glucose dehydrogenase enzymes Platinum | Distinct stream I-shaped 3.0 mm wide/1 mm height | Bottom-most wall | [191,192,194] |
| Phosphate and NaCl (0.1–0.2 M) | Glucose dehydrogenase enzymes Bilirubin oxidase enzyme | Distinct stream I-shape 3.0 mm wide/0.1–1 mm height | Bottom-most wall | [191,195] |
| H2SO4 (2–3 M) | - | Co-laminar flow Y-shaped 2.0 mm/0.123 mm/27.1 mm | Bottom-most wall | [196] |
| H2SO4 (0.3 M) and NaOH (1 M) | Platinum Platinum | Passive electrolyte 0.22 mm/0.07 mm/20 mm | Bottom-most wall | [191,197] |
| NaOH (2.9 M) | Palladium Gold or Palladium | Co-laminar flow T-shaped 3.1 mm/0.34 mm/12.2 mm | Bottom-most wall | [187] |
| H2SO4 (0.1 M) | Platinum/Ruthenium-black Platinum-black | Co-laminar flow F-shaped 1.0 mm/1.0 mm/50 mm | Top-/bottom-most walls | [198] |
| PBS (pH 7.15) | Alcohol dehydrogenase Enzyme Platinum | Distinct stream I-shaped 0.2 mm/0.1 mm/25 mm | Bottom-most wall | [199] |
| H2SO4 (0.5–0.6 M) and KOH (1–2 M) | Platinum /Ruthenium Platinum | Co-laminar flow F-shaped 2.0 mm/3.0 mm/22.1 mm | Top-/bottom-most walls | [200] |
| H2SO4 (0.5 M) | Platinum Platinum | Co-laminar flow I-shaped 0.5 mm/0.051 mm/20.1 mm | Bottom-most wall | [201] |
| KOH (0.2 M) | Nickel hydroxide Silver oxide | Distinct stream I-shaped 0.12 mm high | Bottom-most wall; interdigitated | [202] |
| H2SO4 (0.5–1 M) | Platinum Platinum | Sequential radial flow Circular shaped 25.42 mm diameter | Bottom-most wall | [203] |
| NaOH (0.8 M) H2SO4 (0.4 M) | Platinum Platinum | Co-laminar flow H-shaped 1 mm/0.05 mm/10 mm | Bottom-most wall | [204] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojang, A.A.; Wu, H.-S. Design, Fundamental Principles of Fabrication and Applications of Microreactors. Processes 2020, 8, 891. https://doi.org/10.3390/pr8080891
Bojang AA, Wu H-S. Design, Fundamental Principles of Fabrication and Applications of Microreactors. Processes. 2020; 8(8):891. https://doi.org/10.3390/pr8080891
Chicago/Turabian StyleBojang, Adama A., and Ho-Shing Wu. 2020. "Design, Fundamental Principles of Fabrication and Applications of Microreactors" Processes 8, no. 8: 891. https://doi.org/10.3390/pr8080891
APA StyleBojang, A. A., & Wu, H.-S. (2020). Design, Fundamental Principles of Fabrication and Applications of Microreactors. Processes, 8(8), 891. https://doi.org/10.3390/pr8080891






