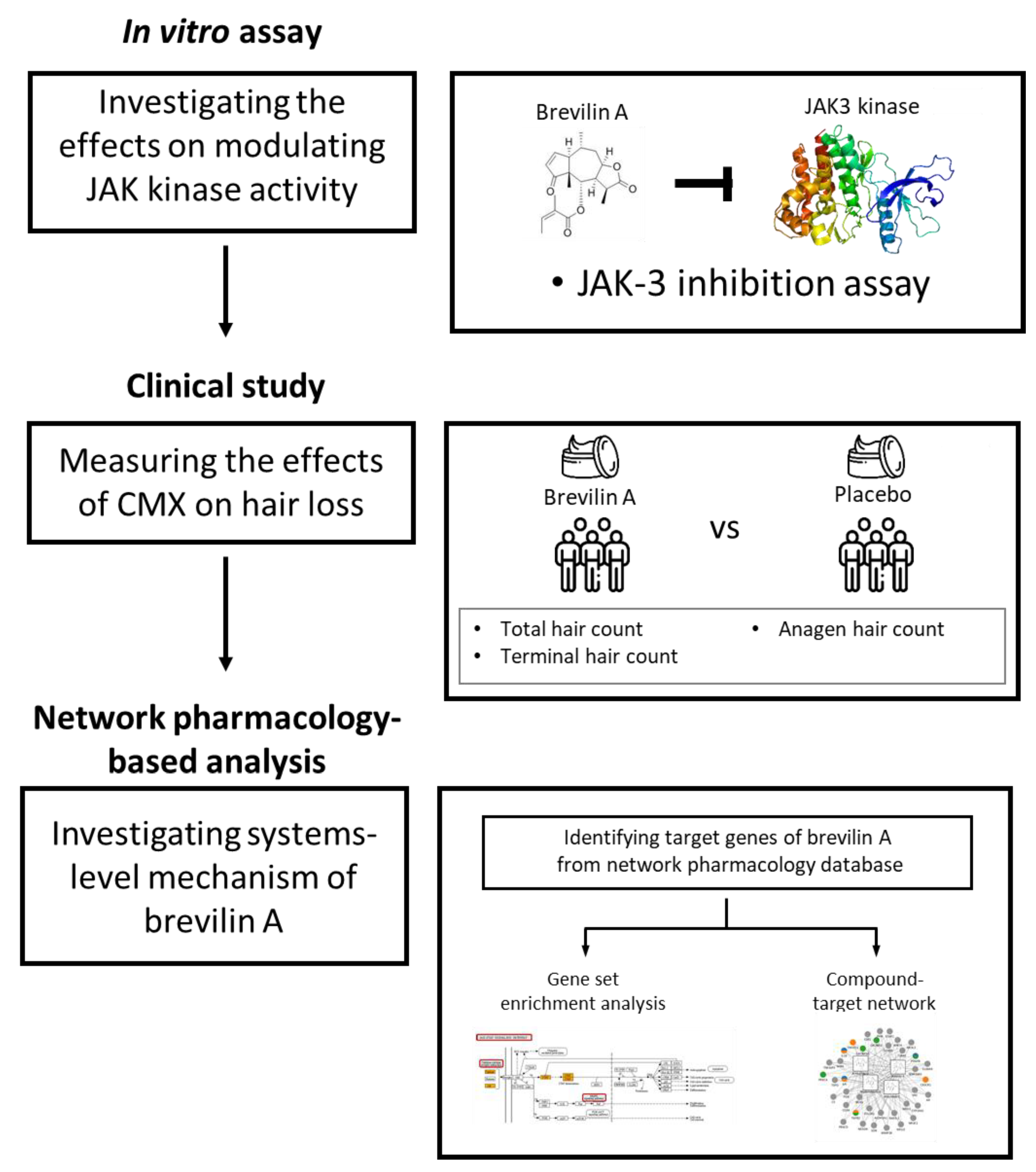
Hair Growth Effect of Emulsion Extracted Brevilin A, a JAK3 Inhibitor, from Centipeda minima
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Preparation of CMX
2.2. HPLC
2.3. Ultra (U)HPLC-Q-TOF-MS Conditions
2.4. JAK3 Inhibition Assay
2.5. Study Population
2.6. Study Design and Hair Counts
2.7. Network Pharmacological Analyses
2.8. Statistical Analysis
3. Results
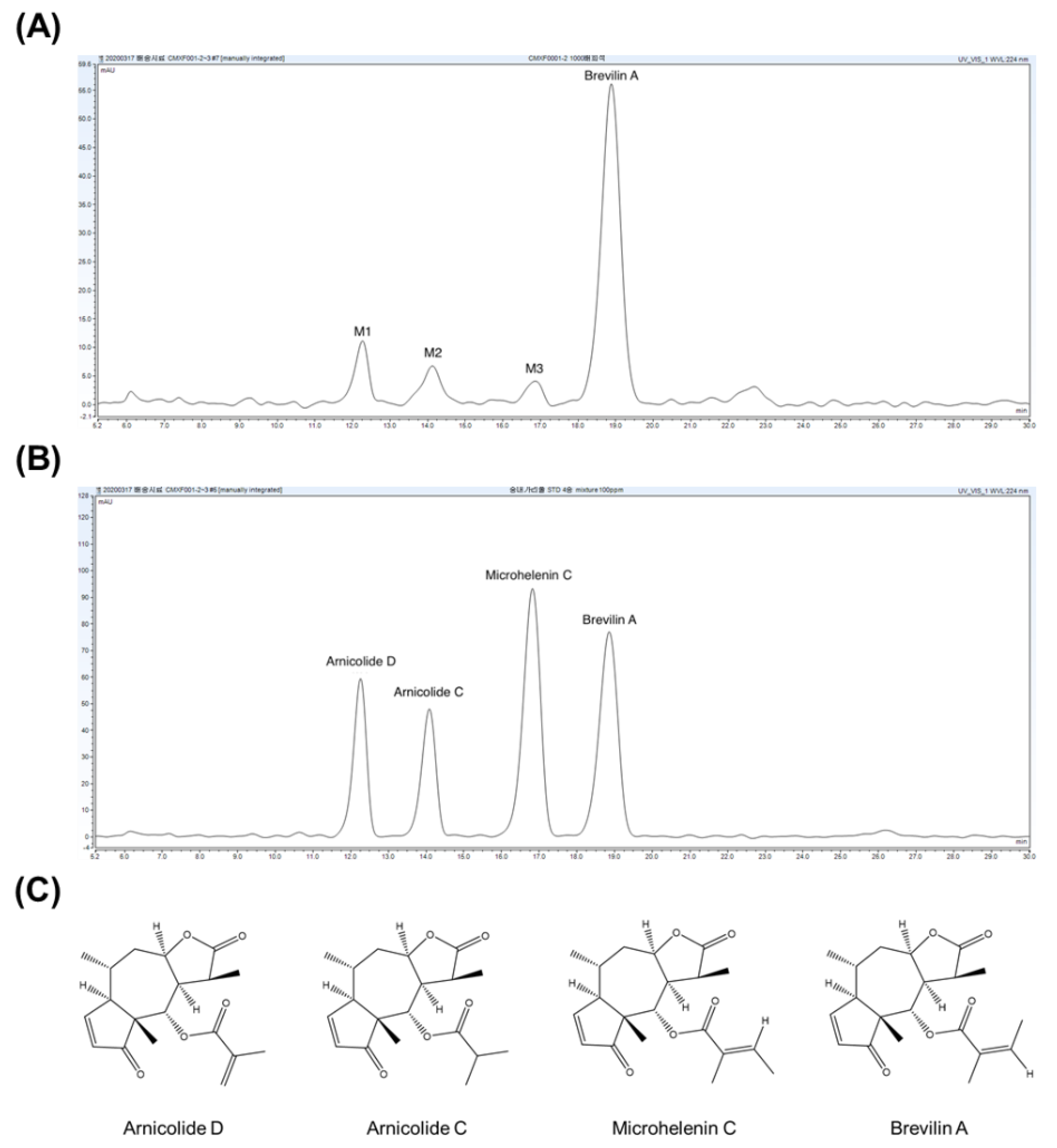
3.1. CMX Analysis
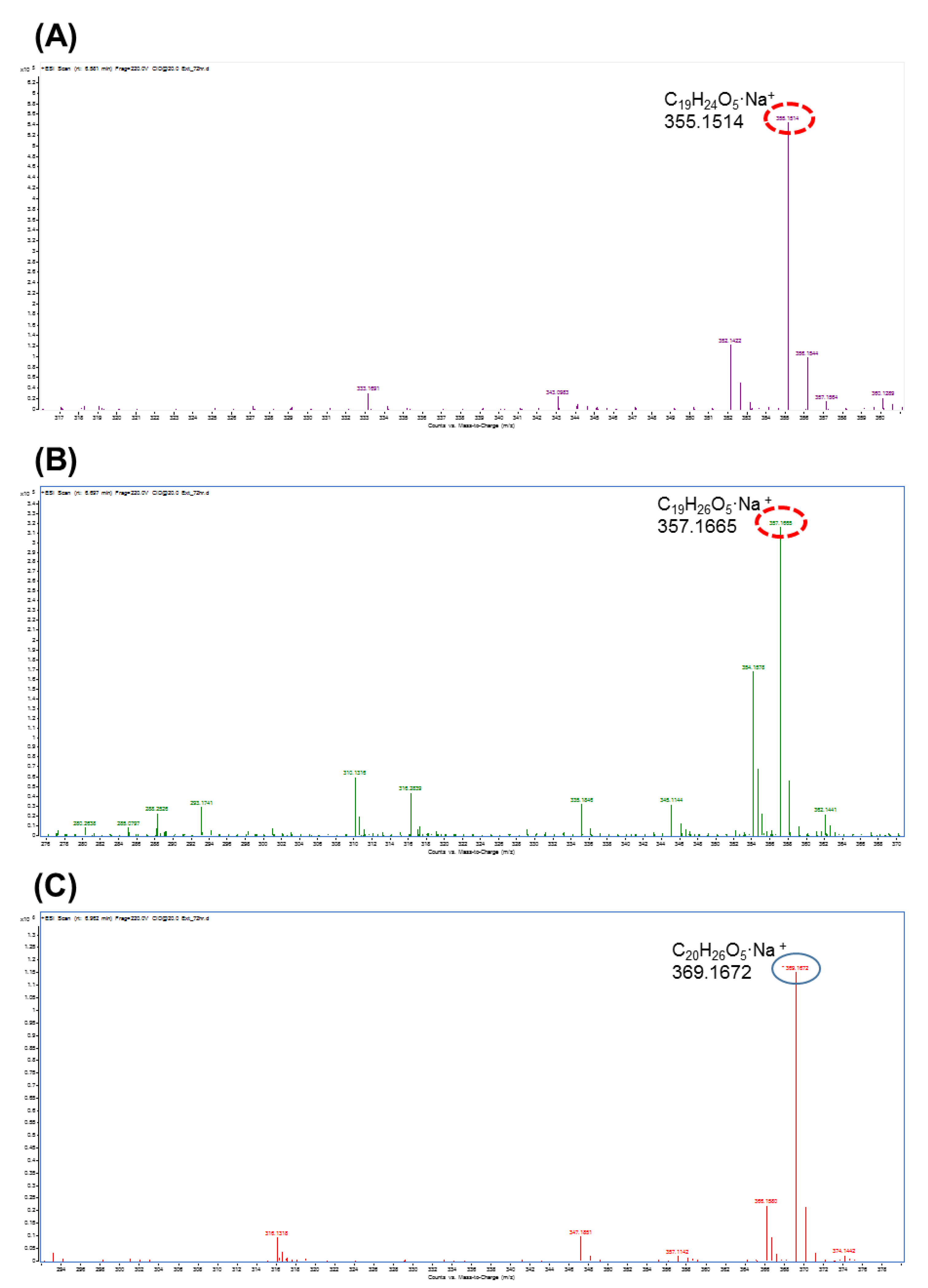
3.2. Compound Identification by LC-Q-TOF
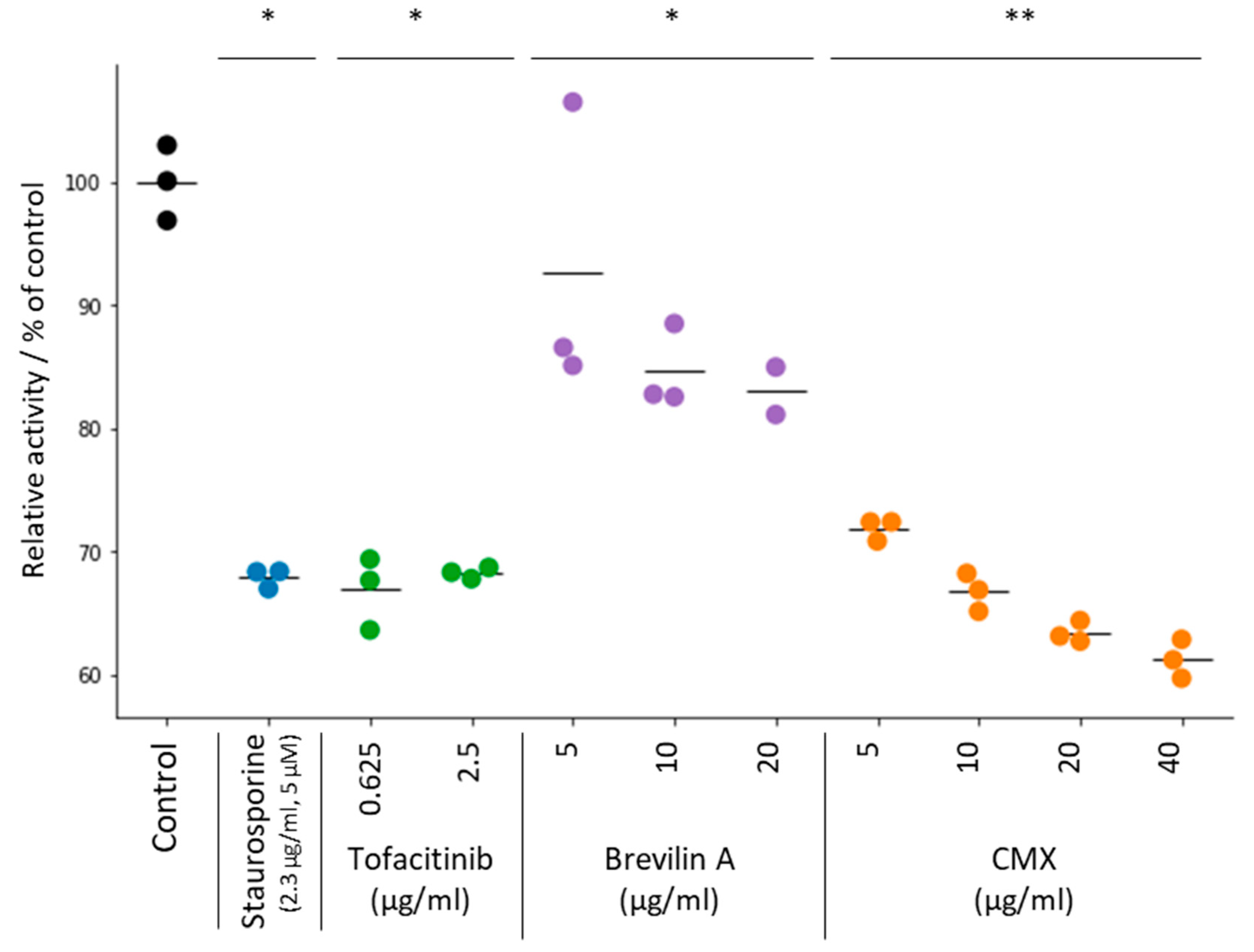
3.3. JAK3 Inhibition Assay
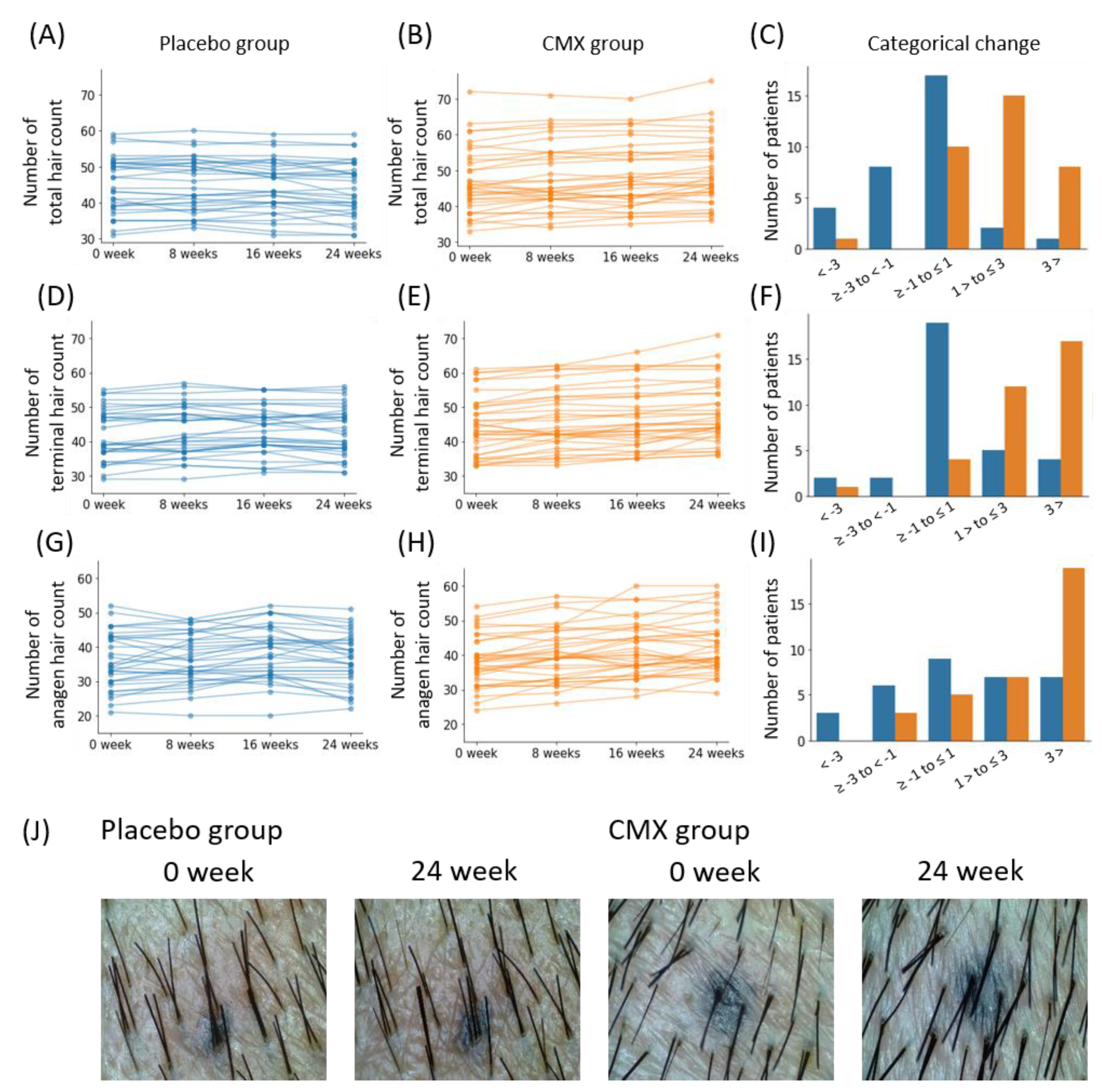
3.4. Study Population
3.5. Network Pharmacological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilhar, A.; Kalish, R.S. Alopecia Areata: A tissue specific autoimmune disease of the hair follicle. Autoimmun. Rev. 2006, 5, 64–69. [Google Scholar] [CrossRef]
- Amos, G.; Amos, E.; Ralf, P. Alopecia Areata. N. Engl. J. Med. 2012, 366, 1515–1525. [Google Scholar]
- Hordinsky, M.K. Overview of alopecia areata. J. Investig. Dermatol. Symp. Proc. 2013, 16, S13–S15. [Google Scholar] [CrossRef] [PubMed]
- Courtois, M.L.G.; Hourseau, C.; Grollier, J.F. Hair cycle and alopecia. Skin Pharmacol. 1994, 7, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.H.; King, L.E., Jr.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef]
- Divito, S.J.; Kupper, T.S. Inhibiting Janus kinases to treat alopecia areata. Nat. Med. 2014, 20, 989–990. [Google Scholar] [CrossRef]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef]
- Iorizzo, M.; Tosti, A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin. Emerg. Drugs 2018, 23, 77–81. [Google Scholar] [CrossRef]
- Crowley, E.L.; Fine, S.C.; Katipunan, K.K.; Gooderham, M.J. The Use of Janus Kinase Inhibitors in Alopecia Areata: A Review of the Literature. J. Cutan. Med. Surg. 2019, 23, 289–297. [Google Scholar] [CrossRef]
- de Oliveira, A.B.; Alpalhao, M.; Filipe, P.; Maia-Silva, J. The role of Janus kinase inhibitors in the treatment of alopecia areata: A systematic review. Dermatol. Ther. 2019, 32, e13053. [Google Scholar] [CrossRef]
- Park, H.; Yu, D.A.; Kwon, O. Janus kinase inhibitors: An innovative treatment for alopecia areata. J. Dermatol. 2019, 46, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Du, Y.; Nan, J.; Zhang, X.; Qin, X.; Wang, Y.; Hou, J.; Wang, Q.; Yang, J. Brevilin A, a novel natural product, inhibits janus kinase activity and blocks STAT3 signaling in cancer cells. PLoS ONE 2013, 8, e63697. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.Z.; Nisar, M.A.; Alshwmi, M.; Din, S.R.U.; Gamallat, Y.; Khan, M.; Ma, T. Brevilin A Inhibits STAT3 Signaling and Induces ROS-Dependent Apoptosis, Mitochondrial Stress and Endoplasmic Reticulum Stress in MCF-7 Breast Cancer Cells. Onco Targets Ther. 2020, 13, 435–450. [Google Scholar] [CrossRef]
- Chan, C.O.; Jin, D.P.; Dong, N.P.; Chen, S.B.; Mok, D.K. Qualitative and quantitative analysis of chemical constituents of Centipeda minima by HPLC-QTOF-MS & HPLC-DAD. J. Pharm. Biomed. Anal. 2016, 125, 400–407. [Google Scholar] [PubMed]
- Robin, S.L.; Taylor, G.H.N.T. Antibacterial constituents of the Nepalese medicinal herb, Centipeda minima. Phytochemistry 1998, 47, 631–634. [Google Scholar]
- Huang, S.S.; Chiu, C.S.; Lin, T.H.; Lee, M.M.; Lee, C.Y.; Chang, S.J.; Hou, W.C.; Huang, G.J.; Deng, J.S. Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. J. Ethnopharmacol. 2013, 147, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Lee, C.Y.; Kim, Y.S.; Kim, C.E. The Methodological Trends of Traditional Herbal Medicine Employing Network Pharmacology. Biomolecules 2019, 9, 362. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Chem. Inf. 2014, 6, 13. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, F.; Wang, Y.; Li, C.; Zhang, X.; Li, H.; Diao, L.; Gu, J.; Wang, W.; Li, D.; et al. BATMAN-TCM: A Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci. Rep. 2016, 6, 21146. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Yu, S.J.; Bai, H.; Ning, K. TCM-Mesh: The database and analytical system for network pharmacology analysis for TCM preparations. Sci. Rep. 2017, 7, 2821. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wang, X.Y.; Hao, X.Y.; Yan, Y.M.; Hong, M.; Wei, S.F.; Zhou, Y.L.; Wang, Q.; Cheng, Y.X.; Liu, Y.Q. Ethanol Extract of Centipeda minima Exerts Antioxidant and Neuroprotective Effects via Activation of the Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2019, 2019, 9421037. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zheng, Z.; Fu, X.; Li, J.; Yin, C.; Chou, J.; Wang, Y.; Liu, Y.; Chen, Y.; Bai, J.; et al. Arnicolide D exerts anti-melanoma effects and inhibits the NF-kappaB pathway. Phytomedicine 2019, 64, 153065. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Wang, Y.P.; Wang, X.N.; Li, C.Y.; Zhu, P.L.; Huang, Y.M.; Yang, Z.Y.; Chen, S.B.; Yu, Z.L. The JAK2/STAT3 pathway is involved in the anti-melanoma effects of brevilin A. Life Sci. 2020, 241, 117169. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Y.; Huang, Q.; Wen, S.; Wei, Y.; Chen, Y.; Zhang, X.; Bai, F.; Lu, Z.; Wei, J.; et al. Helenalin from Centipeda minima ameliorates acute hepatic injury by protecting mitochondria function, activating Nrf2 pathway and inhibiting NF-kappaB activation. Biomed. Pharmacother. 2019, 119, 109435. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, X.; Zou, J.; Shi, Y.; Wang, Y.; Tai, J.; Yang, Y.; Zhou, X.; Guo, D.; Wang, J.; et al. Pharmacology mechanism of Flos magnoliae and Centipeda minima for treating allergic rhinitis based on pharmacology network. Drug Dev. Ind. Pharm. 2019, 45, 1547–1555. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Chan, C.O.; Xie, X.J.; Wan, S.W.; Zhou, G.L.; Yuen, A.C.; Mok, D.K.; Chen, S.B. Qualitative and quantitative analysis of sesquiterpene lactones in Centipeda minima by UPLC-Orbitrap-MS & UPLC-QQQ-MS. J. Pharm. Biomed. Anal. 2019, 174, 360–366. [Google Scholar]
- Omura, S.; Asami, Y.; Crump, A. Staurosporine: New lease of life for parent compound of today’s novel and highly successful anti-cancer drugs. J. Antibiot. (Tokyo) 2018, 71, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.; Fertig, A.C.G.; Evan, D. Sudeep Gaudi, Sexual side effects of 5-α-reductase inhibitors finasteride and dutasteride: A comprehensive review. Dermatol. Online J. 2017, 23, 1–12. [Google Scholar]
- Alfredo, R.; Cantisani, C.; Luca, M.; Alessandra, I.; Elisabetta, S.; Stefano, C. Minoxidil Use in Dermatology, Side Effects and Recent Patents. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 130–136. [Google Scholar]


| Analyte | Concentration (μg/mL) | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (RSD%) | Accuracy (%) | Precision (RSD%) | ||
| Arnicolide D | 1 | 85.3 | 6.0 | 84.6 | 13.6 |
| 10 | 102.0 | 3.6 | 96.3 | 4.9 | |
| 100 | 100.0 | 0.1 | 100.1 | 0.4 | |
| Arnicolide C | 1 | 91.2 | 8.3 | 112.9 | 3.5 |
| 10 | 94.9 | 3.4 | 94.4 | 4.5 | |
| 100 | 100.0 | 0.1 | 99.9 | 1.2 | |
| Microhelenin C | 1 | 105.9 | 4.6 | 115.3 | 12.9 |
| 10 | 102.3 | 0.3 | 103.6 | 2.9 | |
| 100 | 100.0 | 0.1 | 99.7 | 0.7 | |
| Brevilin A | 1 | 86.2 | 7.5 | 87.9 | 15.0 |
| 10 | 100.9 | 1.3 | 99.6 | 3.7 | |
| 100 | 99.9 | 0.1 | 99.1 | 0.9 | |
| Compounds | M1 | M2 | M3 | Brevilin A |
|---|---|---|---|---|
| RT (min) | 12.28 | 14.21 | 16.86 | 18.95 |
| Content (mg/mL) | 14.92 ± 4.25 | 12.24 ± 7.58 | 2.75 ± 0.76 | 62.93 ± 17.00 |
| Compounds | Formula [M+Na]+ | Theoretical Mass (Da) | Measured Mass (Da) | Error (ppm) |
|---|---|---|---|---|
| M1 | (C19H24O5·Na)+ | 355.1516 | 355.1514 | 0.56 |
| M2 | (C19H26O5·Na)+ | 357.1672 | 357.1665 | 1.96 |
| M3 | (C20H26O5·Na)+ | 369.1672 | 369.1672 | 0 |
| Placebo Group (n = 32) | CMX Group (n = 34) | |
|---|---|---|
| Age (mean ± SE) | 46.9 ± 4.0 | 46.2 ± 4.7 |
| Baseline hair count (mean ± SE) | ||
| Total hair count | 45.0 ± 7.8 | 47.0 ± 9.1 |
| Terminal hair count | 42.4 ± 7.4 | 44.6 ± 8.6 |
| Anagen hair count | 36.0 ± 8.1 | 38.1 ± 7.3 |
| Term | Overlap | Adjusted p-Value | Odds Ratio | Combined Score | Genes |
|---|---|---|---|---|---|
| JAK-STAT signaling pathway | 4/162 | 0.0016 | 12.66 | 103.88 | IL10; IL4; STAT3; PDGFB |
| Cytokine-cytokine receptor interaction | 5/294 | 0.0015 | 8.72 | 72.21 | IL10; IL4; CX3CR1; TGFB2; TNFSF11 |
| MAPK signaling pathway | 5/295 | 0.0015 | 8.69 | 8.70 | CACNA1I; TGFB2; PDGFB; PRKCA; CACNA1G |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.H.; Lee, W.-Y.; Trinh, T.A.; Pyo, J.S.; Lee, S.; Kim, C.-E.; Lee, D.H.; Park, E.-S.; Kang, K.S. Hair Growth Effect of Emulsion Extracted Brevilin A, a JAK3 Inhibitor, from Centipeda minima. Processes 2020, 8, 767. https://doi.org/10.3390/pr8070767
Kim BH, Lee W-Y, Trinh TA, Pyo JS, Lee S, Kim C-E, Lee DH, Park E-S, Kang KS. Hair Growth Effect of Emulsion Extracted Brevilin A, a JAK3 Inhibitor, from Centipeda minima. Processes. 2020; 8(7):767. https://doi.org/10.3390/pr8070767
Chicago/Turabian StyleKim, Byoung Ha, Won-Yung Lee, Tuy An Trinh, Jae Sung Pyo, Sooyeun Lee, Chang-Eop Kim, Dong Hwan Lee, Eun-Seok Park, and Ki Sung Kang. 2020. "Hair Growth Effect of Emulsion Extracted Brevilin A, a JAK3 Inhibitor, from Centipeda minima" Processes 8, no. 7: 767. https://doi.org/10.3390/pr8070767
APA StyleKim, B. H., Lee, W.-Y., Trinh, T. A., Pyo, J. S., Lee, S., Kim, C.-E., Lee, D. H., Park, E.-S., & Kang, K. S. (2020). Hair Growth Effect of Emulsion Extracted Brevilin A, a JAK3 Inhibitor, from Centipeda minima. Processes, 8(7), 767. https://doi.org/10.3390/pr8070767







