Hydrothermal–Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Avocado Starch Extraction by MAE
Optimization of Starch Extraction
2.3. Avocado Starch Extraction by a Conventional Method (CONV)
2.4. Starch Characterization
2.4.1. Water Absorption and Solubility of Starch
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Wide-Angle X-Ray Scattering
2.4.7. Molecular Exclusion Chromatography
2.5. Statistical Analyses
3. Results and Discussion
3.1. Microwave-Assisted Extraction of Avocado Starch
3.2. Statistical Analysis and Optimization of Starch Extraction
3.3. Avocado Starch Extraction MAE NO–ISO and Conventional Extraction
3.4. Starch Characterization
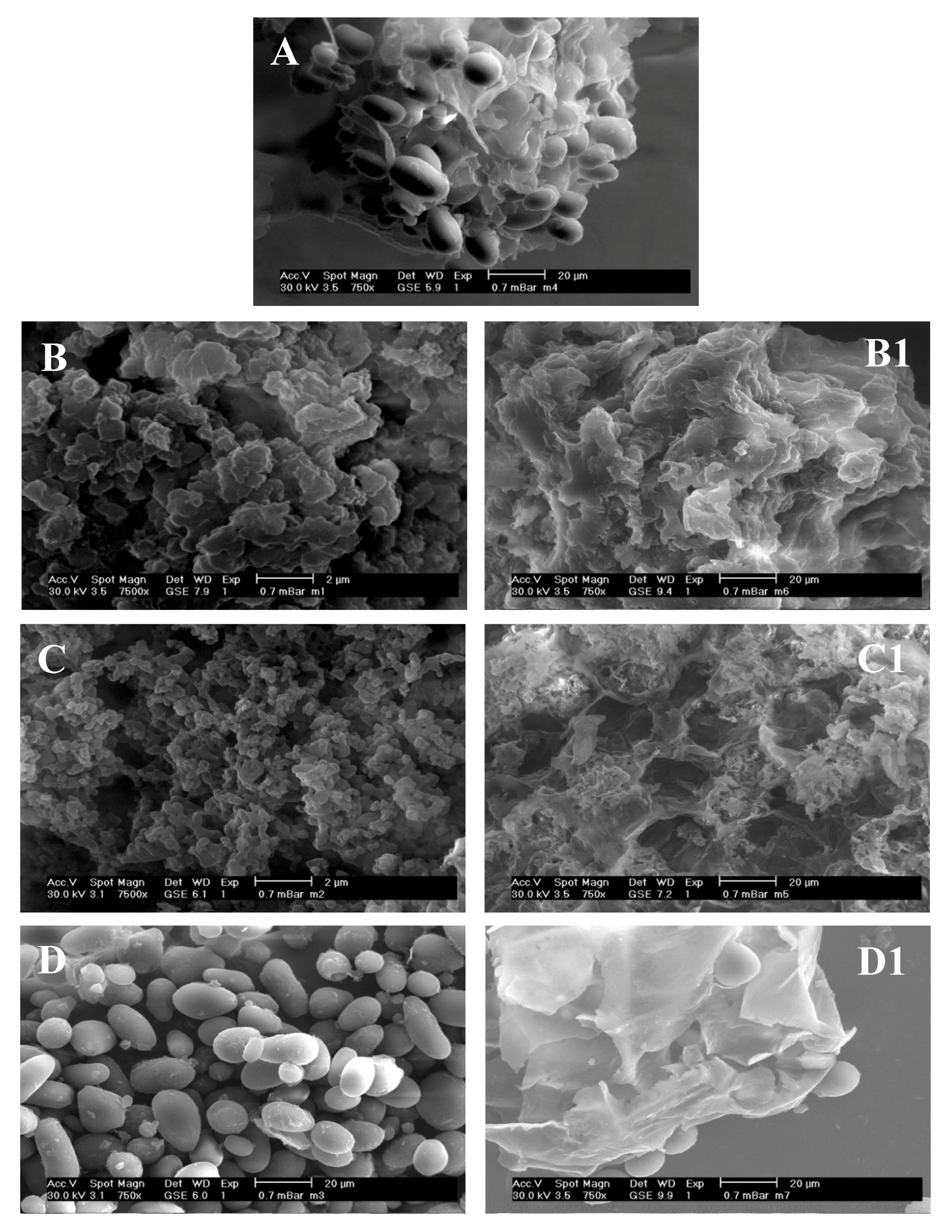
3.4.1. Scanning Electron Microscopy (SEM)
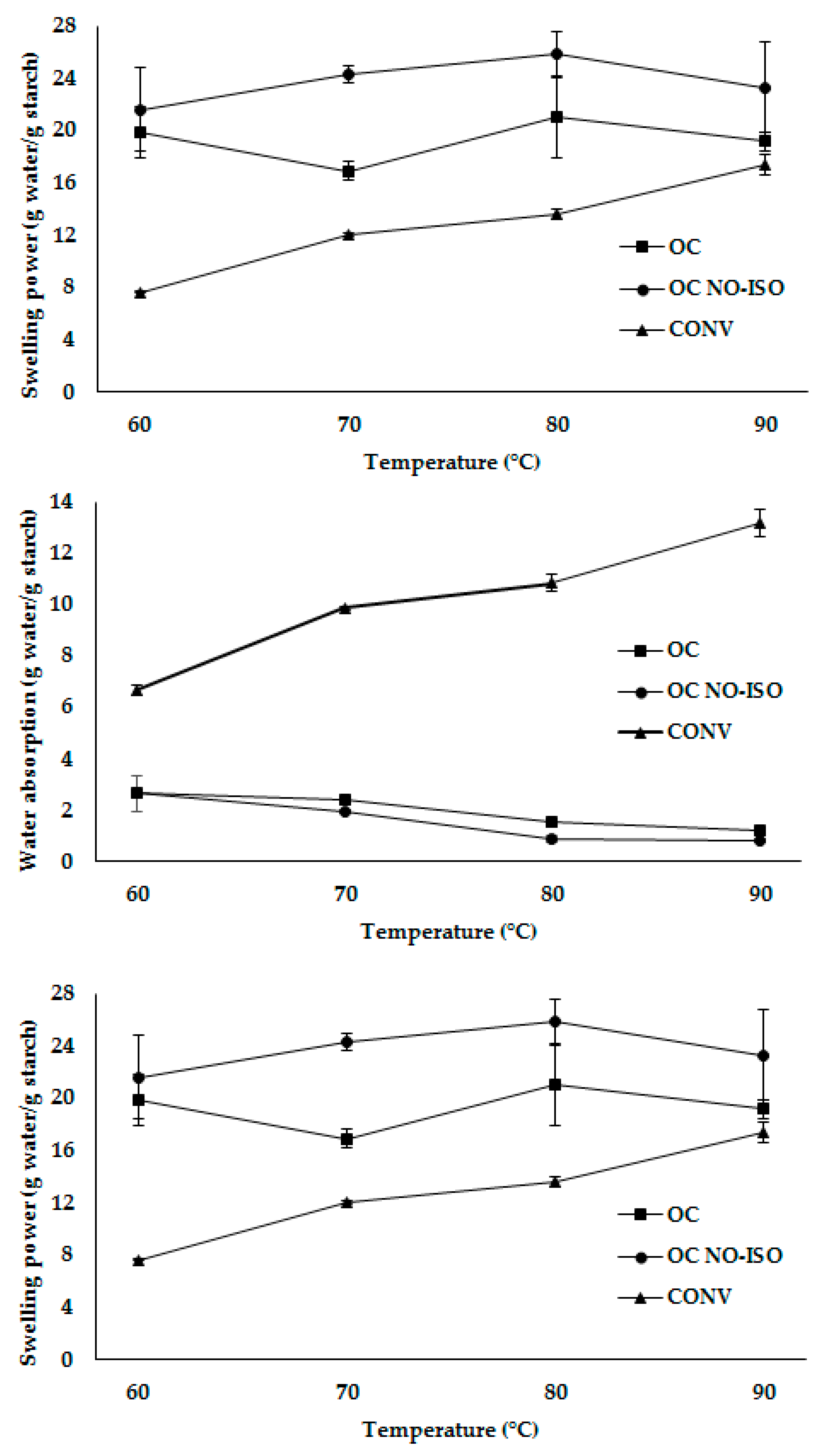
3.4.2. Solubility, Swelling Power (SP) and Water Absorption Capacity (WAC) of Starch
3.4.3. Thermogravimetric Analysis
3.4.4. Differential Scanning Calorimetry
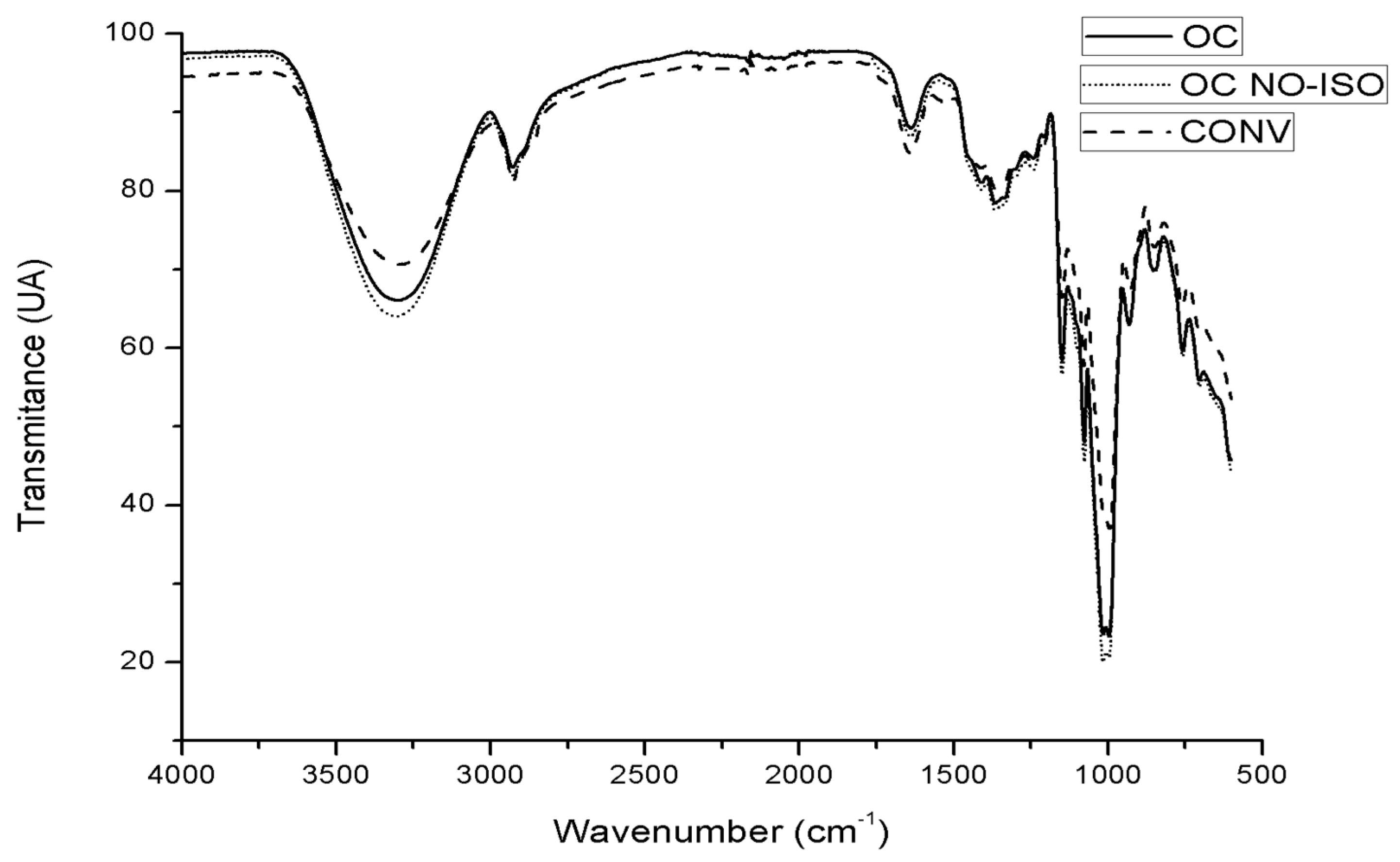
3.4.5. Fourier-Transform Infrared Spectroscopy (FTIR)
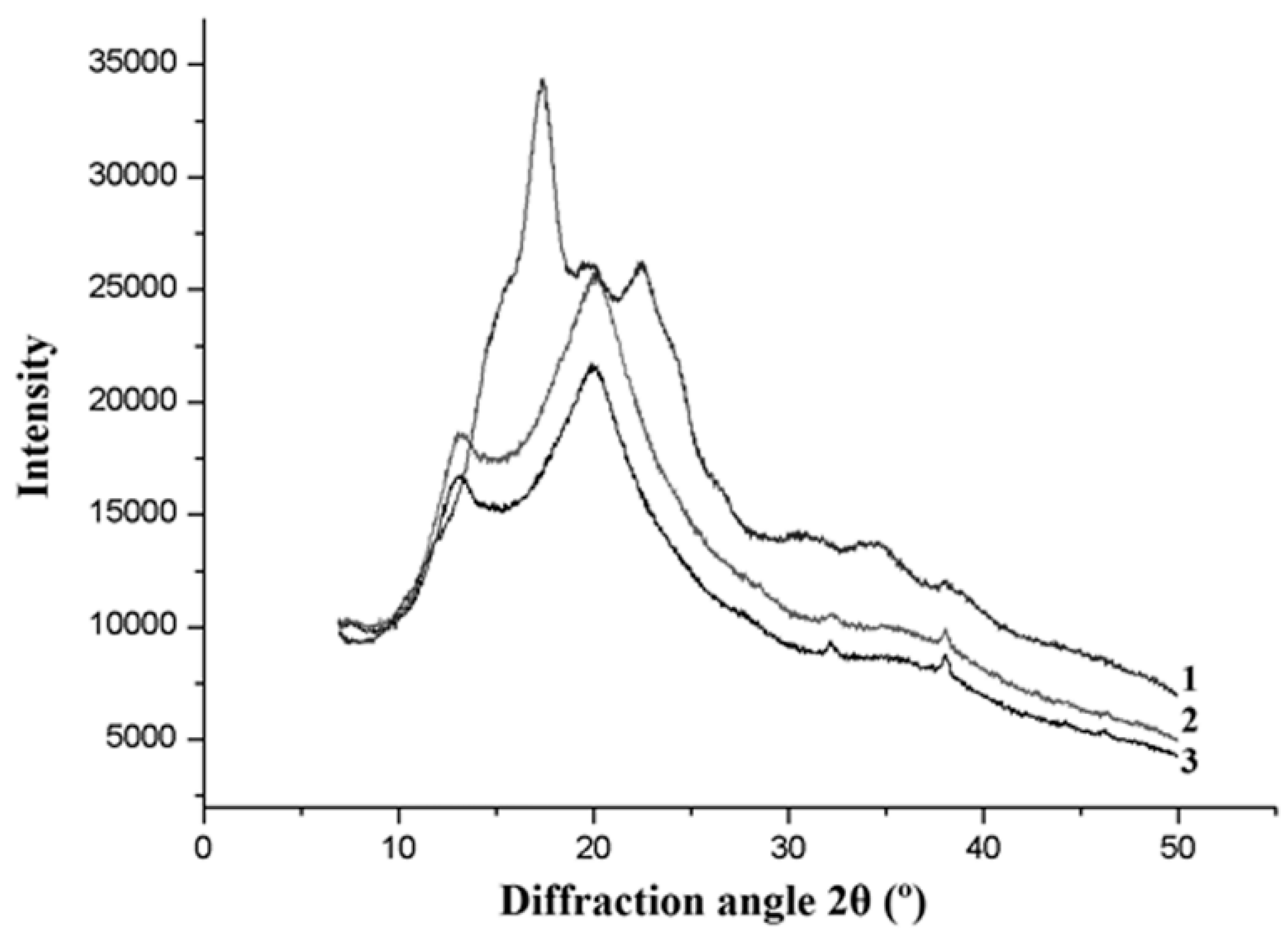
3.4.6. X-Ray Diffraction (XRD)
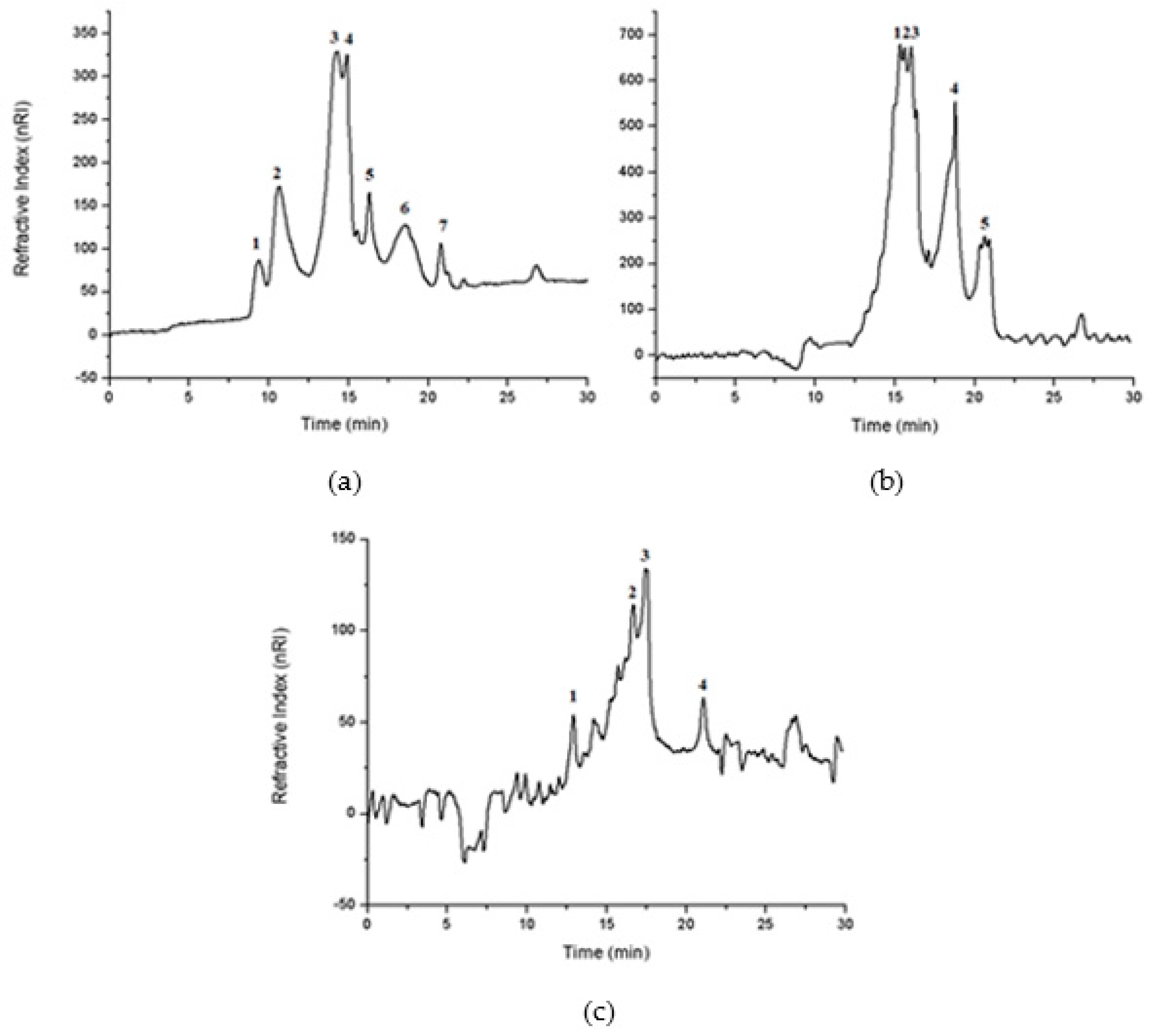
3.4.7. Molecular Exclusion Chromatography (GPC)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvarez, L.D.; Moreno, A.O.; Ochoa, F.G. Avocado. In Tropical and Subtropical Fruits: Postharvest Physiology, Processing and Packaging; Wiley-BlackWell: Hoboken, NJ, USA, 2012; pp. 437–454. [Google Scholar]
- Cowan, A.K.; Wolstenholme, B.N. Avocado. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 294–300. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Agriculture Database. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 11 February 2020).
- SIAP. Servicio de Información Agroalimentaria y Pesquera. 2020. Available online: http://infosiap.siap.gob.mx/aagricola_siap_gb/ientidad/index.jsp (accessed on 27 February 2020).
- Abubakar, A.N.F.; Achmadi, S.S.; Suparto, I.H. Triterpenoid of Avocado (Persea Americana) Seed and Its Cytotoxic Activity toward Breast MCF-7 and Liver HepG2 Cancer Cells. Asian Pac. J. Trop. Biomed. 2017, 7, 397–400. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamac, M.; Estrella, I.; Hernandez, T.; Bartolomé, B.; Dykes, G.A. Phenolic Compound Profiles and Antioxidant Capacity of Persea Americana Mill. Peels and Seeds of Two Varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Andrade, M.-J.; Kylli, P.; Estévez, M. Avocado (Persea Americana Mill.) Phenolics, in Vitro Antioxidant and Antimicrobial Activities, and Inhibition of Lipid and Protein Oxidation in Porcine Patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, N.; Castellani, V.; Sala, S. Current Options for the Valorization of Food Manufacturing Waste: A Review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Lacerda, L.G.; Colman, T.A.D.; Bauab, T.; Da Silva Carvalho Filho, M.A.; Demiate, I.M.; De Vasconcelos, E.C.; Schnitzler, E. Thermal, Structural and Rheological Properties of Starch from Avocado Seeds (Persea Americana, Miller) Modified with Standard Sodium Hypochlorite Solutions. J. Therm. Anal. Calorim. 2014, 115, 1893–1899. [Google Scholar] [CrossRef]
- Khlestkin, V.K.; Peltek, S.E.; Kolchanov, N.A. Review of Direct Chemical and Biochemical Transformations of Starch. Carbohydr. Polym. 2018, 181, 460–476. [Google Scholar] [CrossRef]
- Zhu, F. Structures, Properties, Modifications, and Uses of Oat Starch. Food Chem. 2017, 229, 329–340. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Ascheri, D.P.R.; Bukzem, A.L.; Morais, C.C.; Carvalho, C.W.P.; Ascheri, J.L.R. Physicochemical properties of starch from avocado seed (Persea Americana Mill). Bol. Cent. Pesqui. Process. Aliment. 2016, 34, 1–12. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Blácido, D.R. Isolation and Characterization of Starch from Babassu Mesocarp. Food Hydrocoll. 2016, 55, 47–55. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-Products: Nutritional and Functional Properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.; Barbosa-Martín, E.; Martínez-Antonio, A.; González-Mondragón, E.; Betancur-Ancona, D. Some Physicochemical and Rheological Properties of Starch Isolated from Avocado Seeds. Int. J. Biol. Macromol. 2016, 86, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Structure, Functionality and Applications of Debranched Starch: A Review. Trends Food Sci. Technol. 2017, 63, 70–79. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Whitney, K.; Reuhs, B.L.; Doehlert, D.C.; Simsek, S. Effect of Hydrothermal Treatment on Physicochemical and Digestibility Properties of Oat Starch. Food Res. Int. 2013, 52, 17–25. [Google Scholar] [CrossRef]
- Lacerda, L.G.; Da Silva Carvalho Filho, M.A.; Bauab, T.; Demiate, I.M.; Colman, T.A.D.; Andrade, M.M.P.; Schnitzler, E. The Effects of Heat-Moisture Treatment on Avocado Starch Granules: Thermoanalytical and Structural Analysis. J. Therm. Anal. Calorim. 2015, 120, 387–393. [Google Scholar] [CrossRef]
- Hsieh, C.; Liu, W.; Whaley, J.K.; Shi, Y. Structure, Properties, and Potential Applications of Waxy Tapioca Starches—A Review. Trends Food Sci. Technol. 2019, 83, 225–234. [Google Scholar] [CrossRef]
- Builders, P.F.; Nnurum, A.; Mbah, C.C.; Attama, A.A.; Manek, R. The Physicochemical and Binder Properties of Starch from Persea Americana Miller (Lauraceae). Starch/Staerke 2010, 62, 309–320. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of High Added-Value Components from Food Wastes: Conventional, Emerging Technologies and Commercialized Applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Lara-Flores, A.A.; Araujo, R.G.; Rodrigues, R.M.; Aguedo, M.; Aguilar, C.N.; Trajano, H.L.; Ruiz, H.A. Bioeconomy and Biorefinery: Valorization of Hemicellulose from Lignocellulosic Biomass and Potential Use of Avocado Residues as a Promising Resource of Bioproducts. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 141–170. [Google Scholar]
- Kala, H.K.; Mehta, R.; Sen, K.K.; Tandey, R.; Mandal, V. Critical Analysis of Research Trends and Issues in Microwave Assisted Extraction of Phenolics: Have We Really Done Enough. Trends Anal. Chem. 2016, 85, 140–152. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, X.H.; Wang, Y. Microwave Assisted Extraction of Secondary Metabolites from Plants: Current Status and Future Directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Chemat, F.; Cravotto, G. Microwave-Assisted Extraction for Bioactive Compounds; Theroy and Practise (Volume 4); Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Silva, I.R.A.; Magnani, M.; de Albuquerque, F.S.M.; Batista, K.S.; de Souza Aquino, J.; Queiroga-Neto, V. Characterization of the Chemical and Structural Properties of Native and Acetylated Starches from Avocado (Persea Americana Mill.) Seeds. Int. J. Food Prop. 2017, 20, S279–S289. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Methods 932.06, 925.09, 923.03, 991.43; Association of Official Analytical Chemists: Gaithesburg, MD, USA, 1995. [Google Scholar]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Comparison of Microwave and Conduction-Convection Heating Autohydrolysis Pretreatment for Bioethanol Production. Bioresour. Technol. 2017, 243, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.A.; Cerqueira, M.A.; Silva, H.D.; Rodríguez-Jasso, R.M.; Vicente, A.A.; Teixeira, J.A. Biorefinery Valorization of Autohydrolysis Wheat Straw Hemicellulose to Be Applied in a Polymer-Blend Film. Carbohydr. Polym. 2013, 92, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Abaide, E.R.; Zabot, G.L.; Tres, M.V.; Martins, R.F.; Fagundez, J.L.; Nunes, L.F.; Druzian, S.; Soares, J.F.; Dal Prá, V.; Silva, J.R.F.; et al. Yield, Composition, and Antioxidant Activity of Avocado Pulp Oil Extracted by Pressurized Fluids. Food Bioprod. Process. 2017, 102, 289–298. [Google Scholar] [CrossRef]
- De la Torre-Gutiérrez, L.; Chel-Guerrero, L.A.; Betancur-Ancona, D. Functional Properties of Square Banana (Musa Balbisiana) Starch. Food Chem. 2008, 106, 1138–1144. [Google Scholar] [CrossRef]
- Zortéa-Guidolin, M.E.B.; Demiate, I.M.; de Godoy, R.C.B.; de Paula Scheer, A.; Grewell, D.; Jane, J.-L. Structural and Functional Characterization of Starches from Brazilian Pine Seeds (Araucaria Angustifolia). Food Hydrocoll. 2017, 63, 19–26. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Y.; Li, X.; Chen, L.; Xie, F. Supramolecular Structure of Jackfruit Seed Starch and Its Relationship with Digestibility and Physicochemical Properties. Carbohydr. Polym. 2016, 150, 269–277. [Google Scholar] [CrossRef]
- Song, Y.; Jane, J. Characterization of Barley Starches of Waxy, Normal, and High Amylose Varieties. Carbohydr. Polym. 2000, 41, 365–377. [Google Scholar] [CrossRef]
- Buksa, K. Extraction and Characterization of Rye Grain Starch and Its Susceptibility to Resistant Starch Formation. Carbohydr. Polym. 2018, 194, 184–192. [Google Scholar] [CrossRef]
- Aguilar-González, M.Á.; Gorokhovsky, A.V.; Aguilar-Elguezabal, A. Removal of Lead and Nickel from Aqueous Solutions by SiO2 Doped Potassium Titanate. Mater. Sci. Eng. B 2010, 174, 105–113. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the Structural Order of Native Starch Granules Using Combined FTIR and XRD Analysis. J. Polym. Res. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Luchese, C.; Garrido, T.; Corralo Spada, J.; Cristina Tessaro, I.; de la Caba, K. Development and Characterization of Cassava Starch Films Incorporated with Blueberry Pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, W.; Zhao, X.; Liu, Y.; Gao, W. Study on the Morphology, Crystalline Structure and Thermal Properties of Yellow Ginger Starch Acetates with Different Degrees of Substitution. Thermochim. Acta J. 2009, 495, 57–62. [Google Scholar] [CrossRef]
- Nie, H.; Li, C.; Liu, P.; Lei, C.; Li, J. Retrogradation, Gel Texture Properties, Intrinsic Viscosity and Degradation Mechanism of Potato Starch Paste under Ultrasonic Irradiation. Food Hydrocoll. 2017, 95, 590–600. [Google Scholar] [CrossRef]
- Kolling, W.M. Physical, Thermal and Sorption Profile of Starch Obtained from Tacca Leontopetaloides. Starch/Starke 2005, 57, 55–61. [Google Scholar]
- Fan, D.; Ma, S.; Wang, L.; Zhao, J.; Zhang, H.; Chen, W. Effect of Microwave Heating on Optical and Thermal Properties of Rice Starch. Starch/Starke 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, M.; Yuan, S.; Sun, S.; Huo, Q. Effect of Microwave Treatment on the Physicochemical Properties of Potato Starch Granules. Chem. Cent. J. 2013, 7, 1–7. [Google Scholar] [CrossRef]
- Do Prado Cordoba, L.; Ribeiro, L.S.; Rosa, L.S.; Lacerda, L.G.; Schnitzler, E. Effect of Enzymatic Treatments on Thermal, Rheological and Structural Properties of Pinhão Starch. Thermochim. Acta 2016, 642, 45–51. [Google Scholar] [CrossRef]
- Beninca, C.; Colman, D.; Gustavo, L.; Beninca, C.; Andre, T.; Aure, M.; Bannach, G.; Schnitzler, E. Thermal, Rheological, and Structural Behaviors of Natural and Modified Cassava Starch Granules, with Sodium Hypochlorite Solutions. J. Therm. Anal. Calorim. 2013, 111, 2217–2222. [Google Scholar] [CrossRef]
- Malumba, P.; Doran, L.; Zanmenou, W.; Odjo, S.; Katanga, J.; Blecker, C.; Béra, F. Morphological, Structural and Functional Properties of Starch Granules Extracted from the Tubers and Seeds of Sphenostylis Stenocarpa. Carbohydr. Polym. 2017, 178, 286–294. [Google Scholar] [CrossRef]
- Wijaya, C.; Diem, Q.; Hsu, Y.; Permatasari, S.; Nyoo, J.; Laysandra, L.; Edi, F.; Ismadji, S. Heliyon Isolation and Characterization of Starch from Limnophila Aromatica. Heliyon 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Luo, F.; Huang, Q.; Fu, X.; Zhang, L.; Yu, S. Preparation and Characterisation of Crosslinked Waxy Potato Starch. Food Chem. 2009, 115, 563–568. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared Spectroscopy as a Tool to Characterise Starch Ordered Structure—A Joint FTIR-ATR, NMR, XRD and DSC Study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Norziah, M.H.; Seow, C.C. Methods for the Study of Starch Retrogradation. Food Chem. 2000, 71, 9–36. [Google Scholar] [CrossRef]
- Ruiz, A.; Rodríguez, R.M.; Fernandes, B.D.; Vicente, A.; Teixeira, A. Hydrothermal Processing, as an Alternative for Upgrading Agriculture Residues and Marine Biomass according to the Biorefinery Concept: A Review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef]
- Rostro, M.; Sánchez-González, M.; Rivas, S.; Moure, A.; Domínguez, H.; Carlos, J. Non-Isothermal Autohydrolysis of Nixtamalized Maize Pericarp: Production of Nutraceutical Extracts. LWT Food Sci. Technol. 2014, 58, 550–556. [Google Scholar] [CrossRef]
- Rigual, V.; Santos, T.M.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Combining Autohydrolysis and Ionic Liquid Microwave Treatment to Enhance Enzymatic Hydrolysis of Eucalyptus Globulus Wood. Bioresour. Technol. 2018, 251, 197–203. [Google Scholar] [CrossRef]
- Santos, T.M.; Alonso, M.V.; Oliet, M.; Domínguez, J.C.; Rigual, V.; Rodriguez, F. Effect of Autohydrolysis on Pinus Radiata Wood for Hemicellulose Extraction. Carbohydr. Polym. 2018, 194, 285–293. [Google Scholar] [CrossRef]
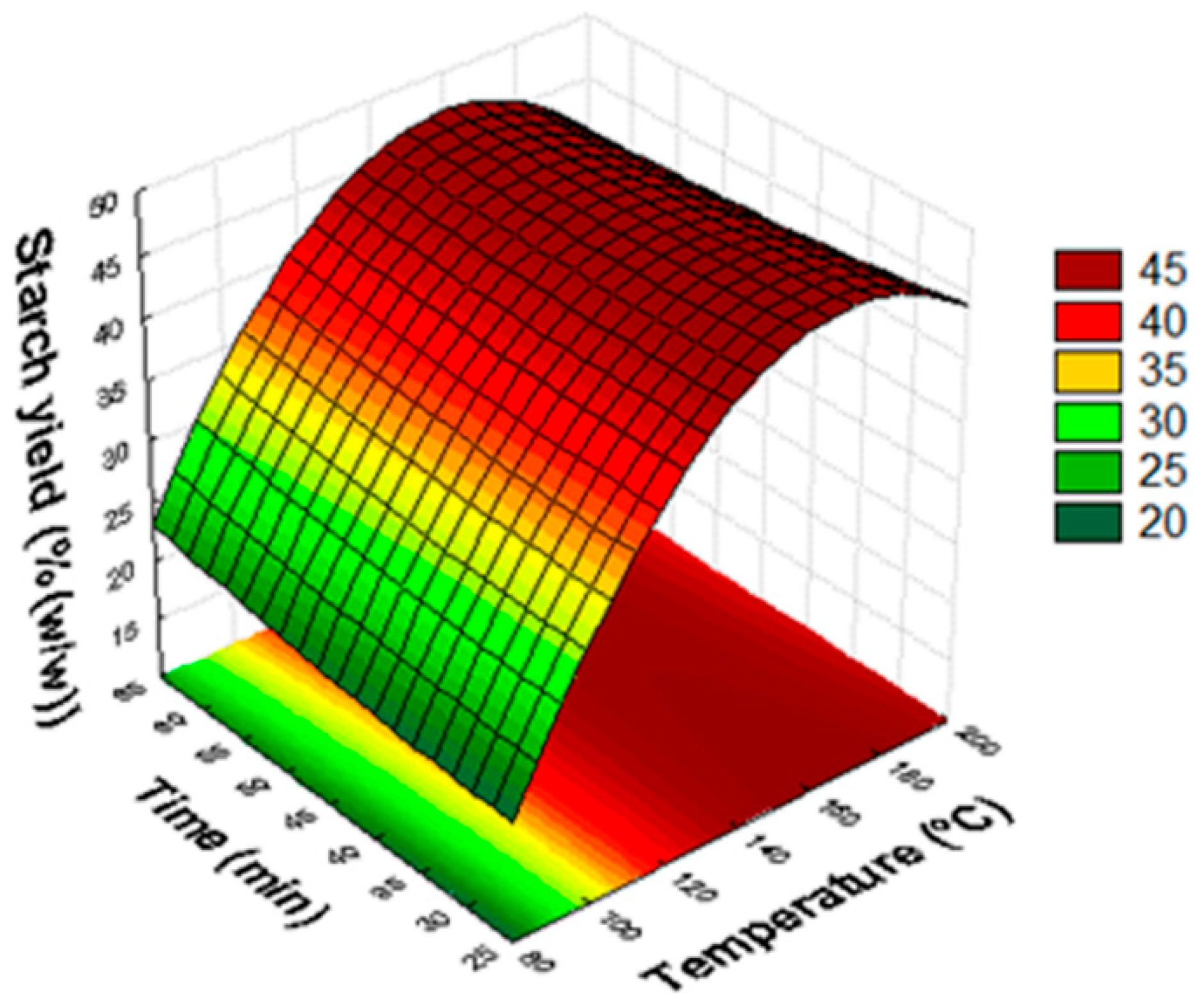
| Assay | Variables | Starch Yield (% (w/w)) | |||
|---|---|---|---|---|---|
| X1 | X2 | ||||
| 1 | 90 | (−1) | 30 | (−1) | 26.13 ± 0.87 |
| 2 | 90 | (–1) | 60 | (1) | 27.48 ± 1.87 |
| 3 | 90 | (–1) | 45 | (0) | 27.36 ± 4.92 |
| 4 | 180 | (1) | 30 | (−1) | 47.31 ± 0.85 |
| 5 | 180 | (1) | 60 | (1) | 44.82 ± 1.00 |
| 6 | 180 | (1) | 45 | (0) | 46.06 ± 0.81 |
| 7 | 135 | (0) | 30 | (−1) | 43.75 ± 0.28 |
| 8 | 135 | (0) | 60 | (1) | 45.48 ± 0.26 |
| 9 | 135 | (0) | 45 | (0) | 42.97 ± 0.53 |
| 10 | 135 | (0) | 45 | (0) | 44.15 ± 1.46 |
| 11 | 135 | (0) | 45 | (0) | 43.88 ± 3.83 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 703.87 | 5 | 140.77 | 196.75 | 0.0001 * |
| x1 | 545.41 | 1 | 545.41 | 759.45 | 0.000001 * |
| x12 | 146.39 | 1 | 146.39 | 203.83 | 0.00003 * |
| x2 | 0.06 | 1 | 0.06 | 0.08 | 0.78802 |
| x22 | 0.24 | 1 | 0.24 | 0.33 | 0.58952 |
| x1 x2 | 3.69 | 1 | 3.69 | 5.15 | 0.07259 |
| Residual | 3.59 | 5 | 0.72 | ||
| Lack of fit | 2.81 | 3 | 0.94 | 2.45 | 0.3027 |
| Pure error | 0.76 | 2 | 0.38 | ||
| Cor total | 707.2153 | 10 | |||
| R2 | 0.9949 | ||||
| Adj R2 | 0.9899 | ||||
| C.V. | 2.12 |
| Parameters | OC Starch | OC NO–ISO Starch | CONV Starch |
|---|---|---|---|
| DSC | |||
| T0 (°C) | 36.86 | 46.62 | 35.94 |
| Tm (°C) | 47.43 | 51.96 | 60.26 |
| Tf (°C) | 58.35 | 63.29 | 74.33 |
| ΔH (J/g) | 113.00 | 218.90 | 562.0 |
| TGA | |||
| 1st | 70.33 | 77.96 | 115.23 |
| 2nd | 280.5 | 280.4 | 299.3 |
| 3rd | 348.28 | 343.08 | 355.74 |
| OC | OC NO–ISO | CONV | ||||
|---|---|---|---|---|---|---|
| Peak | Mp (g/mol) | Pd | Mp (g/mol) | Pd | Mp (g/mol) | Pd |
| 1 | 190,732,143 * | 1.128 | 165,387 | 1.020 | 2261,719 * | 1.037 |
| 2 | 27,003,383 * | 1.201 | 120,484 | 1.011 | 38,172 | 1.021 |
| 3 | 512,653 | 1.194 | 78,030 | 1.053 | 16,601 | 1.086 |
| 4 | 262,398 | 1.036 | 3866 | 1.281 | 324 | 1.062 |
| 5 | 57,883 | 1.065 | 533 | 1.155 | ||
| 6 | 5165 | 1.386 | ||||
| 7 | 441 | 1.049 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, R.G.; Rodríguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Rosas-Flores, W.; Aguilar-González, M.A.; Pintado, M.E.; Lopez-Badillo, C.; Luevanos, C.; Aguilar, C.N. Hydrothermal–Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization. Processes 2020, 8, 759. https://doi.org/10.3390/pr8070759
Araújo RG, Rodríguez-Jasso RM, Ruiz HA, Govea-Salas M, Rosas-Flores W, Aguilar-González MA, Pintado ME, Lopez-Badillo C, Luevanos C, Aguilar CN. Hydrothermal–Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization. Processes. 2020; 8(7):759. https://doi.org/10.3390/pr8070759
Chicago/Turabian StyleAraújo, Rafael G., Rosa M. Rodríguez-Jasso, Héctor A. Ruiz, Mayela Govea-Salas, Walfred Rosas-Flores, Miguel Angel Aguilar-González, Manuela E. Pintado, Claudia Lopez-Badillo, Cynthia Luevanos, and Cristobal Noe Aguilar. 2020. "Hydrothermal–Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization" Processes 8, no. 7: 759. https://doi.org/10.3390/pr8070759
APA StyleAraújo, R. G., Rodríguez-Jasso, R. M., Ruiz, H. A., Govea-Salas, M., Rosas-Flores, W., Aguilar-González, M. A., Pintado, M. E., Lopez-Badillo, C., Luevanos, C., & Aguilar, C. N. (2020). Hydrothermal–Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization. Processes, 8(7), 759. https://doi.org/10.3390/pr8070759











