Compact Heat Integrated Reactor System of Steam Reformer, Shift Reactor and Combustor for Hydrogen Production from Ethanol
Abstract
1. Introduction
2. Modeling and Simulation
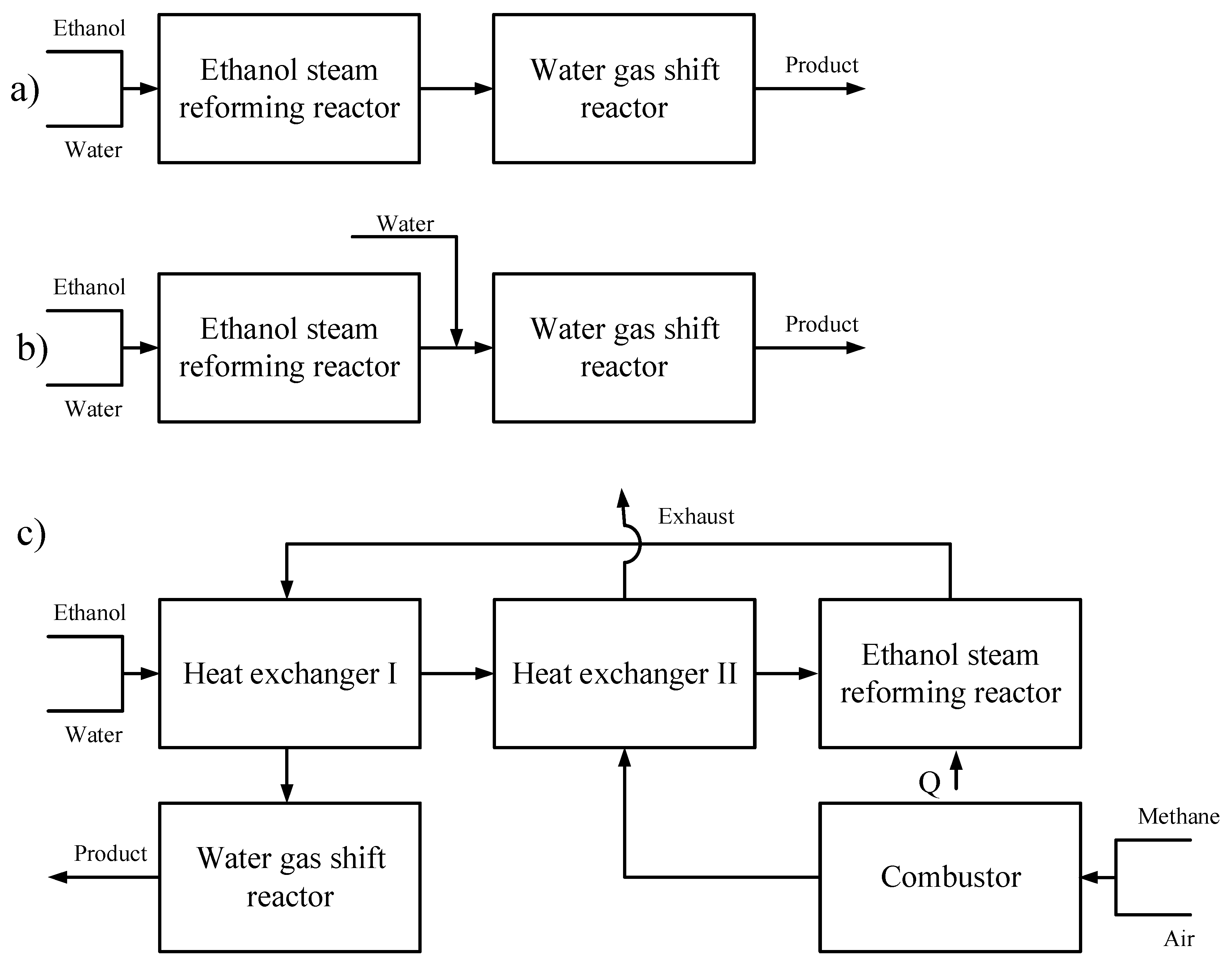
2.1. Description of Reformer Concept Development
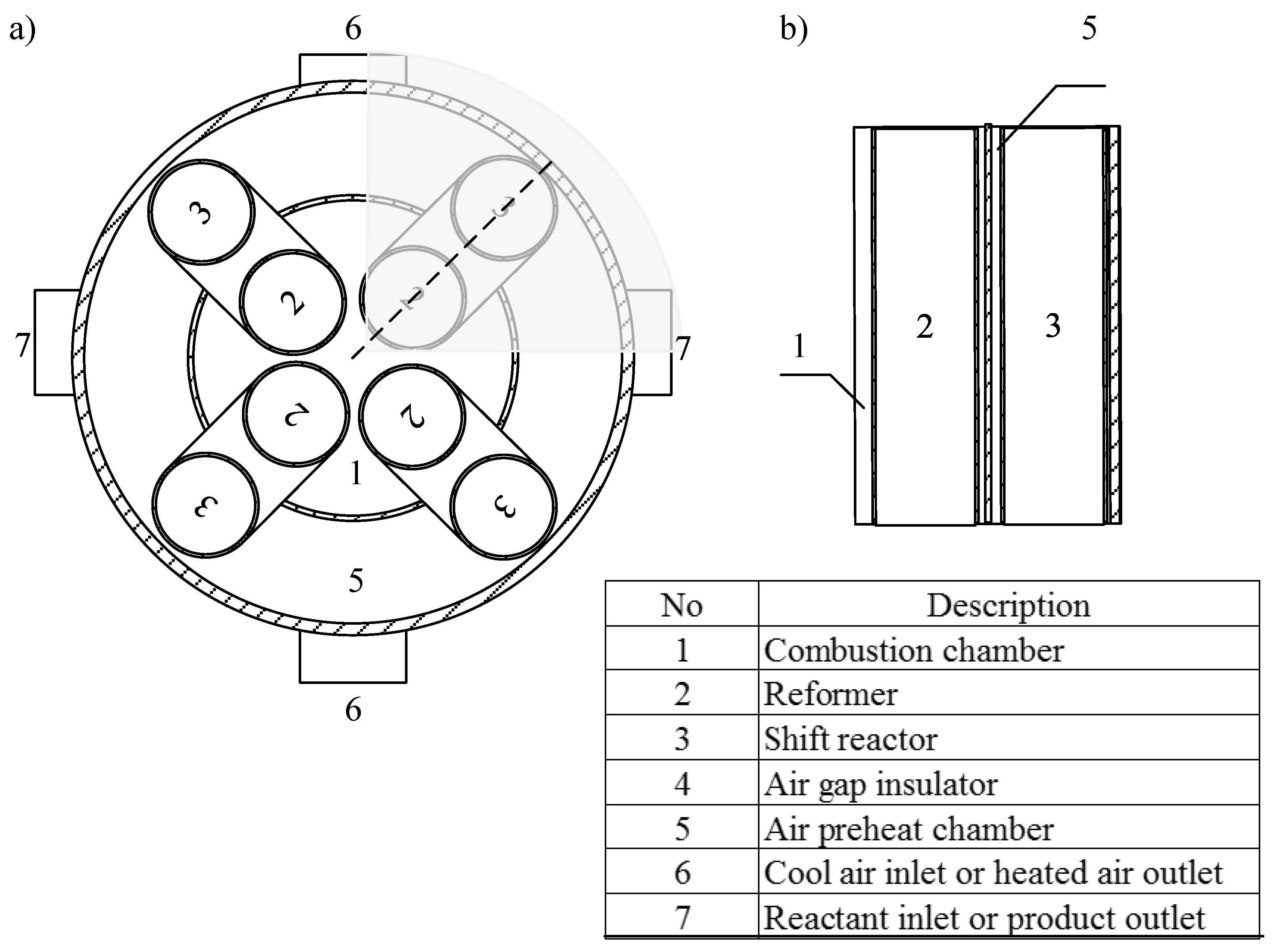
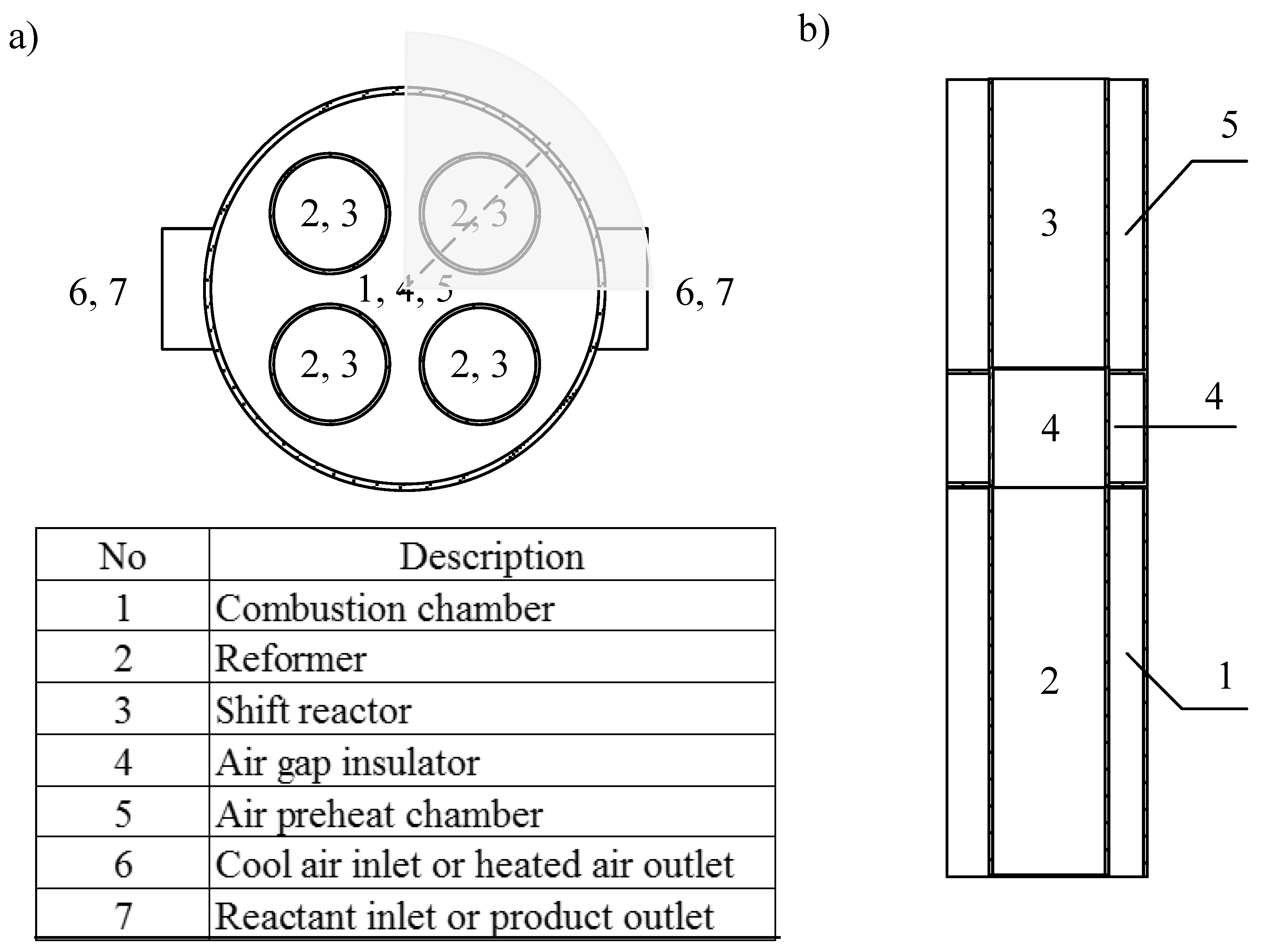
2.2. CHIRS Designs in Detail
3. Results and Discussion
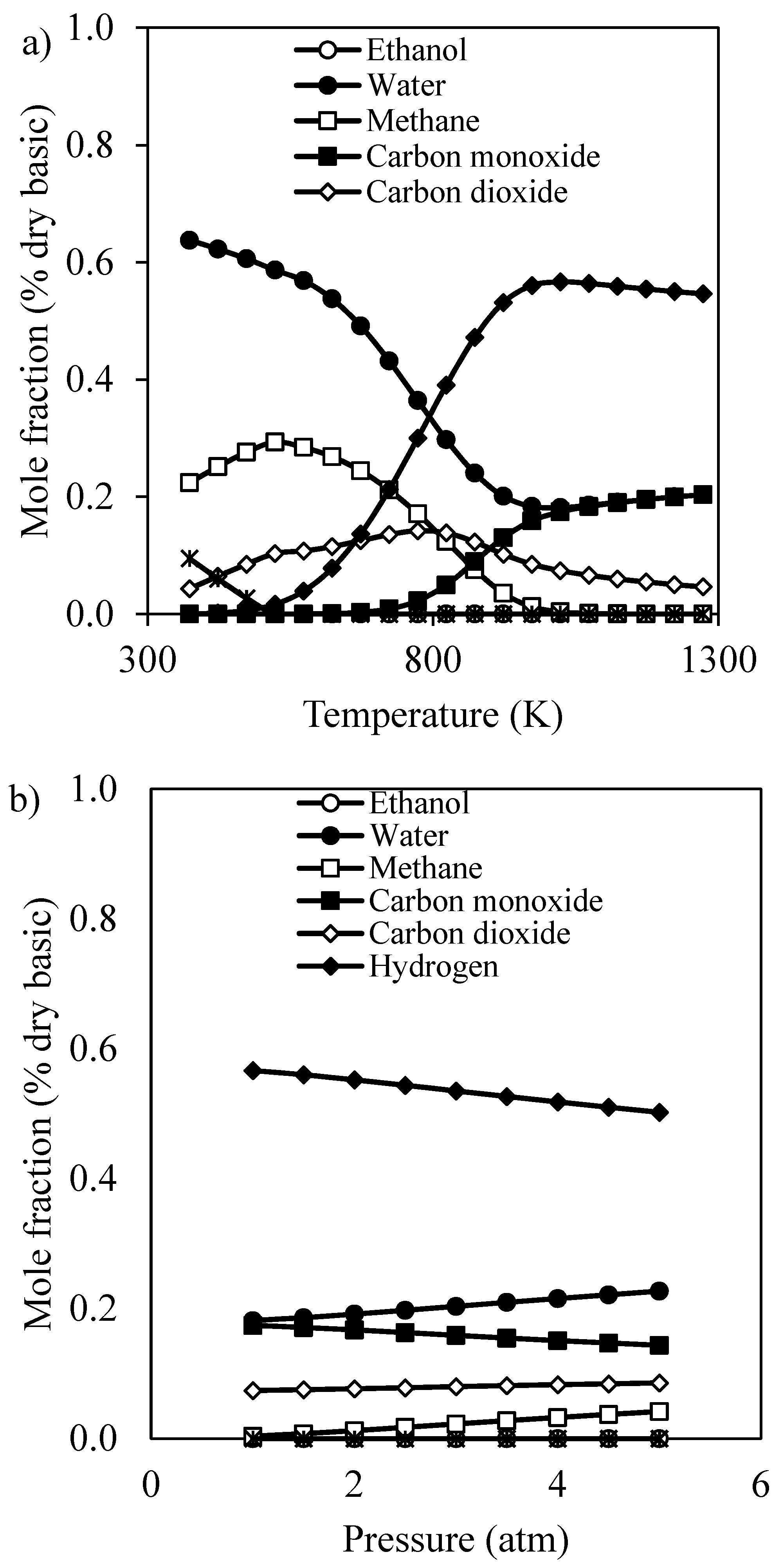
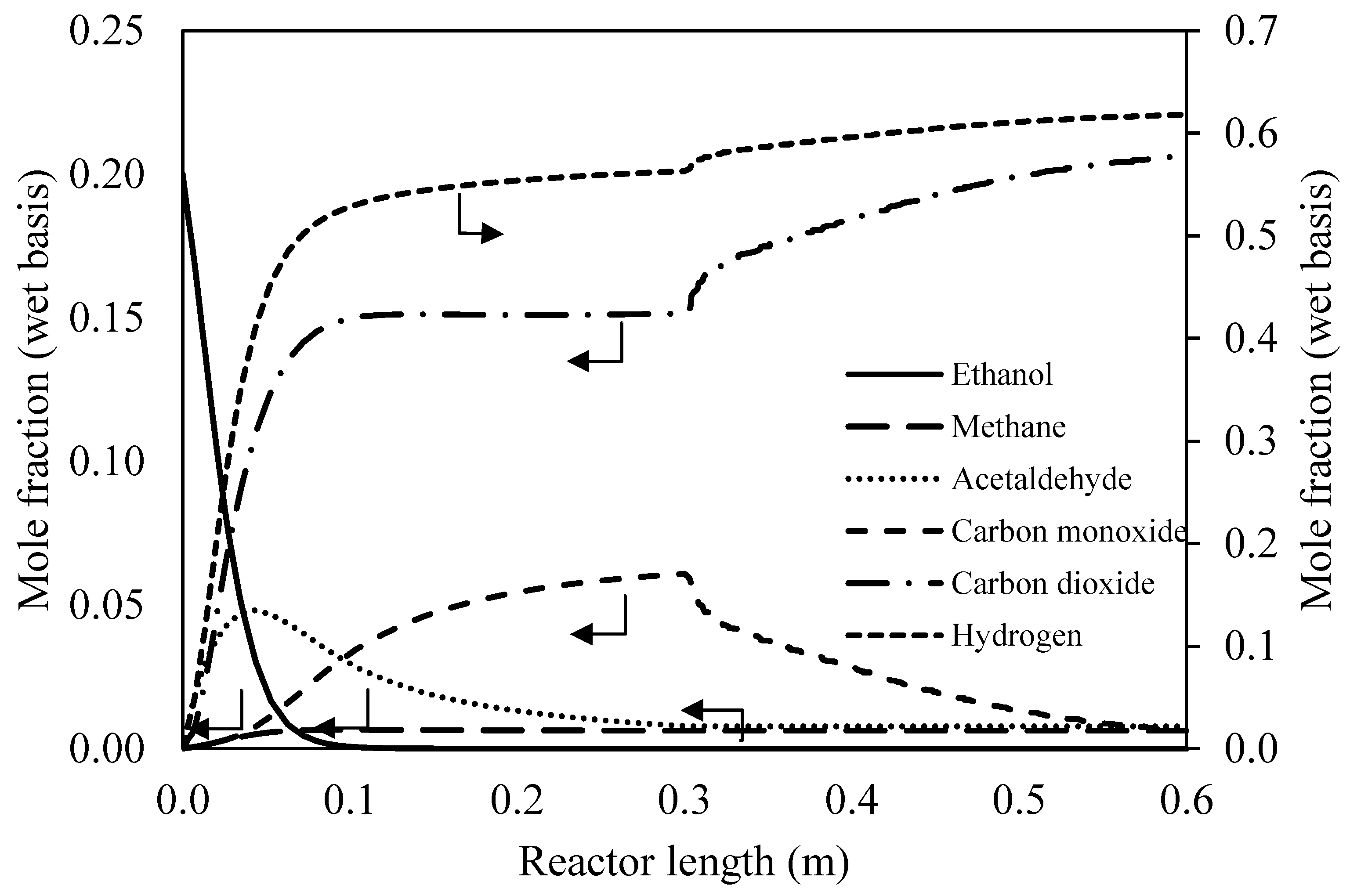
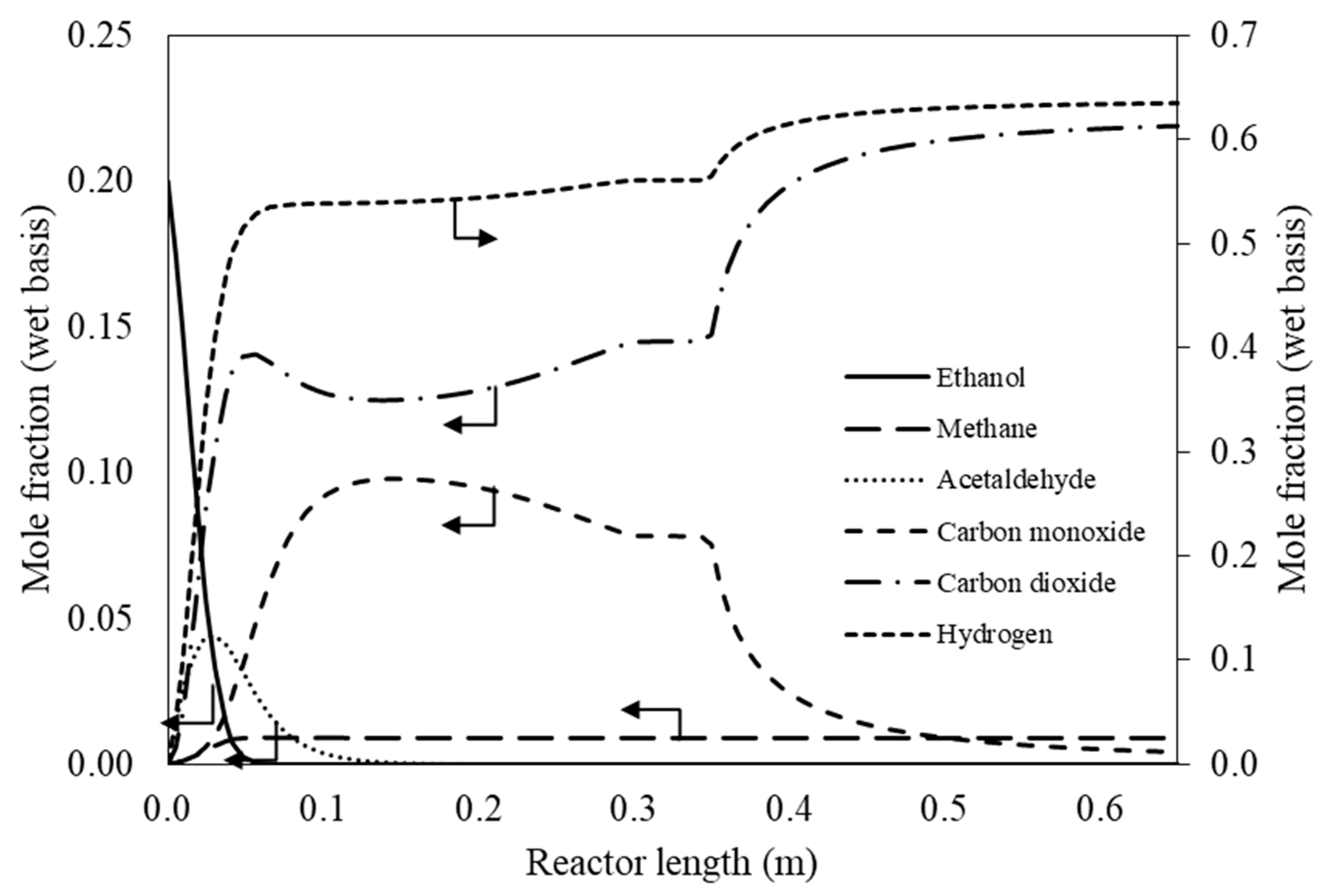
3.1. Preliminary Study of Reformer via Aspen Plus
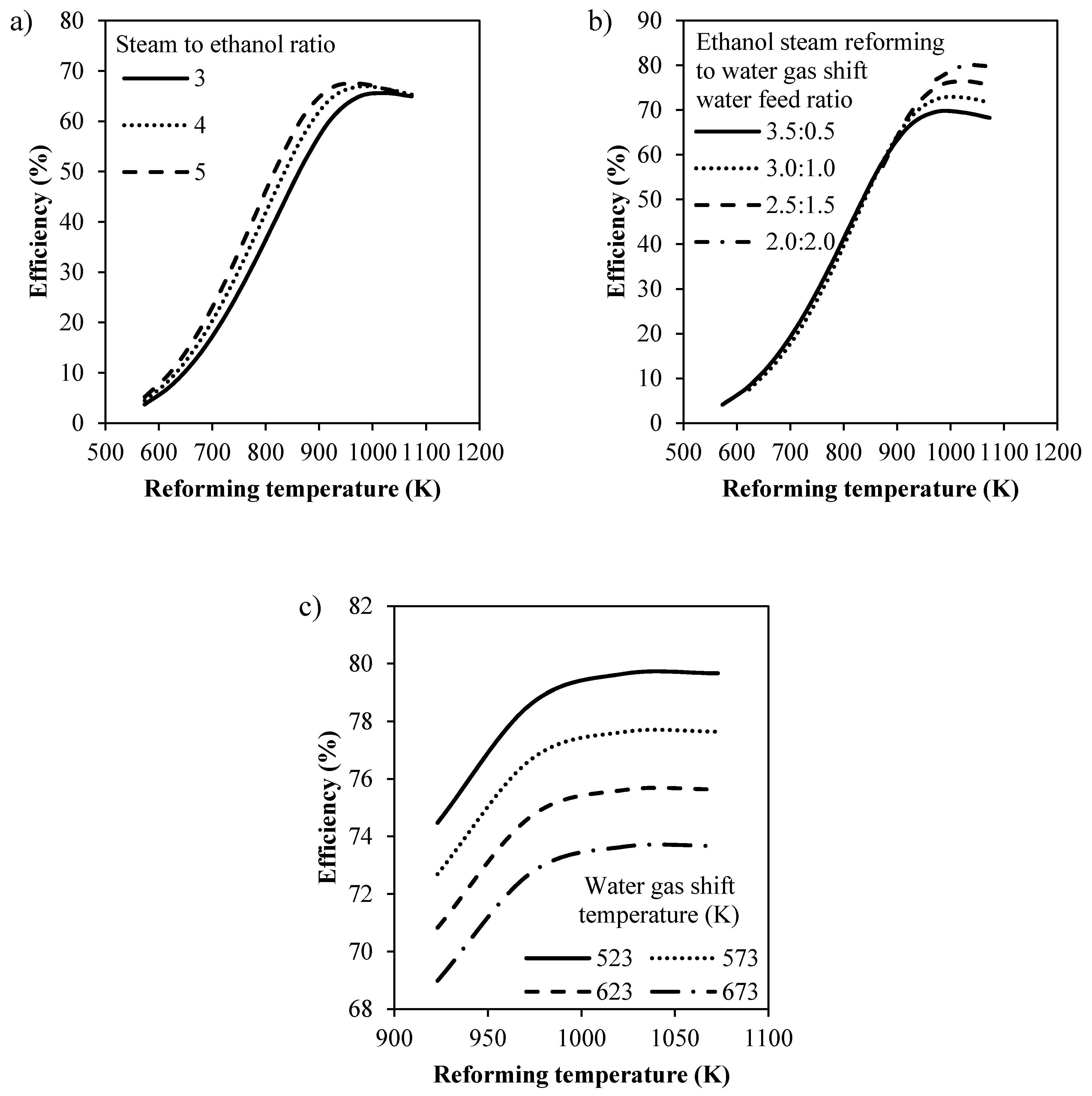
3.1.1. Effect of Operating Conditions on Reaction Performance
3.1.2. Sequential Steam Reformer and Shift Reactor (SRSR)
3.1.3. SRSR with Energy Management by Water Splitting (SRSR-WS)
3.1.4. Compact Heat Integrated Reactor System (CHIRS)
3.2. Study of the Compact Heat Integrated Reactor System (CHIRS) via COMSOL Multiphysics
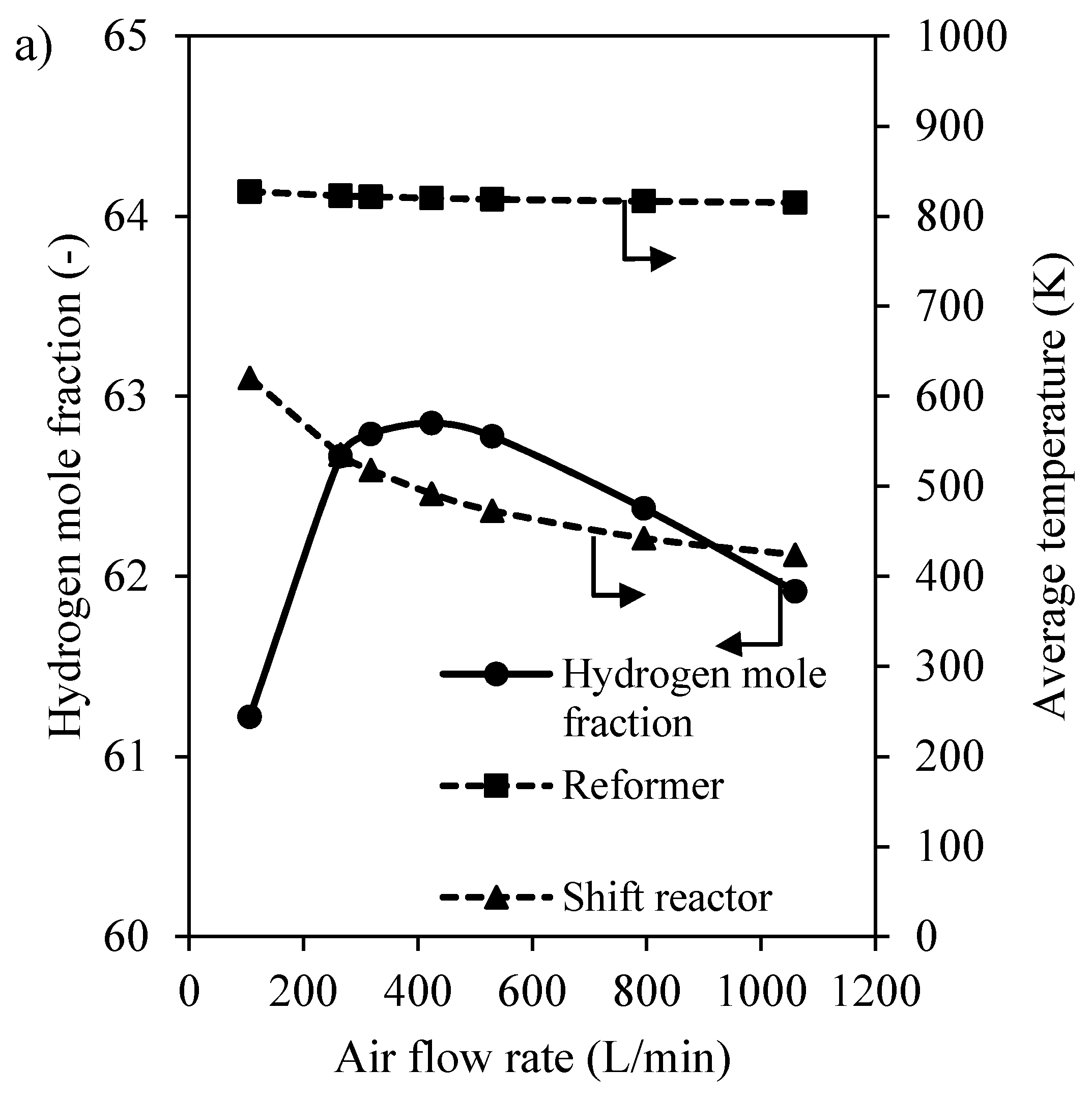
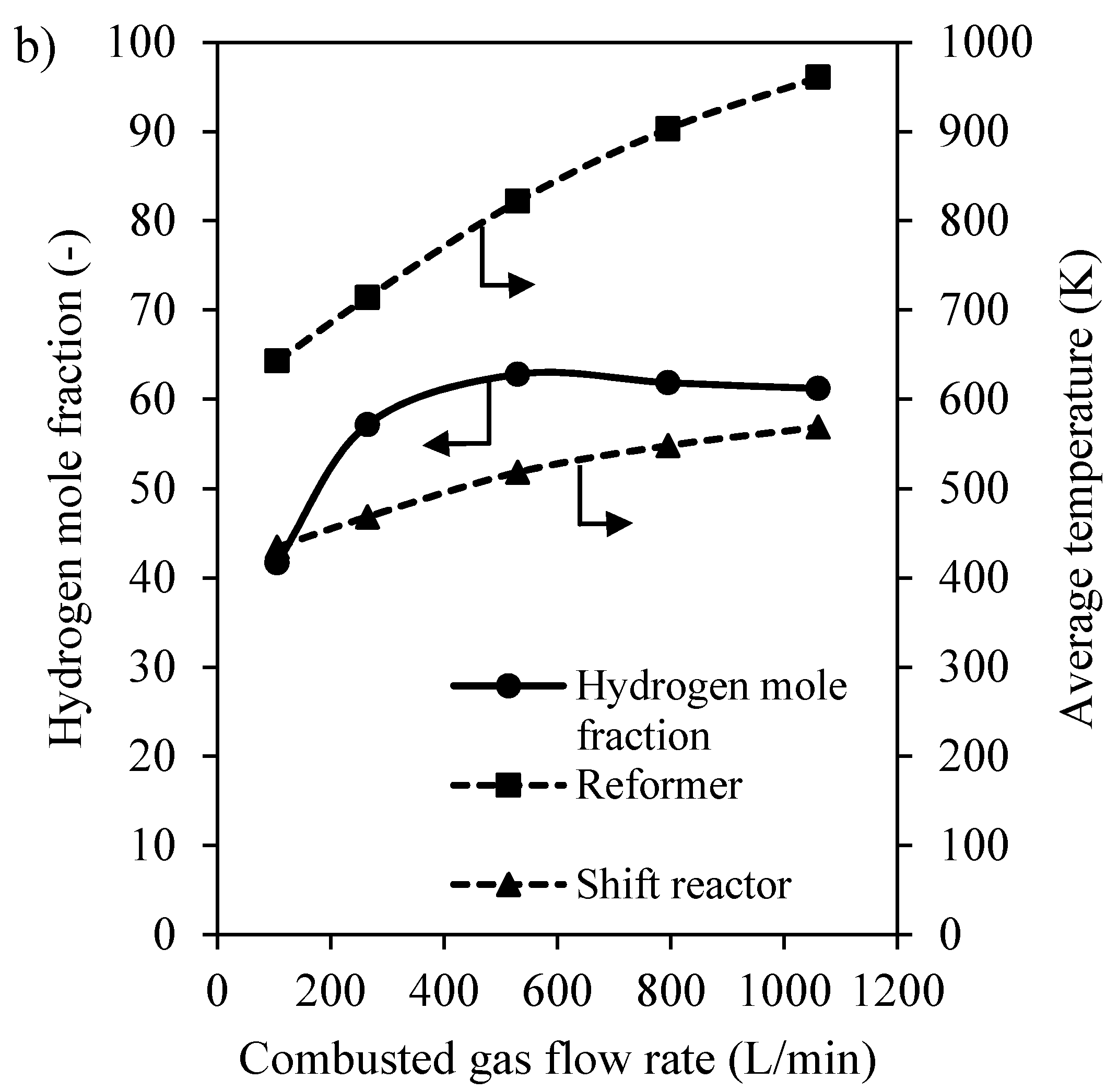
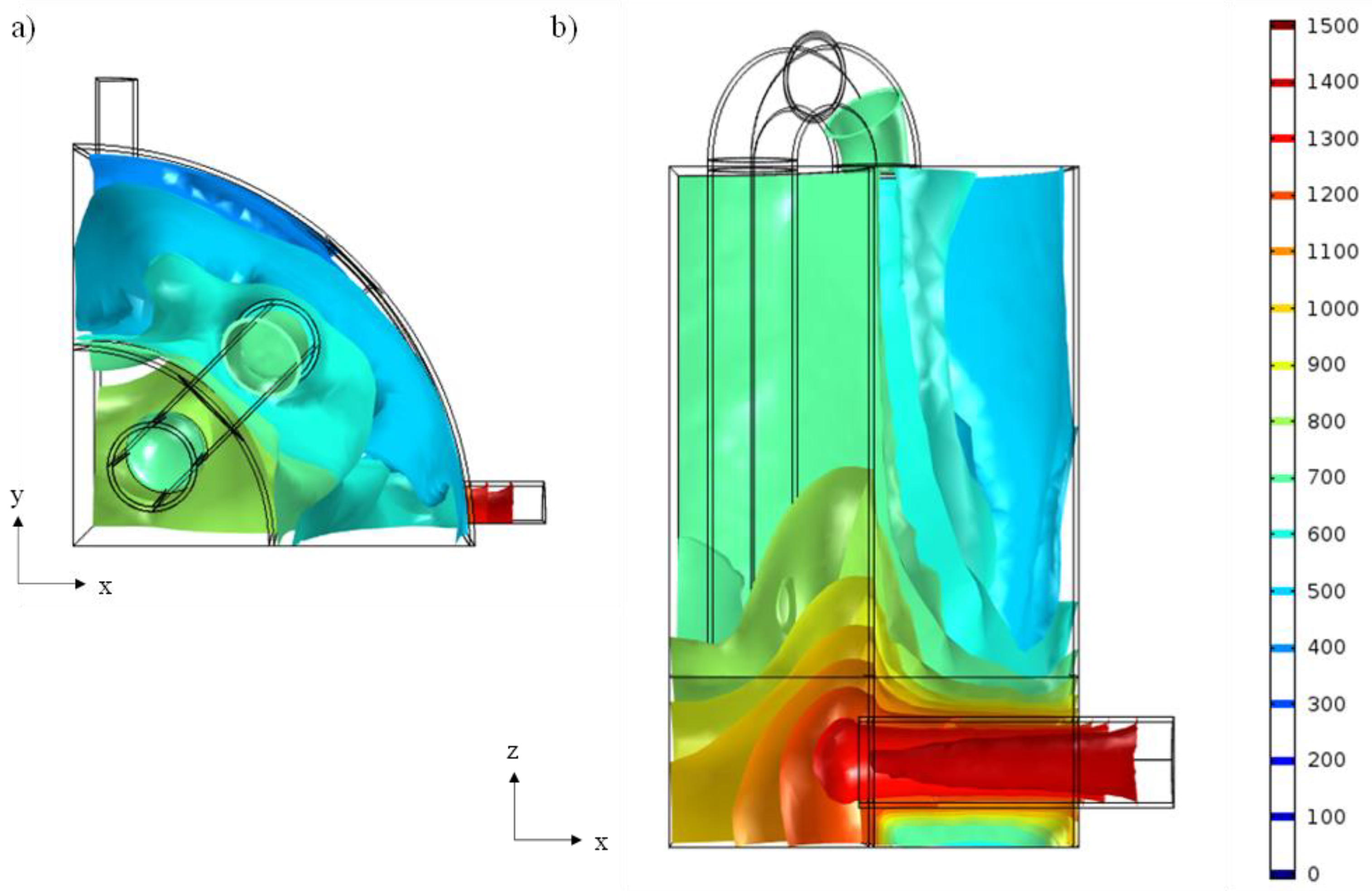
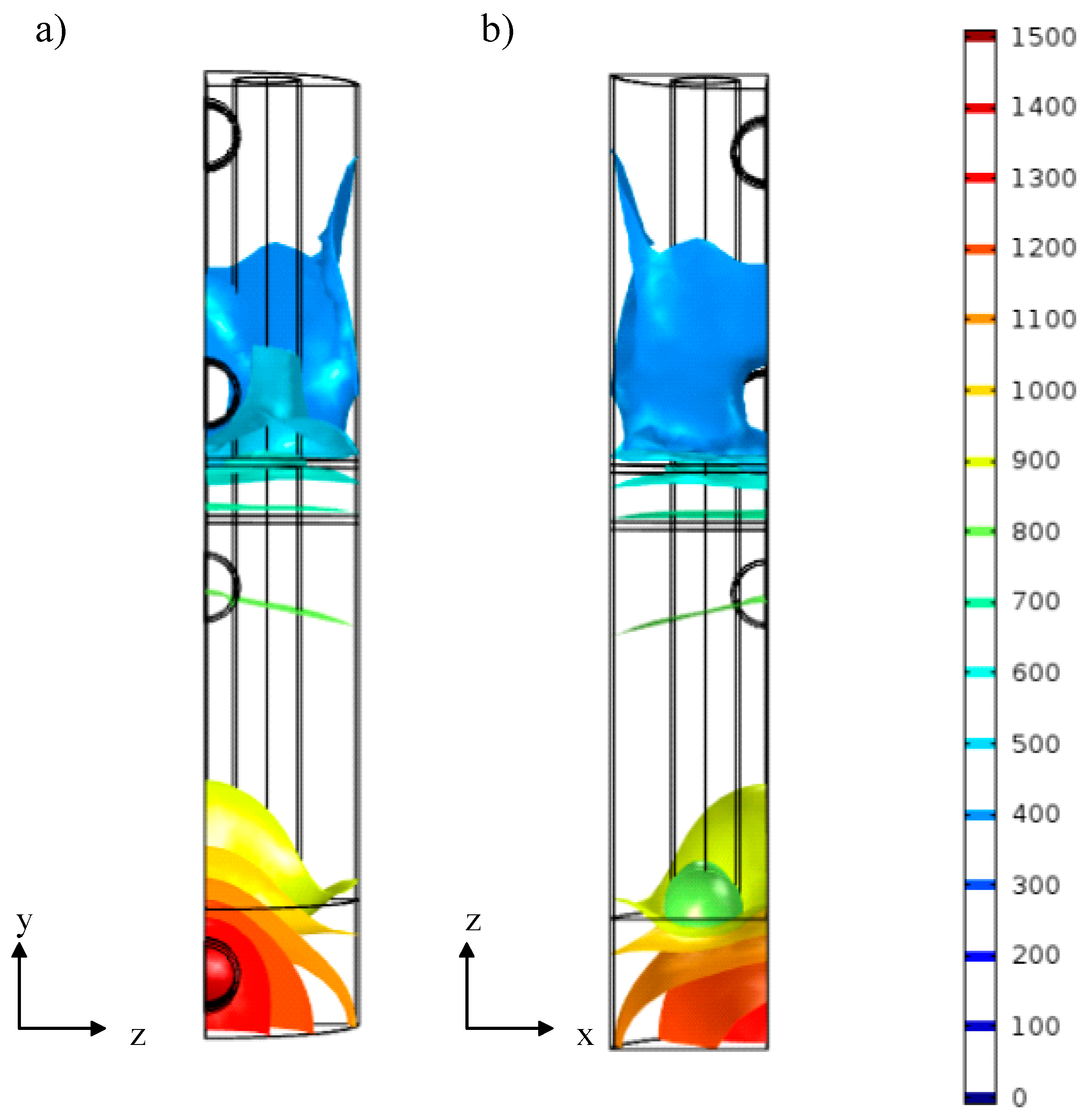
3.2.1. Preliminary Study of CHIRS
3.2.2. Characteristics of CHIRS (I)
3.2.3. Characteristics of CHIRS (II)
3.2.4. Comparison in Process Performance between CHIRS (I) and CHIRS (II)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Specific heat at constant pressure (J kg−1 K−1) | |
| Mass diffusion coefficient of species i in mixture (m2 s−1) | |
| g | Gravity force (m/s2) |
| Heat of reaction j (J/mol) | |
| Heat of combustion of species i (kW mol−1) | |
| Thermal conductivity (W m−1 K−1) | |
| KWGS | Thermodynamics equilibrium constant (-) |
| Molar mass of species i (kg kmol−1) | |
| Mole flow rate (mol s−1) | |
| Pressure (Pa) | |
| Rate of reaction j (mol s−1 m−2) | |
| Temperature (K) | |
| Velocity (m s−1) | |
| Greek symbols | |
| Density (kg m−3) | |
| Dynamic viscosity (Pa s) | |
| Mass fraction of species i | |
References
- Liu, K.; Song, C.; Subramani, V. Hydrogen and Syngas Production and Purification Technologies; Wiley Online Library: Hoboken, NJ, USA, 2010. [Google Scholar]
- Padró, C.; Lau, F. Advances in Hydrogen Energy; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrog. Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Moftakhari Sharifzadeh, M.M.; Rajabi, M.; Aghbashlo, M.; Tabatabai, M.; Hosseinpour, S.; Ramakrishna, S. Design of an integrated process for simultaneous chemical looping hydrogen production and electricity generation with CO2 capture. Int. J. Hydrog. Energy 2017, 42, 8486–8496. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Nahar, G.; Mote, D.; Dupont, V. Hydrogen production from reforming of biogas: Review of technological advances and an Indian perspective. Renew. Sustain. Energy Rev. 2017, 76, 1032–1052. [Google Scholar] [CrossRef]
- Xue, Y.-P.; Yan, C.-F.; Zhao, X.-Y.; Huang, S.-L.; Guo, C.-Q. Ni/La2O3-ZrO2 catalyst for hydrogen production from steam reforming of acetic acid as a model compound of bio-oil. Korean J. Chem. Eng. 2017, 34, 305–313. [Google Scholar] [CrossRef]
- Quan, C.; Xu, S.; Zhou, C. Steam reforming of bio-oil from coconut shell pyrolysis over Fe/olivine catalyst. Energy Convers. Manag. 2017, 141, 40–47. [Google Scholar] [CrossRef]
- Nabgan, W.; Tuan Abdullah, T.A.; Mat, R.; Nabgan, B.; Gambo, Y.; Ibrahim, M.; Ahmad, A.; Jalil, A.A.; Triwahyono, S.; Saeh, I. Renewable hydrogen production from bio-oil derivative via catalytic steam reforming: An overview. Renew. Sustain. Energy Rev. 2017, 79, 347–357. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrog. Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Rodrigues, A.E. Insight into steam reforming of ethanol to produce hydrogen for fuel cells. Chem. Eng. J. 2006, 117, 39–49. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen production from ethanol reforming: Catalysts and reaction mechanism. Renew. Sustain. Energy Rev. 2015, 44, 132–148. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Cui, G.; Wang, Z.; Jiang, B.; Wang, K.; Chen, H.; Xu, Y. Hydrogen production and reduction of Ni-based oxygen carriers during chemical looping steam reforming of ethanol in a fixed-bed reactor. Int. J. Hydrog. Energy 2017, 42, 26217–26230. [Google Scholar] [CrossRef]
- Tripodi, A.; Compagnoni, M.; Ramis, G.; Rossetti, I. Process simulation of hydrogen production by steam reforming of diluted bioethanol solutions: Effect of operating parameters on electrical and thermal cogeneration by using fuel cells. Int. J. Hydrog. Energy 2017, 42, 23776–23783. [Google Scholar] [CrossRef]
- Castedo, A.; Uriz, I.; Soler, L.; Gandía, L.M.; Llorca, J. Kinetic analysis and CFD simulations of the photocatalytic production of hydrogen in silicone microreactors from water-ethanol mixtures. Appl. Catal. B Environ. 2017, 203, 210–217. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.Q. Thermodynamic analysis of steam reforming of ethanol for hydrogen generation. Int. J. Energy Res. 2008, 32, 1432–1443. [Google Scholar] [CrossRef]
- Rabenstein, G.; Hacker, V. Hydrogen for fuel cells from ethanol by steam-reforming, partial-oxidation and combined auto-thermal reforming: A thermodynamic analysis. J. Power Sources 2008, 185, 1293–1304. [Google Scholar] [CrossRef]
- Wanat, E.C.; Venkataraman, K.; Schmidt, L.D. Steam reforming and water–gas shift of ethanol on Rh and Rh–Ce catalysts in a catalytic wall reactor. Appl. Catal. A Gen. 2004, 276, 155–162. [Google Scholar] [CrossRef]
- Utaka, T.; Okanishi, T.; Takeguchi, T.; Kikuchi, R.; Eguchi, K. Water gas shift reaction of reformed fuel over supported Ru catalysts. Appl. Catal. A Gen. 2003, 245, 343–351. [Google Scholar] [CrossRef]
- Amadeo, N.E.; Laborde, M.A. Hydrogen production from the low-temperature water-gas shift reaction: Kinetics and simulation of the industrial reactor. Int. J. Hydrog. Energy 1995, 20, 949–956. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Kaftan, A.; Kusche, M.; Laurin, M.; Wasserscheid, P.; Libuda, J. KOH-promoted Pt/Al2O3 catalysts for water gas shift and methanol steam reforming: An operando DRIFTS-MS study. Appl. Catal. B Environ. 2017, 201, 169–181. [Google Scholar] [CrossRef]
- Andisheh Tadbir, M.; Akbari, M.H. Integrated methanol reforming and oxidation in wash-coated microreactors: A three-dimensional simulation. Int. J. Hydrog. Energy 2012, 37, 2287–2297. [Google Scholar] [CrossRef]
- Grote, M.; Maximini, M.; Yang, Z.; Engelhardt, P.; Köhne, H.; Lucka, K.; Brenner, M. Experimental and computational investigations of a compact steam reformer for fuel oil and diesel fuel. J. Power Sources 2011, 196, 9027–9035. [Google Scholar] [CrossRef]
- Yin, F.; Ji, S.; Mei, H.; Zhou, Z.; Li, C. Coupling of highly exothermic and endothermic reactions in a metallic monolith catalyst reactor: A preliminary experimental study. Chem. Eng. J. 2009, 155, 285–291. [Google Scholar] [CrossRef]
- Jiwanuruk, T.; Putivisutisak, S.; Ponpesh, P.; Kositanont, C.; Tagawa, T.; Yamada, H.; Fukuhara, C.; Assabumrungrat, S. Comparison between parallel and checked arrangements of micro reformer for H2 production from methane. Chem. Eng. J. 2015, 268, 135–143. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Seo, D.-J.; Seo, Y.-T.; Yoon, W.-L. Investigation of the characteristics of a compact steam reformer integrated with a water-gas shift reactor. J. Power Sources 2006, 161, 1208–1216. [Google Scholar] [CrossRef]
- Hayer, F.; Bakhtiary-Davijany, H.; Myrstad, R.; Holmen, A.; Pfeifer, P.; Venvik, H.J. Synthesis of dimethyl ether from syngas in a microchannel reactor—Simulation and experimental study. Chem. Eng. J. 2011, 167, 610–615. [Google Scholar] [CrossRef]
- Lima da Silva, A.; Malfatti, C.d.F.; Müller, I.L. Thermodynamic analysis of ethanol steam reforming using Gibbs energy minimization method: A detailed study of the conditions of carbon deposition. Int. J. Hydrogen Energy 2009, 34, 4321–4330. [Google Scholar] [CrossRef]
- Uriz, I.; Arzamendi, G.; López, E.; Llorca, J.; Gandía, L.M. Computational fluid dynamics simulation of ethanol steam reforming in catalytic wall microchannels. Chem. Eng. J. 2011, 167, 603–609. [Google Scholar] [CrossRef]
- Montero, C.; Remiro, A.; Valle, B.; Oar-Arteta, L.; Bilbao, J.; Gayubo, A.G. Origin and Nature of Coke in Ethanol Steam Reforming and Its Role in Deactivation of Ni/La2O3–αAl2O3 Catalyst. Ind. Eng. Chem. Res. 2019, 58, 14736–14751. [Google Scholar] [CrossRef]
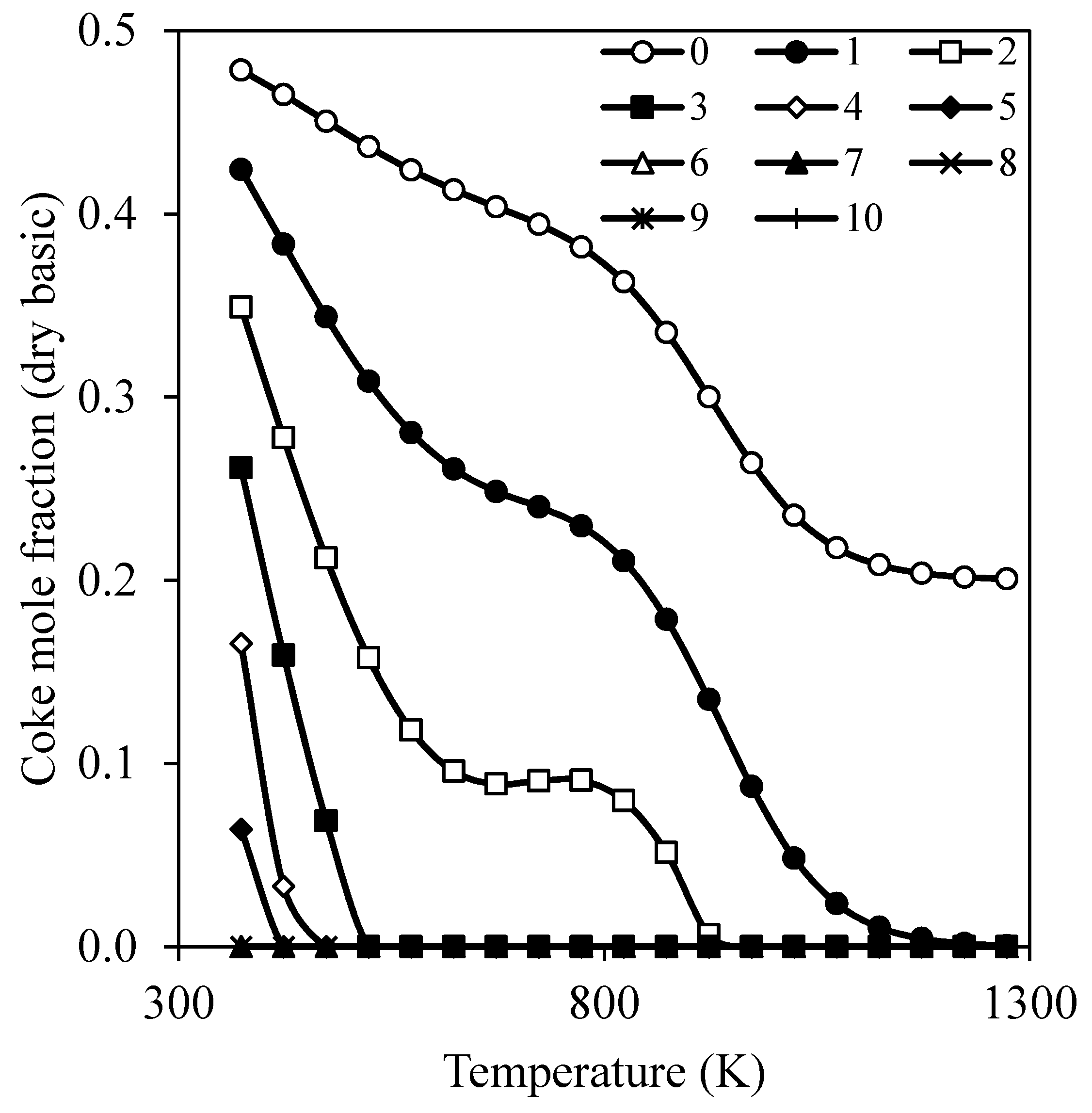
| Parameter | CHIRS (I) | CHIRS (II) | Unit |
|---|---|---|---|
| Combustion chamber inner diameter | 234.644 | 234.644 | mm |
| Combustion chamber outer diameter | 240.644 | 240.644 | mm |
| Air preheat chamber inner diameter | 475.288 | 234.644 | mm |
| Air preheat chamber outer diameter | 481.288 | 240.644 | mm |
| Reactor inner diameter | 47.5 | 47.5 | mm |
| Reactor outer diameter | 53.5 | 53.5 | mm |
| Ethanol steam reforming reactor length | 300 | 300 | mm |
| Water gas shift reactor length | 300 | 300 | mm |
| Air gap height | - | 50 | mm |
| Reactor Performance | CHIRS (I) | CHIRS (II) | Unit |
|---|---|---|---|
| Reactor volume | 72.77 | 34.11 | L |
| Average reforming temperature | 752.15 | 805.44 | K |
| Average water gas shift temperature | 564.12 | 481.94 | K |
| Carbon monoxide selectivity | 0.61 | 0.43 | % |
| Methane selectivity | 0.62 | 0.91 | % |
| Acetaldehyde selectivity | 0.77 | 0.00 | % |
| Hydrogen selectivity | 61.81 | 63.49 | % |
| Hydrogen production | 16.47 | 15.36 | mmol/s |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaodee, W.; Jiwanuruk, T.; Ountaksinkul, K.; Charojrochkul, S.; Charoensuk, J.; Wongsakulphasatch, S.; Assabumrungrat, S. Compact Heat Integrated Reactor System of Steam Reformer, Shift Reactor and Combustor for Hydrogen Production from Ethanol. Processes 2020, 8, 708. https://doi.org/10.3390/pr8060708
Khaodee W, Jiwanuruk T, Ountaksinkul K, Charojrochkul S, Charoensuk J, Wongsakulphasatch S, Assabumrungrat S. Compact Heat Integrated Reactor System of Steam Reformer, Shift Reactor and Combustor for Hydrogen Production from Ethanol. Processes. 2020; 8(6):708. https://doi.org/10.3390/pr8060708
Chicago/Turabian StyleKhaodee, Watcharapong, Tara Jiwanuruk, Khunnawat Ountaksinkul, Sumittra Charojrochkul, Jarruwat Charoensuk, Suwimol Wongsakulphasatch, and Suttichai Assabumrungrat. 2020. "Compact Heat Integrated Reactor System of Steam Reformer, Shift Reactor and Combustor for Hydrogen Production from Ethanol" Processes 8, no. 6: 708. https://doi.org/10.3390/pr8060708
APA StyleKhaodee, W., Jiwanuruk, T., Ountaksinkul, K., Charojrochkul, S., Charoensuk, J., Wongsakulphasatch, S., & Assabumrungrat, S. (2020). Compact Heat Integrated Reactor System of Steam Reformer, Shift Reactor and Combustor for Hydrogen Production from Ethanol. Processes, 8(6), 708. https://doi.org/10.3390/pr8060708







