Fabrication of Biopolymer Based Nanoparticles for the Entrapment of Chromium and Iron Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Nanoparticles
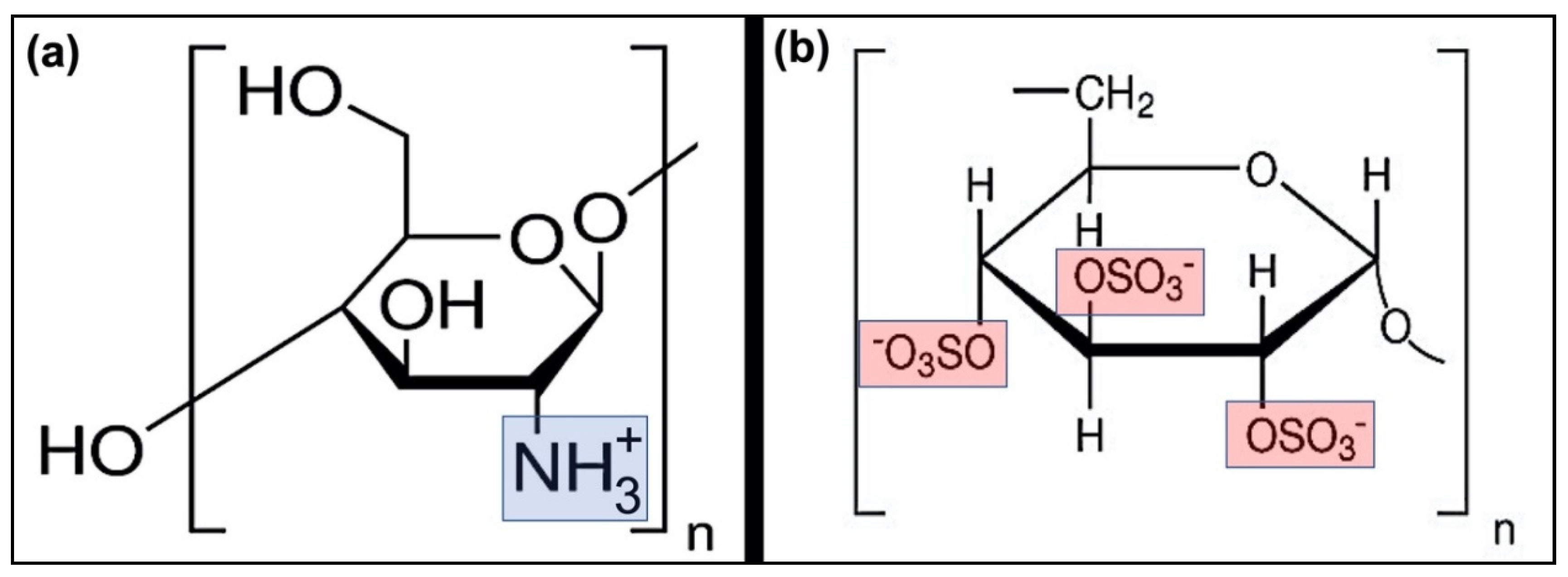
2.2.1. Preparation of CS–DS Nanoparticles
2.2.2. Preparation of Whey Protein-Chitosan Nanoparticles
2.3. Characterization of the Nanoparticles
2.3.1. Particle Size Analysis
2.3.2. Zeta Potential Measurements
2.3.3. Transmission Electron Microscopy (TEM)
2.4. Caco-2 Cell Iron Absorption
2.5. Iron Absorption Analysis and Determination of Loading Efficiency
2.6. Statistical Analysis
3. Results
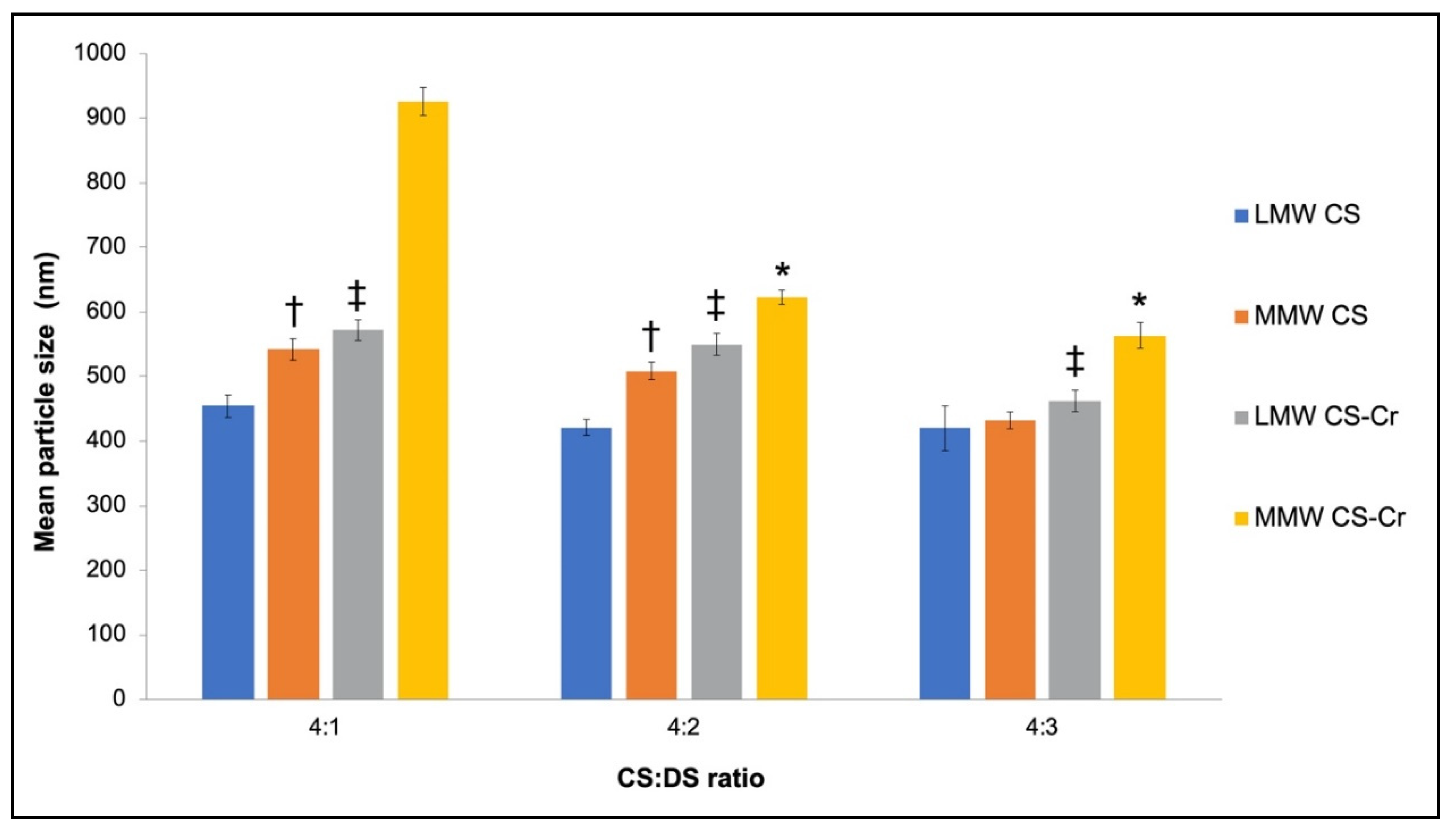
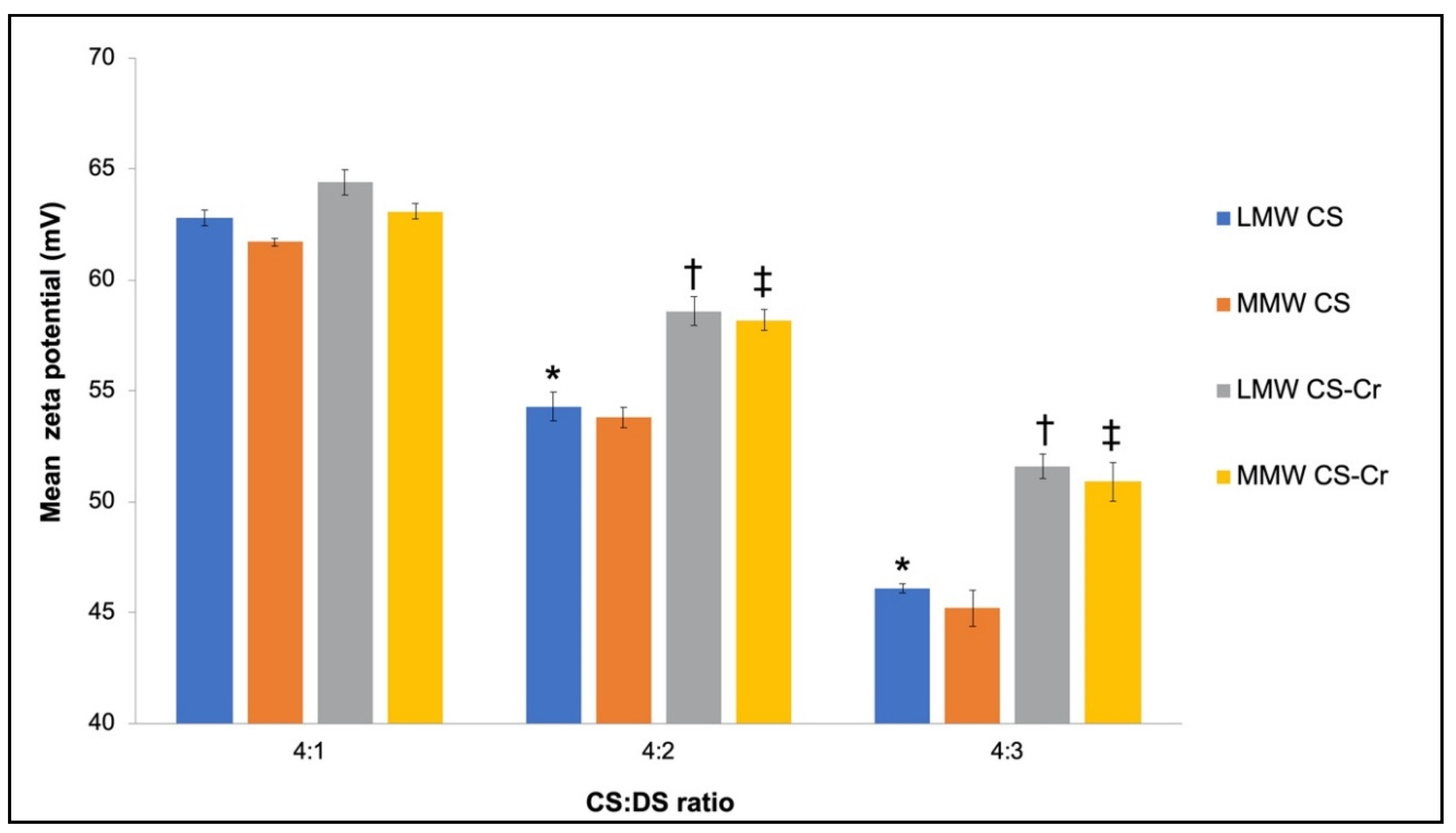
3.1. Physiochemical Characteristics of Chromium-Entrapped CS-DS Nanoparticles
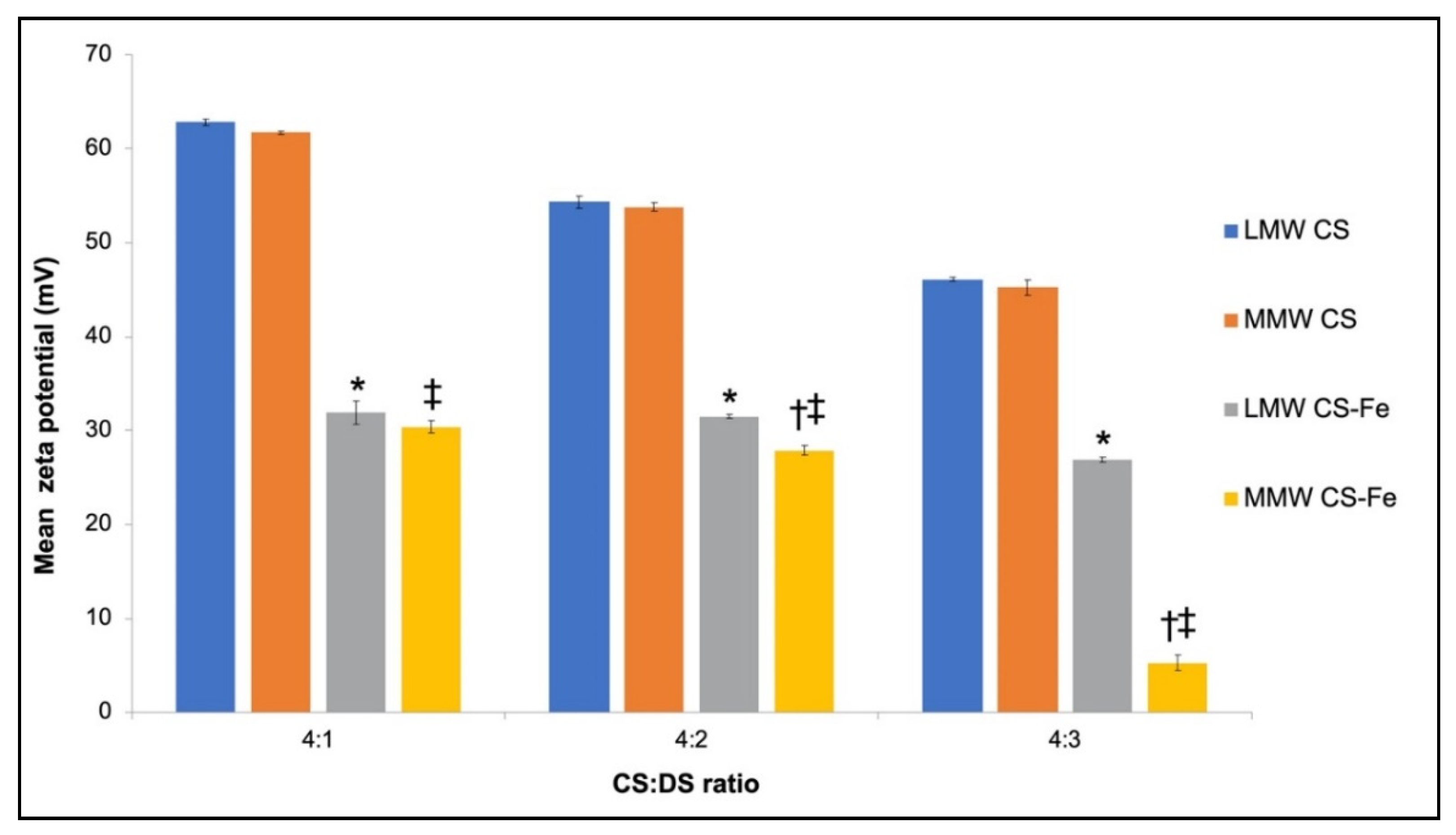
3.2. Physiochemical Characteristics of Iron-Entrapped CS-DS Nanoparticles and Submicron Particles
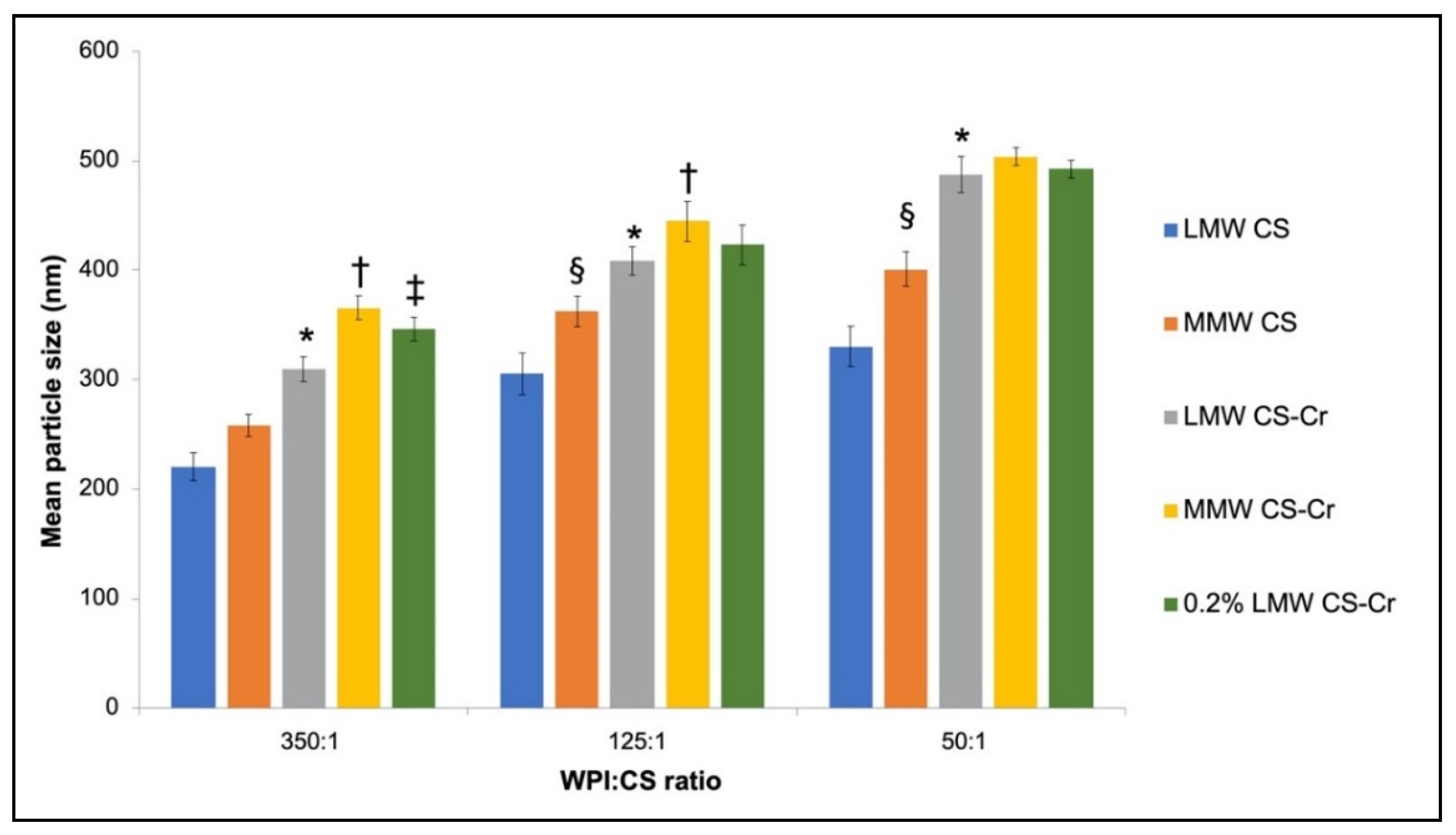
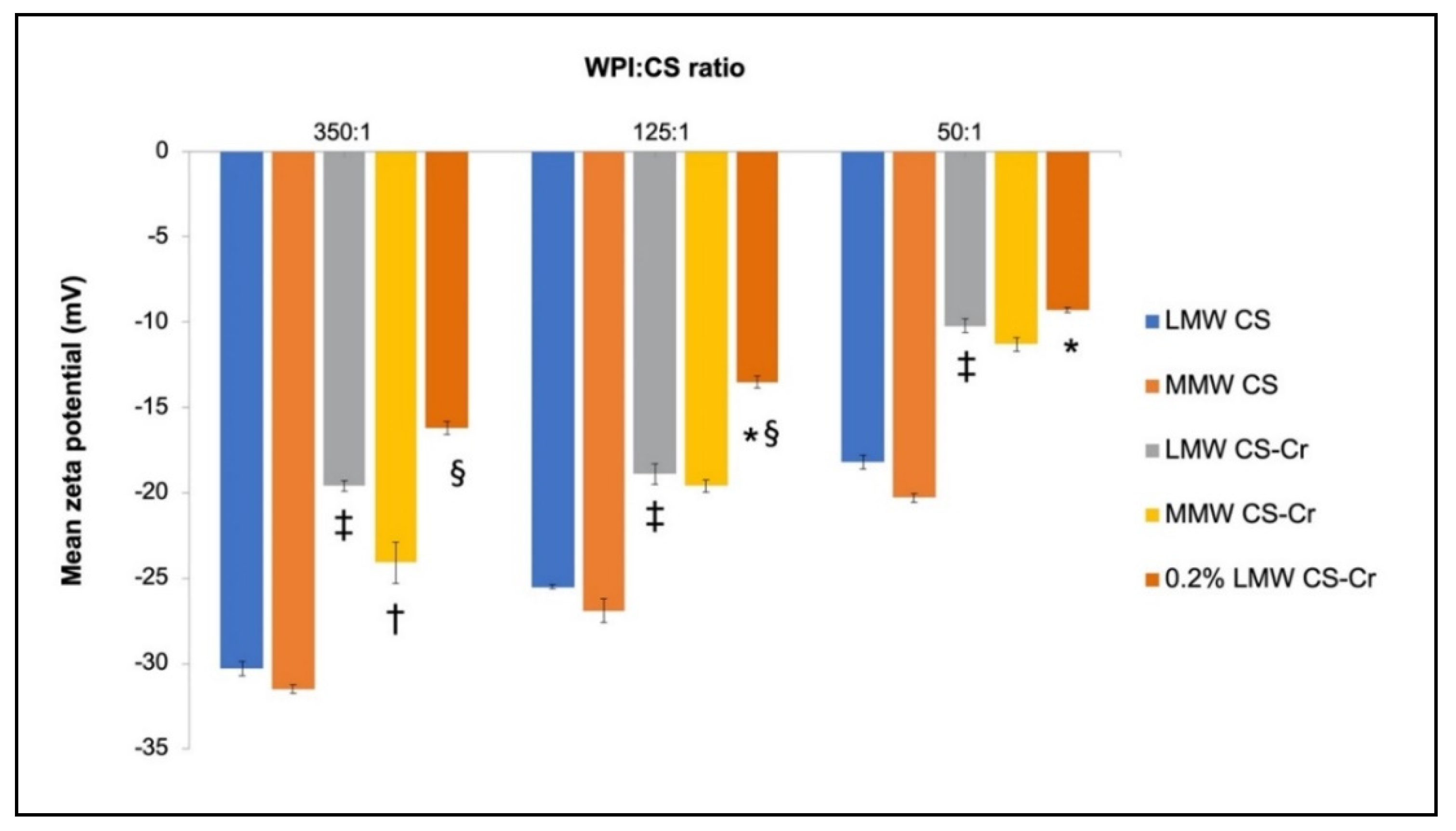
3.3. Physiochemical Characteristics of Chromium-Entrapped WPI-CS Nanoparticles
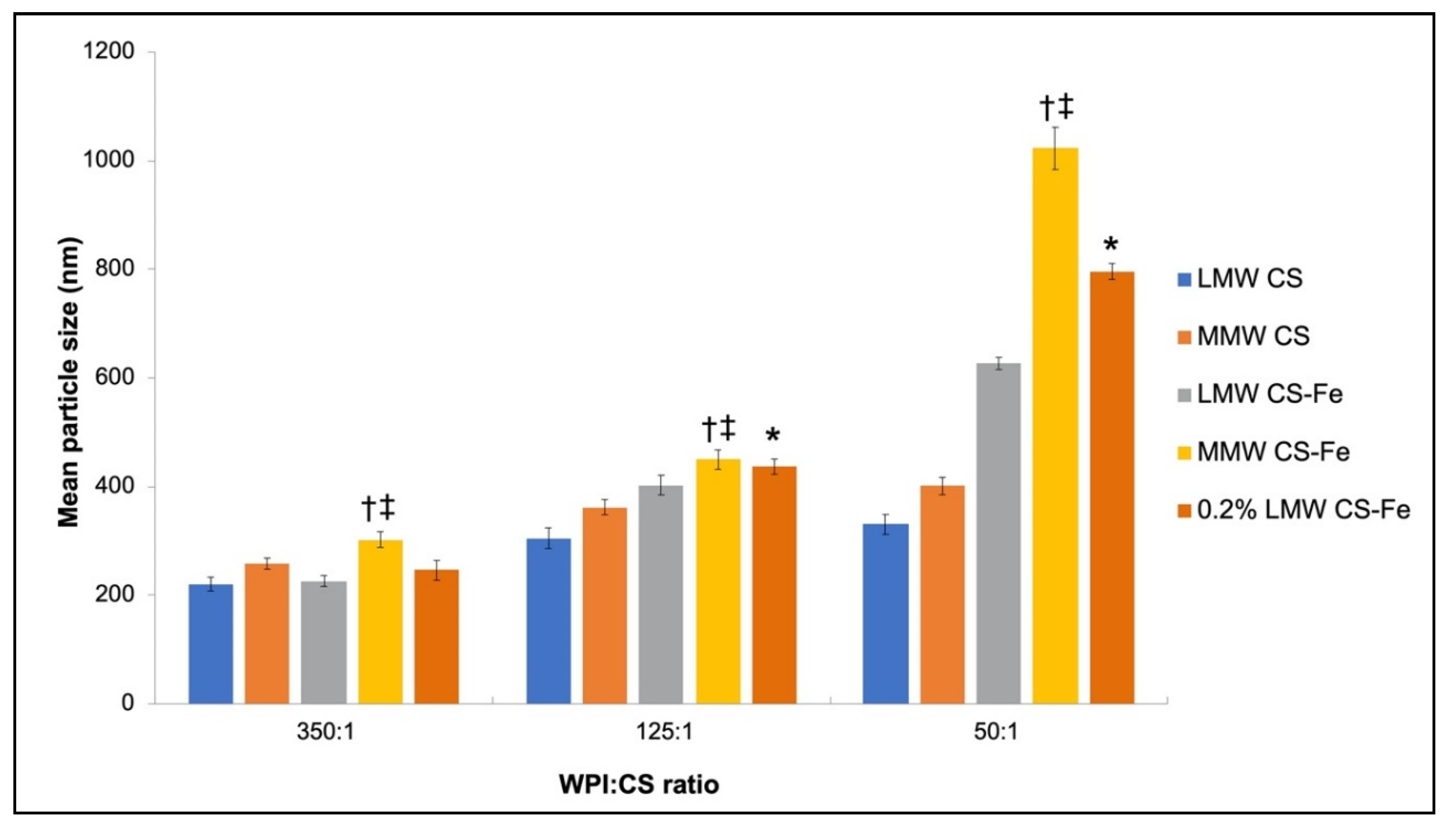
3.4. Physiochemical Characteristics of Iron-Entrapped WPI–CS Nanoparticles
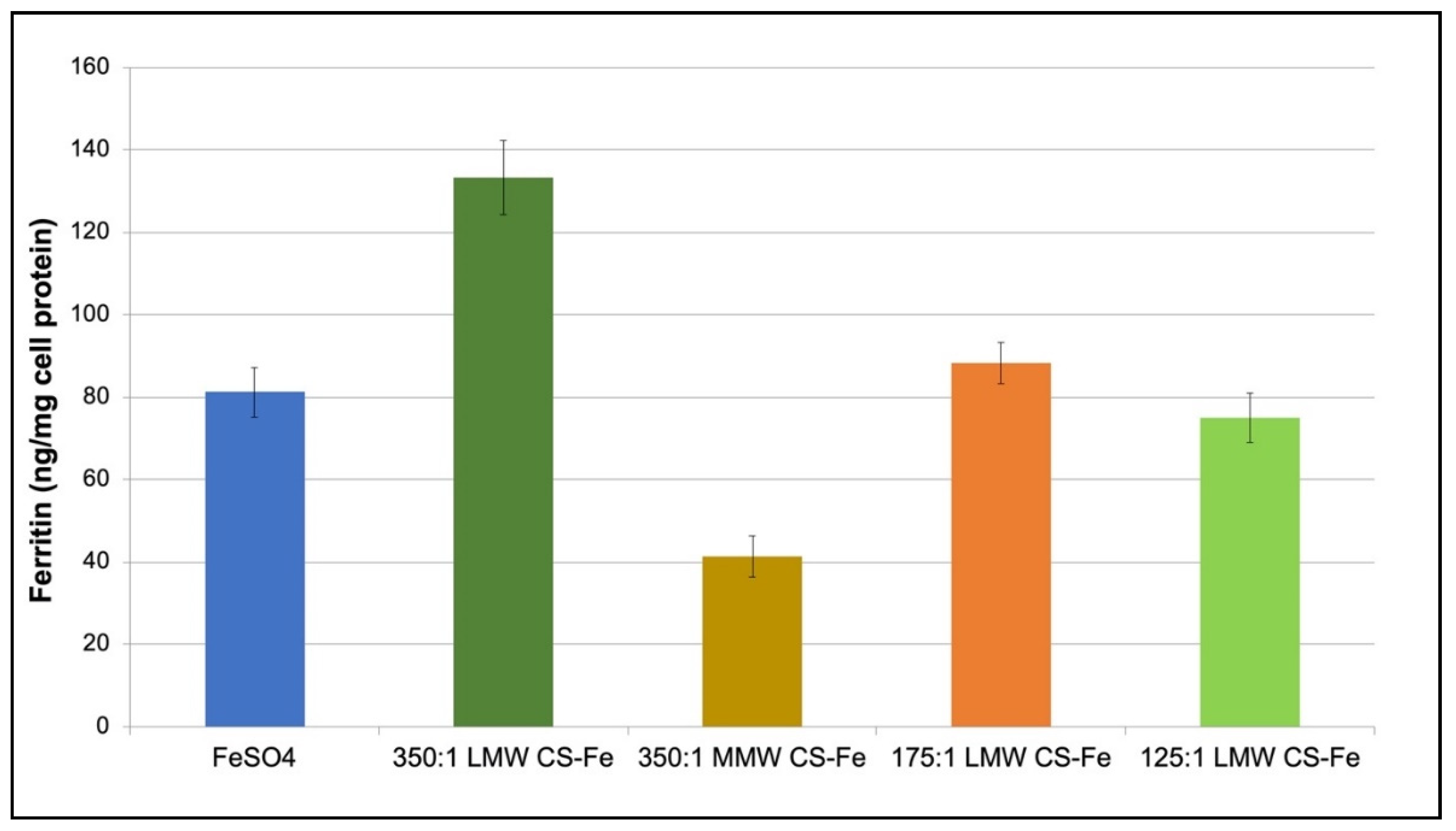
3.5. Caco-2 Cell Iron Permeability Studies
4. Discussion
4.1. Properties of CS–DS Nanoparticles
4.2. Properties of WPI-CS Nanoparticles
4.3. Iron Absorption in Caco-2 Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Singer, G.M.; Geohas, J. The effect of chromium picolinate and biotin supplementation on glycemic control in poorly controlled patients with type 2 diabetes mellitus: A placebo-controlled, double-blinded, randomized trial. Diabetes Technol. Ther. 2006, 8, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Kozlovsky, A.S. Chromium intake, absorption and excretion of subjects consuming self-selected diets. Am. J. Clin. Nutr. 1985, 41, 1177–1183. [Google Scholar] [CrossRef]
- Hex, N.; Bartlett, C.; Wright, D.; Taylor, M.; Varley, D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet. Med. 2012, 29, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Aspects Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Zariwala, M.G.; Somavarapu, S.; Farnaud, S.; Renshaw, D. Comparison study of oral iron preparations using a human intestinal model. Sci. Pharm. 2013, 81, 1123–1139. [Google Scholar] [CrossRef]
- Zariwala, M.G.; Farnaud, S.; Merchant, Z.; Somavarapu, S.; Renshaw, D. Ascorbyl palmitate/DSPE-PEG nanocarriers for oral iron delivery: Preparation, characterisation and in vitro evaluation. Colloids Surf. B Biointerfaces 2014, 115, 86–92. [Google Scholar] [CrossRef]
- Hallberg, L.; Brune, M.; Rossander-Hulthen, L. Is there a physiological role of vitamin C in iron absorption? Ann. N. Y. Acad. Sci. 1987, 498, 324–332. [Google Scholar] [CrossRef]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Jeejeebhoy, K.N. The role of chromium in nutrition and therapeutics and as a potential toxin. Nutr. Rev. 1999, 57, 329–335. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Chen, Y.; Mohanraj, V.J.; Wang, F.; Benson, H.A. Designing chitosan-dextran sulfate nanoparticles using charge ratios. AAPS PharmSciTech 2007, 8, E98. [Google Scholar] [CrossRef]
- Mitra, S.; Gaur, U.; Ghosh, P.C.; Maitra, A.N. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J. Control. Release 2001, 74, 317–323. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Lopez, H.L.; Kedia, A.; Habowski, S.M.; Sandrock, J.E.; Raub, B.; Kerksick, C.M.; Ferrando, A.A. Effects of an amylopectin and chromium complex on the anabolic response to a suboptimal dose of whey protein. J. Int. Soc. Sports Nutr. 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mohanraj, V.J.; Parkin, J.E. Chitosan-dextran sulfate nanoparticles for delivery of an anti-angiogenesis peptide. Lett. Pept. Sci. 2003, 10, 621–629. [Google Scholar] [CrossRef]
- Zamproni, L.N.; Teixeira, D.; Alliegro, A.A.; Maugeri, I.L.; des Rieux, A.; Porcionatto, M.A. Decreased viability and neurite length in neural cells treated with chitosan-dextran sulfate nanocomplexes. Neurotoxicology 2020, 76, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte three layer nanoparticles of chitosan/dextran sulfate/chitosan for dual drug delivery. Colloids Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef] [PubMed]
- Giroux., H.J.; Houde, J.; Britten, M. Preparation of nanoparticles from denatured whey protein by pH-cycling treatment. Food Hydrocoll. 2010, 24, 341–346. [Google Scholar] [CrossRef]
- Stekel, A.; Olivares, M.; Pizarro, F.; Chadud, P.; Lopez, I.; Amar, M. Absorption of fortification iron from milk formulas in infants. Am. J. Clin. Nutr. 1986, 43, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Derman, D.P.; Bothwell, T.H.; MacPhail, A.P.; Torrance, J.D.; Bezwoda, W.R.; Charlton, R.W.; Mayet, F.G. Importance of ascorbic acid in the absorption of iron from infant foods. Scand. J. Haematol. 1980, 25, 193–201. [Google Scholar] [CrossRef]
- Maryam, K.; Avadi, M.; Abbaspour, M.; Jahangiri, A.; Boldaji, S. Effect of different molecular weights of chitosan on preparation and characterization of insulin loaded nanoparticles by ion gelation method. Int. J. Drug Dev. Res. 2012, 4, 271–277. [Google Scholar]
- Chaiyasan, W.; Srinivas, S.P.; Tiyaboonchai, W. Mucoadhesive chitosan-dextran sulfate nanoparticles for sustained drug delivery to the ocular surface. J. Ocul. Pharmacol. Ther. 2013, 29, 200–207. [Google Scholar] [CrossRef]
- Anal, A.K.; Tobiassen, A.; Flanagan, J.; Singh, H. Preparation and characterization of nanoparticles formed by chitosan-caseinate interactions. Colloids Surf. B Biointerfaces 2008, 64, 104–110. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Ullah, N.; Cao, J.; Lan, Y.; Ge, W.; Hackman, R.M.; Li, Z.; Chen, L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef] [PubMed]
- Guzey, D.; McClements, D.J. Characterization of β-lactoglobulin–chitosan interactions in aqueous solutions: A calorimetry, light scattering, electrophoretic mobility and solubility study. Food Hydrocoll. 2006, 20, 124–131. [Google Scholar] [CrossRef]
- Rojas, G.; Silva, J.; Flores, J.; Rodriguez, A.; Ly, M. Adsorption of chromium onto cross-linked chitosan. Sep. Purif. Technol. 2005, 44, 31–36. [Google Scholar] [CrossRef]
- Omar Zaki, S.S.; Ibrahim, M.; Katas, H. Particle Size Affects Concentration-Dependent Cytotoxicity of Chitosan Nanoparticles towards Mouse Hematopoietic Stem Cells. J. Nanotechnol. 2015, 2015, 919658. [Google Scholar] [CrossRef]
- Ma, Z.; Lim, L.Y. Uptake of chitosan and associated insulin in Caco-2 cell monolayers: A comparison between chitosan molecules and chitosan nanoparticles. Pharm. Res. 2003, 20, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Hubert, N.; Hentze, M. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: Implications for regulation and cellular function. Proc. Natl. Acad. Sci. USA 2002, 99, 12345–12350. [Google Scholar] [CrossRef]



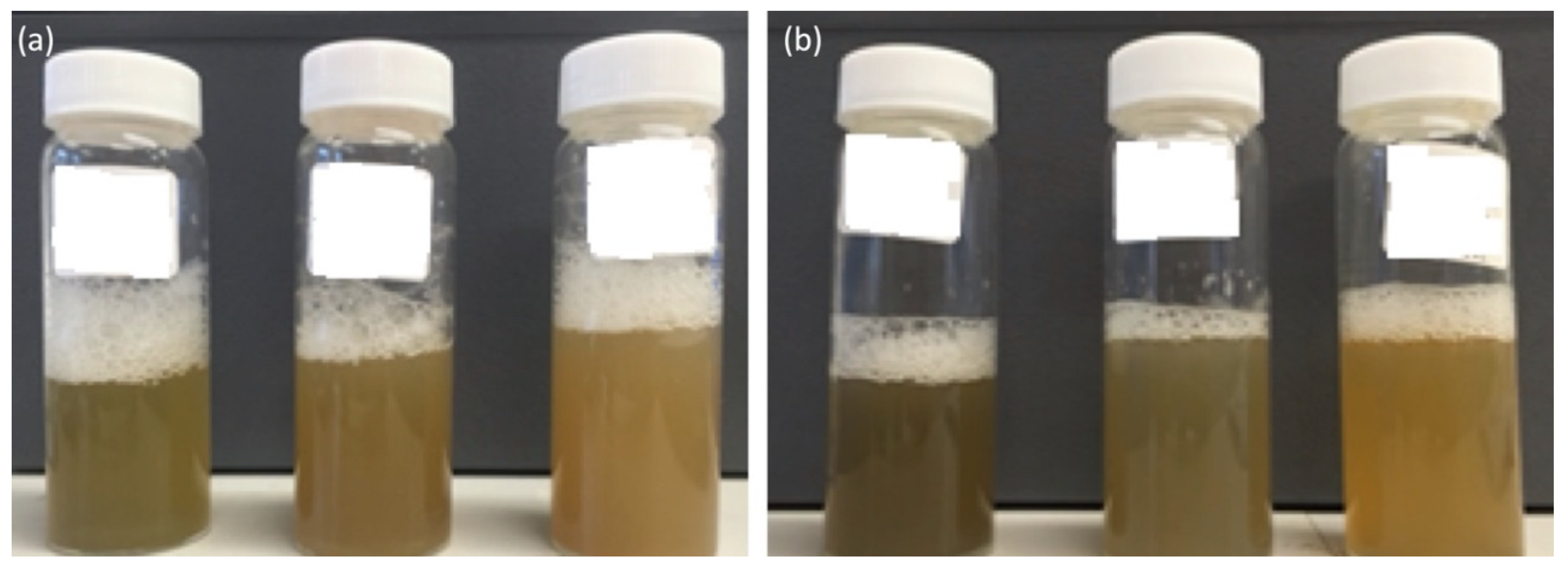
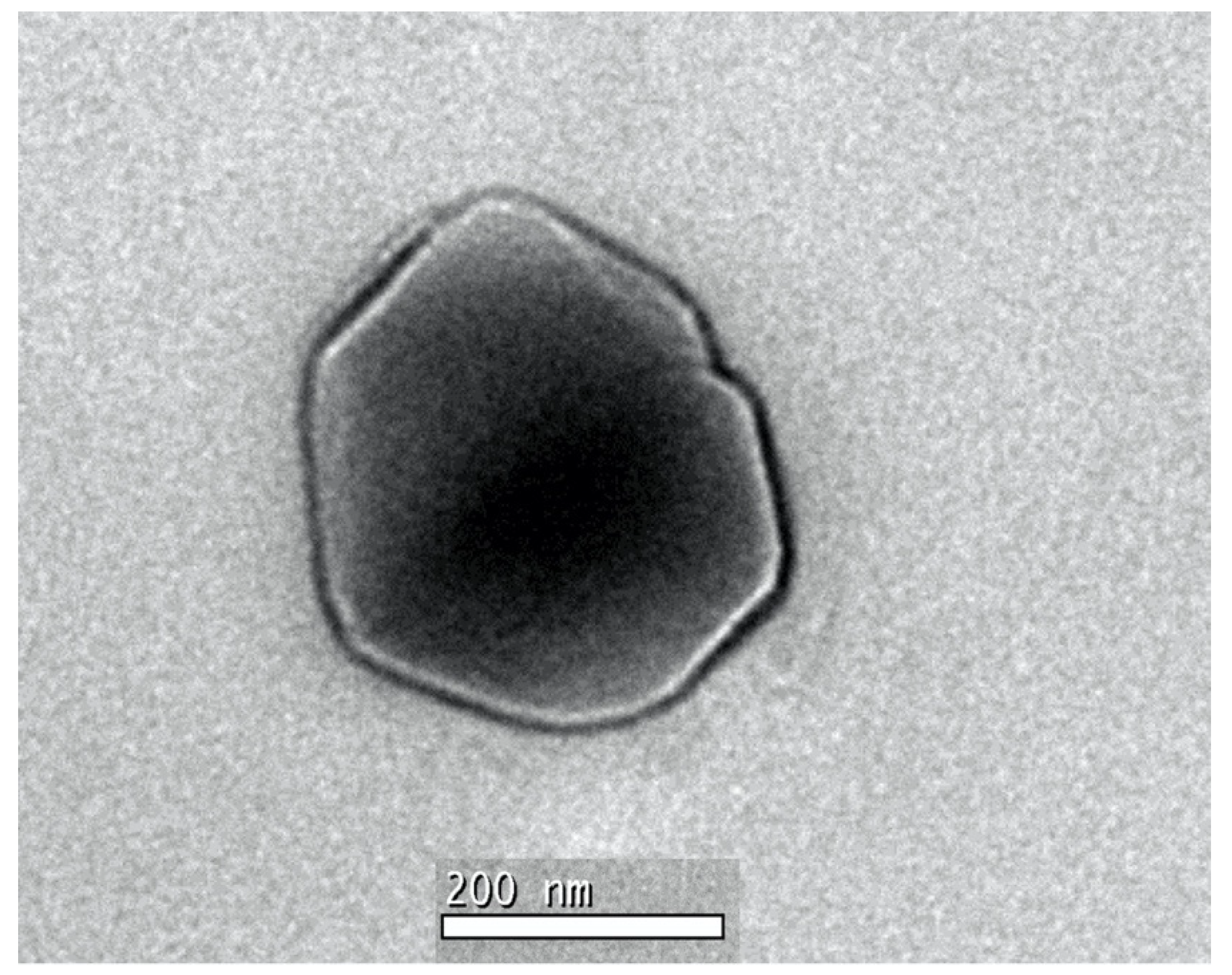
| Type of Nanoparticle | CS:DS Ratio | Chitosan Volume (mL) | Dextran Volume (mL) | Chromium Hydrate (mg) | Ferrous Sulfate (mg) | Loading Efficiency (%) |
|---|---|---|---|---|---|---|
| empty | 4:1 | 10 | 2.5 | - | - | - |
| empty | 4:2 | 10 | 5.0 | - | - | - |
| empty | 4:3 | 10 | 7.5 | - | - | - |
| Cr entrapped | 4:1 | 10 | 2.5 | 12.5 | - | ND |
| Cr entrapped | 4:2 | 10 | 5.0 | 15.0 | - | ND |
| Cr entrapped | 4:3 | 10 | 7.5 | 17.5 | - | ND |
| Fe entrapped | 4:1 | 10 | 2.5 | - | 12.5 | 13 ± 2.3 |
| Fe entrapped | 4:2 | 10 | 5.0 | - | 15.0 | 21 ± 4.5 |
| Fe entrapped | 4:3 | 10 | 7.5 | - | 17.5 | 47 ± 5.2 |
| Sample | WPI:CS Ratio | Whey Protein (mL) | Chitosan (mL) | Chromium Hydrate (mg) |
|---|---|---|---|---|
| empty | 50:1 | 10.0 | 5.0 | - |
| empty | 125:1 | 12.5 | 2.5 | - |
| empty | 350:1 | 14.0 | 1.0 | - |
| Cr loaded | 50:1 | 10.0 | 5.0 | 5 |
| Cr loaded | 125:1 | 12.5 | 2.5 | 5 |
| Cr loaded | 350:1 | 14.0 | 1.0 | 5 |
| WPI:CS Ratio | Whey Protein (mL) | Chitosan (mL) | Ferrous Sulfate (mg) | Ascorbic Acid (mg) | Loading Efficiency (%) |
|---|---|---|---|---|---|
| 50:1 | 10.0 | 5.0 | 10 | 10 | 29 ± 4.3 |
| 125:1 | 12.5 | 2.5 | 10 | 10 | 38 ± 4.1 |
| 350:1 | 14.0 | 1.0 | 10 | 10 | 54 ± 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, N.; Zariwala, M.G.; Al-Obaidi, H. Fabrication of Biopolymer Based Nanoparticles for the Entrapment of Chromium and Iron Supplements. Processes 2020, 8, 707. https://doi.org/10.3390/pr8060707
Patel N, Zariwala MG, Al-Obaidi H. Fabrication of Biopolymer Based Nanoparticles for the Entrapment of Chromium and Iron Supplements. Processes. 2020; 8(6):707. https://doi.org/10.3390/pr8060707
Chicago/Turabian StylePatel, Nishay, Mohammed Gulrez Zariwala, and Hisham Al-Obaidi. 2020. "Fabrication of Biopolymer Based Nanoparticles for the Entrapment of Chromium and Iron Supplements" Processes 8, no. 6: 707. https://doi.org/10.3390/pr8060707
APA StylePatel, N., Zariwala, M. G., & Al-Obaidi, H. (2020). Fabrication of Biopolymer Based Nanoparticles for the Entrapment of Chromium and Iron Supplements. Processes, 8(6), 707. https://doi.org/10.3390/pr8060707





