Iron Oxide/Salicylic Acid Nanoparticles as Potential Therapy for B16F10 Melanoma Transplanted on the Chick Chorioallantoic Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Functionalized Nanoparticles Dispersion
2.2. Cell Preparation
2.3. Animals
2.4. CAM Model
2.5. Histological Analyses
3. Results
3.1. Morphological Effects of SaMNPs on the CAM Assay
3.2. Macroscopic Assessments of B16F10 Xenografts
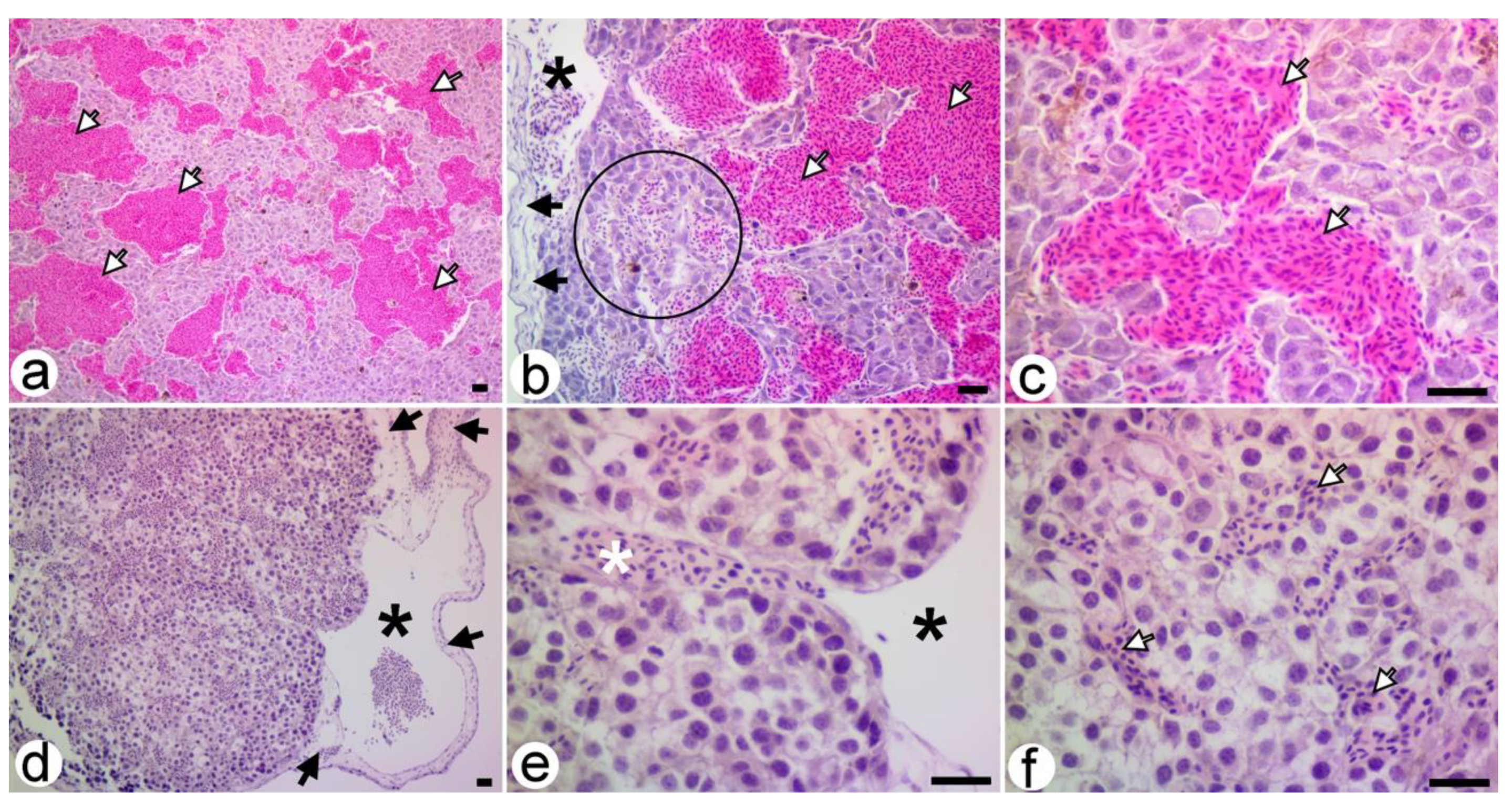
3.2.1. Untreated Xenografts
3.2.2. SaMNP Treated Xenografts
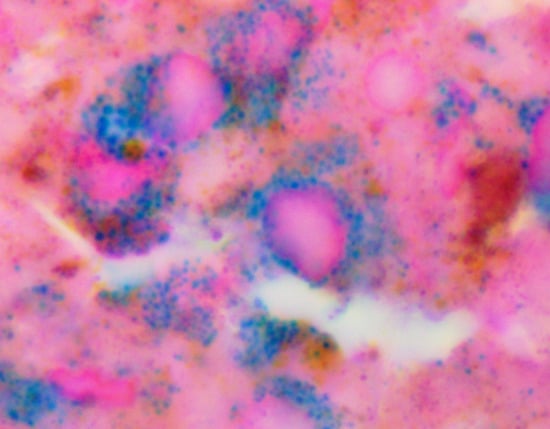
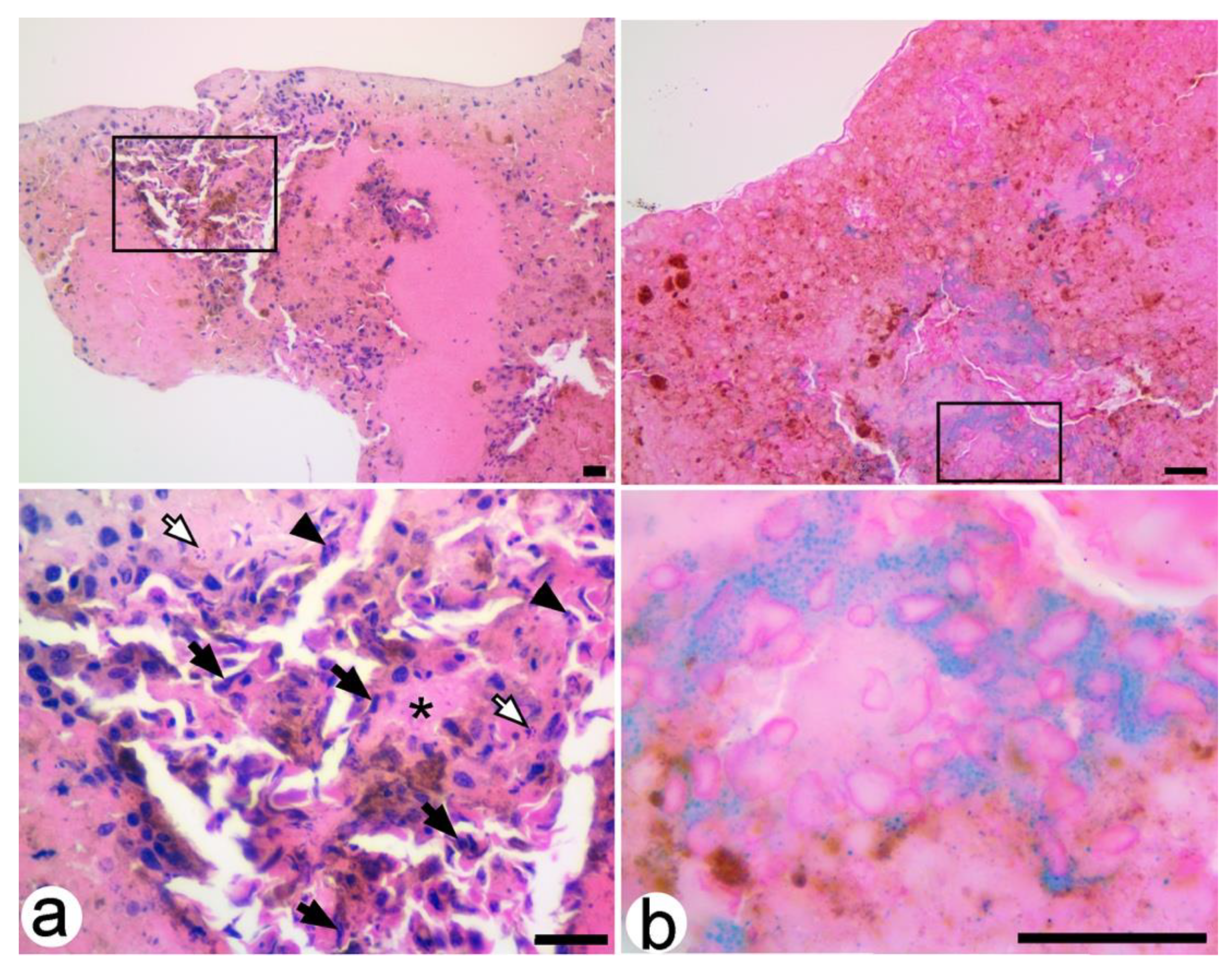
3.3. Microscopic Assessments of B16F10 Xenografts
3.3.1. Untreated Xenografts
3.3.2. SaMNP-Treated Xenografts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Prim. 2015, 1, 15003. [Google Scholar] [CrossRef] [PubMed]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma treatment in review. Immunotargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Eigentler, T.K.; Keilholz, U.; Hauschild, A.; Kirkwood, J.M. Systematic Review of Medical Treatment in Melanoma: Current Status and Future Prospects. Oncologist 2011, 16, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Hughes, H.; Shiatis, A.E.; Pabari, A.; Mosahebi, A.; Seifalian, A. Current and Future Nanotechnology Applications in the Management of Melanoma: A Review. J. Nanomed. Nanotechnol. 2015, 6, 6. [Google Scholar] [CrossRef]
- Bombelli, F.B.; Webster, C.A.; Moncrieff, M.; Sherwood, V. The scope of nanoparticle therapies for future metastatic melanoma treatment. Lancet Oncol. 2014, 15, e22–e32. [Google Scholar] [CrossRef]
- Hafeez, A.; Kazmi, I. Dacarbazine nanoparticle topical delivery system for the treatment of melanoma. Sci. Rep. 2017, 7, 16517. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.; Pinho, J.O.; Soveral, G.; Ascensão, L.; Matela, N.; Reis, C.; Gaspar, M.M. A Novel Hybrid Nanosystem Integrating Cytotoxic and Magnetic Properties as a Tool to Potentiate Melanoma Therapy. Nanomaterials 2020, 10, 693. [Google Scholar] [CrossRef]
- Janko, C.; Ratschker, T.; Nguyen, K.; Zschiesche, L.; Tietze, R.; Lyer, S.; Alexiou, C. Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as Platform for the Targeted Multimodal Tumor Therapy. Front. Oncol. 2019, 9, 59. [Google Scholar] [CrossRef]
- Tang, J.Q.; Hou, X.Y.; Yang, C.S.; Li, Y.X.; Xin, Y.; Guo, W.W.; Wei, Z.P.; Liu, Y.Q.; Jiang, G. Recent developments in nanomedicine for melanoma treatment. Int. J. Cancer 2017, 141, 646–653. [Google Scholar] [CrossRef]
- Park, S. Nano-mechanical phenotype as a promising biomarker to evaluate cancer development, progression, and anti-cancer drug efficacy. J. Cancer Prev. 2016, 21, 73–80. [Google Scholar] [CrossRef]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Sarna, M.; Zadlo, A.; Czuba-Pelech, B.; Urbanska, K. Nanomechanical Phenotype of Melanoma Cells Depends Solely on the Amount of Endogenous Pigment in the Cells. Int. J. Mol. Sci. 2018, 19, 607. [Google Scholar] [CrossRef] [PubMed]
- Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K. Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion. Sci. Rep. 2019, 9, 9280. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Wang, Y.M. Superparamagnetic iron oxide nanoparticulate system: Synthesis, targeting, drug delivery and therapy in cancer. Dalton Trans. 2019, 48, 9490–9515. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, S.; Chen, X. Nanotheranostics for personalized medicine. Expert Rev. Mol. Diagn. 2013, 13, 257–269. [Google Scholar] [CrossRef]
- Rybka, J.D. Radiosensitizing properties of magnetic hyperthermia mediated by superparamagnetic iron oxide nanoparticles (SPIONs) on human cutaneous melanoma cell lines. Rep. Pract. Oncol. Radiother. 2019, 24, 152–157. [Google Scholar] [CrossRef]
- Shiff, S.J.; Shivaprasad, P.; Santini, D.L. Cyclooxygenase inhibitors: Drugs for cancer prevention. Curr. Opin. Pharmacol. 2003, 3, 352–361. [Google Scholar] [CrossRef]
- Sawaoka, H.; Tsuji, S.; Tsujii, M.; Gunawan, E.S.; Sasaki, Y.; Kawano, S.; Hori, M. Cyclooxygenase inhibitors suppress angiogenesis and reduce tumor growth in vivo. Lab. Investig. 1999, 79, 1469–1477. Available online: https://pubmed.ncbi.nlm.nih.gov/10616198/ (accessed on 9 April 2020).
- Thun, M.J.; Henley, S.J.; Patrono, C. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J. Natl. Cancer Inst. 2002, 94, 252–266. [Google Scholar] [CrossRef]
- Chan, A.T.; Ogino, S.; Fuchs, C.S. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 2007, 356, 2131–2142. [Google Scholar] [CrossRef]
- Xu, X.M.; Sansores-Garcia, L.; Chen, X.M.; Matijevic-Aleksic, N.; Du, M.; Wu, K.K. Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proc. Natl. Acad. Sci. USA 1999, 96, 5292–5297. [Google Scholar] [CrossRef]
- Yin, M.J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998, 396, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.C.; Waterhouse, N.J.; Goldstein, J.C.; Schuler, M.; Green, D.R. Aspirin induces apoptosis through release of cytochrome c from mitochondria. Neoplasia 2000, 2, 505–513. [Google Scholar] [CrossRef]
- Nunez, L.; Valero, R.A.; Senovilla, L.; Sanz-Blasco, S.; García-Sancho, J.; Villalobos, C. Cell proliferation depends on mitochondrial Ca2+ uptake: Inhibition by salicylate. J. Physiol. 2006, 571 (Pt 1), 57–73. [Google Scholar] [CrossRef]
- Spitz, G.A.; Furtado, C.M.; Sola-Penna, M.; Zancan, P. Acetylsalicylic acid and salicylic acid decrease tumor cell viability and glucose metabolism modulating 6-phosphofructo-1-kinase structure and activity. Biochem. Pharmacol. 2009, 77, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.I.J.; Kankipati, C.S.; Jones, S.; Newman, R.M.; Safrany, S.T.; Perry, C.J.; Nicholl, I.D. A novel mechanism for the anticancer activity of aspirin and salicylates. Int. J. Oncol. 2019, 54, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, I.E.; Quigley, P.J. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem. Cell Biol. 2008, 130, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane in the study of tumor angiogenesis. Rom. J. Morphol. Embryol. 2008, 49, 131–135. Available online: http://www.rjme.ro/RJME/resources/files/490208131135.pdf (accessed on 9 April 2020). [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Naik, M.; Brahma, P.; Dixit, M.A. Cost-Effective and Efficient Chick Ex-Ovo CAM Assay Protocol to Assess Angiogenesis. Methods Protoc. 2018, 1, 19. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
- DeBord, L.C.; Pathak, R.R.; Villaneuva, M.; Liu, H.C.; Harrington, D.A.; Yu, W.; Lewis, M.T.; Sikora, A.G. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am. J. Cancer Res. 2018, 8, 1642–1660. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6129484/pdf/ajcr0008-1642.pdf (accessed on 9 April 2020). [PubMed]
- Kalirai, H.; Shahidipour, H.; Coupland, S.E.; Luyten, G. Use of the Chick Embryo Model in Uveal Melanoma. Ocul. Oncol. Pathol. 2015, 1, 133–140. [Google Scholar] [CrossRef]
- Buteică, S.A.; Mihaiescu, D.E.; Rogoveanu, I.; Mărgăritescu, D.N.; Mîndrilă, I. Chick Chorioallantoic Membrane Model as a Preclinical Tool for Nanoparticles Biology Study. Rom. Biotechnol. Lett. 2016, 21, 11684–11690. Available online: https://e-repository.org/rbl/vol.21/iss.4/9.pdf (accessed on 9 April 2020).
- Vu, B.T.; Shahin, S.A.; Croissant, J.; Fatieiev, Y.; Matsumoto, K.; Le-Hoang Doan, T.; Yik, T.; Simargi, S.; Conteras, A.; Ratliff, L.; et al. Chick chorioallantoic membrane assay as an in vivo model to study the effect of nanoparticle-based anticancer drugs in ovarian cancer. Sci. Rep. 2018, 8, 8524. [Google Scholar] [CrossRef]
- Mîndrilă, I.; Buteică, S.A.; Mihaiescu, D.E.; Burada, F.; Mîndrilă, B.; Predoi, M.C.; Pirici, I.; Fudulu, A.; Croitoru, O. Magnetic nanoparticles-based therapy for malignant mesothelioma. Rom. J. Morphol. Embryol. 2017, 58, 457–463. Available online: http://www.rjme.ro/RJME/resources/files/580217457463.pdf (accessed on 9 April 2020).
- Mîndrilă, I.; Buteică, S.A.; Mihaiescu, D.E.; Badea, G.; Fudulu, A.; Mărgăritescu, D.N. Fe3O4/salicylic acid nanoparticles versatility in magnetic mediated vascular nanoblockage. J. Nanopart. Res. 2016, 18, 10. [Google Scholar] [CrossRef]
- Mihaiescu, D.E.; Buteică, A.S.; Neamţu, J.; Istrati, D.; Mîndrilă, I. Fe3O4/Salicylic acid nanoparticles behavior on chick CAM vasculature. J. Nanopart. Res. 2013, 15, 1857. [Google Scholar] [CrossRef]
- Lemus, D.; Dabancens, A.; Illanes, J.; Fuenzalida, M.; Guerrero, A.; López, C. Antiangiogenic effect of betamethasone on the chick cam stimulated by TA3 tumor supernatant. Biol. Res. 2001, 34, 227–236. [Google Scholar] [CrossRef]
- Holmes, C.E.; Jasielec, J.; Levis, J.E.; Skelly, J.; Muss, H.B. Initiation of aspirin therapy modulates angiogenic protein levels in women with breast cancer receiving tamoxifen therapy. Clin. Transl. Sci. 2013, 6, 386–390. [Google Scholar] [CrossRef]
- Hu, Y.; Lou, X.; Wang, R.; Sun, C.; Liu, X.; Liu, S.; Wang, Z.; Ni, C. Aspirin, a Potential GLUT1 Inhibitor in a Vascular Endothelial Cell Line. Open Med. (Wars) 2019, 14, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, G.M.; Sarah Johnson, A.; Partington, M.; Burn, J.; Wilson, R.; Arthur, H.M. Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox- independent mechanism. FASEB J. 2006, 20, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Mojid Mondol, M.A. Antiangiogenic Study of Two Nonsteriodal Antiinflammatory Compounds Using Chick Chorioallantoic Membrane Assay. J. Med. Sci. 2006, 6, 609–614. [Google Scholar] [CrossRef]
- Blyakhman, F.A.; Makarova, E.B.; Fadeyev, F.A.; Lugovets, D.V.; Safronov, A.P.; Shabadrov, P.A.; Shklyar, T.F.; Melnikov, G.Y.; Orue, I.; Kurlyandskaya, G.V. The Contribution of Magnetic Nanoparticles to Ferrogel Biophysical Properties. Nanomaterials 2019, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Casco, M.; Olsen, T.; Herbst, A.; Evans, G.; Rothermel, T.; Pruett, L.; Simionescu, D.; Visconti, R.; Alexis, F. Iron Oxide Nanoparticles Stimulates Extra-Cellular Matrix Production in Cellular Spheroids. Bioengineering 2017, 4, 4. [Google Scholar] [CrossRef]
- Buteică, S.A.; Mîndrilă, I.; Mihaiescu, D.E.; Purcaru, S.O.; Dricu, A.; Nicolicescu, C.; Neamțu, J. In vitro and in vivo effects of Fe3O4/salicylic acid magnetic nanoparticles on the human glioblastoma cells. Dig. J. Nanomater. Biostruct. 2014, 9, 959–965. Available online: http://www.chalcogen.ro/959_Buteica.pdf (accessed on 9 April 2020).
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- He, C.; Jiang, S.; Jin, H.; Chen, S.; Lin, G.; Yao, H.; Wang, X.; Mi, P.; Ji, Z.; Lin, Y.; et al. Mitochondrial electron transport chain identified as a novel molecular target of SPIO nanoparticles mediated cancer-specific cytotoxicity. Biomaterials 2016, 83, 102–114. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, M.C.; Mîndrilă, I.; Buteică, S.A.; Purcaru, Ș.O.; Mihaiescu, D.E.; Mărginean, O.M. Iron Oxide/Salicylic Acid Nanoparticles as Potential Therapy for B16F10 Melanoma Transplanted on the Chick Chorioallantoic Membrane. Processes 2020, 8, 706. https://doi.org/10.3390/pr8060706
Predoi MC, Mîndrilă I, Buteică SA, Purcaru ȘO, Mihaiescu DE, Mărginean OM. Iron Oxide/Salicylic Acid Nanoparticles as Potential Therapy for B16F10 Melanoma Transplanted on the Chick Chorioallantoic Membrane. Processes. 2020; 8(6):706. https://doi.org/10.3390/pr8060706
Chicago/Turabian StylePredoi, Maria Cristina, Ion Mîndrilă, Sandra Alice Buteică, Ștefana Oana Purcaru, Dan Eduard Mihaiescu, and Ovidiu Marcel Mărginean. 2020. "Iron Oxide/Salicylic Acid Nanoparticles as Potential Therapy for B16F10 Melanoma Transplanted on the Chick Chorioallantoic Membrane" Processes 8, no. 6: 706. https://doi.org/10.3390/pr8060706
APA StylePredoi, M. C., Mîndrilă, I., Buteică, S. A., Purcaru, Ș. O., Mihaiescu, D. E., & Mărginean, O. M. (2020). Iron Oxide/Salicylic Acid Nanoparticles as Potential Therapy for B16F10 Melanoma Transplanted on the Chick Chorioallantoic Membrane. Processes, 8(6), 706. https://doi.org/10.3390/pr8060706