Challenges towards Targeted Drug Delivery in Cancer Nanomedicines
Abstract
:1. Introduction
2. Drug Delivery Strategies in Cancer Therapy
3. Drug Targeting Strategies in Cancer Therapy
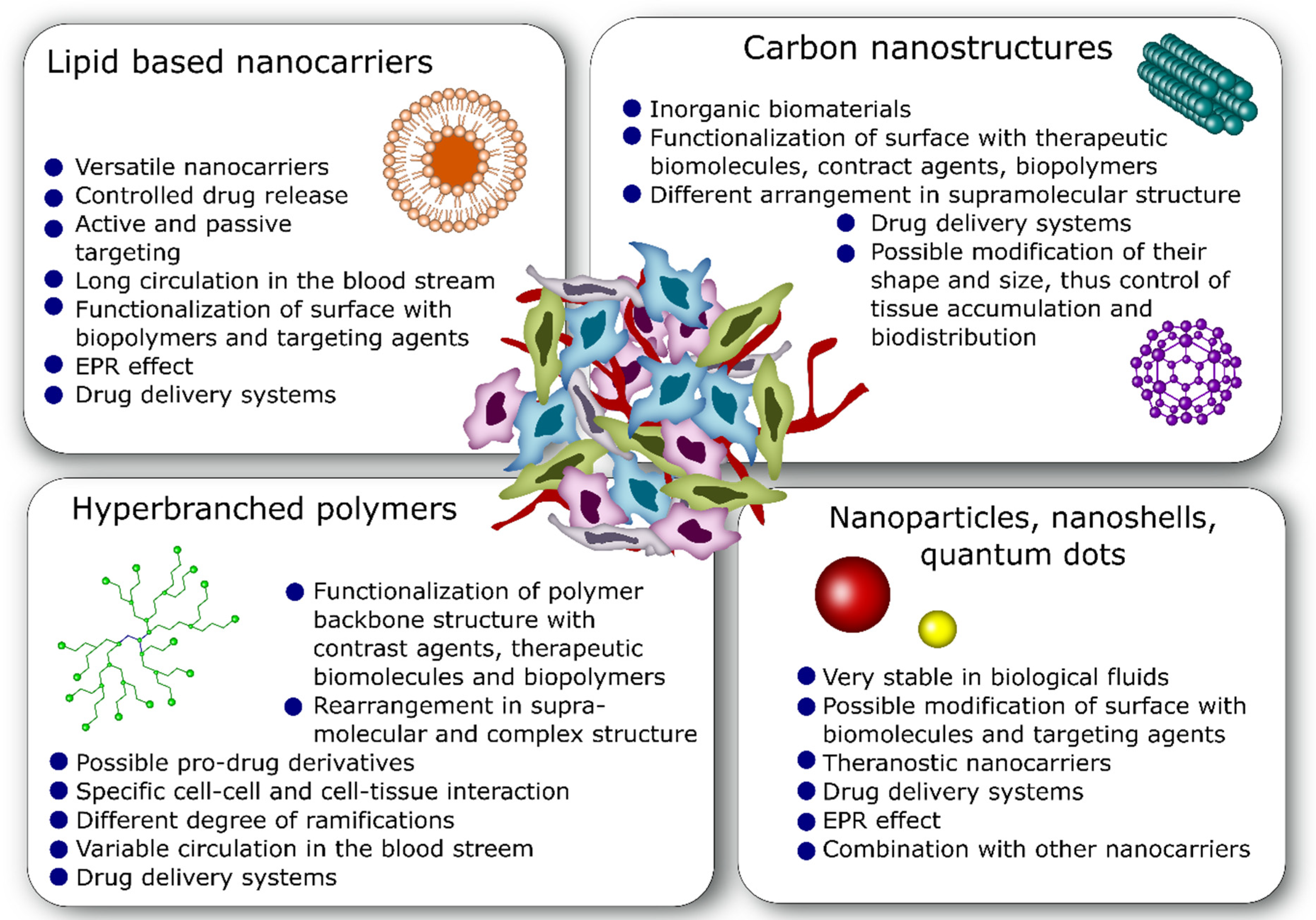
4. Commonly Used Nano-Drug Delivery Systems in Anticancer Therapy
4.1. Lipid-Based Nanocarriers
4.2. Nanoparticles
4.3. Carbon Nanostructures
4.4. Hyperbranched Polymers
5. Challenges toward Drug Delivery Systems in Anticancer Therapy
5.1. Efficacy and Side Effects
5.2. Biopharmaceutical Properties and Anticancer Activity
5.3. Off-Targeting Effects and Other Side Effects
6. Emerging Targeted Drug Delivery Systems
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Wen, Y.; He, D.-X.; Wang, Y.-F.; Liu, X.-L.; Li, C.; Liang, X.-J. Near-Infrared AIEgens as Transformers to Enhance Tumor Treatment Efficacy with Controllable Self-Assembled Redox-Responsive Carrier-Free Nanodrug. Biomaterials 2019, 193, 12–21. [Google Scholar] [CrossRef]
- Zhao, R.; Zheng, G.; Fan, L.; Shen, Z.; Jiang, K.; Guo, Y.; Shao, J.-W. Carrier-Free Nanodrug by Co-Assembly of Chemotherapeutic Agent and Photosensitizer for Cancer Imaging and Chemo-Photo Combination Therapy. Acta Biomater. 2018, 70, 197–210. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Palange, A.L.; Palomba, R.; Rizzuti, I.F.; Ferreira, M.; Decuzzi, P. Deformable Discoidal Polymeric Nanoconstructs for the Precise Delivery of Therapeutic and Imaging Agents. Mol. Ther. 2017, 25, 1514–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.; Han, X.; Wang, R.; Gao, X.; Hu, P.; Yue, W.; Chen, Y.; Shi, J. Nanocatalysts-Augmented and Photothermal-Enhanced Tumor-Specific Sequential Nanocatalytic Therapy in Both NIR-I and NIR-II Biowindows. Adv. Mater. 2019, 31, e1805919. [Google Scholar] [CrossRef]
- Kang, S.; Kim, E.H.; Hwang, J.-E.; Shin, J.-H.; Jeong, Y.S.; Yim, S.Y.; Joo, E.W.; Eun, Y.G.; Lee, D.J.; Sohn, B.H.; et al. Prognostic Significance of High Metabolic Activity in Breast Cancer: PET Signature in Breast Cancer. Biochem. Biophys. Res. Commun. 2019, 511, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rossmanna, C.; Haemmerich, D. Review of Temperature Dependence of Thermal Properties, Dielectric Properties, and Perfusion of Biological Tissues at Hyperthermic and Ablation Temperatures. Crit. Rev. Biomed. Eng. 2014, 42, 467–492. [Google Scholar] [CrossRef] [Green Version]
- Kateb, B.; Yamamoto, V.; Yu, C.; Grundfest, W.; Gruen, J.P. Infrared Thermal Imaging: A Review of the Literature and Case Report. NeuroImage 2009, 47, T154–T162. [Google Scholar] [CrossRef]
- Guaita-Esteruelas, S.; Gumà, J.; Masana, L.; Borràs, J. The Peritumoural Adipose Tissue Microenvironment and Cancer. The Roles of Fatty Acid Binding Protein 4 and Fatty Acid Binding Protein 5. Mol. Cell Endocrinol. 2018, 462, 107–118. [Google Scholar] [CrossRef]
- Vermeulen, L.M.P.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. The Proton Sponge Hypothesis: Fable or Fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.-J. PH-Sensitive Nano-Systems for Drug Delivery in Cancer Therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Kutova, O.M.; Guryev, E.L.; Sokolova, E.A.; Alzeibak, R.; Balalaeva, I.V. Targeted Delivery to Tumors: Multidirectional Strategies to Improve Treatment Efficiency. Cancers 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villaverde, G.; Baeza, A. Targeting Strategies for Improving the Efficacy of Nanomedicine in Oncology. Beilstein J. Nanotechnol. 2019, 10, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Aftab, S.; Shah, A.; Nadhman, A.; Kurbanoglu, S.; Ozkan, S.A.; Dionysiou, D.D.; Shukla, S.S.; Aminabhavi, T.M. Nanomedicine: An Effective Tool in Cancer Therapy. Int. J. Pharm. 2018, 540, 132–149. [Google Scholar] [CrossRef]
- Yang, X.-Z.; Dou, S.; Wang, Y.-C.; Long, H.-Y.; Xiong, M.-H.; Mao, C.-Q.; Yao, Y.-D.; Wang, J. Single-Step Assembly of Cationic Lipid–Polymer Hybrid Nanoparticles for Systemic Delivery of SiRNA. ACS Nano 2012, 6, 4955–4965. [Google Scholar] [CrossRef]
- Beija, M.; Salvayre, R.; Lauth-de Viguerie, N.; Marty, J.-D. Colloidal Systems for Drug Delivery: From Design to Therapy. Trends Biotechnol. 2012, 30, 485–496. [Google Scholar] [CrossRef]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical Interactions with Model Lipid Membranes: Applications in Drug Discovery and Drug Delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef]
- Zhang, R.X.; Cai, P.; Zhang, T.; Chen, K.; Li, J.; Cheng, J.; Pang, K.S.; Adissu, H.A.; Rauth, A.M.; Wu, X.Y. Polymer-Lipid Hybrid Nanoparticles Synchronize Pharmacokinetics of Co-Encapsulated Doxorubicin-Mitomycin C and Enable Their Spatiotemporal Co-Delivery and Local Bioavailability in Breast Tumor. Nanomedicine 2016, 12, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- Pokharkar, V.B.; Jolly, M.R.; Kumbhar, D.D. Engineering of a Hybrid Polymer-Lipid Nanocarrier for the Nasal Delivery of Tenofovir Disoproxil Fumarate: Physicochemical, Molecular, Microstructural, and Stability Evaluation. Eur. J. Pharm. Sci. 2015, 71, 99–111. [Google Scholar] [CrossRef]
- Hadilou, N.; Khoshgenab, A.N.; Amoli-Diva, M.; Sadighi-Bonabi, R. Remote Trice Light, Temperature, and PH-Actuation of Switchable Magneto-Plasmonic Nanocarriers for Combinational Photothermal and Controlled/Targeted Chemotherapies. J. Pharm. Sci. 2018, 107, 3123–3133. [Google Scholar] [CrossRef]
- Dramou, P.; Fizir, M.; Taleb, A.; Itatahine, A.; Dahiru, N.S.; Mehdi, Y.A.; Wei, L.; Zhang, J.; He, H. Folic Acid-Conjugated Chitosan Oligosaccharide-Magnetic Halloysite Nanotubes as a Delivery System for Camptothecin. Carbohydr. Polym. 2018, 197, 117–127. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, K.; Zhou, T.; Jia, X.; Li, J.; Liu, P. PEGylated Multi-Walled Carbon Nanotubes as Versatile Vector for Tumor-Specific Intracellular Triggered Release with Enhanced Anti-Cancer Efficiency: Optimization of Length and PEGylation Degree. Colloids Surf. B Biointerfaces 2018, 168, 43–49. [Google Scholar] [CrossRef]
- Dufès, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in Gene Delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Wang, C.; Guo, L.; Xiao, Z.; Liu, K.; Yan, H. Co-Administration of a Charge-Conversional Dendrimer Enhances Antitumor Efficacy of Conventional Chemotherapy. Eur. J. Pharm. Biopharm. 2018, 127, 371–377. [Google Scholar] [CrossRef]
- Ghorbani, M.; Mahmoodzadeh, F.; Nezhad-Mokhtari, P.; Hamishehkar, H. A Novel Polymeric Micelle-Decorated Fe3O4/Au Core–Shell Nanoparticle for PH and Reduction-Responsive Intracellular Co-Delivery of Doxorubicin and 6-Mercaptopurine. New J. Chem. 2018, 42, 18038–18049. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Chen, T.; Gong, T.; Zhao, T.; Fu, Y.; Zhang, Z.; Gong, T. A Comparison Study between Lycobetaine-Loaded Nanoemulsion and Liposome Using NRGD as Therapeutic Adjuvant for Lung Cancer Therapy. Eur. J. Pharm. Sci. 2018, 111, 293–302. [Google Scholar] [CrossRef]
- Maeda, H.; Khatami, M. Analyses of Repeated Failures in Cancer Therapy for Solid Tumors: Poor Tumor-Selective Drug Delivery, Low Therapeutic Efficacy and Unsustainable Costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the Angiogenic Switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Behr, J.-P. The Proton Sponge: A Trick to Enter Cells the Viruses Did Not Exploit. Chim. Int. J. Chem. 1997, 51, 34–36. [Google Scholar]
- Bikram, M.; West, J.L. Thermo-Responsive Systems for Controlled Drug Delivery. Expert Opin. Drug Deliv. 2008, 5, 1077–1091. [Google Scholar] [CrossRef]
- Song, X.; Wan, Z.; Chen, T.; Fu, Y.; Jiang, K.; Yi, X.; Ke, H.; Dong, J.; Yang, L.; Li, L.; et al. Development of a Multi-Target Peptide for Potentiating Chemotherapy by Modulating Tumor Microenvironment. Biomaterials 2016, 108, 44–56. [Google Scholar] [CrossRef]
- Dassie, J.P.; Hernandez, L.I.; Thomas, G.S.; Long, M.E.; Rockey, W.M.; Howell, C.A.; Chen, Y.; Hernandez, F.J.; Liu, X.Y.; Wilson, M.E.; et al. Targeted Inhibition of Prostate Cancer Metastases with an RNA Aptamer to Prostate-Specific Membrane Antigen. Mol. Ther. 2014, 22, 1910–1922. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin Receptor 1 in Cancer: A New Sight for Cancer Therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Khajavinia, A.; Varshosaz, J.; Dehkordi, A.J. Targeting Etoposide to Acute Myelogenous Leukaemia Cells Using Nanostructured Lipid Carriers Coated with Transferrin. Nanotechnology 2012, 23, 405101. [Google Scholar] [CrossRef]
- Mao, J.; Ran, D.; Xie, C.; Shen, Q.; Wang, S.; Lu, W. EGFR/EGFRvIII Dual-Targeting Peptide-Mediated Drug Delivery for Enhanced Glioma Therapy. ACS Appl. Mater. Interfaces 2017, 9, 24462–24475. [Google Scholar] [CrossRef]
- Nielsen, U.B.; Kirpotin, D.B.; Pickering, E.M.; Hong, K.; Park, J.W.; Shalaby, M.R.; Shao, Y.; Benz, C.C.; Marks, J.D. Therapeutic Efficacy of Anti-ErbB2 Immunoliposomes Targeted by a Phage Antibody Selected for Cellular Endocytosis. Biochim. Biophys. Acta 2002, 1591, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug. Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Koshkaryev, A.; Sawant, R.; Deshpande, M.; Torchilin, V. Immunoconjugates and Long Circulating Systems: Origins, Current State of the Art and Future Directions. Adv. Drug Deliv. Rev. 2013, 65, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The Big Picture on Nanomedicine: The State of Investigational and Approved Nanomedicine Products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug Deliv. 2017, 2017, 9090325. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Banala, V.T.; Sharma, S.; Barnwal, P.; Urandur, S.; Shukla, R.P.; Ahmad, N.; Mittapelly, N.; Pandey, G.; Dwivedi, M.; Kalleti, N.; et al. Synchronized Ratiometric Codelivery of Metformin and Topotecan through Engineered Nanocarrier Facilitates In Vivo Synergistic Precision Levels at Tumor Site. Adv. Healthc. Mater. 2018, 7, e1800300. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Ma, Y.; Che, M.; Zhang, B.; Zhang, Y.; Li, Y.; Zhang, W.; Sang, S. Fluorescent Carbon Dots as Carriers for Intracellular Doxorubicin Delivery and Track. J. Drug Deliv. Sci. Technol. 2019, 49, 527–533. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Silva, J.O.; Mussi, S.V.; Lopes, S.C.A.; Leite, E.A.; Cassali, G.D.; Cardoso, V.N.; Townsend, D.M.; Colletti, P.M.; Ferreira, L.A.M.; et al. Nanostructured Lipid Carrier Co-Loaded with Doxorubicin and Docosahexaenoic Acid as a Theranostic Agent: Evaluation of Biodistribution and Antitumor Activity in Experimental Model. Mol. Imaging Biol. 2018, 20, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.N.; Derbali, R.M.; Yang, C.; Superstein, R.; Hamel, P.; Chain, J.L.; Hardy, P. Co-Delivery of MiR-181a and Melphalan by Lipid Nanoparticles for Treatment of Seeded Retinoblastoma. J. Control. Release 2019, 298, 177–185. [Google Scholar] [CrossRef]
- Amoli-Diva, M.; Sadighi-Bonabi, R.; Pourghazi, K.; Hadilou, N. Tunable Surface Plasmon Resonance–Based Remote Actuation of Bimetallic Core-Shell Nanoparticle-Coated Stimuli Responsive Polymer for Switchable Chemo-Photothermal Synergistic Cancer Therapy. J. Pharm. Sci. 2018, 107, 2618–2627. [Google Scholar] [CrossRef]
- Poudel, B.K.; Soe, Z.C.; Ruttala, H.B.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Gautam, M.; Ou, W.; Nguyen, H.T.; Jeong, J.-H.; et al. In Situ Fabrication of Mesoporous Silica-Coated Silver-Gold Hollow Nanoshell for Remotely Controllable Chemo-Photothermal Therapy via Phase-Change Molecule as Gatekeepers. Int. J. Pharm. 2018, 548, 92–103. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Ganganboina, A.B.; Tsai, Y.; Chiu, H.; Doong, R. Multifunctional GQDs-Concanavalin A@Fe3O4 Nanocomposites for Cancer Cells Detection and Targeted Drug Delivery. Anal. Chim. Acta 2018, 1027, 109–120. [Google Scholar] [CrossRef]
- Gui, W.; Zhang, J.; Chen, X.; Yu, D.; Ma, Q. N-Doped Graphene Quantum Dot@mesoporous Silica Nanoparticles Modified with Hyaluronic Acid for Fluorescent Imaging of Tumor Cells and Drug Delivery. Microchim. Acta 2017, 185, 66. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, L.; Zhong, Y.; Shen, Y.; Li, C.; An, N. Fabrication of Ultrasmall WS2 Quantum Dots-Coated Periodic Mesoporous Organosilica Nanoparticles for Intracellular Drug Delivery and Synergistic Chemo-Photothermal Therapy. Onco Targets Ther. 2018, 11, 1949–1960. [Google Scholar] [CrossRef] [Green Version]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Elhissi, A.M.A.; Ahmed, W.; Hassan, I.U.; Dhanak, V.R.; D’Emanuele, A. Carbon Nanotubes in Cancer Therapy and Drug Delivery. J. Drug Deliv. 2012, 2012, 837327. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Lacerda, L.; Partidos, C.D.; Prato, M.; Bianco, A. Carbon Nanotube-Mediated Delivery of Peptides and Genes to Cells: Translating Nanobiotechnology to Therapeutics. J. Drug Deliv. Sci. Technol. 2005, 15, 41–47. [Google Scholar] [CrossRef]
- Charbgoo, F.; Nikkhah, M.; Behmanesh, M. Size of Single-Wall Carbon Nanotube Affects the Folate Receptor-Mediated Cancer Cell Targeting. Biotechnol. Appl. Biochem. 2018, 65, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Heidari Khoee, M.; Khoee, S.; Lotfi, M. Synthesis of Titanium Dioxide Nanotubes with Liposomal Covers for Carrying and Extended Release of 5-FU as Anticancer Drug in the Treatment of HeLa Cells. Anal. Biochem. 2019, 572, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Mohammadnejad, J.; Yavari, K. Synthesis and Evaluation of Polyethylene Glycol- and Folic Acid-Conjugated Polyamidoamine G4 Dendrimer as Nanocarrier. J. Drug Deliv. Sci. Technol. 2019, 50, 278–286. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Asgari, S.; Hosseini, S.H.; Akhlaghi, M. Codelivery of Hydrophobic and Hydrophilic Drugs by Graphene-Decorated Magnetic Dendrimers. Langmuir 2018, 34, 15304–15318. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Danhier, F. To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Nayak, P.P.; Nijil, S.; Narayanan, A.; Badekila, A.K.; Kini, S. Nanomedicine in Cancer Clinics: Are We There Yet? Curr. Pathobiol. Rep. 2021, 9, 43–55. [Google Scholar] [CrossRef]
- Home-ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ (accessed on 12 April 2021).
- EU Clinical Trials Register-Update. Available online: https://www.clinicaltrialsregister.eu/ (accessed on 12 April 2021).
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Multidisciplinary: Nanomedicines. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/multidisciplinary/multidisciplinary-nanomedicines (accessed on 11 April 2021).
- Narang, A.S.; Chang, R.-K.; Hussain, M.A. Pharmaceutical Development and Regulatory Considerations for Nanoparticles and Nanoparticulate Drug Delivery Systems. J. Pharm. Sci. 2013, 102, 3867–3882. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [Green Version]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug Targeting to Tumors: Principles, Pitfalls and (Pre-) Clinical Progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Morgan, P.; Brown, D.G.; Lennard, S.; Anderton, M.J.; Barrett, J.C.; Eriksson, U.; Fidock, M.; Hamrén, B.; Johnson, A.; March, R.E.; et al. Impact of a Five-Dimensional Framework on R&D Productivity at AstraZeneca. Nat. Rev. Drug Discov. 2018, 17, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and Strategies in Anti-Cancer Nanomedicine Development: An Industry Perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zhou, S.; Gao, W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano 2020, 14, 12281–12290. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer Nanomedicine: A Review of Recent Success in Drug Delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of Pegylated Liposomal Doxorubicin: Review of Animal and Human Studies. Clin. Pharm. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticulate Systems for Brain Delivery of Drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Park, I.H.; Sohn, J.H.; Kim, S.B.; Lee, K.S.; Chung, J.S.; Lee, S.H.; Kim, T.Y.; Jung, K.H.; Cho, E.K.; Kim, Y.S.; et al. An Open-Label, Randomized, Parallel, Phase III Trial Evaluating the Efficacy and Safety of Polymeric Micelle-Formulated Paclitaxel Compared to Conventional Cremophor EL-Based Paclitaxel for Recurrent or Metastatic HER2-Negative Breast Cancer. Cancer Res. Treat. 2016, 49, 569–577. [Google Scholar] [CrossRef]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged Circulation Time and Enhanced Accumulation in Malignant Exudates of Doxorubicin Encapsulated in Polyethylene-Glycol Coated Liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [Green Version]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Tanaka, N.; Kanatani, S.; Tomer, R.; Sahlgren, C.; Kronqvist, P.; Kaczynska, D.; Louhivuori, L.; Kis, L.; Lindh, C.; Mitura, P.; et al. Whole-Tissue Biopsy Phenotyping of Three-Dimensional Tumours Reveals Patterns of Cancer Heterogeneity. Nat. Biomed. Eng. 2017, 1, 796–806. [Google Scholar] [CrossRef]
- Natfji, A.A.; Ravishankar, D.; Osborn, H.M.I.; Greco, F. Parameters Affecting the Enhanced Permeability and Retention Effect: The Need for Patient Selection. J. Pharm. Sci. 2017, 106, 3179–3187. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Ashford, M.; Hennink, W.; Crommelin, D.; Storm, G. Cancer Nanomedicine: Is Targeting Our Target? Nat. Rev. Mater. 2016, 1, 16069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchilin, V. Tumor Delivery of Macromolecular Drugs Based on the EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Yuan, H.; Song, Y.; Hu, H.; Wen, B.; He, M.; Zhang, H.; Li, Y.; Li, F.; Shu, P.; et al. Reappraisal of Anticancer Nanomedicine Design Criteria in Three Types of Preclinical Cancer Models for Better Clinical Translation. Biomaterials 2021, 275, 120910. [Google Scholar] [CrossRef]
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced Cardiotoxicity and Comparable Efficacy in a Phase III Trial of Pegylated Liposomal Doxorubicin HCl (CAELYX/Doxil) versus Conventional Doxorubicin for First-Line Treatment of Metastatic Breast Cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Sparreboom, A.; Scripture, C.D.; Trieu, V.; Williams, P.J.; De, T.; Yang, A.; Beals, B.; Figg, W.D.; Hawkins, M.; Desai, N. Comparative Preclinical and Clinical Pharmacokinetics of a Cremophor-Free, Nanoparticle Albumin-Bound Paclitaxel (ABI-007) and Paclitaxel Formulated in Cremophor (Taxol). Clin. Cancer Res. 2005, 11, 4136–4143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, I.C.; Bhatia, V. Nab-Paclitaxel for Breast Cancer: A New Formulation with an Improved Safety Profile and Greater Efficacy. Expert Rev. Anticancer Ther. 2007, 7, 919–943. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Office of the Commissioner. Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considering-whether-fda-regulated-product-involves-application-nanotechnology (accessed on 13 June 2021).
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.D.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine Applied to Translational Oncology: A Future Perspective on Cancer Treatment. Nanomedicine 2016, 12, 81–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced Nanomedicine and Cancer: Challenges and Opportunities in Clinical Translation. Int. J. Pharm. 2021, 599, 120438. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Poh, M.-Z.; Insin, N.; Bawendi, M.G.; Fukumura, D.; Munn, L.L.; Jain, R.K. Diffusion of Particles in the Extracellular Matrix: The Effect of Repulsive Electrostatic Interactions. Biophys. J. 2010, 99, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Hadjidemetriou, M.; Al-Ahmady, Z.; Mazza, M.; Collins, R.F.; Dawson, K.; Kostarelos, K. In Vivo Biomolecule Corona around Blood-Circulating, Clinically Used and Antibody-Targeted Lipid Bilayer Nanoscale Vesicles. ACS Nano 2015, 9, 8142–8156. [Google Scholar] [CrossRef]
- Hadjidemetriou, M.; Al-Ahmady, Z.; Kostarelos, K. Time-Evolution of In Vivo Protein Corona onto Blood-Circulating PEGylated Liposomal Doxorubicin (DOXIL) Nanoparticles. Nanoscale 2016, 8, 6948–6957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minchinton, A.I.; Tannock, I.F. Drug Penetration in Solid Tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer Nanotechnology: The Impact of Passive and Active Targeting in the Era of Modern Cancer Biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Arranja, A.G.; Pathak, V.; Lammers, T.; Shi, Y. Tumor-Targeted Nanomedicines for Cancer Theranostics. Pharmacol. Res. 2017, 115, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for Poorly Water-Soluble Anticancer Drugs—Barriers of Translation and Solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in Anti-Cancer Therapy: An Overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Luchini, A.; Vitiello, G. Understanding the Nano-Bio Interfaces: Lipid-Coatings for Inorganic Nanoparticles as Promising Strategy for Biomedical Applications. Front. Chem. 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Pavel, I.-A.; Girardon, M.; Hajj, S.E.; Parant, S.; Amadei, F.; Kaufmann, S.; Tanaka, M.; Fierro, V.; Celzard, A.; Canilho, N.; et al. Lipid-Coated Mesoporous Silica Microparticles for the Controlled Delivery of β-Galactosidase into Intestines. J. Mater. Chem. B 2018, 6, 5633–5639. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Goren, D.; Horowitz, A.T.; Tzemach, D.; Lossos, A.; Siegal, T. Long-Circulating Liposomes for Drug Delivery in Cancer Therapy: A Review of Biodistribution Studies in Tumor-Bearing Animals. Adv. Drug Deliv. Rev. 1997, 24, 337–344. [Google Scholar] [CrossRef]
- Rizk, M.; Zou, L.; Savic, R.; Dooley, K. Importance of Drug Pharmacokinetics at the Site of Action. Clin. Transl. Sci. 2017, 10, 133–142. [Google Scholar] [CrossRef]
- Sandritter, T.L.; McLaughlin, M.; Artman, M.; Lowry, J. The Interplay between Pharmacokinetics and Pharmacodynamics. Pediatrics Rev. 2017, 38, 195–206. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Wright, F.A.; Guo, D.; Wang, X.; Wang, D.; Wojcikiewicz, R.J.H.; Luo, J. Multifunctional Telodendrimer Nanocarriers Restore Synergy of Bortezomib and Doxorubicin in Ovarian Cancer Treatment. Cancer Res. 2017, 77, 3293–3305. [Google Scholar] [CrossRef] [Green Version]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The Regulation of Nanomaterials and Nanomedicines for Clinical Application: Current and Future Perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- European Medicines Agency Sinerem: Withdrawn Application. Available online: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/sinerem (accessed on 13 June 2021).
- Tarhan, Ö. 16-Safety and regulatory issues of nanomaterials in foods. In Handbook of Food Nanotechnology; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 655–703. ISBN 978-0-12-815866-1. [Google Scholar]
- Refine Nanomed—Regulatory Science Framework. Available online: http://refine-nanomed.eu/ (accessed on 11 March 2021).
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Yan, F.; Yu, S.; Shen, P. Efficacy and Cardiotoxicity of Liposomal Doxorubicin-Based Chemotherapy in Advanced Breast Cancer: A Meta-Analysis of Ten Randomized Controlled Trials. PLoS ONE 2015, 10, e0133569. [Google Scholar] [CrossRef] [Green Version]
- Lyass, O.; Uziely, B.; Ben-Yosef, R.; Tzemach, D.; Heshing, N.I.; Lotem, M.; Brufman, G.; Gabizon, A. Correlation of Toxicity with Pharmacokinetics of Pegylated Liposomal Doxorubicin (Doxil) in Metastatic Breast Carcinoma. Cancer 2000, 89, 1037–1047. [Google Scholar] [CrossRef]
- Ibrahim, N.K.; Desai, N.; Legha, S.; Soon-Shiong, P.; Theriault, R.L.; Rivera, E.; Esmaeli, B.; Ring, S.E.; Bedikian, A.; Hortobagyi, G.N.; et al. Phase I and Pharmacokinetic Study of ABI-007, a Cremophor-Free, Protein-Stabilized, Nanoparticle Formulation of Paclitaxel. Clin. Cancer Res. 2002, 8, 1038–1044. [Google Scholar]
- Hamid, R.; Manzoor, I. Nanomedicines: Nano Based Drug Delivery Systems Challenges and Opportunities; IntechOpen: London, UK, 2020; ISBN 978-1-83962-333-2. [Google Scholar]
- Brand, W.; Noorlander, C.W.; Giannakou, C.; De Jong, W.H.; Kooi, M.W.; Park, M.V.; Vandebriel, R.J.; Bosselaers, I.E.; Scholl, J.H.; Geertsma, R.E. Nanomedicinal Products: A Survey on Specific Toxicity and Side Effects. Int. J. Nanomed. 2017, 12, 6107–6129. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A Critical Review of the Biological Mechanisms Underlying the in Vivo and in Vitro Toxicity of Carbon Nanotubes: The Contribution of Physico-Chemical Characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Halamoda-Kenzaoui, B.; Bremer-Hoffmann, S. Main Trends of Immune Effects Triggered by Nanomedicines in Preclinical Studies. Int. J. Nanomed. 2018, 13, 5419–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornaguera, C.; García-Celma, M.J. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Tang, L.; Cai, K.; Yang, X.; Yin, L.; Zhang, Y.; Dobrucki, L.W.; Helferich, W.G.; Fan, T.M.; Cheng, J. Albumin as a “Trojan Horse” for Polymeric Nanoconjugates Transendothelial Transport across Tumor Vasculatures for Improved Cancer Targeting. Biomater. Sci. 2018, 6, 1189–1200. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Pirollo, K.F.; Nemunaitis, J.; Leung, P.K.; Nunan, R.; Adams, J.; Chang, E.H. Safety and Efficacy in Advanced Solid Tumors of a Targeted Nanocomplex Carrying the P53 Gene Used in Combination with Docetaxel: A Phase 1b Study. Mol. Ther. 2016, 24, 1697–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, M.N.; Celia, C.; Petrikaite, V. Challenges towards Targeted Drug Delivery in Cancer Nanomedicines. Processes 2021, 9, 1527. https://doi.org/10.3390/pr9091527
Hafeez MN, Celia C, Petrikaite V. Challenges towards Targeted Drug Delivery in Cancer Nanomedicines. Processes. 2021; 9(9):1527. https://doi.org/10.3390/pr9091527
Chicago/Turabian StyleHafeez, Muhammad Nadeem, Christian Celia, and Vilma Petrikaite. 2021. "Challenges towards Targeted Drug Delivery in Cancer Nanomedicines" Processes 9, no. 9: 1527. https://doi.org/10.3390/pr9091527
APA StyleHafeez, M. N., Celia, C., & Petrikaite, V. (2021). Challenges towards Targeted Drug Delivery in Cancer Nanomedicines. Processes, 9(9), 1527. https://doi.org/10.3390/pr9091527







