High-Solids Anaerobic Digestion Followed by Ultrasonication of Digestate and Wet-Type Anaerobic Digestion for Enhancing Methane Yield from OFMSW
Abstract
1. Introduction
2. Materials and Method
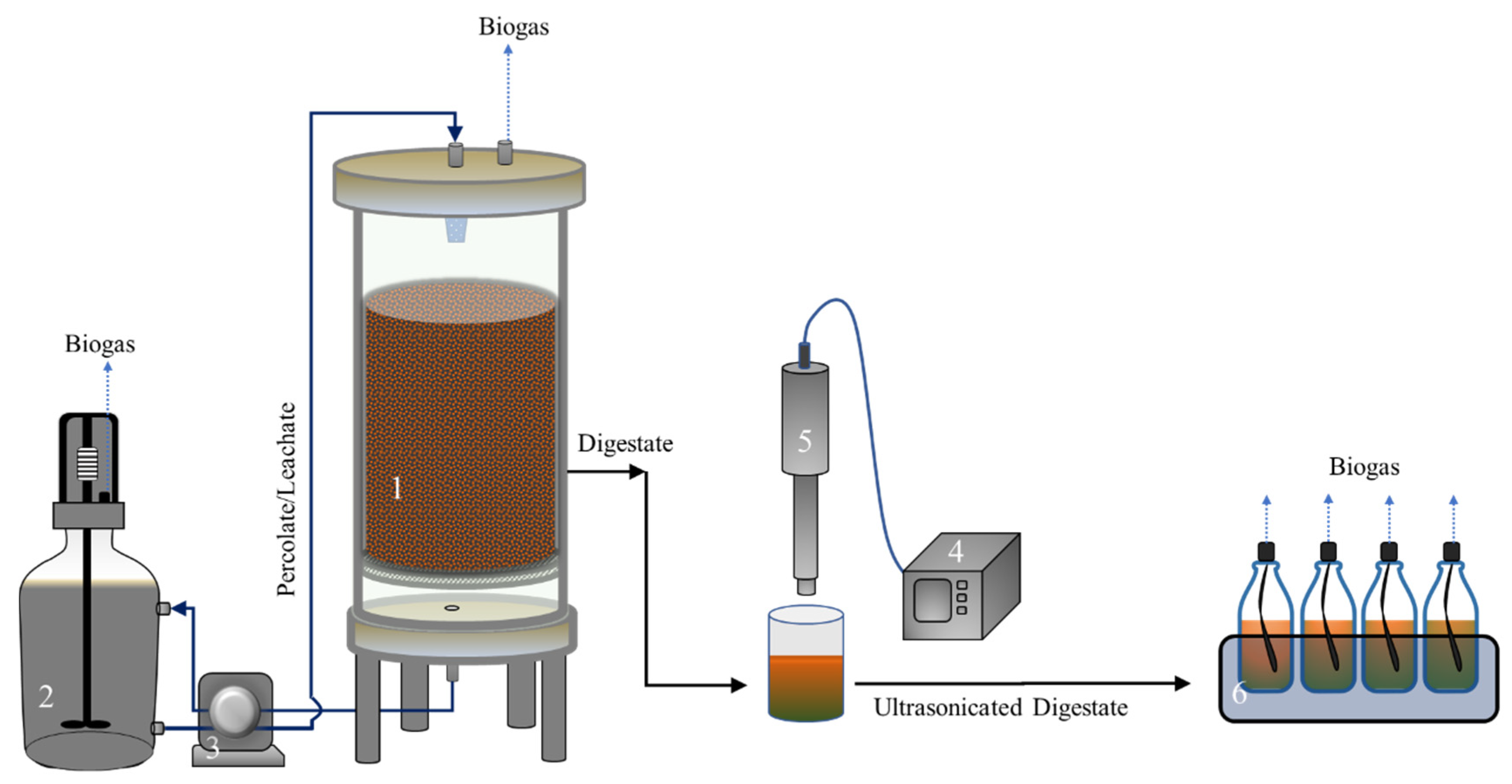
2.1. High-Solids Anaerobic Digestion of OFMSW
2.2. Ultrasound Treatment and BMP Test of HSAD Digestate
2.3. Analytical Method
2.4. Kinetic and Statistical Analyses
3. Results and Discussion
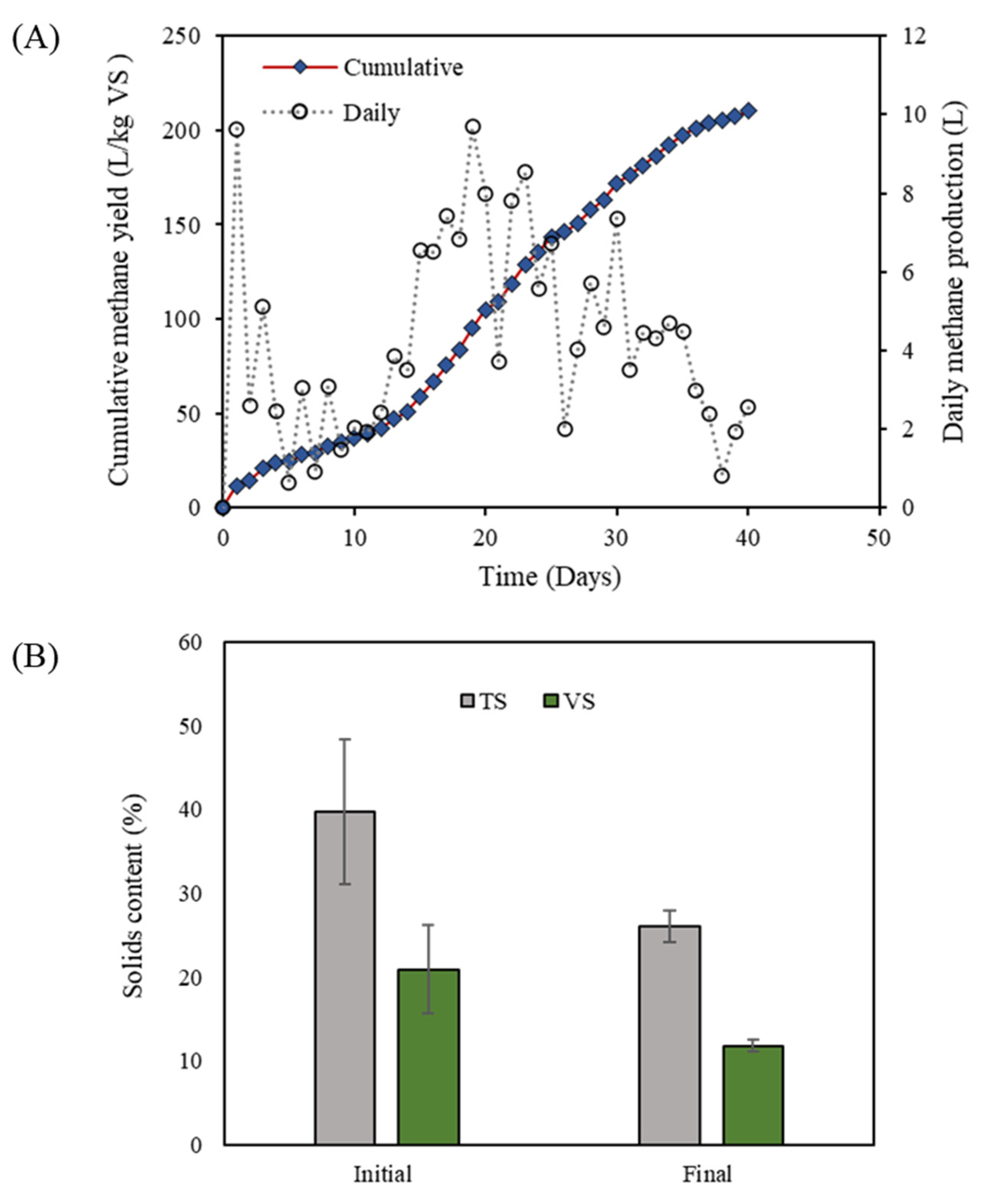
3.1. Performance of HSAD
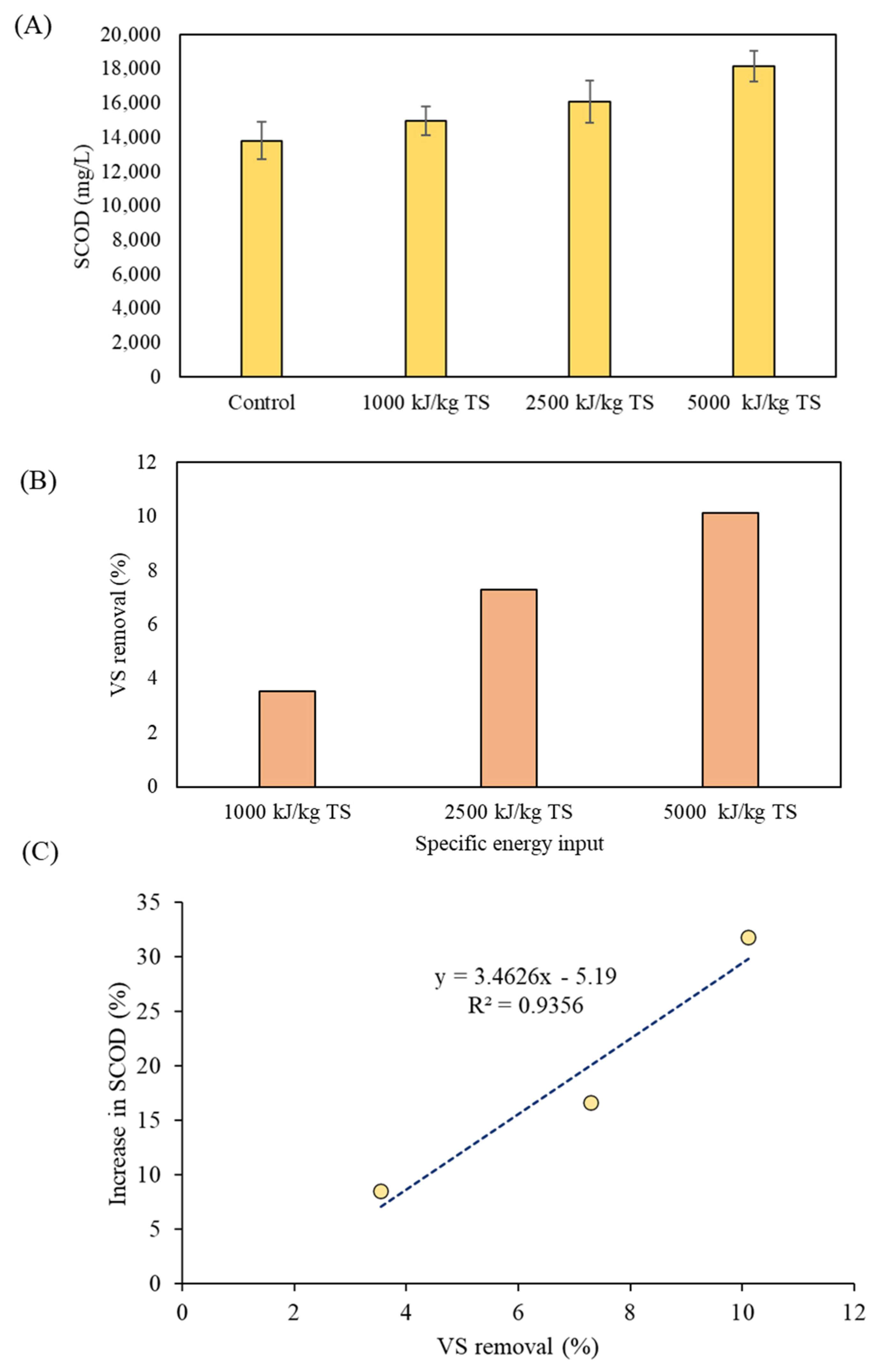
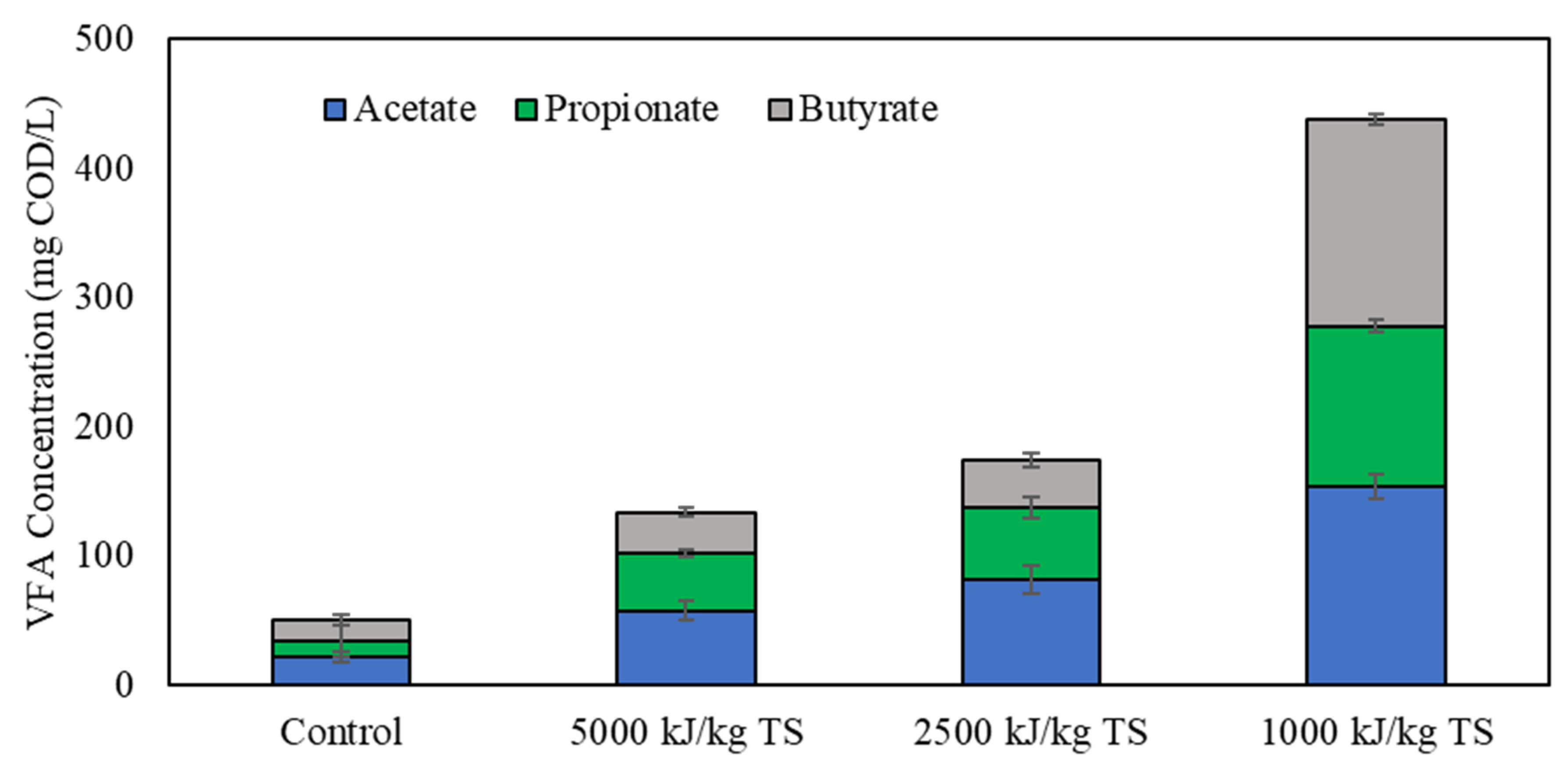
3.2. Effects of Ultrasonication of Digestate
3.3. Biomethane Recovery from Digestate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kawai, K.; Tasaki, T. Revisiting estimates of municipal solid waste generation per capita and their reliability. J. Mater. Cycles Waste Manag. 2016, 18, 1–13. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Woerden, F. Van What a Waste 2.0 Everything You Should Know About Solid Waste Management; The World Bank: Washington, DC, USA, 2018; ISBN 978-1-4648-1347-4. [Google Scholar]
- Aghbashlo, M.; Tabatabaei, M.; Soltanian, S.; Ghanavati, H. Biopower and biofertilizer production from organic municipal solid waste: An exergoenvironmental analysis. Renew. Energy 2019, 143, 64–76. [Google Scholar] [CrossRef]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Bilgili, M.S.; Demir, A.; Varank, G. Evaluation and modeling of biochemical methane potential (BMP) of landfilled solid waste: A pilot scale study. Bioresour. Technol. 2009, 100, 4976–4980. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Apul, O.G.; Sanin, F.D. Ultrasonic pretreatment and subsequent anaerobic digestion under different operational conditions. Bioresour. Technol. 2010, 101, 8984–8992. [Google Scholar] [CrossRef]
- Michele, P.; Giuliana, D.; Carlo, M.; Sergio, S.; Fabrizio, A. Optimization of solid state anaerobic digestion of the OFMSW by digestate recirculation: A new approach. Waste Manag. 2015, 35, 111–118. [Google Scholar] [CrossRef]
- Pastor-Poquet, V.; Papirio, S.; Trably, E.; Rintala, J.; Escudié, R.; Esposito, G. High-solids anaerobic digestion requires a trade-off between total solids, inoculum-to-substrate ratio and ammonia inhibition. Int. J. Environ. Sci. Technol. 2019, 16, 7011–7024. [Google Scholar] [CrossRef]
- Lee, E.; Bittencourt, P.; Casimir, L.; Jimenez, E.; Wang, M.; Zhang, Q.; Ergas, S.J. Biogas production from high solids anaerobic co-digestion of food waste, yard waste and waste activated sludge. Waste Manag. 2019, 95, 432–439. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Europe—Status, Experience and Prospects. Available online: https://www.researchgate.net/publication/284229577_Anaerobic_digestion_of_the_organic_fraction_of_municipal_solid_waste_in_Europe_-_Status_experience_and_prospects (accessed on 8 April 2020).
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Brown, D.; Shi, J.; Li, Y. Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour. Technol. 2012, 124, 379–386. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V.; Naddeo, V. Comparative technology assessment of anaerobic digestion of organic fraction of MSW. WIT Trans. Ecol. Environ. 2010, 142, 355–366. [Google Scholar]
- Lindner, J.; Zielonka, S.; Oechsner, H.; Lemmer, A. Effects of mechanical treatment of digestate after anaerobic digestion on the degree of degradation. Bioresour. Technol. 2015, 178, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Boni, M.R.; D’Amato, E.; Polettini, A.; Pomi, R.; Rossi, A. Effect of ultrasonication on anaerobic degradability of solid waste digestate. Waste Manag. 2016, 48, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Somers, M.H.; Azman, S.; Sigurnjak, I.; Ghyselbrecht, K.; Meers, E.; Meesschaert, B.; Appels, L. Effect of digestate disintegration on anaerobic digestion of organic waste. Bioresour. Technol. 2018, 268, 568–576. [Google Scholar] [CrossRef]
- Cesaro, A.; Velten, S.; Belgiorno, V.; Kuchta, K. Enhanced anaerobic digestion by ultrasonic pretreatment of organic residues for energy production. J. Clean. Prod. 2014, 74, 119–124. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Aldin, S.; Hafez, H.; Nakhla, G.; Ray, M. Impact of ultrasonication of hog manure on anaerobic digestability. Ultrason. Sonochem. 2011, 18, 164–171. [Google Scholar] [CrossRef]
- Chowdhury, B.; Lin, L.; Dhar, B.R.; Islam, M.N.; McCartney, D.; Kumar, A. Enhanced biomethane recovery from fat, oil, and grease through co-digestion with food waste and addition of conductive materials. Chemosphere 2019, 236, 124362. [Google Scholar] [CrossRef]
- Dhar, B.R.; Nakhla, G.; Ray, M.B. Techno-economic evaluation of ultrasound and thermal pretreatments for enhanced anaerobic digestion of municipal waste activated sludge. Waste Manag. 2012, 32, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mondal, C. Comparative Kinetic Study of Anaerobic Treatment of Thermally Pretreated Source-Sorted Organic Market Refuse. J. Eng. 2015, 2015, 684749. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, Y.; Li, R.; Nelles, M.; Stinner, W.; Li, Y. Effects of Percolate Recirculation on Dry Anaerobic Co-digestion of Organic Fraction of Municipal Solid Waste and Corn Straw. Energy Fuels 2017, 31, 12183–12191. [Google Scholar] [CrossRef]
- Wenjing, L.; Chao, P.; Lama, A.; Xindi, F.; Rong, Y.; Dhar, B.R. Effect of pre-treatments on biological methane potential of dewatered sewage sludge under dry anaerobic digestion. Ultrason. Sonochem. 2019, 52, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Bougrier, C.; Carrère, H.; Delgenès, J.P. Solubilisation of waste-activated sludge by ultrasonic treatment. Chem. Eng. J. 2005, 106, 163–169. [Google Scholar] [CrossRef]
- Lizama, A.C.; Figueiras, C.C.; Herrera, R.R.; Pedreguera, A.Z.; Ruiz Espinoza, J.E. Effects of ultrasonic pretreatment on the solubilization and kinetic study of biogas production from anaerobic digestion of waste activated sludge. Int. Biodeterior. Biodegrad. 2017, 123, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, L.; Zhou, Y.L.; Chen, J.C.; Liang, Y.M.; Wei, L. Pilot-scale operation of enhanced anaerobic digestion of nutrient-deficient municipal sludge by ultrasonic pretreatment and co-digestion of kitchen garbage. J. Environ. Chem. Eng. 2013, 1, 73–78. [Google Scholar] [CrossRef]
- Khanal, S.K.; Grewell, D.; Sung, S.; Van Leeuwen, J. Ultrasound applications in wastewater sludge pretreatment: A review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 277–313. [Google Scholar] [CrossRef]
- Garoma, T.; Pappaterra, D. An investigation of ultrasound effect on digestate solubilization and methane yield. Waste Manag. 2017, 71, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Elbeshbishy, E.; Hafez, H.; Ranjan Dhar, B.; Nakhla, G. Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int. J. Hydrogen Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Xiong, H.; Chen, J.; Wang, H.; Shi, H. Influences of volatile solid concentration, temperature and solid retention time for the hydrolysis of waste activated sludge to recover volatile fatty acids. Bioresour. Technol. 2012, 119, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; Belgiorno, V. Sonolysis and ozonation as pretreatment for anaerobic digestion of solid organic waste. Ultrason. Sonochem. 2013, 20, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Chen, J.; Du, G.; Chen, J. Enhancement of solubilization and acidification of waste activated sludge by pretreatment. Waste Manag. 2008, 28, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Quarmby, J.; Scott, J.R.; Mason, A.K.; Davies, G.; Parsons, S.A. The application of ultrasound as a pre-treatment for anaerobic digestion. Environ. Technol. (United Kingdom) 1999, 20, 1155–1161. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Arumugam, A.; Ponnusami, V. Biodiesel production from Calophyllum inophyllum oil a potential non-edible feedstock: An overview. Renew. Energy 2019, 131, 459–471. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Shi, X.; Lin, J.; Zuo, J.; Li, P.; Li, X.; Guo, X. Effects of free ammonia on volatile fatty acid accumulation and process performance in the anaerobic digestion of two typical bio-wastes. J. Environ. Sci. 2017, 55, 49–57. [Google Scholar] [CrossRef]

| Condition | Modified Gompertz Model | |||
|---|---|---|---|---|
| CH4 Potential (L CH4/kg VS) | Maximum CH4 Production Rate (L CH4/kg VS/d) | Lag Phase (d) | R2 | |
| Control | 74 ± 7 | 1.5 ± 0.4 | 2 ± 1 | 0.9734 |
| 1000 kJ/kg TS | 127 ± 11 | 4.7 ± 1 | 1 ± 1 | 0.9822 |
| 2500 kJ/kg TS | 132 ± 8 | 4.8 ± 0.8 | 1 ± 1 | 0.9945 |
| 5000 kJ/kg TS | 158 ± 15 | 6 ± 1 | 0 | 0.9832 |
| Control | 1000 kJ/kg TS | 2500 kJ/kg TS | 5000 kJ/kg TS | |
|---|---|---|---|---|
| Total ammonia nitrogen (mg N/L) | 1787 ± 33 | 1977 ± 32 | 2056 ± 54 | 2181 ± 28 |
| Free ammonia nitrogen (mg N/L) | 179 ± 3 | 210 ± 3 | 200 ± 3 | 183 ± 2 |
| Total volatile fatty acids (mg COD/L) | 73 ± 6 | 148 ± 23 | 158 ± 13 | 239 ± 13 |
| Acetate (mg COD/L) | 39 ± 4 | 79 ± 26 | 88 ± 2 | 124 ± 6 |
| Propionate (mg COD/L) | 34 ± 2 | 28 ± 8 | 34 ± 7 | 46 ± 4 |
| Butyrate (mg COD/L) | - | 41 ± 9 | 36 ± 4 | 69 ± 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, B.; Magsi, S.B.; Ting, H.N.J.; Dhar, B.R. High-Solids Anaerobic Digestion Followed by Ultrasonication of Digestate and Wet-Type Anaerobic Digestion for Enhancing Methane Yield from OFMSW. Processes 2020, 8, 555. https://doi.org/10.3390/pr8050555
Chowdhury B, Magsi SB, Ting HNJ, Dhar BR. High-Solids Anaerobic Digestion Followed by Ultrasonication of Digestate and Wet-Type Anaerobic Digestion for Enhancing Methane Yield from OFMSW. Processes. 2020; 8(5):555. https://doi.org/10.3390/pr8050555
Chicago/Turabian StyleChowdhury, Bappi, Sarmad Bilal Magsi, Hok Nam Joey Ting, and Bipro Ranjan Dhar. 2020. "High-Solids Anaerobic Digestion Followed by Ultrasonication of Digestate and Wet-Type Anaerobic Digestion for Enhancing Methane Yield from OFMSW" Processes 8, no. 5: 555. https://doi.org/10.3390/pr8050555
APA StyleChowdhury, B., Magsi, S. B., Ting, H. N. J., & Dhar, B. R. (2020). High-Solids Anaerobic Digestion Followed by Ultrasonication of Digestate and Wet-Type Anaerobic Digestion for Enhancing Methane Yield from OFMSW. Processes, 8(5), 555. https://doi.org/10.3390/pr8050555






