Phytochemical Profile, Antioxidant and Antitumor Activities of Green Grape Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Preparation of the Samples
2.2. Qualitative Phytochemical Screening
2.2.1. Total Alkaloid Content (TAC) Determination
2.2.2. Estimation of Total Tannins Content (TTC)
2.2.3. Estimation of Total Phenolic Content (TPC)
2.2.4. Estimation of Total Flavonoid Content (TFC)
2.2.5. DPPH_Assay
2.3. Cell and Cell Culture
2.3.1. Treatment of Cells
2.3.2. Evaluation of the Anti-Proliferative Activity
2.4. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Screening
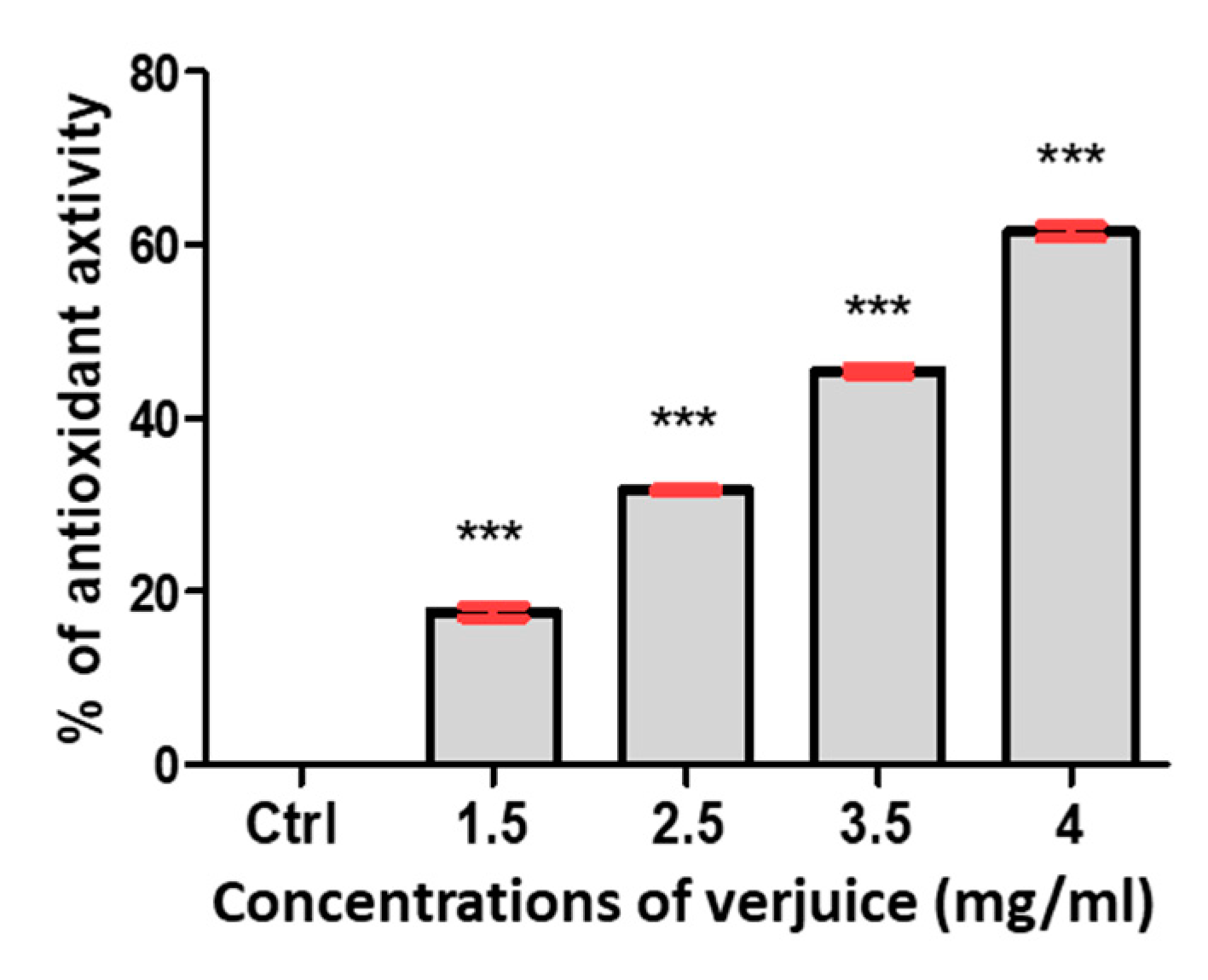
3.2. Antioxidant Activity of Verjuice
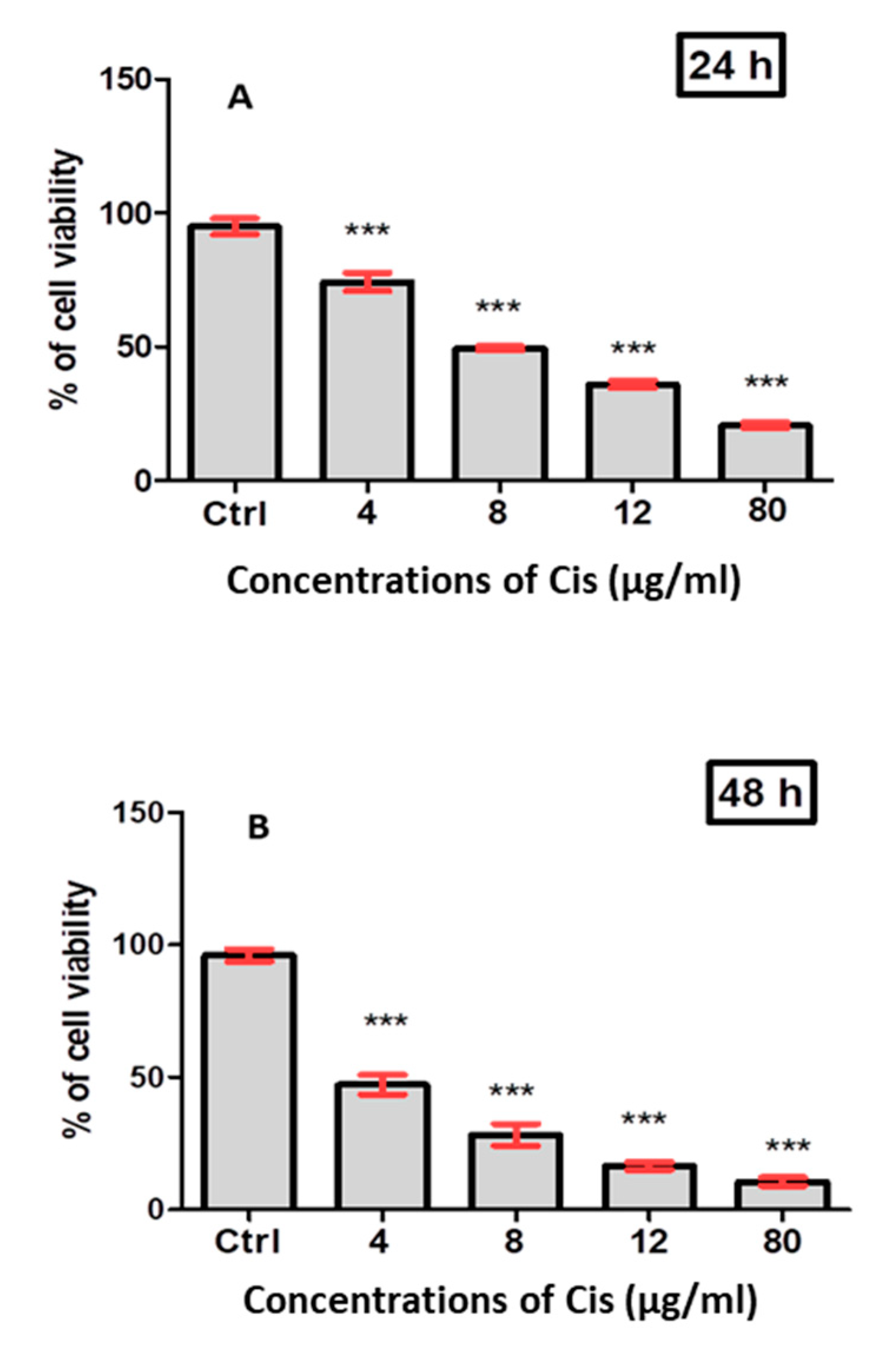
3.3. Cytotoxic Effect of Verjuice Extract on A549 Cancer Cells
3.4. Co-Treatment with Cisplatin and Verjuice Has Superior Inhibitory Effects on A549 Cell Viability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sayed Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Larsen, J.E.; Minna, J.D. Molecular biology of lung cancer: Clinical implications. Clin. Chest Med. 2011, 32, 703–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer statistics. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Koh, W.; Kim, B.; Kim, S.H. Are there new therapeutic options for treating lung cancer based on herbal medicines and their metabolites. J. Ethnopharmacol 2011, 138, 652–661. [Google Scholar] [CrossRef]
- Zhao, T.; Pan, H.; Feng, Y.; Li, H.; Zhao, Y. Petroleum ether extract of Chenopodium album L. prevents cell growth and induces apoptosis of human lung cancer cells. Therap. Med. 2016, 12, 3301–3307. [Google Scholar] [CrossRef] [Green Version]
- Nehme, M. Wild Flowers of Lebanon; National Council for Scientific Research: Beirut, Lebanon, 1978; p. 238. [Google Scholar]
- Post, G.E. Flora f Syria, Palestine and Sinaio; American University of Beirut: Beirut, Lebanon, 1932; p. 658. [Google Scholar]
- Kaliora, A.C.; Kountouri, A.M.; Karathanos, V.T. Antioxidant properties of raisins (Vitis vinifera L.). J. Med. Food 2009, 12, 1302–1309. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Bai, B.R.; Devaraj, S.N. Efficacy of grape seed proanthocyanidins on cardioprotection during isoproterenol-induced myocardial injury in rats. J. Cardiovas. Pharm. 2009, 53, 109–115. [Google Scholar] [CrossRef]
- Setorki, M.; Asgary, S.; Eidi, A.; Haeri Rohani, A. Effects of acute verjuice consumption with a high-cholesterol diet on some biochemical risk factors of atherosclerosis in rabbits. Med. Sci. Monit. 2010, 16, 124–130. [Google Scholar]
- Saad, K. Phytochemical investigation of Fruits and Seeds of Grape (Vitis vinifera L.) grown in Iraq. Int. J. Pharm. Sci. Rev. Res. 2017, 42, 65–66. [Google Scholar]
- Alipour, M.; Davoudi, P.; Davoudi, Z. Effects of unripe grape juice (verjuice) on plasma lipid profile, blood pressure, malondialdehyde and total antioxidant capacity in normal, hyperlipidemic and hyperlipidemic with hypertensive human volunteers. J. Med. Plant Res. 2012, 6, 5677–5683. [Google Scholar] [CrossRef]
- Morris, J.R. Factors influencing grape juice quality. Hort. Technol. 1998, 8, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Harborne, A.J. Phytochemical Methods, A Guide to Modern Techniques of Plant Analysis; Springer: Berlin/Heidelberg, Germany, 2005; pp. 54–84. [Google Scholar]
- Sayed Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, H.; Cerny, M.; Chokr, A.; Kanaan, H.; Merah, O. Fennel seed oil and by-products characterization and their potential applications. Ind. Crops Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Farhan, H.; Rammal, H.; Hijazi, A.; Daher, A.; Reda, M.; Annan, H.; Chokr, A.; Bassal, A.; Badran, B. Chemical composition and antioxidant activity of a Lebanese plant Euphorbia macroclada schyzoceras. Asian Pac. J. Trop. Biomed. 2013, 3, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.P.S. Measurement of Total Phenolics and Tannins Using Folin-Ciocalteu Method. In Quantification of Tannins in Tree and Shrub Foliage; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Farhan, H.; Rammal, H.; Hijazi, A.; Hamad, H.; Daher, A.; Reda, M.; Badran, B. In vitro antioxidant activity of ethanolic and aqueous extracts from crude Malva parviflora L. grown in Lebanon. Asian J. Pharm. Clin. Res. 2012, 5, 234–238. [Google Scholar]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Malek, S.N.A.; Lee, G.S.; Hong, S.L.; Yaacob, H.; Wahab, N.A.; Faizal Weber, J.F.; Shah, S.A.A. Phytochemical and cytotoxic investigations of Curcuma mangga rhizomes. Molecules 2011, 16, 4539–4548. [Google Scholar] [CrossRef] [Green Version]
- Nasreddine, S.; Salameh, F.; Hassan, K.H.; Daher, A.; Nasser, M.; Rammal, H.; Hijazi, A.; Cheaib, A.; Hadadeh, O.; Fayyad-Kazan, H. Valproic acid induces apoptosis and increases CXCR7 expression in epithelial ovarian cancer cell line SKOV-3. Eur. Sci. J. 2015, 126, 171–174. [Google Scholar]
- Mahato, S.B.; Sen, S. Advances in triterpenoid research, 1990–1994. Phytochemistry 1997, 44, 1185–1236. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- NithyaJayanthi, T.G.; Ragunathan, M.G. Antioxidant activity, total phenol, flavonoid, alkaloid, tannin, and saponin contents of leaf extracts of Salvinia molesta. Asian J. Pharm. Clin. Res. 2016, 9, 185–188. [Google Scholar]
- Matute, A.; Tabart, J.; Cheramy-Bien, J.P.; Pirotte, B.; Kevers, C.; Auger, C.; Schini-Kerth, V.; Dommes, J.; Defraigne, J.O.; Pincemail, J. Compared Phenolic Compound Contents of 22 Commercial Fruit and Vegetable Juices: Relationship to Ex-Vivo Vascular Reactivity and Potential in Vivo Projection. Antioxidants 2020, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prommajak, T.; Noppol, L.; Rattanapanone, N. Tannins in Fruit Juices and their Removal. JNS 2020, 19, 76. [Google Scholar] [CrossRef]
- Valenzuela, M.; Bastias, L.; Montenegro, I.; Werner, E.; Madrid, A.; Godoy, P.; Parraga, M.; Villena, J. Autumn Royal and Ribier Grape Juice Extracts Reduced Viability and Metastatic Potential of Colon Cancer Cells. Evid.-Based Complement. Altern. Med. 2018, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Lippard, S.J. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I.; Kocyigit, A.; Gonel, A.; Arslan, E.; Durgun, M. The Protective Effect of Naringenin-Oxime on Cisplatin-Induced Toxicity in Rats. Biochem. Res. Int. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lin, B.; Wu, J.; Zhang, H.; Wu, B. Metformin inhibits the proliferation of A549/CDDP cells by activating p38 mitogen-activated protein kinase. Oncol. Lett. 2014, 8, 1269–1274. [Google Scholar] [CrossRef]
- Ramadan, W.S.; Sait, K.H.; Anfinan, N.M. Anticancer activity of aqueous myrrh extract alone and in combination with cisplatin in HeLa cells. Trop. J. Pharm. Res. 2017, 16, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Nasser, M.; Hijazi, A.; Sayed-Ahmad, B.; Jamal Eddine, Z.; Ibrahim, S.; Rammal, H.; Al-Rekaby, A.-E.-A.; Nasser, M. Efficiency of combining pomegranate juice with low-doses of cisplatin and taxotere on A549 human lung adenocarcinoma cells. Asian Pac. J. Trop. Biomed. 2018, 8, 19–24. [Google Scholar]
- Setorki, M.; Nazari, B.; Asgary, S.; Azadbakht, L.; Rafieian-Kopaei, M. Anti-atherosclerotic effects of verjuice on hypocholesterolemic rabbits. Afr. J. Pharm. Pharm. 2011, 5, 1038–1045. [Google Scholar]
- Zhou, K.; Raffoul, J.J. Potential Anticancer Properties of Grape Antioxidants. J. Oncol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Washington, A.V.; Cubano, L.A.; Dharmawardhane, S.F. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl. Oncol. 2008, 1, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.; Verghese, M.; Walker, L.T.; Shackelford, L.; Chawan, C.B. Grape Products Reduce Colon Cancer in Azoxymethane-induced Aberrant Crypt Foci in Fisher 344 Rats. Sci. Alert 2014. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Prasad, R.; Rosenthal, E.; Katiyar, S.K. Grape seed proanthocyanidins inhibit the invasive potential of head and neck cutaneous squamous cell carcinoma cells by targeting EGFR expression and epithelial-to-mesenchymal transition. BMC Complement. Altern. Med. 2011, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Res. Int. 2018, 113, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Lee, Y.H.; Choi, K.C.; Seong, A.R.; Choi, H.K.; Lee, O.H.; Hwang, H.J.; Yoon, H.G. Grape seed extract regulates androgen receptor-mediated transcription in prostate cancer cells through potent anti-histone acetyltransferase activity. J. Med. Food 2011, 14, 9–16. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, W.G.; Wu, Y.J.; Zheng, T.Z.; Li, W.; Qu, S.Y.; Liu, N.F. Proanthocyanidin from grape seeds potentiates anti-tumor activity of doxorubicin via immunomodulatory mechanism. Int. Immunopharmacol. 2005, 5, 1247–1257. [Google Scholar] [CrossRef]
- Uchino, R.; Madhyastha, R.; Madhyastha, H.; Dhungana, S.; Nakajima, Y.; Omura, S.; Maruyama, M. NFκB-dependent regulation of urokinase plasminogen activator by proanthocyanidin-rich grape seed extract: Effect on invasion by prostate cancer cells. Blood Coagul. Fibrin. 2010, 21, 528–533. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.D.L.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández- Arroyo, S.; Segura-Carretero, A. Cocoa and grape seed byproducts as a source of antioxidant and anti-inflammatory proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]


| Active Compounds | Total Amounts |
|---|---|
| Total alkaloids content (TAC) | 0.057 g (5.7%) |
| Total phenols content (TPC; mg GAE /mL) | 2.82 mg/mL |
| Total flavonoids content (TFC; mg RE/mL) | 2.6 mg/mL |
| Total tannins content (TTC) | 19.9 mg/mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, M.; Cheikh-Ali, H.; Hijazi, A.; Merah, O.; Al-Rekaby, A.E.-A.N.; Awada, R. Phytochemical Profile, Antioxidant and Antitumor Activities of Green Grape Juice. Processes 2020, 8, 507. https://doi.org/10.3390/pr8050507
Nasser M, Cheikh-Ali H, Hijazi A, Merah O, Al-Rekaby AE-AN, Awada R. Phytochemical Profile, Antioxidant and Antitumor Activities of Green Grape Juice. Processes. 2020; 8(5):507. https://doi.org/10.3390/pr8050507
Chicago/Turabian StyleNasser, Mohamad, Hoda Cheikh-Ali, Akram Hijazi, Othmane Merah, Abd El-Ameer N. Al-Rekaby, and Rana Awada. 2020. "Phytochemical Profile, Antioxidant and Antitumor Activities of Green Grape Juice" Processes 8, no. 5: 507. https://doi.org/10.3390/pr8050507
APA StyleNasser, M., Cheikh-Ali, H., Hijazi, A., Merah, O., Al-Rekaby, A. E.-A. N., & Awada, R. (2020). Phytochemical Profile, Antioxidant and Antitumor Activities of Green Grape Juice. Processes, 8(5), 507. https://doi.org/10.3390/pr8050507






