Hydrodynamic and Heat Transfer Study of a Fluidized Bed by Discrete Particle Simulations
Abstract
:1. Introduction
2. Governing Equations
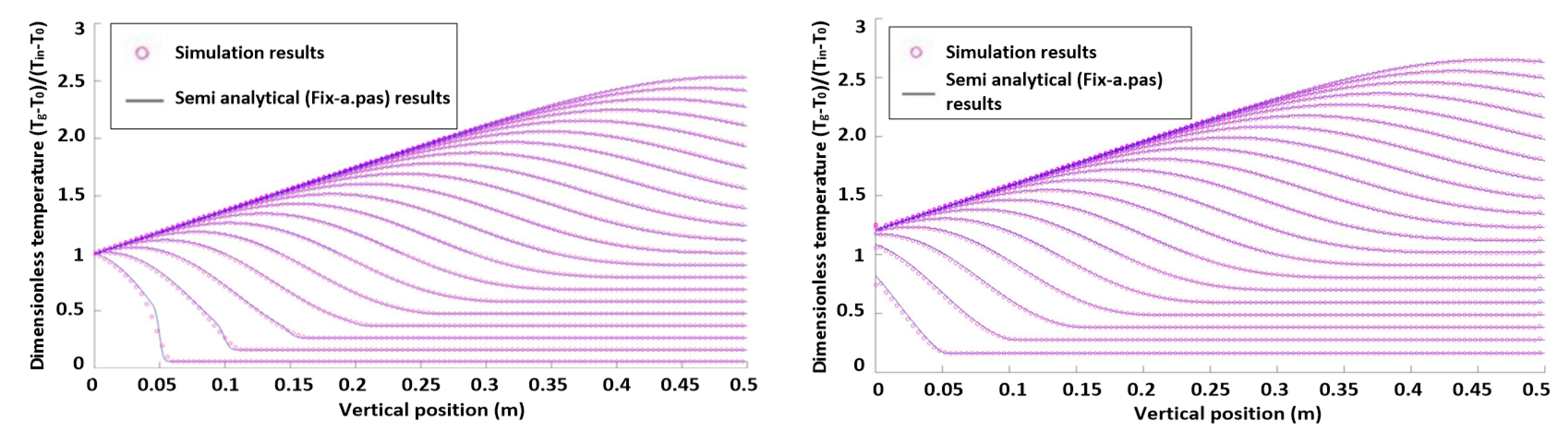
3. Model Verification
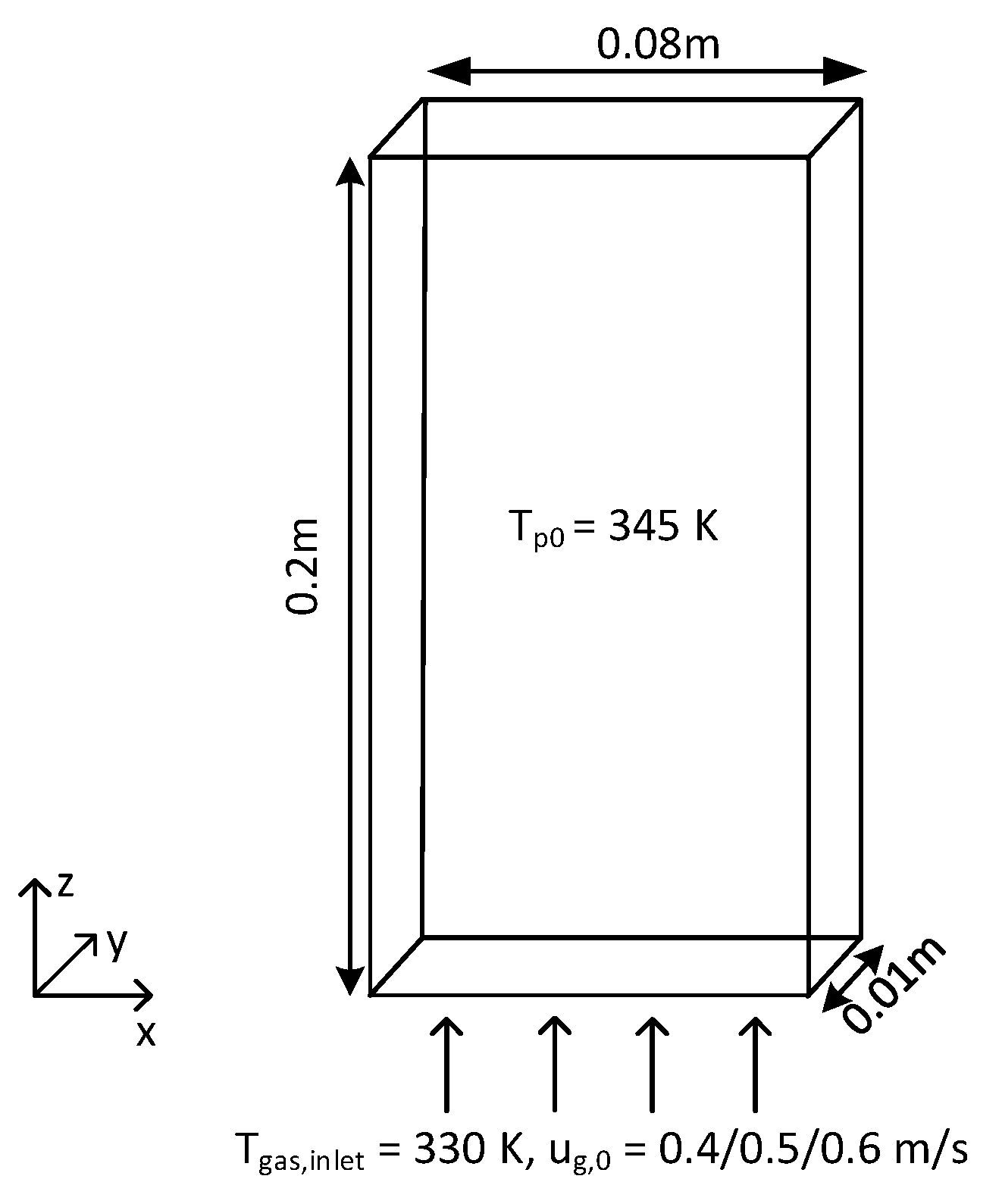
4. Simulation Settings
5. Results
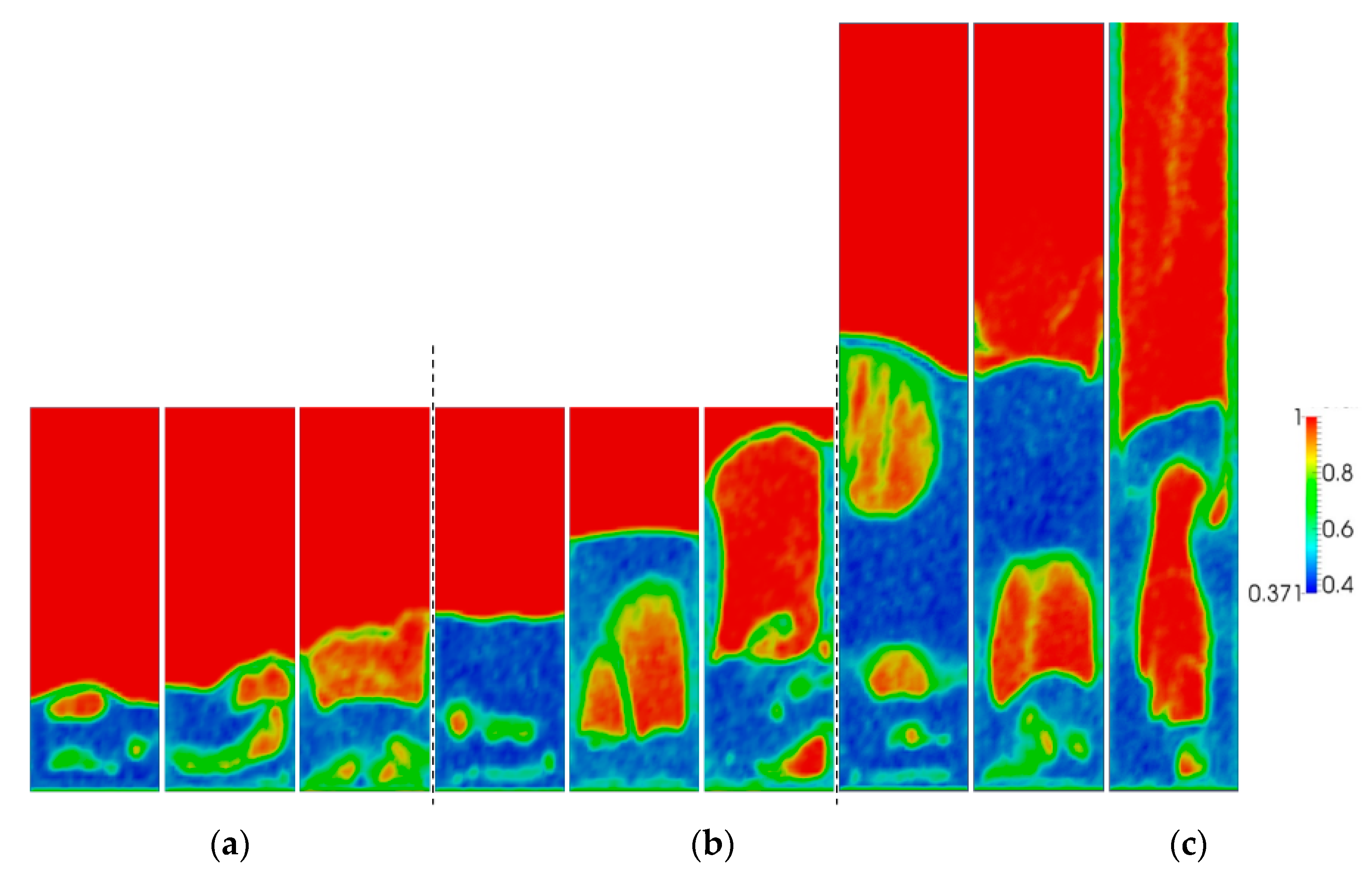
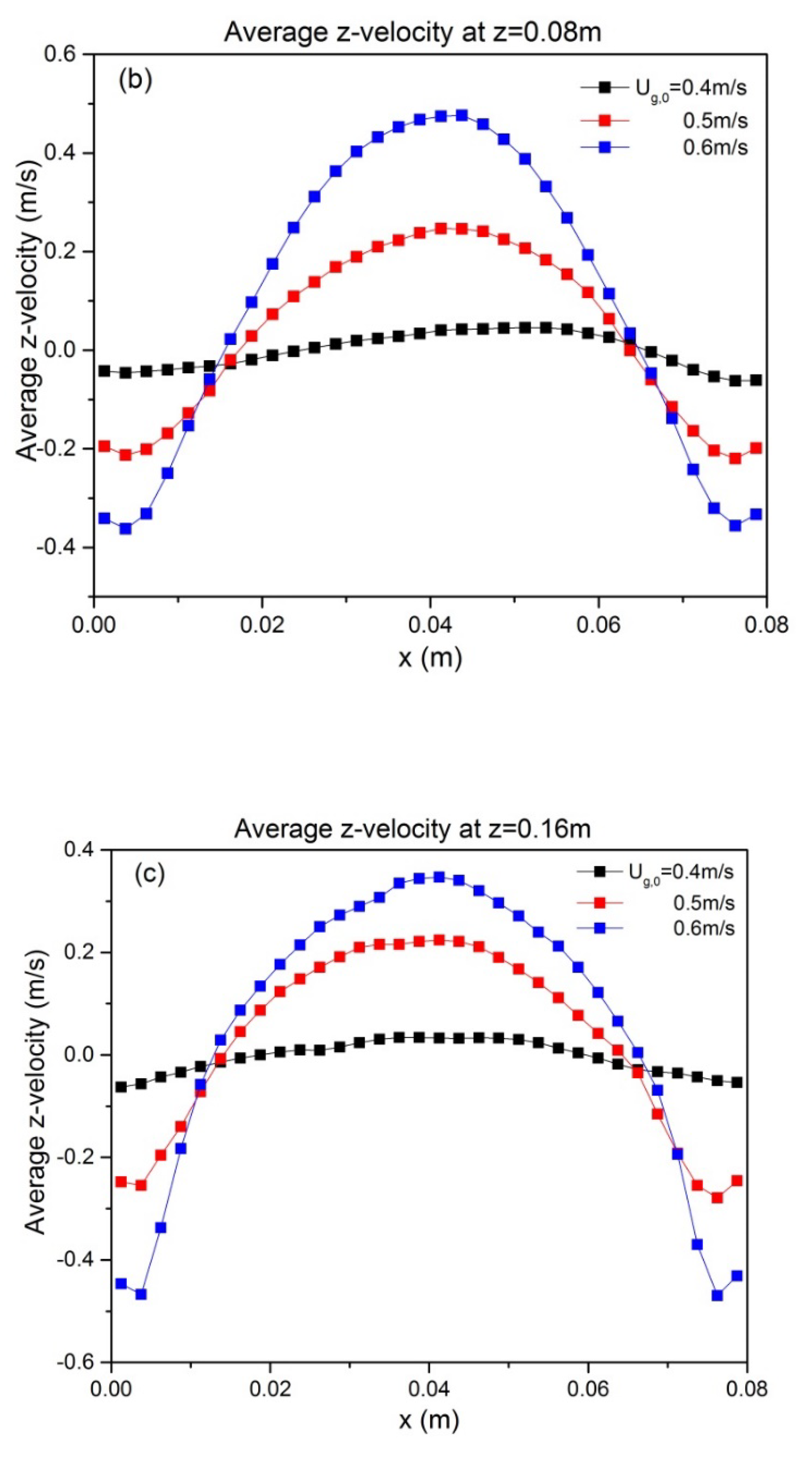
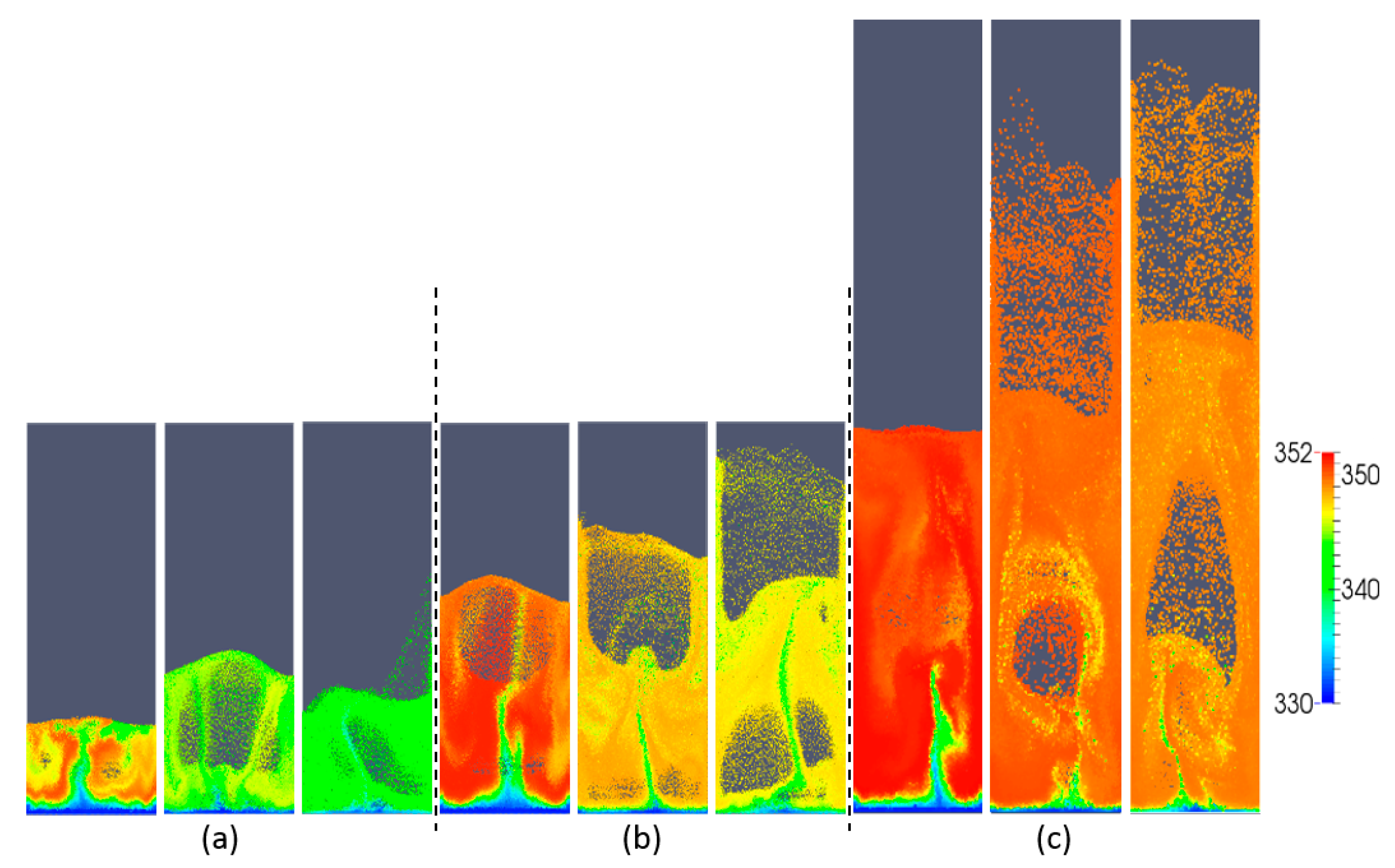
5.1. Effect of Superficial Velocity and Aspect Ratio on Bed Hydrodynamic Behavior
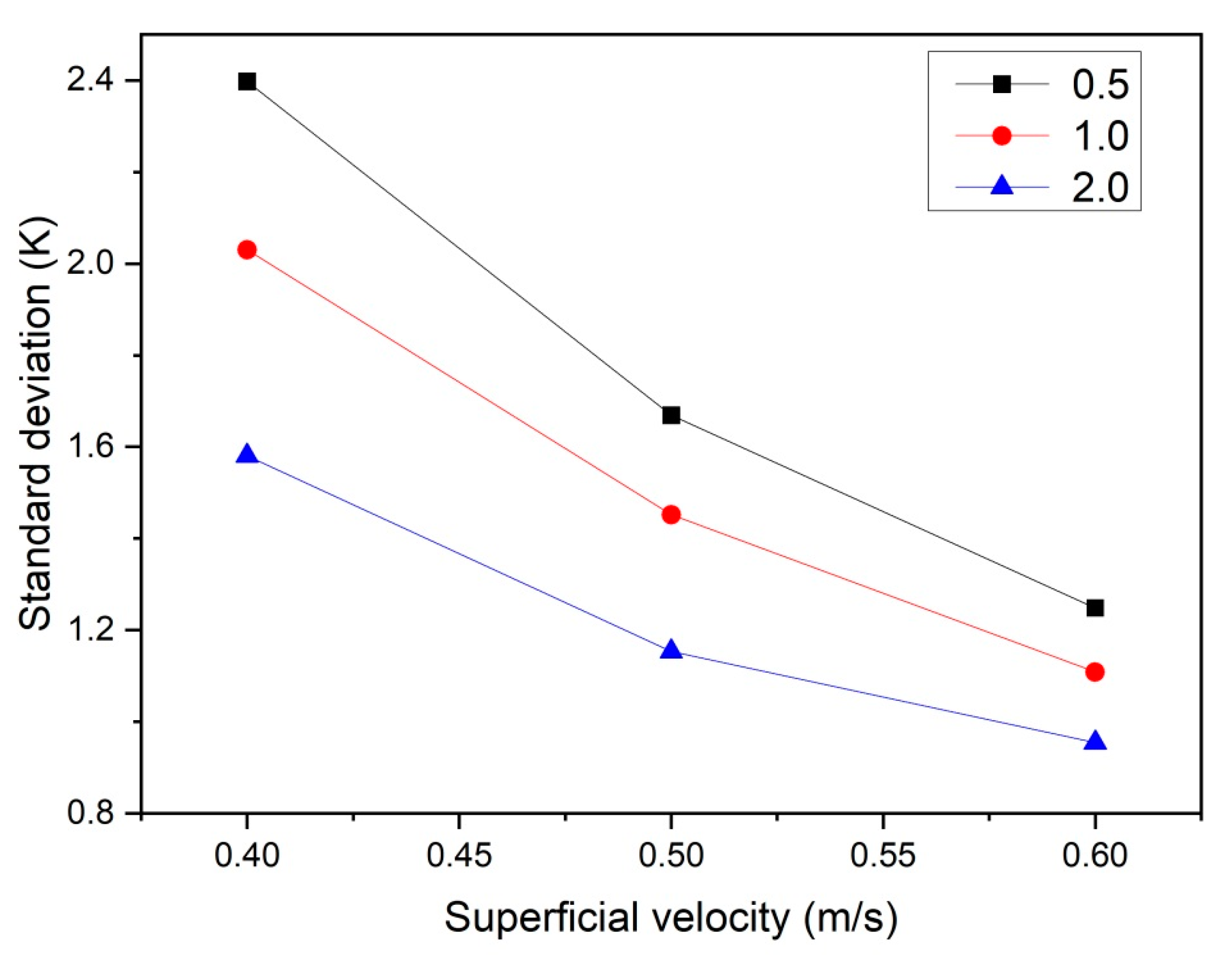
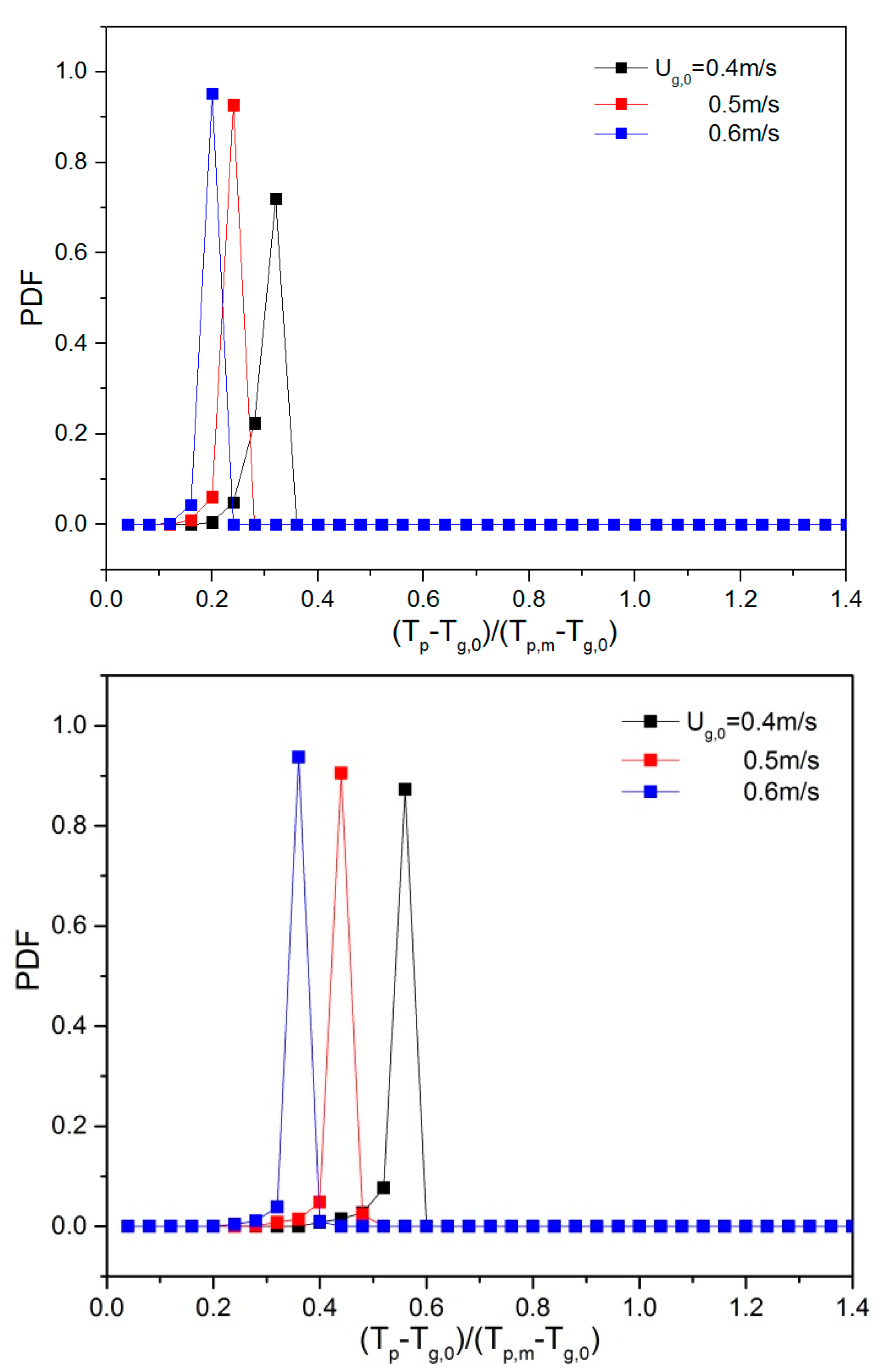
5.2. Effect of Superficial Velocity and Aspect Ratio on Heat Transfer Behavior
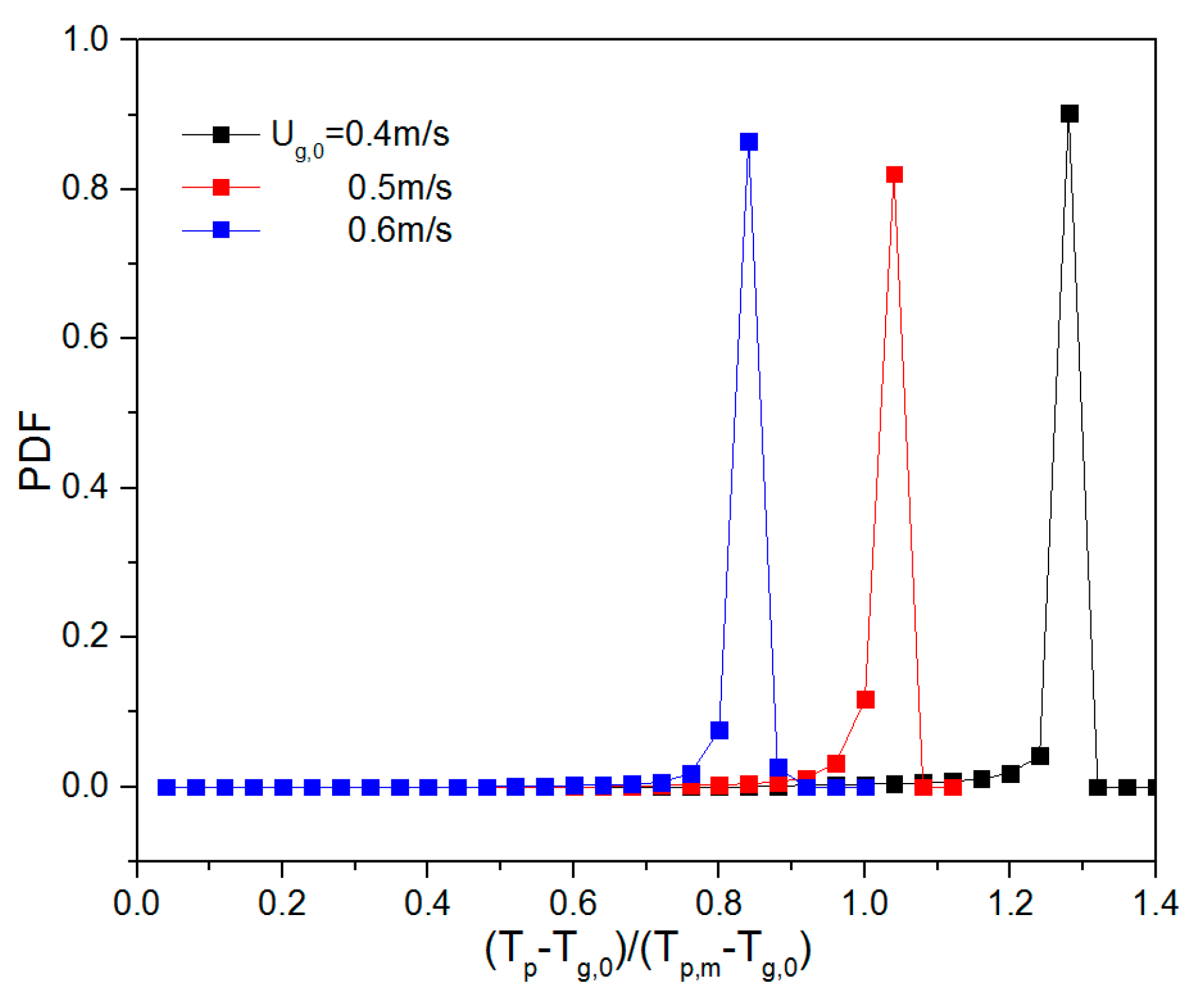
5.3. Effect of the Heat Source Term on Heat Transfer Behavior
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Notation
| Cp | Heat capacity, J·kg−1·K−1 |
| dp | Particle diameter, m |
| g | Gravitational acceleration constant, m·s−2 |
| h | Effective interfacial heat transfer coefficient, W·m−2·K−1 |
| k | Fluid thermal conductivity, W·m−1·K−1 |
| Np | Particle number, - |
| Nup | Particle Nusselt number, - |
| Pr | Prandtl number, - |
| Q | Source term for the interphase heat transfer exchange, W·m−3 |
| q | Heat production, W |
| Rep | Particle Reynolds number, - |
| t | Time, s |
| T | Temperature, K |
| Greek symbols | |
| Δξ | Width of grid cell, m |
| Δψ | Depth of grid cell, m |
| Δζ | Height of grid cell, m |
| ε | Volume fraction, - |
| ρ | Density, kg·m−3 |
| µ | Dynamic viscosity, kg·m−1·s−1 |
| σ | Standard deviation of particle temperature, K |
| Subscripts | |
| p | Particle phase |
| g | Gas phase |
References
- Kunii, D.; Levenspiel, O. Fluidization engineering. In Butterworth-Heinemann Series in Chemical Engineering; Butterworth-Heinemann Limited: Oxford, UK, 1991. [Google Scholar]
- Basu, P.; Nag, P. An investigation into heat transfer in circulating fluidized beds. Int. J. Heat Mass Transf. 1987, 30, 2399–2409. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, A.; Zulli, P. Particle scale study of heat transfer in packed and bubbling fluidized beds. AIChE J. 2009, 55, 868–884. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, A.; Zulli, P. A new computational method for studying heat transfer in fluid bed reactors. Powder Technol. 2010, 197, 102–110. [Google Scholar] [CrossRef]
- Borodulya, V.A.; Ganzha, V.L.; Teplitskii, Y.S.; Epanov, Y.G. Heat transfer in fluidized beds. J. Eng. Phys. 1985, 49, 1197–1202. [Google Scholar] [CrossRef]
- Valenzuela, J.; Glicksman, L. An experimental study of solids mixing in a freely bubbling two-dimensional fluidized bed. Powder Technol. 1984, 38, 63–72. [Google Scholar] [CrossRef]
- Limtrakul, S.; Boonsrirat, A.; Vatanatham, T. DEM modeling and simulation of a catalytic gas–solid fluidized bed reactor: A spouted bed as a case study. Chem. Eng. Sci. 2004, 59, 5225–5231. [Google Scholar] [CrossRef]
- Zhou, H.; Flamant, G.; Gauthier, D. DEM-LES of coal combustion in a bubbling fluidized bed. Part i: Gas-particle turbulent flow structure. Chem. Eng. Sci. 2004, 59, 4193–4203. [Google Scholar] [CrossRef]
- Patil, A.V.; Peters, E.; Kolkman, T.; Kuipers, J. Modeling bubble heat transfer in gas–solid fluidized beds using DEM. Chem. Eng. Sci. 2014, 105, 121–131. [Google Scholar] [CrossRef]
- Deen, N.G.; Annaland, M.V.S.; Van Der Hoef, M.; Kuipers, J. Review of discrete particle modeling of fluidized beds. Chem. Eng. Sci. 2007, 62, 28–44. [Google Scholar] [CrossRef]
- Van Der Hoef, M.A.; Annaland, M.V.S.; Deen, N.G.; Kuipers, J. Numerical Simulation of Dense Gas-Solid Fluidized Beds: A Multiscale Modeling Strategy. Annu. Rev. Fluid Mech. 2008, 40, 47–70. [Google Scholar] [CrossRef]
- Hoomans, B.; Kuipers, J.; Briels, W.; Van Swaaij, W. Discrete particle simulation of bubble and slug formation in a two-dimensional gas-fluidised bed: A hard-sphere approach. Chem. Eng. Sci. 1996, 51, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Annaland, M.V.S.; Kuipers, J.; Deen, N.G. Effect of superficial gas velocity on the particle temperature distribution in a fluidized bed with heat production. Chem. Eng. Sci. 2016, 140, 279–290. [Google Scholar] [CrossRef]
- Beetstra, R.; Van Der Hoef, M.; Kuipers, J. Numerical study of segregation using a new drag force correlation for polydisperse systems derived from lattice-Boltzmann simulations. Chem. Eng. Sci. 2007, 62, 246–255. [Google Scholar] [CrossRef]
- Syamlal, M.; Gidaspow, D. Hydrodynamics of fluidization: Prediction of wall to bed heat transfer coefficients. AIChE J. 1985, 31, 127–135. [Google Scholar] [CrossRef]
- Gunn, D.J. Transfer of heat or mass to particles in fixed and fluidized beds. Int. J. Heat Mass Transf. 1978, 21, 467–476. [Google Scholar] [CrossRef]
- Annaland, M.V.S.; Deen, N.G.; Kuipers, J. Numerical simulation of gas–liquid–solid flows using a combined front tracking and discrete particle method. Chem. Eng. Sci. 2005, 60, 6188–6198. [Google Scholar] [CrossRef]
- Bird, R.; Stewart, W.; Lightfoot, E. Transport Phenomena; John Wiley and Sons: Hoboken, NJ, USA, 2001. [Google Scholar]



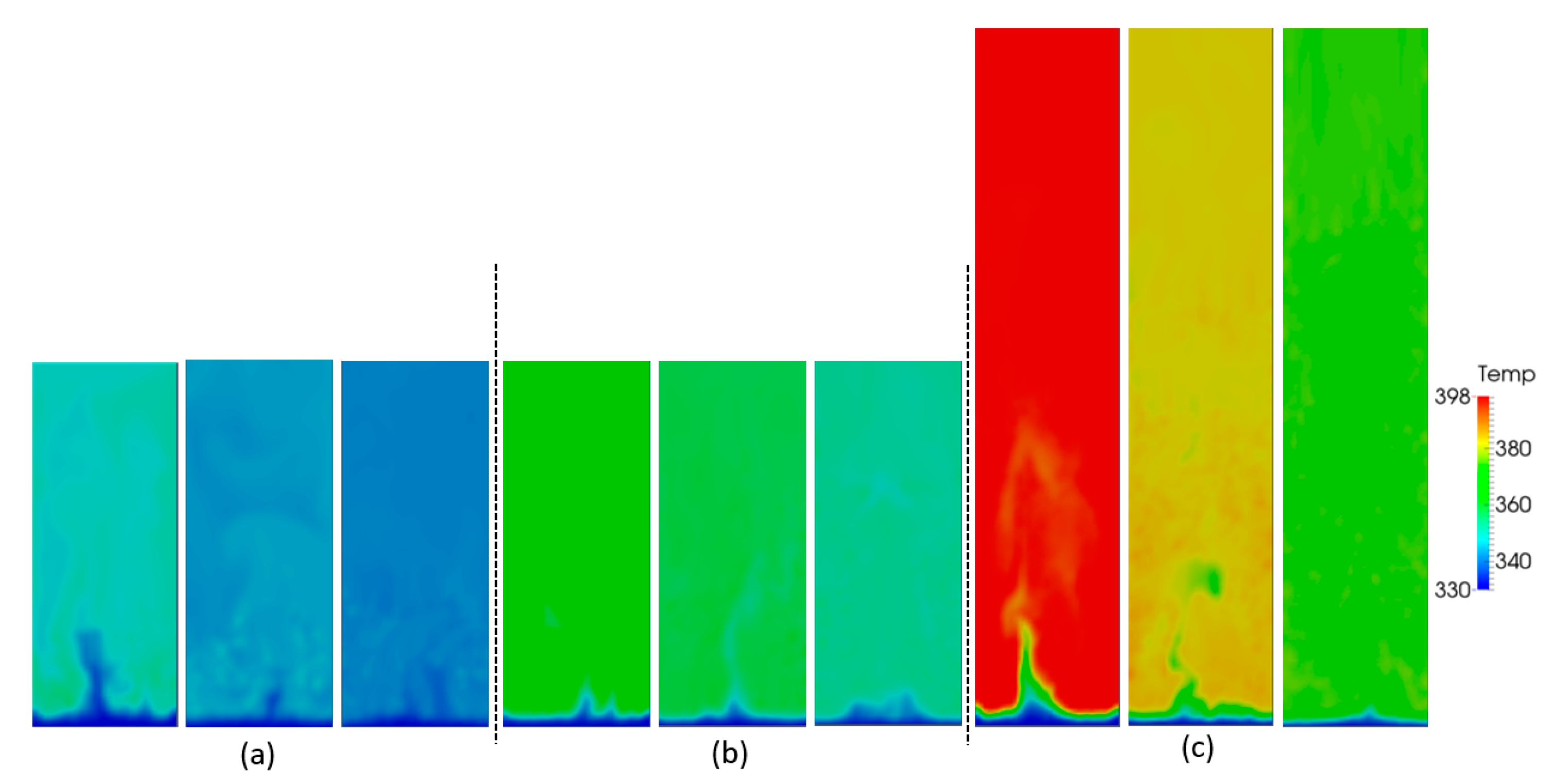
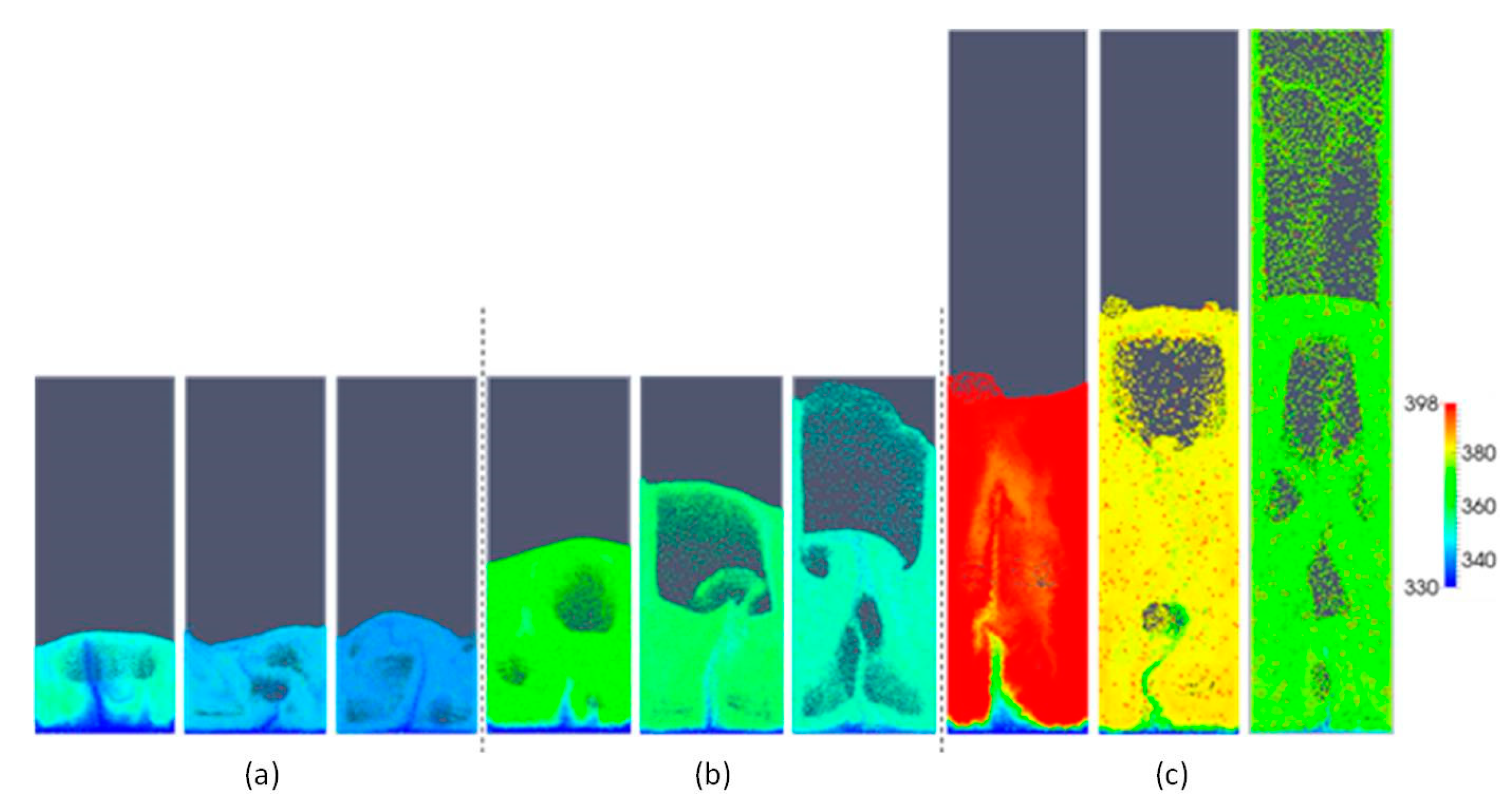
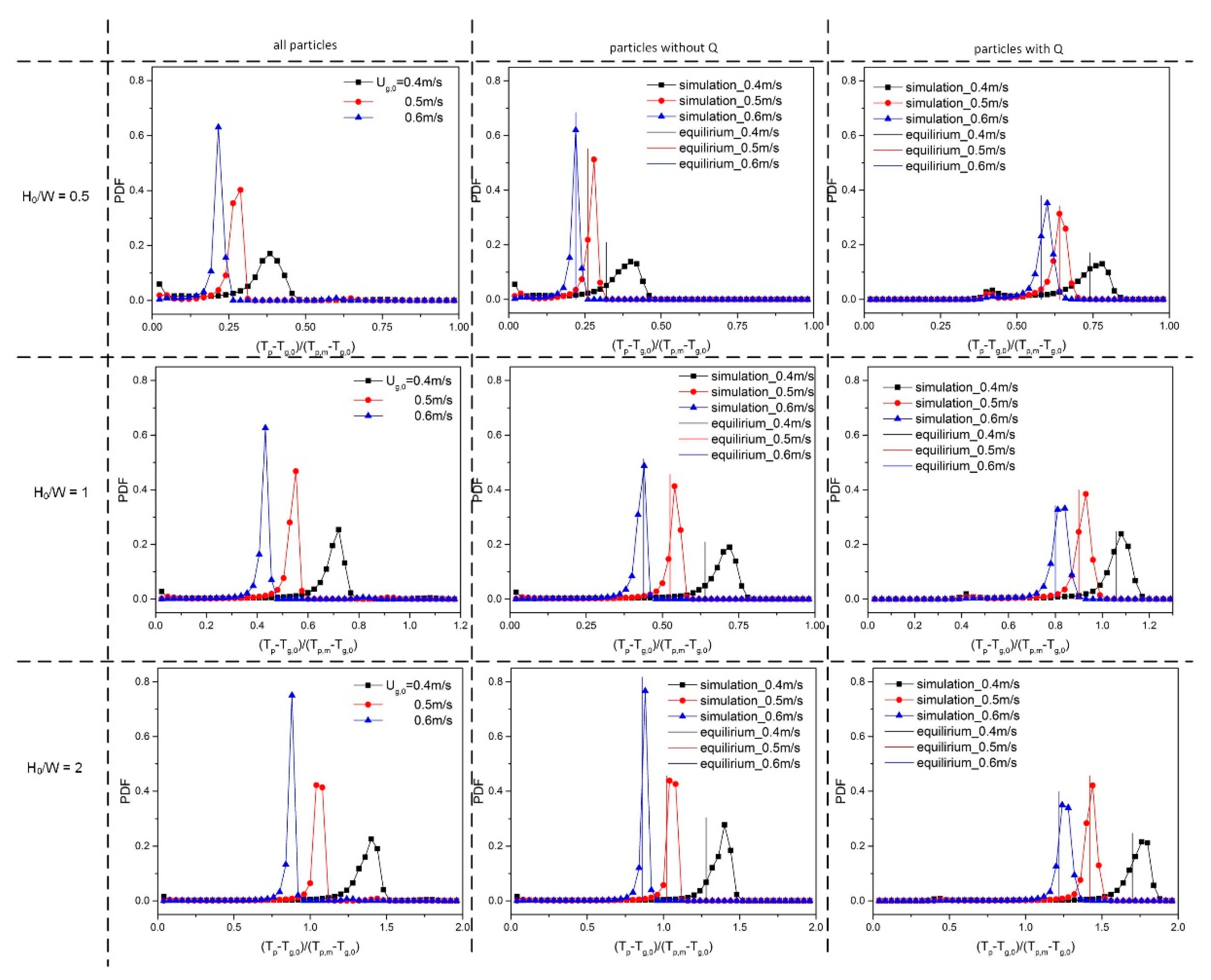
| Property (Unit), Symbol | Value |
|---|---|
| Gas density (kg/m3), ρg | 1000 |
| Inlet gas temperature (K), Tg,0 | 373 |
| Gas viscosity (Pa s), µg | 1.0 × 10−3 |
| Gas heat capacity (J/kg/K), Cpg | 4187 |
| Gas thermal conductivity (W/m/K), kg | 0.5 |
| Particle diameter (m), dp | 3.95 × 10−3 |
| Particle density (kg/m3), ρp | 8400 |
| Initial temperature of gas and particles (K) | 273 |
| Particle heat capacity (J/kg/K), Cpp | 385 |
| Setting | Value |
|---|---|
| Width of the bed (m) | 0.1 |
| Depth of the bed (m) | 0.1 |
| Initial particle bed height (m) | 0.5 |
| Particle number | 78,125 (25 × 25 × 125) |
| Δx = Δy = Δz (m) | 0.004 |
| Time step of particle phase (s) | 0.0001 |
| Time step of gas phase (s) | 0.002 |
| Setting | Value |
|---|---|
| Width of the bed (m) | 0.08 |
| Depth of the bed (m) | 0.01 |
| Initial particle bed height (m) | 0.04/0.08/0.16 |
| Particle number | 37,225/74,450/148,900 |
| Δx = Δy = Δz (m) | 0.0025 |
| Time step of particle phase (s) | 1.0 × 10−5 |
| Time step of gas phase (s) | 2.0 × 10−4 |
| Property (Unit), Value | Value |
|---|---|
| Gas density (kg/m3), ρg | 1.49 |
| Inlet gas temperature (K), Tg,0 | 330 |
| Gas viscosity (Pa s), µg | 1.0 × 10−5 |
| Gas heat capacity (J/kg/K), Cpg | 1670 |
| Gas thermal conductivity (W/m/K), kg | 2.09 × 10−2 |
| Particle diameter (m), dp | 9.95 × 10−4 |
| Particle density (kg/m3), ρp | 667 |
| Particle heat capacity (J/kg/K), Cpp | 1670 |
| Normal coefficient of restitution (particle–particle), e | 0.6 |
| Youngs Modulus | 5.0 × 105 |
| Poisson ratio | 0.45 |
| Non-Uniform Cases | Uniform Cases | |||||
|---|---|---|---|---|---|---|
| Tg | Tp1 | Tp2 | (Tp1-Tp2)/(Tp1-Tg,in) | Tg | Tp | |
| 0.5/0.4 | 346 | 346 | 367 | −1.28 | 346 | 346 |
| 0.5/0.5 | 343 | 343 | 362 | −1.5 | 343 | 343 |
| 0.5/0.6 | 341 | 341 | 359 | −1.7 | 341 | 341 |
| 1.0/0.4 | 362 | 362 | 383 | −0.64 | 362 | 363 |
| 1.0/0.5 | 356 | 356 | 375 | −0.75 | 356 | 356 |
| 1.0/0.6 | 351 | 351 | 370 | −0.85 | 351 | 352 |
| 2.0/0.4 | 394 | 394 | 415 | −0.32 | 394 | 395 |
| 2.0/0.5 | 381 | 381 | 401 | −0.37 | 381 | 382 |
| 2.0/0.6 | 373 | 373 | 391 | −0.42 | 373 | 373 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, L.; Buist, K.A.; Kuipers, J.A.M.; Deen, N.G. Hydrodynamic and Heat Transfer Study of a Fluidized Bed by Discrete Particle Simulations. Processes 2020, 8, 463. https://doi.org/10.3390/pr8040463
Mu L, Buist KA, Kuipers JAM, Deen NG. Hydrodynamic and Heat Transfer Study of a Fluidized Bed by Discrete Particle Simulations. Processes. 2020; 8(4):463. https://doi.org/10.3390/pr8040463
Chicago/Turabian StyleMu, Lijing, Kay A. Buist, J. A. M. Kuipers, and Niels G. Deen. 2020. "Hydrodynamic and Heat Transfer Study of a Fluidized Bed by Discrete Particle Simulations" Processes 8, no. 4: 463. https://doi.org/10.3390/pr8040463
APA StyleMu, L., Buist, K. A., Kuipers, J. A. M., & Deen, N. G. (2020). Hydrodynamic and Heat Transfer Study of a Fluidized Bed by Discrete Particle Simulations. Processes, 8(4), 463. https://doi.org/10.3390/pr8040463






