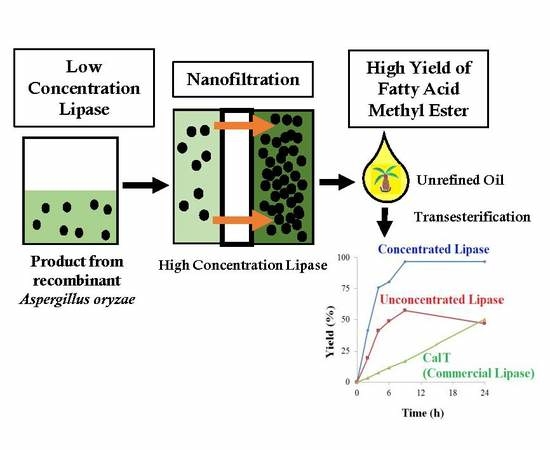
Concentration of Lipase from Aspergillus oryzae Expressing Fusarium heterosporum by Nanofiltration to Enhance Transesterification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microorganisms
2.2. Lipase Production
2.3. Nanofiltration Membrane Separation Processes
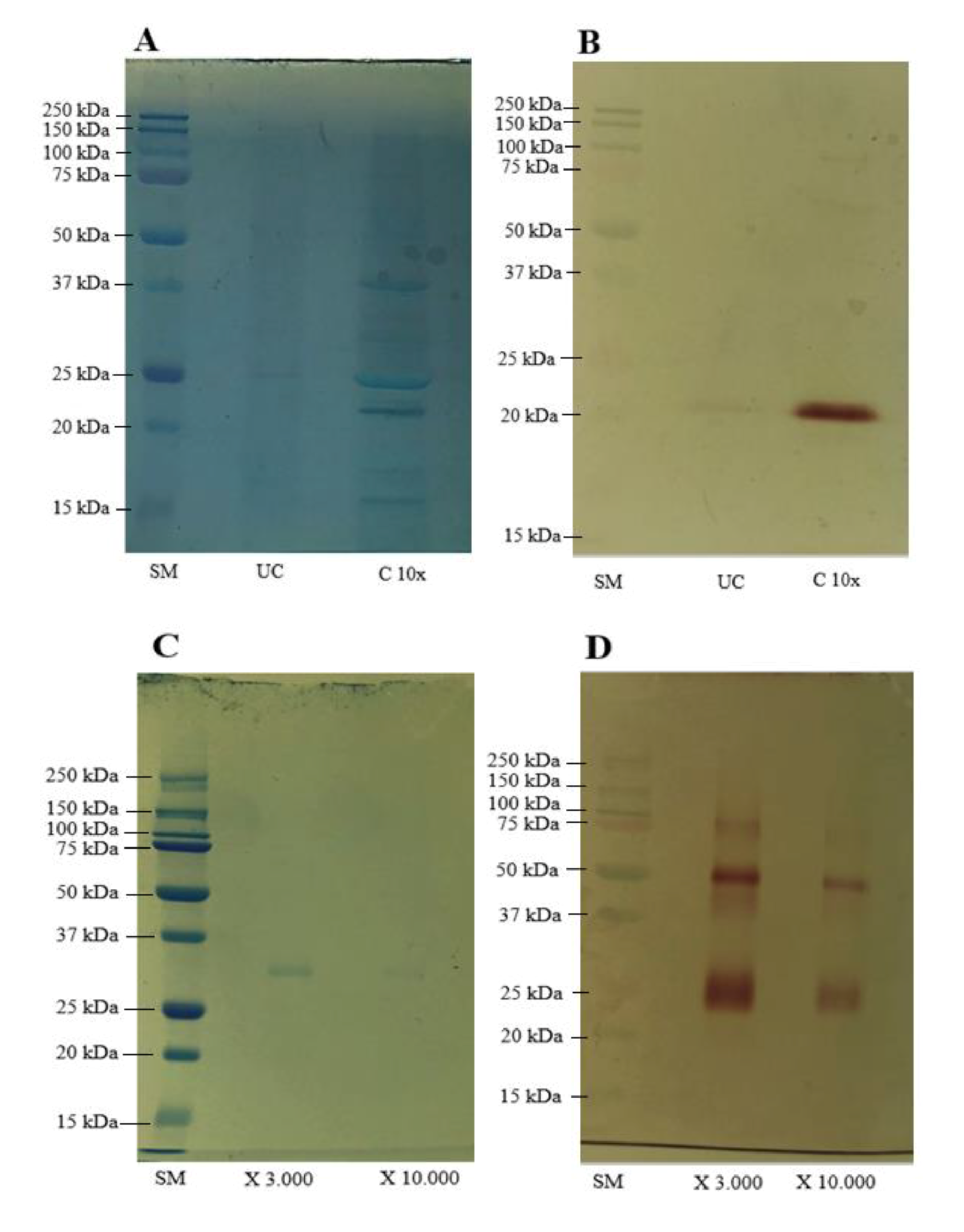
2.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Zymography
2.5. Measurement of Lipase Activity and Protein Assay
2.6. FAME Production by Enzyme
2.7. Analytical Methods
3. Results and Discussion
3.1. Characterization of Lipases before and after Membrane Concentration
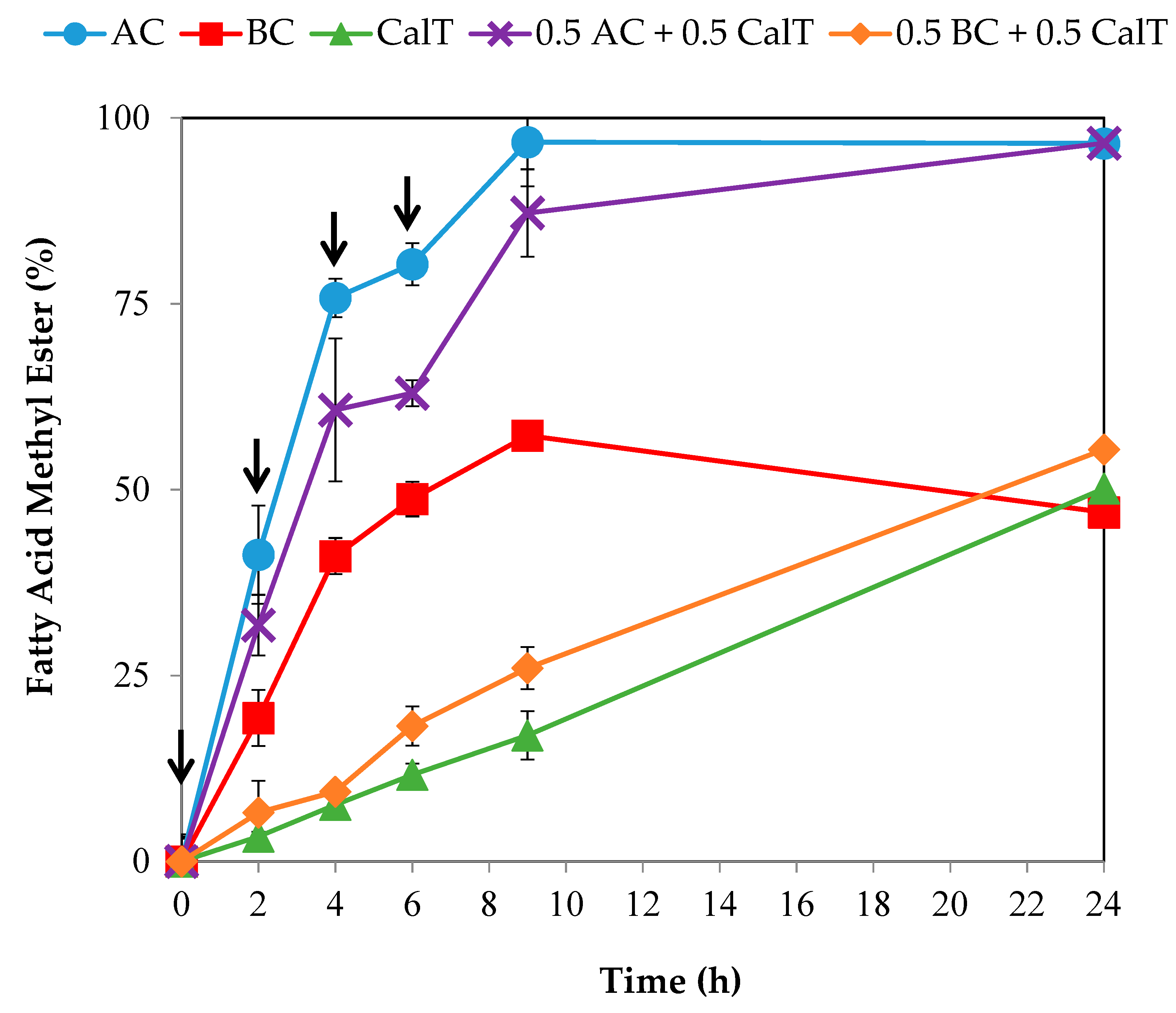
3.2. Efficient FAME Production by Concentrated Lipase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Optimization of palm oil biodiesel blends and engine operating parameters to improve performance and PM morphology in a common rail direct injection diesel engine. Fuel 2020, 260, 116326. [Google Scholar] [CrossRef]
- Lam, M.K.; Jamalluddin, N.A.; Lee, K.T. Production of Biodiesel Using Palm Oil, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128168561. [Google Scholar]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Bhangu, S.K.; Gupta, S.; Ashokkumar, M. Ultrasonic enhancement of lipase-catalysed transesterification for biodiesel synthesis. Ultrason. Sonochem. 2017, 34, 305–309. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification—Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Narwal, S.K.; Gupta, R. Biodiesel production by transesterification using immobilized lipase. Biotechnol. Lett. 2013, 35, 479–490. [Google Scholar] [CrossRef]
- Hama, S.; Noda, H.; Kondo, A. How lipase technology contributes to evolution of biodiesel production using multiple feedstocks. Curr. Opin. Biotechnol. 2018, 50, 57–64. [Google Scholar] [CrossRef]
- Bajaj, A.; Lohan, P.; Jha, P.N.; Mehrotra, R. Biodiesel production through lipase catalyzed transesterification: An overview. J. Mol. Catal. B Enzym. 2010, 62, 9–14. [Google Scholar] [CrossRef]
- Amoah, J.; Quayson, E.; Hama, S.; Yoshida, A.; Hasunuma, T.; Ogino, C.; Kondo, A. Simultaneous conversion of free fatty acids and triglycerides to biodiesel by immobilized Aspergillus oryzae expressing Fusarium heterosporum lipase. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef]
- Amoah, J.; Ho, S.H.; Hama, S.; Yoshida, A.; Nakanishi, A.; Hasunuma, T.; Ogino, C.; Kondo, A. Lipase cocktail for efficient conversion of oils containing phospholipids to biodiesel. Bioresour. Technol. 2016, 211, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Amoah, J.; Ho, S.H.; Hama, S.; Yoshida, A.; Nakanishi, A.; Hasunuma, T.; Ogino, C.; Kondo, A. Converting oils high in phospholipids to biodiesel using immobilized Aspergillus oryzae whole-cell biocatalysts expressing Fusarium heterosporum lipase. Biochem. Eng. J. 2016, 105, 10–15. [Google Scholar] [CrossRef]
- He, Q.; Xu, Y.; Teng, Y.; Wang, D. Biodiesel Production Catalyzed by Whole-Cell Lipase from Rhizopus chinensis. Chin. J. Catal. 2008, 29, 41–46. [Google Scholar] [CrossRef]
- Menoncin, S.; Domingues, N.M.; Freire, D.M.G.; Toniazzo, G.; Cansian, R.L.; Oliveira, J.V.; Di Luccio, M.; de Oliveira, D.; Treichel, H. Study of the extraction, concentration, and partial characterization of lipases obtained from Penicillium verrucosum using solid-state fermentation of soybean bran. Food Bioprocess Technol. 2010, 3, 537–544. [Google Scholar] [CrossRef]
- Preczeski, K.P.; Kamanski, A.B.; Scapini, T.; Camargo, A.F.; Modkoski, T.A.; Rossetto, V.; Venturin, B.; Mulinari, J.; Golunski, S.M.; Mossi, A.J.; et al. Efficient and low-cost alternative of lipase concentration aiming at the application in the treatment of waste cooking oils. Bioprocess Biosyst. Eng. 2018, 41, 851–857. [Google Scholar] [CrossRef]
- Reinehr, C.O.; Treichel, H.; Tres, M.V.; Steffens, J.; Brião, V.B.; Colla, L.M. Successive membrane separation processes simplify concentration of lipases produced by Aspergillus niger by solid-state fermentation. Bioprocess Biosyst. Eng. 2017, 40, 843–855. [Google Scholar] [CrossRef]
- Varkkey, H.; Tyson, A.; Choiruzzad, S.A.B. Palm oil intensification and expansion in Indonesia and Malaysia: Environmental and socio-political factors influencing policy. For. Policy Econ. 2018, 92, 148–159. [Google Scholar] [CrossRef]
- Takaya, T.; Koda, R.; Adachi, D.; Nakashima, K.; Wada, J.; Bogaki, T.; Ogino, C.; Kondo, A. Highly efficient biodiesel production by a whole-cell biocatalyst employing a system with high lipase expression in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2011, 90, 1171–1177. [Google Scholar] [CrossRef]
- Hama, S.; Tamalampudi, S.; Suzuki, Y.; Yoshida, A.; Fukuda, H.; Kondo, A. Preparation and comparative characterization of immobilized Aspergillus oryzae expressing Fusarium heterosporum lipase for enzymatic biodiesel production. Appl. Microbiol. Biotechnol. 2008, 81, 637–645. [Google Scholar] [CrossRef]
- Sasaki, K.; Tsuge, Y.; Sasaki, D.; Teramura, H.; Inokuma, K.; Hasunuma, T.; Ogino, C.; Kondo, A. Mechanical milling and membrane separation for increased ethanol production during simultaneous saccharification and co-fermentation of rice straw by xylose-fermenting Saccharomyces cerevisiae. Bioresour. Technol. 2015, 185, 263–268. [Google Scholar] [CrossRef]
- Febriani; Hertadi, R.; Kahar, P.; Akhmaloka; Madayanti, F. Isolation and purification of novel thermostable alkaline lipase from local thermophilic microorganism. Biosci. Biotechnol. Res. Asia 2010, 7, 617–622. [Google Scholar]
- Amza, R.L.; Kahar, P.; Juanssilfero, A.B.; Miyamoto, N.; Otsuka, H.; Kihira, C.; Ogino, C.; Kondo, A. High cell density cultivation of Lipomyces starkeyi for achieving highly efficient lipid production from sugar under low C/N ratio. Biochem. Eng. J. 2019, 149, 107236. [Google Scholar] [CrossRef]
- Priji, P.; Sajith, S.; Faisal, P.A.; Benjamin, S. Pseudomonas sp. BUP6 produces a thermotolerant alkaline lipase with trans-esterification efficiency in producing biodiesel. 3 Biotech 2017, 7, 369. [Google Scholar] [CrossRef]
- Sharma, A.; Meena, K.R.; Kanwar, S.S. Molecular characterization and bioinformatics studies of a lipase from Bacillus thermoamylovorans BHK67. Int. J. Biol. Macromol. 2018, 107, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Okamoto, M.; Shirai, T.; Tsuge, Y.; Fujino, A.; Sasaki, D.; Morita, M.; Matsuda, F.; Kikuchi, J.; Kondo, A. Toward the complete utilization of rice straw: Methane fermentation and lignin recovery by a combinational process involving mechanical milling, supporting material and nanofiltration. Bioresour. Technol. 2016, 216, 830–837. [Google Scholar] [CrossRef]
- Marie-Olive, M.N.; Athes, V.; Combes, D. Combined effects of pressure and temperature on enzyme stability. High Press. Res. 2000, 19, 317–322. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Ortiz, C.; Segura, R.L.; Fernández-Lafuente, R.; Guisán, J.M.; Palomo, J.M. Purification of different lipases from Aspergillus niger by using a highly selective adsorption on hydrophobic supports. Biotechnol. Bioeng. 2005, 92, 773–779. [Google Scholar] [CrossRef]
- Golunski, S.; Astolfi, V.; Carniel, N.; De Oliveira, D.; Di Luccio, M.; Mazutti, M.A.; Treichel, H. Ethanol precipitation and ultrafiltration of inulinases from Kluyveromyces marxianus. Sep. Purif. Technol. 2011, 78, 261–265. [Google Scholar] [CrossRef]
- Gottschalk, L.M.F.; Bon, E.P.S.; Nobrega, R. Lignin Peroxidase from Streptomyces viridosporus T7A: Enzyme Concentration Using Ultrafiltration. Biotechnol. Appl. Biochem. 2008, 147, 23–32. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Loha, V.; Tia, S.; Bunnag, B. Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour. Technol. 2011, 102, 7159–7164. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Tres, M.V.; Jahn, S.L.; de Oliveira, J.V. Lipases in liquid formulation for biodiesel production: Current status and challenges. Biotechnol. Appl. Biochem. 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
| Process | Volume (mL) | Lipase Activity (U/mL) | Protein (mg/mL) |
|---|---|---|---|
| Recombinant lipase before concentration by NTR7410 | 350 | 6.4 ± 0.1 | 0.7 ± 0.0 |
| Recombinant lipase after concentration by NTR7410 | 65 | 32.6 ± 3.1 | 4.0 ± 0.2 |
| Permeate from NTR7410 | 280 | 0 | 0 |
| CalleraTM Trans L (CalT) | 8396.7 ± 378.4 | 25.1 ± 1.4 |
| Protein Concentrated | Process Used | Concentration Factor Obtained | Activity Loss | Ref. |
|---|---|---|---|---|
| Lipase (recombinant Aspergillus oryzae) | Nanofiltration (3 kDa) | 5 | 5.5% | This study |
| Lipase (Aspergillus niger) | Sequential micro- and ultrafiltration | 3 | 17% in microfiltration and 22% in ultrafiltration | [17] |
| Phytase (Aspergillus niger) | Ultrafiltration (10 kDa) | 4.3 | 14% | [28] |
| Inulinase (Kluyveromyces marxianus) | Ultrafiltration (100 kDa) | 5.5 | 18.4% | [29] |
| Lignin-peroxidase (Streptomyces viridosporus) | Ultrafiltration (10 kDa) | 10 | 10% | [30] |
| Phycocyanin (Spirulina sp.) | Sequential micro- and ultrafiltration | 2 | 13.6% in 1 µm pore size | [31] |
| Lipase Variations | FAME Compositions (%) | ||||
|---|---|---|---|---|---|
| C14:1 | C16:0 | C18:0 | C18:1 cis | C18:2 cis | |
| AC | 0.79 ± 0.15 | 34.46 ± 0.37 | 5.41 ± 1.01 | 45.90 ± 1.71 | 12.46 ± 0.29 |
| BC | 0.89 ± 0.04 | 35.45 ± 0.12 | 5.84 ± 0.08 | 44.88 ± 0.03 | 12.32 ± 0.09 |
| CalT | 0.81 ± 0.04 | 34.75 ± 0.44 | 5.85 ± 0.18 | 45.06 ± 0.12 | 12.48 ± 0.19 |
| 0.5 AC + 0.5 CalT | 0.79 ± 0.01 | 34.46 ± 0.03 | 5.75 ± 0.08 | 45.14 ± 0.09 | 12.94 ± 0.12 |
| 0.5 BC + 0.5 CalT | 0.76 ± 0.08 | 34.71 ± 0.57 | 5.76 ± 0.11 | 45.24 ± 0.44 | 12.66 ± 0.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijaya, H.; Sasaki, K.; Kahar, P.; Quayson, E.; Rachmadona, N.; Amoah, J.; Hama, S.; Ogino, C.; Kondo, A. Concentration of Lipase from Aspergillus oryzae Expressing Fusarium heterosporum by Nanofiltration to Enhance Transesterification. Processes 2020, 8, 450. https://doi.org/10.3390/pr8040450
Wijaya H, Sasaki K, Kahar P, Quayson E, Rachmadona N, Amoah J, Hama S, Ogino C, Kondo A. Concentration of Lipase from Aspergillus oryzae Expressing Fusarium heterosporum by Nanofiltration to Enhance Transesterification. Processes. 2020; 8(4):450. https://doi.org/10.3390/pr8040450
Chicago/Turabian StyleWijaya, Hans, Kengo Sasaki, Prihardi Kahar, Emmanuel Quayson, Nova Rachmadona, Jerome Amoah, Shinji Hama, Chiaki Ogino, and Akihiko Kondo. 2020. "Concentration of Lipase from Aspergillus oryzae Expressing Fusarium heterosporum by Nanofiltration to Enhance Transesterification" Processes 8, no. 4: 450. https://doi.org/10.3390/pr8040450
APA StyleWijaya, H., Sasaki, K., Kahar, P., Quayson, E., Rachmadona, N., Amoah, J., Hama, S., Ogino, C., & Kondo, A. (2020). Concentration of Lipase from Aspergillus oryzae Expressing Fusarium heterosporum by Nanofiltration to Enhance Transesterification. Processes, 8(4), 450. https://doi.org/10.3390/pr8040450