Understanding the Operating Mode of Fe0/Fe-Sulfide/H2O Systems for Water Treatment
Abstract
1. Introduction
2. The Fe0/Fe-Sulfide/H2O System
3. Contaminant Removal in Fe0/Fe-Sulfide/H2O Systems
4. Characterizing the Efficiency of Fe0/FeS2 Systems
5. Extending the Application of Fe0/FeS2 Systems
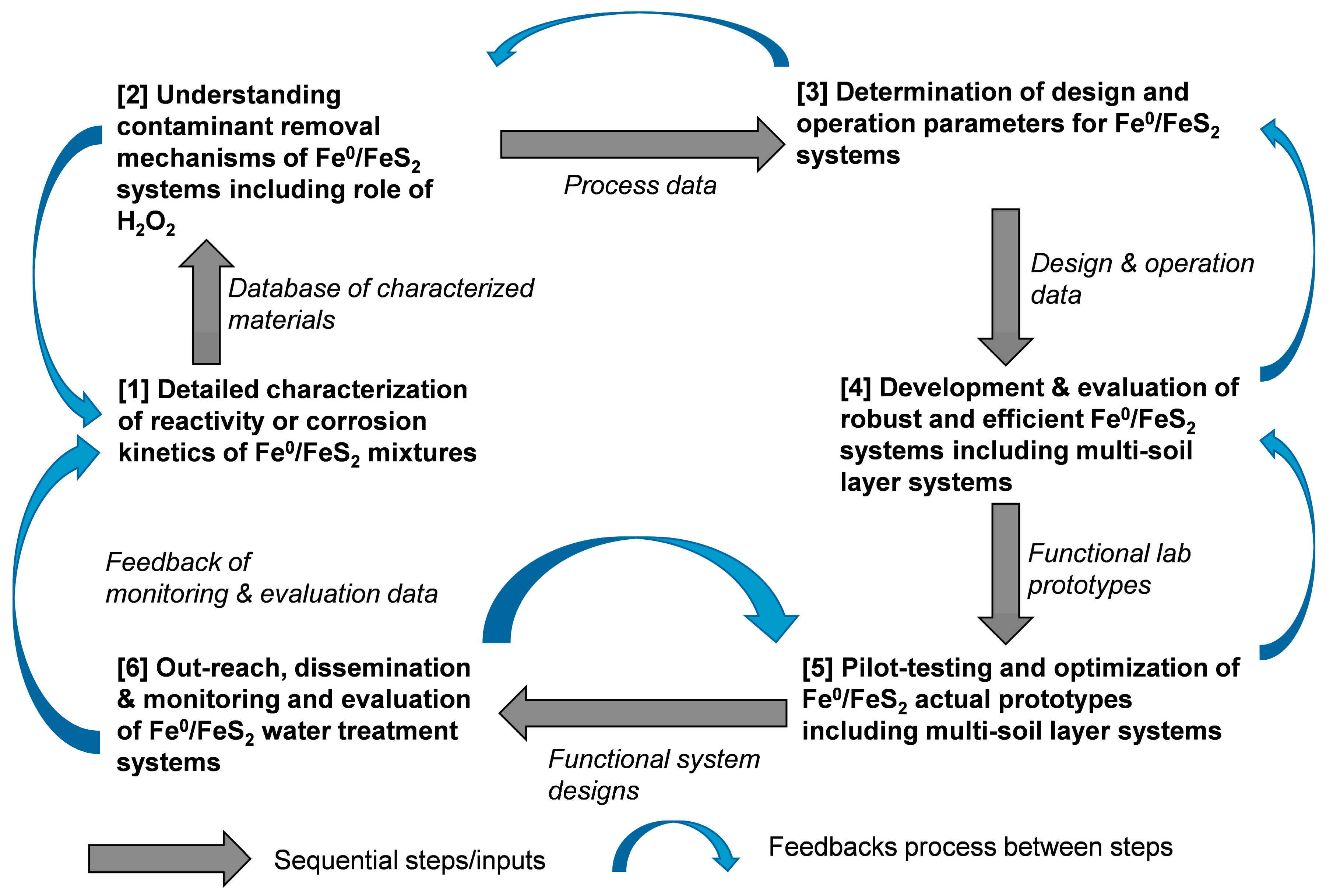
6. Knowledge Gaps and Future Directions
6.1. Characterization of Reactivity of Materials
6.2. Design and Operation Principles of Fe0/FeS2/H2O Systems
6.3. Laboratory-Scale Development Functional Prototypes
6.4. Pilot-Scale and Field Testing and Evaluation
6.5. Outreach and Dissemination of the Fe0/FeS2/H2O Technology
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Craenenbroeck, W. Easton & Anderson and the water supply of Antwerp (Belgium). Ind. Archaeol. Rev. 1998, 20, 105–116. [Google Scholar]
- Mwakabona, H.T.; Ndé-Tchoupé, A.I.; Njau, K.N.; Noubactep, C.; Wydra, K.D. Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res. 2017, 117, 127–142. [Google Scholar] [CrossRef]
- Anderson, M.A. Fundamental Aspects of Selenium Removal by Harza Process. Rep San Joaquin Valley Drainage Program; U.S. Department of the Interior: Sacramento, CA, USA, 1989.
- Tucker, W.G. The purification of water by chemical treatment. Science 1892, 20, 34–38. [Google Scholar] [CrossRef]
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- Davis, F. An Elementary Handbook on Potable Water; Silver, Burdett & Company: New York, NY, USA; Boston, MA, USA; Chicago, IL, USA, 1891. [Google Scholar]
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ. Res. 1992, 64, 699–705. [Google Scholar] [CrossRef]
- Murphy, A.P. Removal of selenate from water by chemical reduction. Ind. Eng. Chem. Res. 1988, 27, 187–191. [Google Scholar] [CrossRef]
- Lauderdale, R.A.; Emmons, A.H. A method for decontaminating small volumes of radioactive water. J. Am. Water Works Assoc. 1951, 43, 327–331. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Antia, D.D.J. Sustainable zero-valent metal (ZVM) water treatment associated with diffusion, infiltration, abstraction, and recirculation. Sustainability 2010, 2, 2988–3073. [Google Scholar] [CrossRef]
- Tellen, V.; Nkeng, G.; Dentel, S. Improved filtration technology for pathogen reduction in rural water supplies. Water 2010, 2, 285–306. [Google Scholar] [CrossRef]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freib. Online Geosci. 2015, 38, 1–80. [Google Scholar]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Gheju, M. Progress in understanding the mechanism of CrVI Removal in Fe0-based filtration systems. Water 2018, 10, 651. [Google Scholar] [CrossRef]
- Guan, Q.; Li, F.; Chen, X.; Tian, C.; Liu, C.; Liu, D. Assessment of the use of a zero-valent iron permeable reactive barrier for nitrate removal from groundwater in the alluvial plain of the Dagu River, China. Environ. Earth Sci. 2019, 78, 244. [Google Scholar] [CrossRef]
- Lü, Y.; Li, J.; Li, Y.; Liang, L.; Dong, H.; Chen, K.; Yao, C.; Li, Z.; Li, J.; Guan, X. The roles of pyrite for enhancing reductive removal of nitrobenzene by zero-valent iron. Appl. Catal. B Environ. 2019, 242, 9–18. [Google Scholar] [CrossRef]
- Khan, A.H.; Rasul, S.B.; Munir, A.K.M.; Habibuddowla, M.; Alauddin, M.; Newaz, S.S.; Hussam, A. Appraisal of a simple arsenic removal method for ground water of Bangladesh. J. Environ. Sci. Health Part A 2000, 35, 1021–1041. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Murcott, S.; Shrestha, R.R.; Dangol, B.; Maharjan, M. Development and dissemination of Kanchan™ Arsenic Filter in rural Nepal. Water Sci. Technol. Water Supply 2006, 6, 137–146. [Google Scholar] [CrossRef]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health Part A 2007, 42, 1869–1878. [Google Scholar] [CrossRef]
- Hussam, A. Contending with a development disaster: SONO filters remove arsenic from well water in Bangladesh (innovations case discussion: SONO filters). Innov. Technol. Gov. Glob. 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Noubactep, C. A critical review on the process of contaminant removal in Fe0-H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Banerji, T.; Chaudhari, S. A cost-effective technology for arsenic removal: Case study of zerovalent iron-based IIT Bombay arsenic filter in West Bengal. In Water and Sanitation in the New Millennium; Springer: New Delhi, India, 2017; pp. 127–137. [Google Scholar]
- Banerji, T.; Kalawapudi, K.; Salana, S.; Vijay, R. Review of processes controlling arsenic retention and release in soils and sediments of Bengal basin and suitable iron based technologies for its removal. Groundw. Sustain. Dev. 2019, 8, 358–367. [Google Scholar] [CrossRef]
- Nanseu-Njiki, C.P.; Gwenzi, W.; Pengou, M.; Rahman, M.A.; Noubactep, C. Fe0/H2O filtration systems for decentralized safe drinking water: Where to from here? Water 2019, 11, 429. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; Noubactep, C.; Nassi, A.; Ruppert, H. Characterizing a newly designed steel-wool-based household filter for safe drinking water provision: Hydraulic conductivity and efficiency for pathogen removal. Processes 2019, 7, 966. [Google Scholar] [CrossRef]
- Hu, R.; Yang, H.; Tao, R.; Cui, X.; Xiao, M.; Amoah, B.K.; Cao, V.; Lufingo, M.; Soppa-Sangue, N.P.; Ndé-Tchoupé, A.I.; et al. Metallic iron for environmental remediation: Starting an overdue progress in knowledge. Water 2020, 12, 641. [Google Scholar] [CrossRef]
- Kenneke, J.F.; McCutcheon, S.C. Use of pretreatment zones and zero-valent iron for the remediation of chloroalkenes in an oxic aquifer. Environ. Sci. Technol. 2003, 37, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.-H.; Kim, K.-W.; Bang, S.; Kim, M.G. Reduction and adsorption mechanisms of selenate by zero-valent iron and related iron corrosion. Appl. Catal. B Environ. 2011, 104, 185–192. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- Miyajima, K.; Noubactep, C. Characterizing the impact of sand addition on the efficiency of granular iron for contaminant removal in batch systems. Chem. Eng. J. 2015, 262, 891–896. [Google Scholar] [CrossRef]
- Makota, S.; Nde-Tchoupe, A.I.; Mwakabona, H.T.; Tepong-Tsindé, R.; Noubactep, C.; Nassi, A.; Njau, K.N. Metallic iron for water treatment: Leaving the valley of confusion. Appl. Water Sci. 2017, 7, 4177–4196. [Google Scholar] [CrossRef]
- Naseri, E.; Ndé-Tchoupé, A.I.; Mwakabona, H.T.; Nanseu-Njiki, C.P.; Noubactep, C.; Njau, K.N.; Wydra, K.D. Making Fe0-based filters a universal solution for safe drinking water provision. Sustainability 2017, 9, 1224. [Google Scholar] [CrossRef]
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water treatment using metallic iron: A tutorial review. Processes 2019, 7, 622. [Google Scholar] [CrossRef]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E.; Harms, S.; Milburn, R.; Sprah, G.; Nadarajah, N. Degradation of carbon tetrachloride in the presence of iron and sulphur containing compounds. Chemosphere 1994, 29, 1477–1489. [Google Scholar] [CrossRef]
- Ghauch, A.; Assi, H.A.; Bdeir, S. Aqueous removal of diclofenac by plated elemental iron: Bimetallic systems. J. Hazard. Mater. 2010, 182, 64–74. [Google Scholar] [CrossRef]
- Ghauch, A.; Abou Assi, H.; Baydoun, H.; Tuqan, A.M.; Bejjani, A. Fe0-based trimetallic systems for the removal of aqueous diclofenac: Mechanism and kinetics. Chem. Eng. J. 2011, 172, 1033–1044. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I.; Vancea, C. An investigation of Cr (VI) removal with metallic iron in the co-presence of sand and/or MnO2. J. Environ. Manag. 2016, 170, 145–151. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Sustaining the efficiency of the Fe(0)/H2O system for Cr (VI) removal by MnO2 amendment. Chemosphere 2019, 214, 389–398. [Google Scholar] [CrossRef]
- Han, S.; Huang, Y.; Liu, Z. Bacterial indicator reduction in dairy manure using hybrid zero-valent iron (h-ZVI) system. Environ. Sci. Pollut. Res. 2019, 26, 10790–10799. [Google Scholar] [CrossRef]
- He, X.; Min, X.; Peng, T.; Ke, Y.; Zhao, F.; Sillanpää, M.; Wang, Y. Enhanced adsorption of antimonate by ball-milled microscale zero valent iron/pyrite composite: Adsorption properties and mechanism insight. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, P.D.; Horney, D.P.; Sivavec, T.M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 1999, 68, 1–17. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Han, L.; Li, J.; Lü, Y.; Yao, C.; Dong, H.; Wang, L.; Li, Y. Pyrite enhanced the reactivity of zero valent iron for reductive removal of dyes. J. Chem. Technol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Zeng, X.; Kuang, H.; Huang, S. Enhancement of ball-miling on pyrite/zero-valent iron for arsenic removal in water: A mechanistic study. Chemosphere 2020, 249, 126130. [Google Scholar] [CrossRef]
- Butler, E.C.; Hayes, K.F. Effects of solution composition and pH on the reductive dechlorination of hexachloroethane by iron sulfide. Environ. Sci. Technol. 1998, 32, 1276–1284. [Google Scholar] [CrossRef]
- Kenneke, J.F.; Weber, E.J. Reductive dehalogenation of halomethanes in iron-and sulfate-reducing sediments. 1. Reactivity pattern analysis. Environ. Sci. Technol. 2003, 37, 713–720. [Google Scholar] [CrossRef]
- Köber, R.; Welter, E.; Ebert, M.; Dahmke, A. Removal of arsenic from groundwater by zerovalent iron and the role of sulfide. Environ. Sci. Technol. 2005, 39, 8038–8044. [Google Scholar] [CrossRef]
- Lü, Y.; Li, Z.; Li, J.; Chen, K.; Dong, H.; Shou, J.; Li, Y. Synergetic effect of pyrite on Cr (VI) removal by zero valent iron in column experiments: An investigation of mechanisms. Chem. Eng. J. 2018, 349, 522–529. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Hussain, I.; Du, X.; Huang, S.; Wen, W. Effect of pyrite on enhancement of zero-valent iron corrosion for arsenic removal in water: A mechanistic study. Chemosphere 2019, 233, 744–753. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling; Walter de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Noubactep, C.; Schöner, A.; Meinrath, G. Mechanism of uranium removal from the aqueous solution by elemental iron. J. Hazard. Mater. 2006, 132, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Merkel, B. Mitigating uranium in groundwater: Prospects and limitations. Environ. Sci. Technol. 2003, 37, 4304–4308. [Google Scholar] [CrossRef] [PubMed]
- Cui, X. Study on the Material Selection of Zero Valent Iron Permeable Reactive Barrier and the Effect of Pyrite on Its Treatment Efficiency; Hohai University: Nanjing, China, 2020. [Google Scholar]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of single heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3050–3056. [Google Scholar] [CrossRef]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of multiple heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3057–3062. [Google Scholar] [CrossRef]
- Noubactep, C. Relevant reducing agents in remediation Fe0/H2O systems. Clean Soil Air Water 2013, 41, 493–502. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Xiao, M.; Qiu, P.; Lufingo, M.; Gwenzi, W.; Noubactep, C. Characterizing the suitability of granular Fe0 for the water treatment industry. Processes 2019, 7, 652. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I.; Nanseu-Njiki, C.P.; Hu, R.; Nassi, A.; Noubactep, C.; Licha, T. Characterizing the reactivity of metallic iron for water defluoridation in batch studies. Chemosphere 2019, 219, 855–863. [Google Scholar] [CrossRef]
- Lee, G.; Rho, S.; Jahng, D. Design considerations for groundwater remediation using reduced metals. Korean J. Chem. Eng. 2004, 21, 621–628. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for environmental remediation: A review of reviews. Water Res. 2015, 85, 114–123. [Google Scholar] [CrossRef]
- Noubactep, C.; Schoner, A.; Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean-Soil Air Water 2009, 37, 930–937. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Comba, S.; Di Molfetta, A.; Sethi, R. A comparison between field applications of nano-, micro-, and millimetric zero-valent iron for the remediation of contaminated aquifers. Water Air Soil Pollut. 2011, 215, 595–607. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “iron wall” for remediation of VOCs. Groundwater 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Kriegman-King, M.R.; Reinhard, M. Transformation of carbon tetrachloride in the presence of sulfide, biotite, and vermiculite. Environ. Sci. Technol. 1992, 26, 2198–2206. [Google Scholar] [CrossRef]
- Kriegman-King, M.R.; Reinhard, M. Transformation of carbon tetrachloride by pyrite in aqueous solution. Environ. Sci. Technol. 1994, 28, 692–700. [Google Scholar] [CrossRef]
- Patterson, R.R.; Fendorf, S.; Fendorf, M. Reduction of hexavalent chromium by amorphous iron sulfide. Environ. Sci. Technol. 1997, 31, 2039–2044. [Google Scholar] [CrossRef]
- Butler, E.C.; Hayes, K.F. Kinetics of the transformation of trichloroethylene and tetrachloroethylene by iron sulfide. Environ. Sci. Technol. 1999, 33, 2021–2027. [Google Scholar] [CrossRef]
- Butler, E.C.; Hayes, K.F. Factors influencing rates and products in the transformation of trichloroethylene by iron sulfide and iron metal. Environ. Sci. Technol. 2001, 35, 3884–3891. [Google Scholar] [CrossRef]
- Zwank, L.; Elsner, M.; Aeberhard, A.; Schwarzenbach, R.P.; Haderlein, S.B. Carbon isotope fractionation in the reductive dehalogenation of carbon tetrachloride at iron (hydr) oxide and iron sulfide minerals. Environ. Sci. Technol. 2005, 39, 5634–5641. [Google Scholar] [CrossRef]
- Choi, K.; Lee, W. Ex-situ Reductive Dechlorination of Carbon Tetrachloride by Iron Sulfide in Batch Reactor. Environ. Eng. Res. 2008, 13, 177–183. [Google Scholar] [CrossRef]
- Hua, B.; Deng, B. Reductive immobilization of uranium (VI) by amorphous iron sulfide. Environ. Sci. Technol. 2008, 42, 8703–8708. [Google Scholar] [CrossRef] [PubMed]
- Bone, S.E.; Bargar, J.R.; Sposito, G. Mackinawite (FeS) reduces mercury (II) under sulfidic conditions. Environ. Sci. Technol. 2014, 48, 10681–10689. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Dai, C.; Zhou, X. Performance and mechanism of pyrite for nitrobenzene removal in aqueous solution. Chem. Eng. Sci. 2014, 111, 135–141. [Google Scholar] [CrossRef]
- Dhanasekara, S.; Attanayake, A.N.B.; Herath, A.C.; Nanayakkara, N.; Senaratne, A.; Indrarathne, S.P.; Weerasooriya, R. Partial degradation of carbofuran by natural pyrite. Environ. Nanotechnol. Monit. Manag. 2015, 4, 51–57. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef]
- Murphy, R.; Strongin, D.R. Surface reactivity of pyrite and related sulfides. Surf. Sci. Rep. 2009, 64, 1–45. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Merkel, B.J. Investigating the mechanism of uranium removal by zerovalent iron. Environ. Chem. 2005, 2, 235–242. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, M.; Ma, B.; Xie, J.; Chen, F.; Charlet, L.; Liu, C. Inhibition of U (VI) reduction by synthetic and natural pyrite. Environ. Sci. Technol. 2014, 48, 10716–10724. [Google Scholar] [CrossRef]
- Weber, E.J. Iron-mediated reductive transformations: Investigation of reaction mechanism. Environ. Sci. Technol. 1996, 30, 716–719. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Permeability of iron sulfide (FeS)-based materials for groundwater remediation. Water Res. 2013, 47, 1267–1276. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Impact of solids formation and gas production on the permeability of ZVI PRBs. J. Environ. Eng. 2011, 137, 689–696. [Google Scholar] [CrossRef]
- Hu, R.; Ndé-Tchoupé, A.I.; Lufingo, M.; Xiao, M.; Nassi, A.; Noubactep, C.; Njau, K.N. The impact of selected pretreatment procedures on iron dissolution from metallic iron specimens used in water treatment. Sustainability 2019, 11, 671. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Volke, P.; Peter, H.J.; Dietrich, P.; Merkel, B. Mechanism of uranium fixation by zero valent iron: The importance of co-precipitation. In Uranium in the Aquatic Environment; Springer: Berlin/Heidelberg, Germany, 2002; pp. 577–586. [Google Scholar]
- Richardson, J.P.; Nicklow, J.W. In situ permeable reactive barriers for groundwater contamination. Soil Sediment Contam. 2002, 11, 241–268. [Google Scholar] [CrossRef]
- Newton, B.T. Noble Gases as Applied Tracers for Investigating Hydrologic and Chemical Processes in Permeable Reactive Barriers. PhD Dissertation, Queens University Belfast, Belfast, UK, 2013. [Google Scholar]
- Kim, H.; Yang, H.; Kim, J. Standardization of the reducing power of zero-valent iron using iodine. J. Environ. Sci. Health Part A 2014, 49, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Btatkeu-K, B.D.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Designing metallic iron based water filters: Light from methylene blue discoloration. J. Environ. Manag. 2016, 166, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, Y.; Wang, W.; Lei, H. A facile method for determining the Fe (0) content and reactivity of zero valent iron. Anal. Methods 2016, 8, 1239–1248. [Google Scholar] [CrossRef]
- Gatcha-Bandjun, N.; Noubactep, C.; Loura, B.B. Mitigation of contamination in effluents by metallic iron: The role of iron corrosion products. Environ. Technol. Innov. 2017, 8, 71–83. [Google Scholar] [CrossRef]
- Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freib. Online Geosci. 2012, 32, 1–10. [Google Scholar]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Characterizing the impact of MnO2 on the, efficiency of Fe0-based filtration systems. Chem. Eng. J. 2014, 250, 416–422. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Determining the optimum Fe0 ratio for sustainable granular Fe0/sand water filters. Chem. Eng. J. 2014, 247, 265–274. [Google Scholar] [CrossRef]
- Phukan, M. Characterizing the Fe0/sand system by the extent of dye discoloration. Freib. Online Geosci. 2015, 40, 1–70. [Google Scholar]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using azo dyes. Chem. Eng. J. 2015, 259, 481–491. [Google Scholar] [CrossRef]
- Min, X.; Li, Y.; Ke, Y.; Shi, M.; Chai, L.; Xue, K. Fe-FeS2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal. Water Sci. Technol. 2017, 76, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Seng, S.; Tabelin, C.B.; Kojima, M.; Hiroyoshi, N.; Ito, M. Galvanic microencapsulation (GME) using zero-valent aluminum and zero-valent iron to suppress pyrite oxidation. Mater. Trans. 2019, 60, 277–286. [Google Scholar] [CrossRef]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A novel and facile method to characterize the suitability of metallic iron for water treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef]
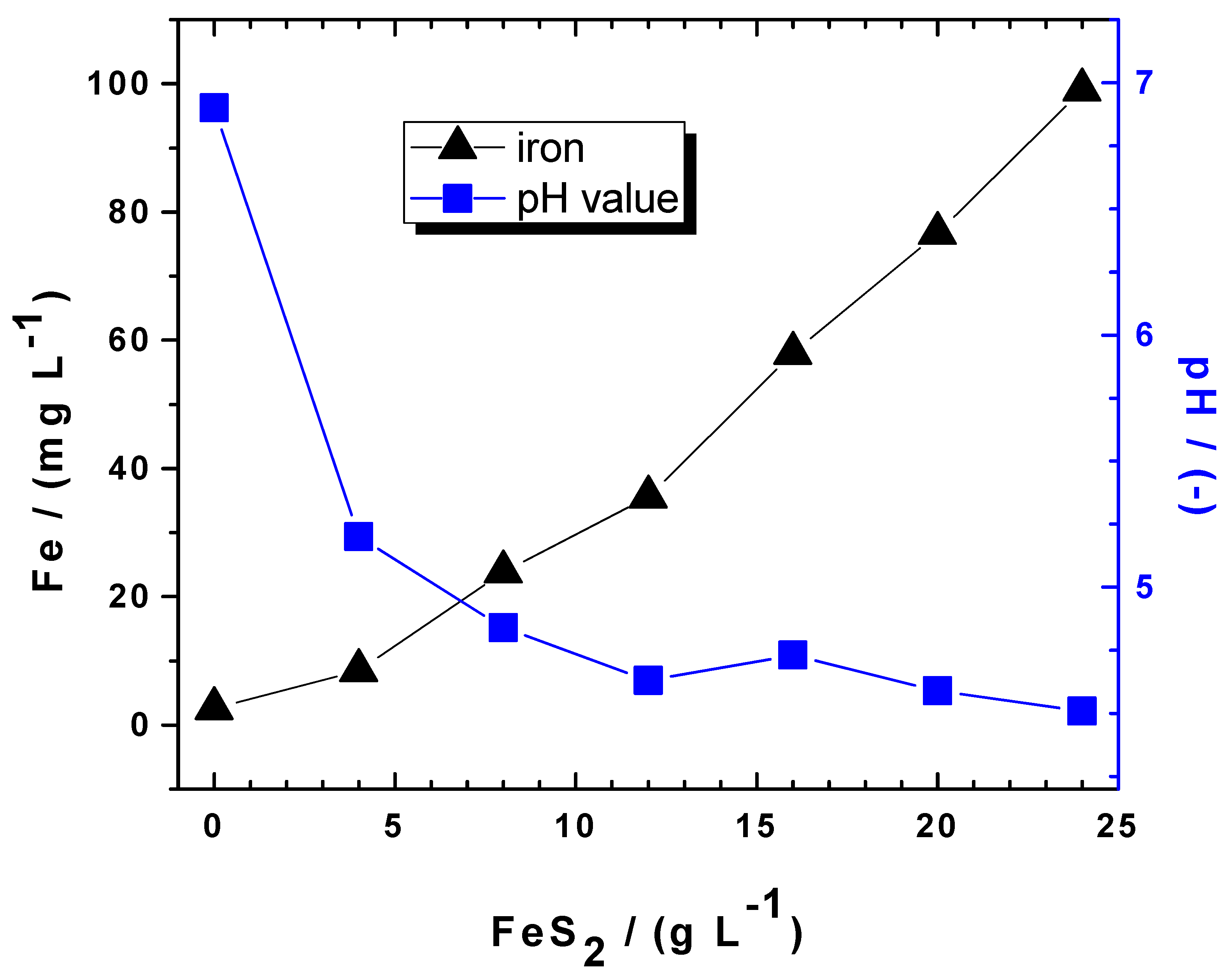
| Contaminant Nature | Pyrite | Metallic Iron | Stirring | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| pH0 (-) | V (mL) | d (mm) | ρ(g L−1) | d (mm) | ρ(g L−1) | (rpm) | ||
| CCl4 | 6.0 | 25 | < 0.841 | 200 | 0.150 | 200 | 170 | 38 |
| Arsenic | 3.0 to 9.0 | 500 | n.s. | 0.20 or 2.0 | n.s. | 0.20 or 2.0 | 400 | 53 |
| Uranium | 7.2 | 20 | 200 to 630 | 25 | 1.6 to 2.5 | 15 | 0.0 | 54, 55 |
| Nitrobenzene | 5.0 to 10.0 | 150 | 40 to 75 | 0.5 to 3.0 | 40 to 75 | 0.5 | 200 | 52 |
| Orange II | 7.0 | 150.0 | 38 to 50 | 0.25 or 2.0 | 0.25 to 2.0 | 0.25 or 0.50 | 200 | 47 |
| RR X-3B | 7.0 | 150.0 | 38 to 50 | 0.25 or 2.0 | 0.25 to 2.0 | 0.25 or 0.50 | 200 | 47 |
| Amido Black 10B | 7.0 | 150.0 | 38 to 50 | 0.25 or 2.0 | 0.25 to 2.0 | 0.25 or 0.50 | 200 | 47 |
| Methylorange | 6.9 | 22 | 38 to 50 | 0.25 or 2.0 | 1.0 | 0.5 | 0.0 | 57 |
| Methylene blue | 7.0 | 22 | 38 to 50 | 0.25 or 2.0 | 1.0 | 0.5 | 0.0 | 57 |
| Title | Journal | Year | Citation | Citation/Year |
|---|---|---|---|---|
| Processes of contaminant removal in “Fe0–H2O” systems revisited. The importance of coprecipitation. | Open Environ. Sci. | 2007 | n.a. | n.a. |
| A critical review on the mechanism of contaminant removal in Fe0–H2O systems | Environ. Technol. | 2008 | 242 | 20.2 |
| Fe0-based alloys for environmental remediation: Thinking outside the box | J. Hazard. Mater. | 2009 | 23 | 2.1 |
| An analysis of the evolution of reactive species in Fe0/H2O systems | J. Hazard. Mater. | 2009 | 108 | 9.8 |
| On the operating mode of bimetallic systems for environmental remediation | J. Hazard. Mater. | 2009 | 29 | 2.6 |
| On the validity of specific rate constants (kSA) in Fe0/H2O systems | J. Hazard. Mater. | 2009 | 14 | 1.6 |
| On nanoscale metallic iron for groundwater remediation | J. Hazard. Mater. | 2010 | 45 | 4.5 |
| The fundamental mechanism of aqueous contaminant removal by metallic iron | Water SA | 2010 | 125 | 12.5 |
| The suitability of metallic iron for environmental remediation | Environ. Prog Sustain. | 2010 | 55 | 5.5 |
| Aqueous contaminant removal by metallic iron: Is the paradigm shifting? | Water SA | 2011 | 59 | 6.6 |
| Metallic iron for environmental remediation: Back to textbooks | Fresenius Environ. Bull. | 2012 | 13 | 1.6 |
| Flaws in the design of Fe0-based filtration systems? | Chemosphere | 2014 | 24 | 4.0 |
| Metallic iron for environmental remediation: A review of reviews | Water Research | 2015 | 72 | 14.4 |
| No scientific debate in the zero-valent iron literature | CLEAN—Soil, Air, Water | 2016 | 8 | 2.0 |
| Research on metallic iron for environmental remediation: Stopping growing sloppy science | Chemosphere | 2016 | 21 | 5.3 |
| Predicting the hydraulic conductivity of metallic iron filters: Modeling gone astray | Water | 2016 | 18 | 4.5 |
| Metallic iron for water treatment: Leaving the valley of confusion | Applied Water Science | 2017 | n.a. | n.a. |
| Rescuing Fe0 remediation research from its systemic flaws | Res. Rev. Insights | 2017 | n.a. | n.a. |
| Metallic iron for environmental remediation: How experts maintain a comfortable status quo | Fresenius Environ. Bull. | 2018 | n.a. | n.a. |
| Iron corrosion: Scientific heritage in jeopardy | Sustainability | 2018 | 5 | 2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Hu, R.; Cui, X.; Gwenzi, W.; Noubactep, C. Understanding the Operating Mode of Fe0/Fe-Sulfide/H2O Systems for Water Treatment. Processes 2020, 8, 409. https://doi.org/10.3390/pr8040409
Xiao M, Hu R, Cui X, Gwenzi W, Noubactep C. Understanding the Operating Mode of Fe0/Fe-Sulfide/H2O Systems for Water Treatment. Processes. 2020; 8(4):409. https://doi.org/10.3390/pr8040409
Chicago/Turabian StyleXiao, Minhui, Rui Hu, Xuesong Cui, Willis Gwenzi, and Chicgoua Noubactep. 2020. "Understanding the Operating Mode of Fe0/Fe-Sulfide/H2O Systems for Water Treatment" Processes 8, no. 4: 409. https://doi.org/10.3390/pr8040409
APA StyleXiao, M., Hu, R., Cui, X., Gwenzi, W., & Noubactep, C. (2020). Understanding the Operating Mode of Fe0/Fe-Sulfide/H2O Systems for Water Treatment. Processes, 8(4), 409. https://doi.org/10.3390/pr8040409








