Evaluation of Polymeric Materials for Chemical Enhanced Oil Recovery
Abstract
1. Introduction
2. Polymeric Materials for Enhanced Oil Recovery
3. Evaluation of Polymeric Materials for EOR
3.1. Fundamental Polymer Properties
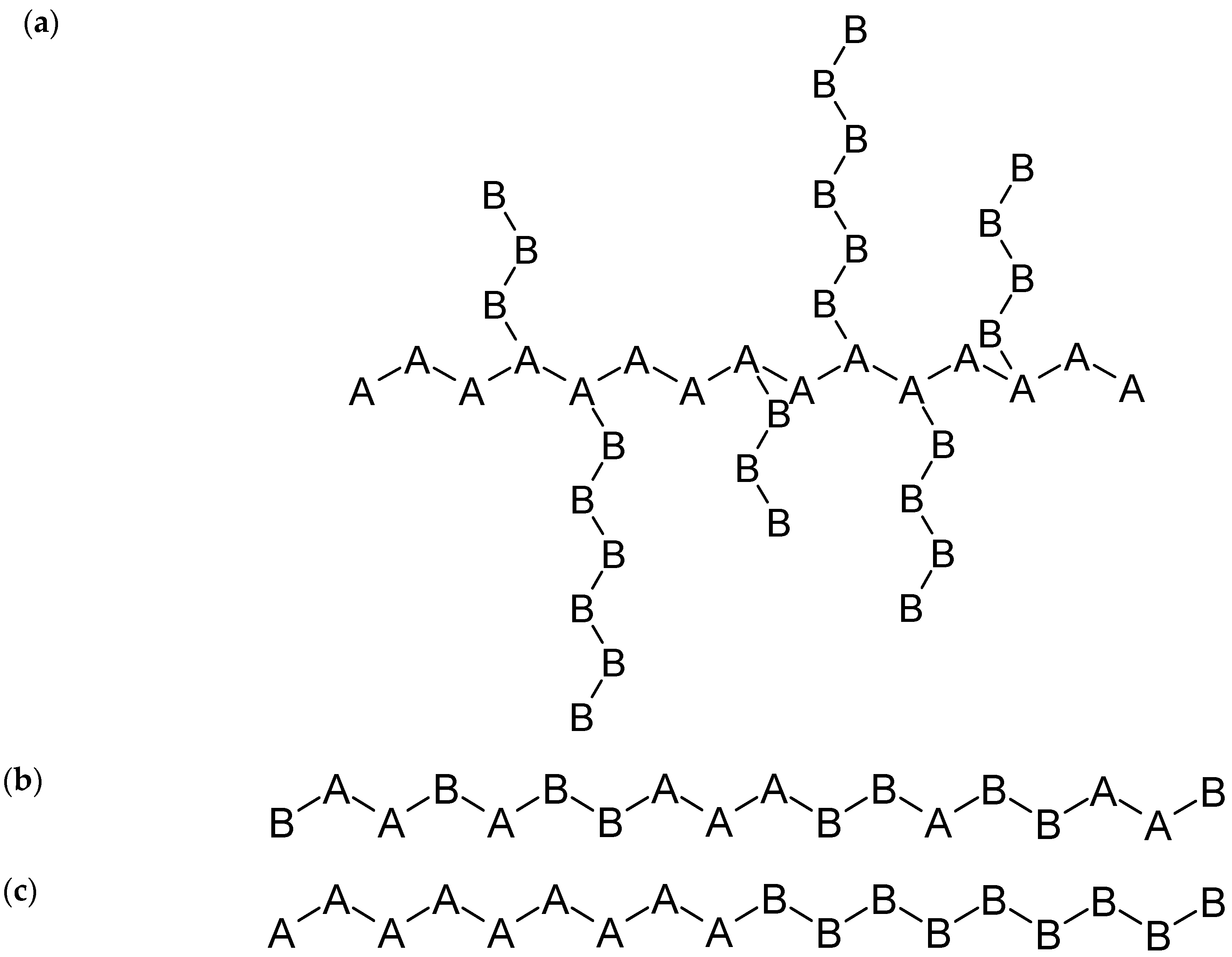
3.1.1. Polymer Composition
3.1.2. Polymer Microstructure
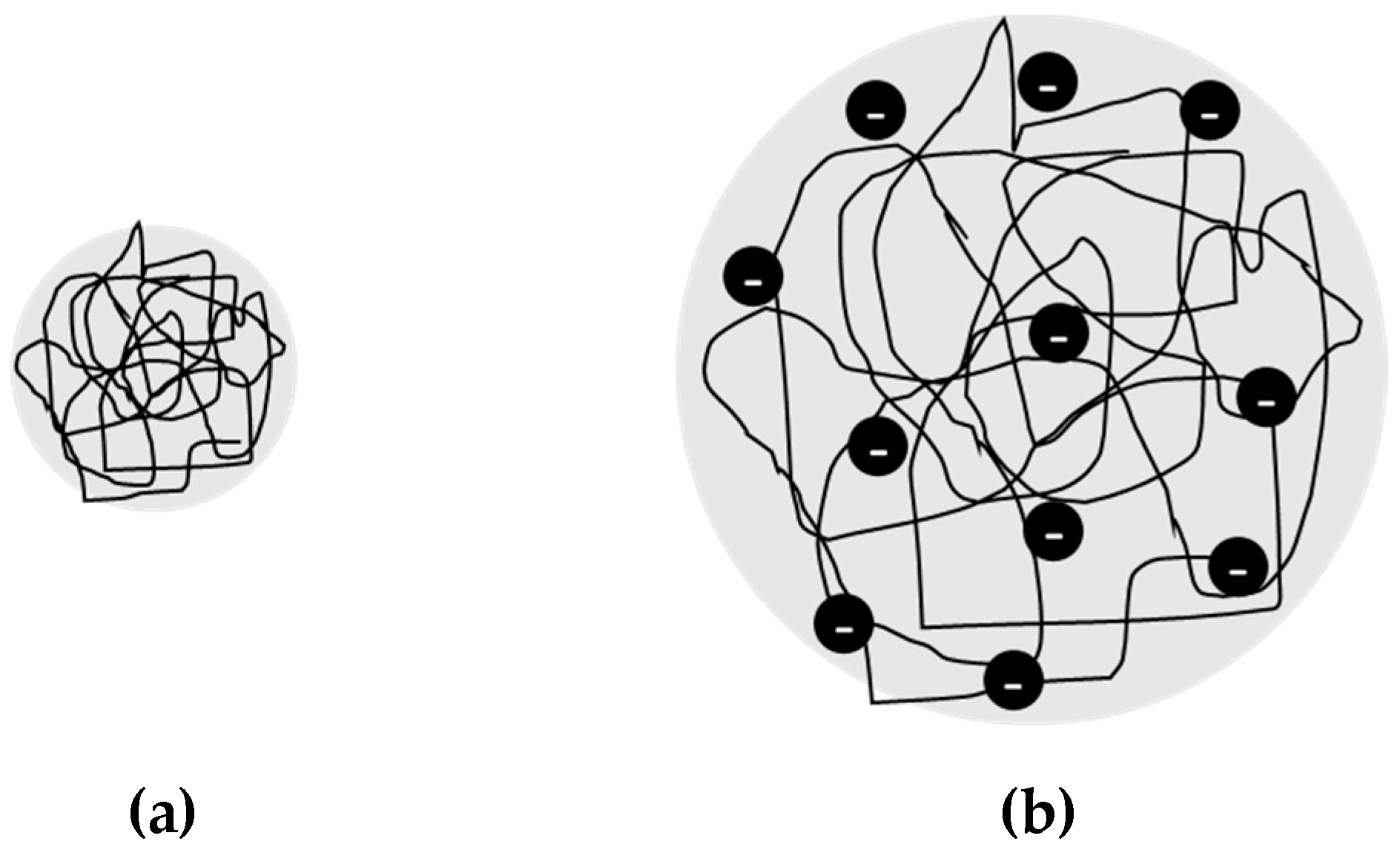
3.1.3. Molecular Weight Averages
3.2. Polymer Degradation
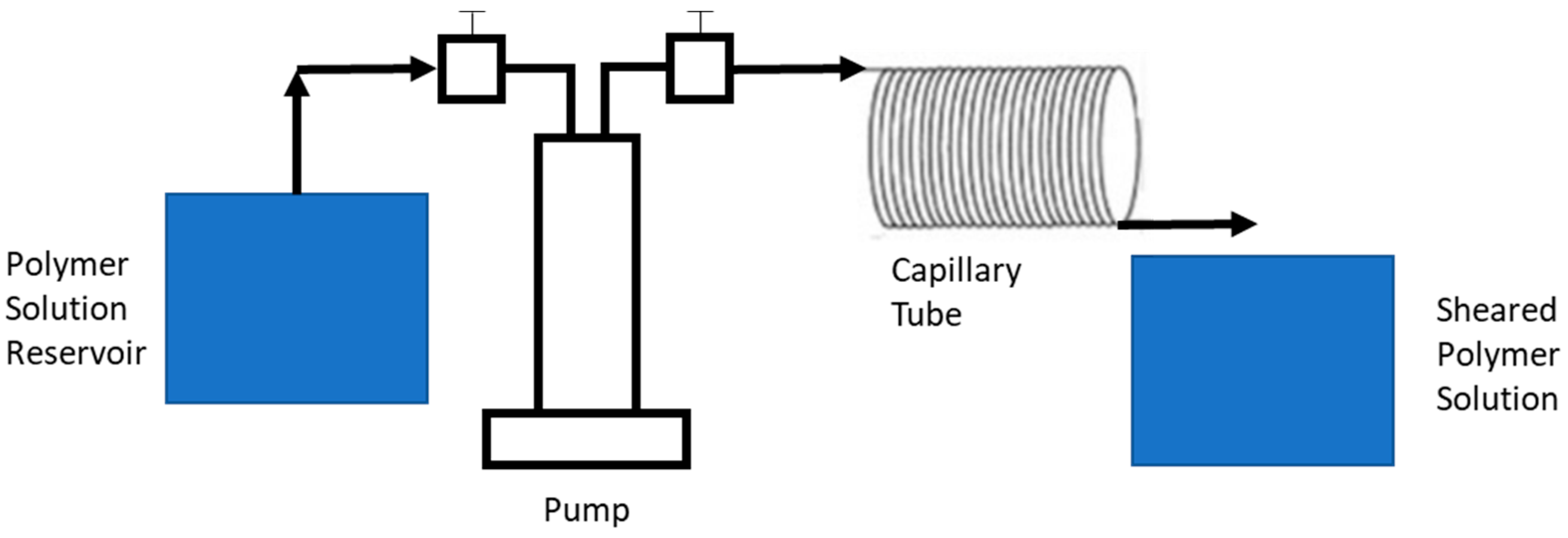
3.2.1. Shear Degradation
3.2.2. Thermal Degradation
3.2.3. Chemical Degradation
3.3. Impacts of Salinity and Hardness
3.3.1. Viscosity Loss
3.3.2. Polymer Precipitation
4. Evaluation of Polymer-Flooding Performance
4.1. Flow Behavior
4.1.1. Rheological Properties
4.1.2. Flow Behavior of Polymers through Porous Media
4.1.3. Polymer Injectivity, Mobility, and Propagation through Porous Media
4.1.4. Polymer Retention and Relative Permeability Reduction
4.1.5. Experimental Techniques for the Evaluation of Polymer Flooding (Flow Behavior)
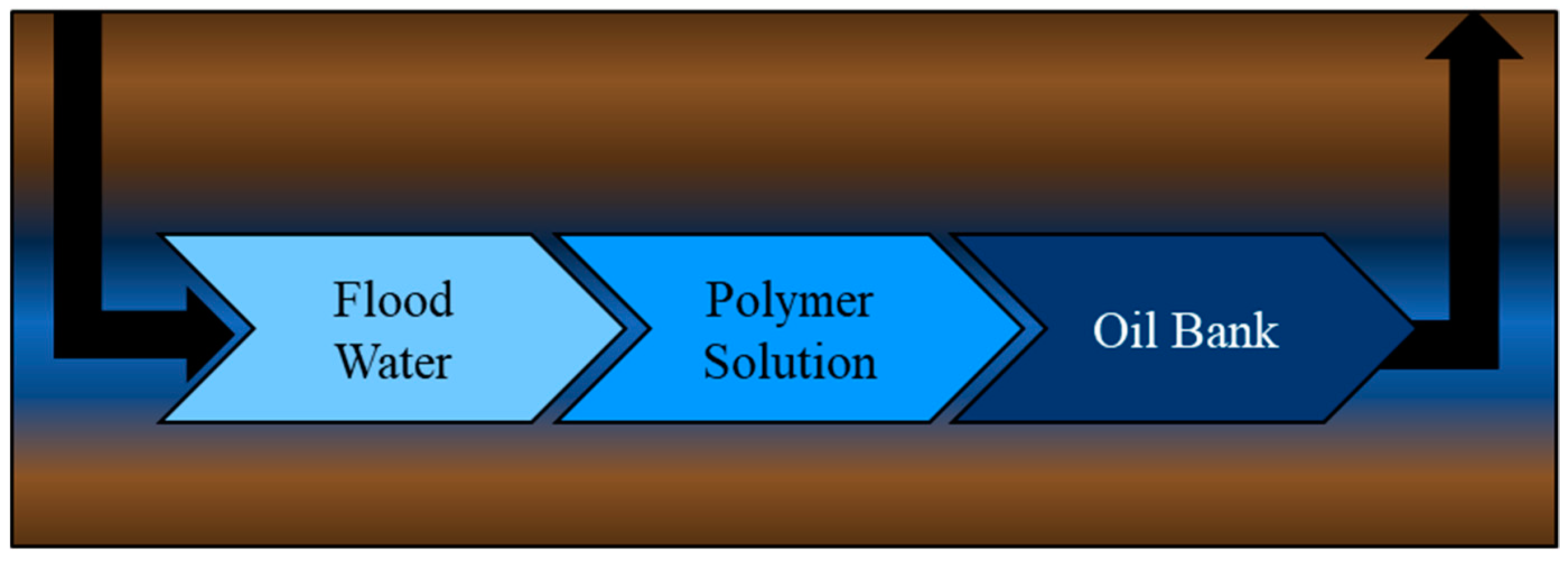
4.2. Oil Recovery Potential
4.2.1. Lab-Scale Polymer-Flooding Testing
- The greater the viscosity of the polymer solution, the higher the oil recovery.
- The molecular weight (MW) of the polymer must be carefully considered in terms of rock permeability. Polymers having very high molecular weights may plug the porous media or cannot flow through low permeability zones (i.e., inaccessible pore volumes). In other words, the higher the permeability of the rock, the higher the MW of the polymers that could be applied.
- For a constant polymer MW, incremental oil recovery increases as polymer concentration increases, as long as proper injectivity of the polymer solution is observed.
- Higher polymer concentrations are required at elevated reservoir temperatures and brines with high salinity and hardness concentrations.
- The higher the polymer concentration, the higher the oil recovery factor [123].
- Higher polymer concentrations (i.e., >2000 ppm) are required for the polymer flooding of viscous oils (i.e., heavy oil).
- The larger the polymer slug size, the larger the oil recovery.
Reduction of Water/Oil Ratios
4.2.2. Extensions to Larger Systems
Pilot-Testing of Polymer Flooding
Field Applications of Polymer Flooding
- Determination of the low sweep efficiency problem of the reservoir to establish if polymer flooding is the appropriate treatment.
- Characterization of the reservoir properties: lithology, stratigraphy, heterogeneities (i.e., fractures and high permeability channels), and reservoir fluid saturation, among others.
- Establishment of the benefits of applying profile modification treatments (i.e., gel treatments) before the implementation of polymer flooding [142]. For instance, the presence of fractures, channels, or high-permeability strata in the reservoir can cause severe channeling of the polymer solution; therefore, “the application of a gel treatment can enhance reservoir sweep if applied before the injection of large volumes of expensive polymer” [9].
- Determination of the reservoir production strategies such, as well patterns and well distance.
- Establishment of the polymer-flooding strategy, such as a “graded or tapered [injection] scheme, in which the polymer concentration is initially high, and subsequent concentrations are reduced step by step” [17]. Another injection scheme is the constant-concentration scheme. This approach can be applied in two modes: injection of a small slug volume of high polymer concentration or a large slug volume of low polymer concentration. In Sheng’s view [17], the most practical scheme is a large slug volume of low polymer concentration; after polymer adsorption has been satisfied, the flowing polymer would have a higher viscosity, which would offer better mobility control in the reservoir. In field applications, the optimum amount of polymer injected, which is expressed by the product of the polymer concentration (in ppm) and slug volume in the pore volume (PV) in the fraction, is 400 ppm·PV.
- Determination of the requirement for zone isolation is important in reservoirs displaying a significant permeability differential between layers (>2.5 times), in low permeability-layer thickness (i.e., if the low permeability layer is > 30% of the total thickness of the pay zone), if the layers are separated by at least 1 m and there is no crossflow between layers [17].
- Determination of polymer properties: cost per unit of viscosity; polymer MW; polymer stability (i.e., mechanical, thermal, chemical, and biological), according to the reservoir conditions (i.e., temperature, formation brine salinity and hardness, reservoir mineralogy, and permeability). In Buciak’s view, divalent cations (i.e., Ca2+ and Mg2+) are the main agents responsible for polymer degradation that drastically reduces the solution viscosity; therefore, the brine employed to prepare the polymer should be treated to reduce the concentration of these divalent cations [142,144]. Furthermore, the polymer MW selected must allow the effective propagation of the polymer solution through the reservoir rock.
- Formulation of optimum polymer solutions (i.e., polymer concentration and MW) and evaluation of the polymer performance in representative reservoir plugs (e.g., polymer retention, determination of the inaccessible pore volume (IPV), and permeability dependence performance): As Seright indicates, the viscosity of a polymer solution increases approximately with the square of the polymer concentration [22]. At the Daqing polymer-flooding project, the average polymer concentration was 0.1 wt % (1000 mg/L), and the MW of the polymers ranged from 12 × 106 to 38 × 106 g/mol. At the Diadem Reservoir, San Jorge Gulf Basin (Argentina), the injection of HPAM at a concentration of 0.15% (1500 mg/L) was successful [142].
- Optimization of the polymer-bank volume. Polymer flooding at the Daqing Oil Field (China) demonstrated that larger polymer-bank volumes are more effective, with an optimum polymer injection volume of 0.7 PV.
- Determination of the optimum injection rates according to the reservoir properties. At the Daqing polymer flooding, injection rates ranged from 0.14 to 0.2 PV/year, depending on well spacing.
- Evaluation of efficient oil/water demulsification processes at surface facilities to overcome the emulsion stabilizing effect of the back-produced polymer solutions, which has been reported to be problematic in polymer-flooding field applications [17].
5. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Speight, J. General methods of oil recovery. In Introduction to Enhanced Recovery Methods for Heavy Oil and Tar Sands, 2nd ed.; Gulf Professional Publishing: Cambridge, MA, USA, 2016; pp. 253–322. [Google Scholar]
- Behnoudfar, P.; Rostami, A.; Hemmati-Sarapardeh, A. Miscible gas injection processes. In Fundamentals of Enhanced Oil and Gas Recovery from Conventional and Unconventional Reservoirs; Bahadori, A., Ed.; Gulf Professional Publishing: Cambridge, MA, USA, 2018; pp. 101–138. [Google Scholar]
- Thomas, S. Enhanced oil recovery—An overview. Oil Gas Sci. Technol. 2008, 63, 9–19. [Google Scholar] [CrossRef]
- Kokal, S.; Al-Kaabi, A. Enhanced oil recovery: Challenges & opportunities. World Pet. Counc. Off. Publ. 2010, 64–69. [Google Scholar]
- Mokheimer, E.; Hamdy, M.; Abubakar, Z.; Shakeel, M.; Habib, M.; Mahmoud, M. A comprehensive review of thermal enhanced oil recovery: Techniques evaluation. J. Energy Resour. 2018, 141, 030801. [Google Scholar] [CrossRef]
- Speight, J. Nonthermal methods of recovery. In Introduction to Enhanced Recovery Methods for Heavy Oil and Tar Sands, 2nd ed.; Gulf Professional Publishing: Cambridge, MA, USA, 2016; pp. 369–386. [Google Scholar]
- Gbadamosi, A.; Junin, R.; Manan, M.; Agi, A.; Yusuff, A. An overview of chemical enhanced oil recovery: Recent advances and prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.; Zhao, J. Successful polymer flooding and surfactant-polymer flooding projects at Shengli Oilfield from 1992 to 2012. J. Pet. Explor. Prod. Technol. 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Wang, D.; Seright, R.; Shao, Z.; Wang, J. Key aspects of project design for polymer flooding at the Daqing Oilfield. SPE Reserv. Eval. Eng. 2008, 11, 1117–1124. [Google Scholar] [CrossRef]
- Rellegadla, S.; Prajapat, G.; Agrawal, A. Polymers for enhanced oil recovery: Fundamentals and selection. Appl. Microbiol. Biotechnol. 2017, 101, 4387–4402. [Google Scholar] [CrossRef]
- Ryles, R. Chemical stability limits of water-soluble polymers used in oil recovery processes. SPE Reserv. Eng. 1988, 3, 23–34. [Google Scholar] [CrossRef]
- Wei, B.; Romero-Zerón, L.; Rodrigue, D. Mechanical properties and flow behavior of polymers for enhanced oil recovery. J. Macromol. Sci. Part B Phys. 2014, 53, 625–644. [Google Scholar] [CrossRef]
- Sheng, J. Modern Chemical Enhanced Oil Recovery—Theory and Practice; Gulf Professional Publishing: Burlington, VT, USA, 2011. [Google Scholar]
- Speight, J. Introduction to Enhanced Recovery Methods for Heavy Oil and Tar Sands, 2nd ed.; Gulf Professional Publishing: Cambridge, MA, USA, 2016. [Google Scholar]
- Abidin, A.; Puspasari, T.; Nugroho, W. Polymers for enhanced oil recovery technology. Proced. Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef]
- Kamal, M.; Sultan, A.; Al-Mybaiyedh, U.; Hussein, I. Review on polymer flooding: Rheology, adsorption, stability, and field applications of various polymer systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Sheng, J.; Leonhardt, B.; Azri, N. Status of polymer-flooding technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Pu, W.; Shen, C.; Wei, B.; Yang, Y.; Li, Y. A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J. Ind. Eng. Chem. 2018, 61, 1–11. [Google Scholar] [CrossRef]
- Wever, D.; Picchioni, F.; Broekhuis, A. Polymers for enhanced oil recovery: A paradigm for structure-property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Thomas, A.; Gaillard, N.; Favero, C. Some key features to consider when studying acrylamide-based polymers for chemical enhanced oil recovery. Oil Gas Sci. Technol. 2012, 67, 887–902. [Google Scholar] [CrossRef]
- Wei, B.; Romero-Zerón, L.; Rodrigue, D. Oil displacement mechanisms of viscoelastic polymers in enhanced oil recovery (EOR): A review. J. Pet. Explor. Prod. Technol. 2014, 4, 113–121. [Google Scholar] [CrossRef]
- Seright, R. Potential for polymer flooding reservoirs with viscous oils. SPE Reserv. Eval. Eng. 2010, 13, 730–740. [Google Scholar] [CrossRef]
- Firozjaii, A.; Saghafi, H. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2019. [Google Scholar] [CrossRef]
- Wever, D.; Picchionia, F.; Broekhuis, A. Branched polyacrylamides: Synthesis and effect of molecular architecture on solution rheology. Eur. Polym. J. 2013, 49, 3289–3301. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, W.; Jian, G.; Wang, C.; Hou, Q.; Niu, J. Development and performance of water soluble salt-resistant polymers for chemical flooding. Adv. Mater. Res. 2012, 476–478, 227–235. [Google Scholar] [CrossRef]
- Sabhapondit, A.; Borthakur, A.; Haque, I. Characterization of acrylamide polymers for enhanced oil recovery. J. Appl. Polym. Sci. 2003, 87, 1869–1878. [Google Scholar] [CrossRef]
- Zaitoun, A.; Makakou, P.; Blin, N.; Al-Maamari, R.; Al-Hashmi, A.; Abdel-Goad, M.; Al-Sharji, H. Shear stability of EOR polymers. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 11–13 April 2011. [Google Scholar]
- Sabhapondit, A.; Borthakur, A.; Haque, I. Water soluble acrylamidomethyl propane sulfonate (AMPS) copolymer as an enhanced oil recovery chemical. Energy Fuels 2003, 17, 683–688. [Google Scholar] [CrossRef]
- Zhong, C.; Luo, P.; Ye, Z.; Chen, H. Characterization and solution properties of a novel water-soluble terpolymer for enhanced oil recovery. Polym. Bull. 2009, 62, 79–89. [Google Scholar] [CrossRef]
- Han, M.; Zhou, X.; Fuseni, A.; Al-Zahrani, B.; AlSofi, A. Laboratory investigation of the injectivity of sulfonated polyacrylamide solutions into carbonate reservoir rocks. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar]
- Gaillard, N.; Giovannetti, B.; Favero, C.; Caritey, J.-P.; Dupuis, G.; Zaitoun, A. New water soluble anionic NVP acrylamide terpolymers for use in harsh EOR conditions. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Ye, Z.; Gou, G.; Gou, S.; Jiang, W.; Liu, T. Synthesis and characterization of a water-soluble sulfonates copolymer of acrylamide and N-allylbenzamide as enhanced oil recovery chemical. J. Appl. Polym. Sci. 2013, 128, 2003–2011. [Google Scholar] [CrossRef]
- Ujjwal, R.; Sharma, T.; Sangwai, J.; Ojha, U. Rheological investigation of a random copolymer of polyacrylamide and polyacryloyl hydrazide (PAM-ran-PAH) for oil recovery applications. J. Appl. Polym. Sci. 2017, 134, 44648. [Google Scholar]
- Ye, Z.; Feng, M.; Gou, S.; Liu, M.; Huang, Z.; Liu, T. Hydrophobically associating acrylamide-based copolymer for chemically enhanced oil recovery. J. Appl. Polym. Sci. 2013, 130, 2901–2911. [Google Scholar] [CrossRef]
- El Hoshoudy, A.; Desouky, S.; Al-sabagh, A.; El-kady, M.; Betiha, M.; Mahmoud, S. Synthesis and characterization of polyacrylamide crosslinked copolymer for enhanced oil recovery and rock wettability alteration. Int. J. Oil Gas Coal Eng. 2015, 3, 47–59. [Google Scholar] [CrossRef]
- El Hoshoudy, A.; Desouky, S.; Al-sabagh, A.; Betiha, M.; El-kady, M.; Mahmoud, S. Evaluation of solution and rheological properties for hydrophobically associated polyacrylamide copolymer as a promised enhanced oil recovery candidate. Egypt. J. Pet. 2017, 26, 779–785. [Google Scholar] [CrossRef]
- Wu, G.; Yu, L.; Jiang, X. Synthesis and properties of an acrylamide-based polymer for enhanced oil recovery: A preliminary study. Adv. Polym. Technol. 2018, 37, 2763–2773. [Google Scholar] [CrossRef]
- Lai, N.; Dong, W.; Ye, Z.; Dong, J.; Qin, X.; Chen, W.; Chen, K. A water-soluble acrylamide hydrophobically associating polymer: Synthesis, characterization, and properties as EOR chemical. J. Appl. Polym. Sci. 2012, 129, 1888–1896. [Google Scholar] [CrossRef]
- Olajire, A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Sheng, J. A comprehensive review of alkaline-surfactant-polymer (ASP) flooding. Asia-Pac. J. Chem. Eng. 2014, 9, 471–489. [Google Scholar] [CrossRef]
- Needham, R.; Doe, P. Polymer flooding review. J. Pet. Technol. 1987, 39, 1503–1507. [Google Scholar] [CrossRef]
- Silva, I.; Aguiar, A.; Rezende, V.; Monsores, A.; Lucas, E. A polymer flooding mechanism for mature oil fields: Laboratory measurements and field results interpretation. J. Pet. Sci. Eng. 2018, 161, 468–475. [Google Scholar] [CrossRef]
- Stegemeier, G. Mechanisms of entrapment and mobilization of oil in porous media. In Improved Oil Recovery by Surfactant and Polymer Flooding; Shah, D., Schechter, R., Eds.; Academic Press: New York, NY, USA, 1977; pp. 55–92. [Google Scholar]
- Lake, L. Enhanced Oil Recovery; Prentice Hall: New Jersey, NJ, USA, 1989. [Google Scholar]
- Wang, D.; Cheng, J.; Yang, Q.; Wenchao, G.; Qun, L.; Chen, F. Viscous-elastic polymer can increase microscale displacement efficiency in cores. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 1–4 October 2000. [Google Scholar]
- Huh, C.; Pope, G. Residual oil saturation from polymer floods: Laboratory measurements and theoretical interpretation. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Xia, H.; Wang, D.; Wang, G.; Ma, W.; Deng, H.; Liu, J. Mechanism of the effect of micro-forces on residual oil in chemical flooding. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Hou, J.; Li, Z.; Zhang, S.; Cao, X.; Du, Q.; Song, X. Computerized tomography study of the microscopic flow mechanism of polymer flooding. Transp. Porous Media 2009, 79, 407–418. [Google Scholar] [CrossRef]
- Meybodi, H.; Kharrat, R.; Wang, X. Study of microscopic and macroscopic displacement behaviors of polymer solution in water-wet and oil-wet media. Transp. Porous Media 2011, 89, 97–120. [Google Scholar] [CrossRef]
- Chanda, M. Chain copolymerization. In Introduction to Polymer Science and Chemistry; CRC Press: Boca Raton, FL, USA, 2006; pp. 436–437. [Google Scholar]
- Mothé, C.; Correia, D.; de Franca, F.; Riga, A. Thermal and rheological study of polysaccharides for enhanced oil recovery. J. Therm. Anal. Calorim. 2006, 85, 31–36. [Google Scholar] [CrossRef]
- Kulawardana, E.; Koh, H.; Kim, D.; Liyanage, P.; Upamali, K.; Huh, C.; Weerasooriya, U.; Pope, G. Rheology and transport of improved EOR polymers under harsh reservoir conditions. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Kulicke, W.; Böse, N.; Bouldin, M. The role of polymers in enhanced oil recovery. In Water-Soluble Polymers for Petroleum Recovery; Stahl, G., Schulz, D., Eds.; Springer: Boston, MA, USA, 1988; pp. 1–17. [Google Scholar]
- Thomas, A. Polymer flooding. In Chemical Enhanced Oil Recovery (cEOR): A Practical Overview; InTech Open: Rijeka, Croatia, 2016; pp. 55–99. [Google Scholar]
- Mishra, S.; Bera, A.; Mandal, A. Effect of polymer adsorption on permeability reduction in enhanced oil recovery. J. Pet. Eng. 2014, 2014, 395857. [Google Scholar] [CrossRef]
- Zaitoun, A.; Bertin, H.; Lasseux, D. Two-phase flow property modifications by polymer adsorption. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–22 April 1998. [Google Scholar]
- Al-Sharji, H.; Grattoni, C.; Dawe, R.; Zimmerman, R. Disproportionate permeability reduction due to polymer adsorption entanglement. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 21–22 May 2001. [Google Scholar]
- Zou, C.; Zhao, P.; Ge, J.; Lei, Y.; Luo, P. β-cyclodextrin modified anionic and cationic acrylamide polymers for enhancing oil recovery. Carbohydr. Polym. 2012, 87, 607–613. [Google Scholar] [CrossRef]
- Fernandez, I. Evaluation of cationic water-soluble polymers with improved thermal stability. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar]
- Smets, G.; Hesbain, A. Hydrolysis of polyacrylamide and acrylic acid-acrylamide copolymers. J. Polym. Sci. 1959, 40, 217–226. [Google Scholar] [CrossRef]
- Riahinezhad, M.; Romero-Zerón, L.; McManus, N.; Penlidis, A. Design of tailor-made water-soluble copolymers for enhanced oil recovery polymer flooding applications. Macromol. React. Eng. 2017, 11, 1600020. [Google Scholar] [CrossRef]
- Choi, J.; Ka, D.; Chung, T.; Jung, J.; Koo, G.; Uhm, T.; Jung, S.; Park, S.; Jung, H. Evaluation of highly stable ultrahigh-molecular-weight partially hydrolyzed polyacrylamide for enhanced oil recovery. Macromol. Res. 2015, 23, 518–524. [Google Scholar] [CrossRef]
- Scott, A.J.; Duever, T.; Penlidis, A. The role of pH, ionic strength and monomer concentration on the terpolymerization of 2-acrylamido-2-methylpropane sulfonic acid, acrylamide and acrylic acid. Polymer 2019, 177, 214–230. [Google Scholar] [CrossRef]
- Halverson, F.; Lancaster, J.; O’Connor, N. Sequence distribution of carboxyl groups in hydrolyzed polyacrylamide. Macromolecules 1985, 18, 1139–1144. [Google Scholar] [CrossRef]
- Nagase, K.; Sakaguchi, K. Alkaline hydrolysis of polyacrylamide. J. Polym. Sci. Part A 1965, 3, 2475–2482. [Google Scholar] [CrossRef]
- Shawki, S.; Hamielec, A. Estimation of the reactivity ratios in the copolymerization of acrylic acid and acrylamide from composition-conversion measurements by an improved nonlinear least-squares method. J. Appl. Polym. Sci. 1979, 23, 3155–3166. [Google Scholar] [CrossRef]
- Oliveira, P.; Costa, J.; Oliveira, L.; Mota, L.; de Oliveira, L.; Mansur, C. Hydrolysis and thermal stability of partially hydrolyzed polyacrylamide in high-salinity environments. J. Appl. Polym. Sci. 2019, 136, 47793. [Google Scholar] [CrossRef]
- Preusser, C.; Ezenwajiaku, I.; Hutchinson, R. The combined influence of monomer concentration and ionization on acrylamide/acrylic acid composition in aqueous solution radical batch copolymerization. Macromolecules 2016, 49, 4746–4756. [Google Scholar] [CrossRef]
- Scott, A.J.; Penlidis, A. Designing optimal terpolymers for enhanced oil recovery (polymer flooding). Ind. Eng. Chem. Res. 2020, in press. [Google Scholar]
- Riahinezhad, M.; McManus, N.; Penlidis, A. Effect of monomer concentration and pH on reaction kinetics and copolymer microstructure of acrylamide/acrylic acid copolymer. Macromol. React. Eng. 2015, 9, 100–113. [Google Scholar] [CrossRef]
- Riahinezhad, M.; McManus, N.; Penlidis, A. Shear viscosity of poly (acrylamide/acrylic acid) solutions. Macromol. Symp. 2016, 360, 179–184. [Google Scholar] [CrossRef]
- Kazemi, N.; Duever, T.; Penlidis, A. Reactivity ratio estimation from cumulative copolymer composition data. Macromol. React. Eng. 2012, 5, 385–403. [Google Scholar] [CrossRef]
- Kazemi, N.; Duever, T.; Penlidis, A. Demystifying the estimation of reactivity ratios for terpolymerization systems. AIChE J. 2014, 60, 1752–1766. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Lu, Z.; Feng, Y. Thermoviscosifying polymer used for enhanced oil recovery: Rheological behaviors and core flooding test. Polym. Bull. 2013, 70, 391–401. [Google Scholar] [CrossRef]
- Cochin, D.; Candau, F.; Zana, R.; Talmon, Y. Direct imaging of microstructures formed in aqueous solutions of polyamphiphiles. Macromolecules 1992, 25, 4220–4223. [Google Scholar] [CrossRef]
- McCormick, C. Water-soluble random and graft copolymers for utilization in enhanced oil recovery. J. Macromol. Sci. Part A Chem. 1985, 22, 955–982. [Google Scholar] [CrossRef]
- Chang, Y.; McCormick, C. Water-soluble copolymers. 49. Effect of the distribution of the hydrophobic cationic monomer dimethyldodecyl(2-acrylamidoethyl)ammonium bromide on the solution behavior of associating acrylamide copolymers. Macromolecules 1993, 26, 6121–6126. [Google Scholar] [CrossRef]
- McCormick, C.; Chen, G. Water-soluble copolymers. IV. Random copolymers of acrylamide with sulfonated comonomers. J. Polym. Sci. Polym. Chem. Ed. 1982, 20, 817–838. [Google Scholar] [CrossRef]
- Koenig, J. Chemical Microstructure of Polymer Chains; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Riahinezhad, M. Clarifying Multi-Component Polymerization Kinetics for Tailoring Properties of Acrylamide/Acrylic Acid Copolymers for Enhanced Oil Recovery. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2016. [Google Scholar]
- Brar, A.; Hekmatyar, S. Microstructure determination of the acrylonitrile-styrene-methyl methacrylate terpolymers by NMR spectroscopy. J. Appl. Polym. Sci. 1999, 74, 3026–3032. [Google Scholar] [CrossRef]
- Brar, A.; Sunita. Compositional sequence determination of acrylonitrile-butyl acrylate copolymers by 13C-NMR spectroscopy. Polymer 1993, 34, 3391–3396. [Google Scholar] [CrossRef]
- Scott, A.J.; Gabriel, V.; Dubé, M.; Penlidis, A. Making the most of parameter estimation: Terpolymerization troubleshooting tips. Processes 2019, 7, 444. [Google Scholar] [CrossRef]
- Wu, M.; Ball, L. Block Copolymers for Enhanced Oil Recovery. U.S. Patent 4540498, 10 September 1985. [Google Scholar]
- Shaikh, S.; Ali, S.; Hamad, E.; Abu-Sharkh, B. Synthesis and solution properties of poly(acrylamide-styrene) block copolymers with high hydrophobic content. Polym. Eng. Sci. 1999, 39, 1962–1968. [Google Scholar] [CrossRef]
- Raffa, P.; Broekhuis, A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Kujawa, P.; Audibert-Hayet, A.; Selb, J.; Candau, F. Rheological properties of multisticker associative polyelectrolytes in semidilute aqueous solutions. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1640–1655. [Google Scholar] [CrossRef]
- Seright, R.; Seheult, M.; Talashek, T. Injectivity characteristics of EOR polymers. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar]
- Born, K.; Langendorff, V.; Boulenguer, P. Xanthan. In Biopolymers Online: Biology Chemistry Biotechnology Applications; Wiley: New York, NY, USA, 2005; pp. 259–269. [Google Scholar]
- Stokke, B.; Christensen, B.; Smidsrod, O. Macromolecular properties of xanthan. In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1998; pp. 433–472. [Google Scholar]
- Messaud, F.; Sanderson, R.; Runyon, J.; Otte, T.; Pasch, H.; Williams, S. An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers. Prog. Polym. Sci. 2009, 34, 351–368. [Google Scholar] [CrossRef]
- Flory, P.; Fox, T. Treatment of intrinsic viscosities. J. Am. Chem. Soc. 1951, 73, 1904–1908. [Google Scholar] [CrossRef]
- Zeynali, M.; Rabii, A.; Baharvand, H. Synthesis of partially hydrolyzed polyacrylamide and investigation of solution properties (viscosity behaviour). Iran. Polym. J. 2004, 13, 479–484. [Google Scholar]
- Scott, A.J.; Kazemi, N.; Penlidis, A. AMPS/AAm/AAc terpolymerization: Experimental verification of the EVM framework for ternary reactivity ratio estimation. Processes 2017, 5, 9. [Google Scholar] [CrossRef]
- Jouenne, S.; Anfray, J.; Cordelier, P.; Mateen, K.; Levitt, D.; Souilem, I.; Marchal, P.; Lemaitre, C.; Choplin, L.; Nesvick, J.; et al. Degradation (or lack thereof) and drag reduction of HPAM solutions during transport in turbulent flow in pipelines. Oil Gas Facil. 2015, 4, 80–92. [Google Scholar] [CrossRef]
- Morel, D.; Zaugg, E.; Jouenne, S.; Danquigny, J.; Cordelier, P. Dalia/Camelia polymer injection in deep offshore field Angola learnings and in situ polymer sampling results. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015. [Google Scholar]
- Saleh, L.; Wei, M.; Bai, B. Data analysis and novel screening criteria for polymer flooding based on a comprehensive database. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Muller, G. Thermal stability of high-molecular-weight polyacrylamide aqueous solutions. Polym. Bull. 1981, 5, 31–37. [Google Scholar] [CrossRef]
- Moradi-Araghi, A.; Doe, P. Hydrolysis and precipitation of polyacrylamides in hard brines at elevated temperatures. SPE Reserv. Eng. 1987, 2, 189–198. [Google Scholar] [CrossRef]
- Bjørkum, P.; Nadeau, P. Temperature controlled porosity/permeability reduction, fluid migration, and petroleum exploration in sedimentary basins. APPEA J. 1998, 38, 453–464. [Google Scholar] [CrossRef]
- Liang, K.; Han, P.; Chen, Q.; Su, X.; Feng, Y. Comparative study on enhancing oil recovery under high temperature and high salinity: Polysaccharides versus synthetic polymer. ACS Omega 2019, 4, 10620–10628. [Google Scholar] [CrossRef]
- Stahl, G.; Moradi-Araghi, A.; Doe, P. High temperature and hardness stable copolymers of vinylpyrrolidone and acrylamide. In Water-Soluble Polymers for Petroleum Recovery; Stahl, G., Schulz, D., Eds.; Springer: Boston, MA, USA, 1988; pp. 121–130. [Google Scholar]
- Vermolen, E.; Van Haasterecht, M.; Masalmeh, S.; Faber, M.; Boersma, D.; Gruenenfelder, M. Pushing the envelope for polymer flooding towards high-temperature and high-salinity reservoirs with polyacrylamide based ter-polymers. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011. [Google Scholar]
- Masalmeh, S.; AlSumaiti, A.; Gaillard, N.; Daguerre, F.; Skauge, T.; Skuage, A. Extending polymer flooding towards high-temperature and high-salinity carbonate reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 11–14 November 2019. [Google Scholar]
- Levitt, D.; Pope, G. Selection and screening of polymers for enhanced-oil recovery. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Gaillard, N.; Sanders, D.; Favero, C. Improved oil recovery using thermally and chemically protected compositions based on co- and ter-polymers containing acrylamide. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Seright, R.; Skjevrak, I. Effect of dissolved iron and oxygen on stability of hydrolyzed polyacrylamide polymers. SPE J. 2015, 20, 433–441. [Google Scholar] [CrossRef]
- Jouenne, S.; Klimenko, A.; Levitt, D. Polymer flooding: Establishing specifications for dissolved oxygen and iron in injection water. SPE J. 2017, 22, 438–446. [Google Scholar] [CrossRef]
- Ikegami, A.; Imai, N. Precipitation of polyelectrolytes by salts. J. Polym. Sci. 1962, 56, 133–152. [Google Scholar] [CrossRef]
- De Melo, M.; Lucas, E. Characterization and selection of polymers for future research on enhanced oil recovery. Chem. Chem. Technol. 2008, 2, 295–303. [Google Scholar]
- Urbissinova, T.; Kuru, E. Effect of elasticity during viscoelastic polymer flooding: A possible mechanism of increasing the sweep efficiency. J. Can. Pet. Technol. 2010, 49, 49–56. [Google Scholar] [CrossRef]
- Jouenne, S.; Heurteux, G. Correlation of mobility reduction of HPAM solutions at high velocity in porous medium with ex-situ measurements of elasticity. SPE J. 2019. [Google Scholar] [CrossRef]
- Li, K.; Sun, W.; Li, F.; Qu, Y.; Yang, Y. Novel method for characterizing single-phase polymer flooding. SPE J. 2014, 19, 695–702. [Google Scholar] [CrossRef]
- Li, Z.; Delshad, M. Development of an analytical injectivity model for non-Newtonian polymer solutions. SPE J. 2014, 19, 381–389. [Google Scholar] [CrossRef]
- Seright, R.; Fan, T.; Wavrik, K.; Balaban, R. New insights into polymer rheology in porous media. SPE J. 2011, 16, 35–42. [Google Scholar] [CrossRef]
- Zhou, Y.; Muggeridge, A.; Berg, C.; King, P. Effect of layering on incremental oil recovery from tertiary polymer flooding. SPE Reserv. Eval. Eng. 2019, 22, 941–951. [Google Scholar] [CrossRef]
- Azad, M.; Trivedi, J. Quantification of the viscoelastic effects during polymer flooding: A critical review. SPE J. 2019, 24, 2731–2757. [Google Scholar] [CrossRef]
- Daripa, P.; Dutta, S. Modeling and simulation of surfactant–polymer flooding using a new hybrid method. J. Comput. Phys. 2017, 335, 249–282. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, J.; Liu, L.; Zhou, K.; Zhang, Y.; Dai, T.; Guo, L.; Cao, W. An inversion method of relative permeability curves in polymer flooding considering physical properties of polymer. SPE J. 2018, 23, 1929–1943. [Google Scholar] [CrossRef]
- Daripa, P.; Ding, X. A numerical study of instability control for the design of an optimal policy of enhanced oil recovery by tertiary displacement processes. Transp. Porous Media. 2012, 93, 675–703. [Google Scholar] [CrossRef]
- Alsofi, A.M.; Liu, J.S.; Han, M.; Aramco, S. Numerical simulation of surfactant–polymer coreflooding experiments for carbonates. J. Pet. Sci. Eng. 2013, 111, 184–196. [Google Scholar] [CrossRef]
- Khodaverdian, M.F.; Sorop, T.; Postif, S.J.; Van den Hoek, P.J. Polymer flooding in unconsolidated-sand formations: Fracturing and geomechanical considerations. SPE Prod. Oper. 2010, 25, 211–222. [Google Scholar] [CrossRef]
- Azad, M.; Trivedi, J. Does polymer’s viscoelasticity influence heavy-oil sweep efficiency and injectivity at 1 ft/D? SPE Reserv. Eval. Eng. 2019. [Google Scholar] [CrossRef]
- Torrealba, V.A.; Hoteit, H. Improved polymer flooding injectivity and displacement by considering compositionally-tuned slugs. J. Pet. Sci. Eng. 2019, 178, 14–26. [Google Scholar] [CrossRef]
- Choi, S.; Sharma, M.; Bryant, S.; Huh, C. pH-Sensitive polymers for novel conformance-control and polymer-flood applications. SPE Reserv. Eval. Eng. 2010, 13, 926–939. [Google Scholar] [CrossRef]
- Manichand, R.; Seright, R. Field vs. laboratory polymer-retention values for a polymer flood in the Tambaredjo Field. SPE Reserv. Eval. Eng. 2014, 17, 314–325. [Google Scholar] [CrossRef]
- Zhang, G.; Seright, R. Effect of concentration on HPAM retention in porous media. SPE J. 2014, 19, 373–380. [Google Scholar] [CrossRef]
- Liang, J.; Seright, R. Wall-effect/gel-droplet model of disproportionate permeability reduction. SPE J. 2001, 6, 268–272. [Google Scholar] [CrossRef]
- Ferreira, V.; Moreno, R. Polyacrylamide adsorption and readsorption in sandstone porous media. SPE J. 2019. [Google Scholar] [CrossRef]
- Seright, R. Disproportionate permeability reduction with pore-filling gels. SPE J. 2009, 14, 5–13. [Google Scholar] [CrossRef]
- Liang, J.; Sun, H.; Seright, R. Why do gels reduce water permeability more than oil permeability? SPE Reserv. Eng. 1995, 10, 282–286. [Google Scholar] [CrossRef]
- Wan, H.; Seright, R. Is polymer retention different under anaerobic vs. aerobic conditions? SPE J. 2017, 22, 431–437. [Google Scholar] [CrossRef]
- Juárez-Morejón, J.; Bertin, H.; Omari, A.; Hamon, G.; Cottin, C.; Morel, D.; Romero, C.; Bourdarot, G. A new approach to polymer flooding: Effects of early polymer injection and wettability on final oil recovery. SPE J. 2019, 24, 129–139. [Google Scholar] [CrossRef]
- Riahinezhad, M.; Romero-Zerón, L.; McManus, N.; Penlidis, A. Evaluating the performance of tailor-made water-soluble copolymers for enhanced oil recovery polymer flooding applications. Fuel 2017, 203, 269–278. [Google Scholar] [CrossRef]
- Romero-Zerón, L.; Banthong, S. Viscoelasticity of a supramolecular polymer network and its relevance for enhanced oil recovery. In Polymer Rheology; InTech Open: Rijeka, Croatia, 2018; pp. 95–118. [Google Scholar]
- Jang, H.; Zhang, K.; Chon, B.; Choi, H. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.; Yu, L.; Gong, H.; Dong, M.; Li, Y. The displacement efficiency and rheology of welan gum for enhanced heavy oil recovery. Polym. Adv. Technol. 2014, 25, 1122–1129. [Google Scholar] [CrossRef]
- Buchgraber, M.; Clemens, T.; Castanier, L.; Kovscek, A. A microvisual study of the displacement of viscous oil by polymer solutions. SPE Reserv. Eval. Eng. 2011, 14, 269–280. [Google Scholar] [CrossRef]
- Hou, Q.; Zhu, Y.; Luo, Y.; Weng, R.; Guoqing, J. Studies on nitrogen foam flooding for conglomerate reservoir. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar]
- Manichand, R.; Moe Soe Let, K.; Gil, L.; Quillien, B.; Seright, R. Effective propagation of HPAM solutions through the Tambaredjo Reservoir during a polymer flood. SPE Prod. Oper. 2013, 28, 358–368. [Google Scholar] [CrossRef]
- Wang, D.; Han, P.; Shao, Z.; Hou, W.; Seright, R. Sweep-improvement options for the Daqing oil field. SPE Reserv. Eval. Eng. 2008, 11, 18–26. [Google Scholar] [CrossRef]
- Buciak, J.; Fondevila Sancet, G.; Del Pozo, L. Polymer-flooding-pilot learning curve: Five-plus years’ experience to reduce cost per incremental barrel of oil. SPE Reserv. Eval. Eng. 2015, 18, 11–19. [Google Scholar] [CrossRef]
- Seright, R.; Wang, D.; Lerner, N.; Nguyen, A.; Sabid, J.; Tochor, R. Can 25-cp polymer solution efficiently displace 1,600-cp oil during polymer flooding? SPE J. 2018, 23, 2260–2278. [Google Scholar] [CrossRef]
- Seright, R.; Campbell, A.; Mozley, P.; Han, P. Stability of partially hydrolyzed polyacrylamides at elevated temperatures in the absence of divalent cations. SPE J. 2010, 15, 341–348. [Google Scholar] [CrossRef]
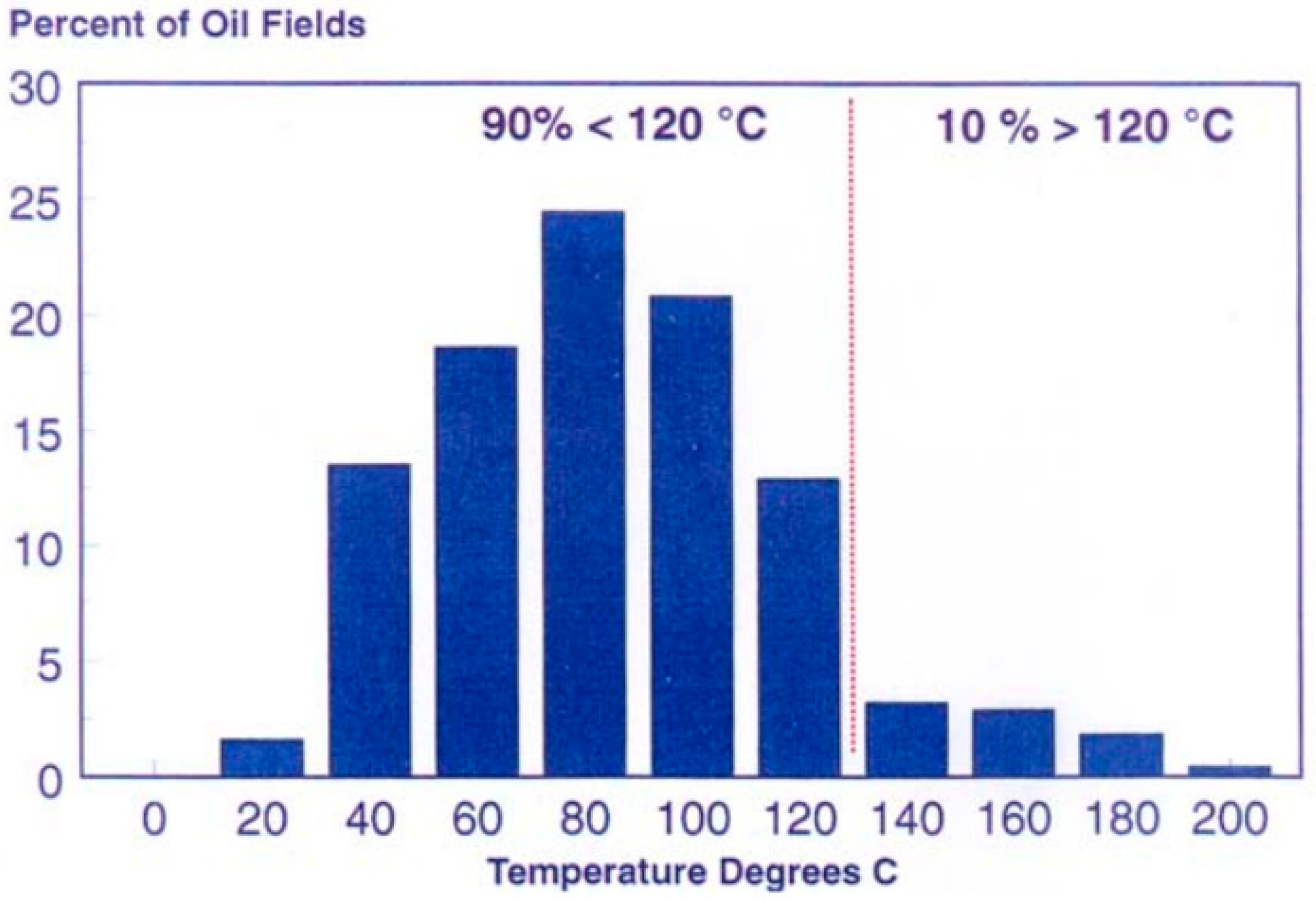
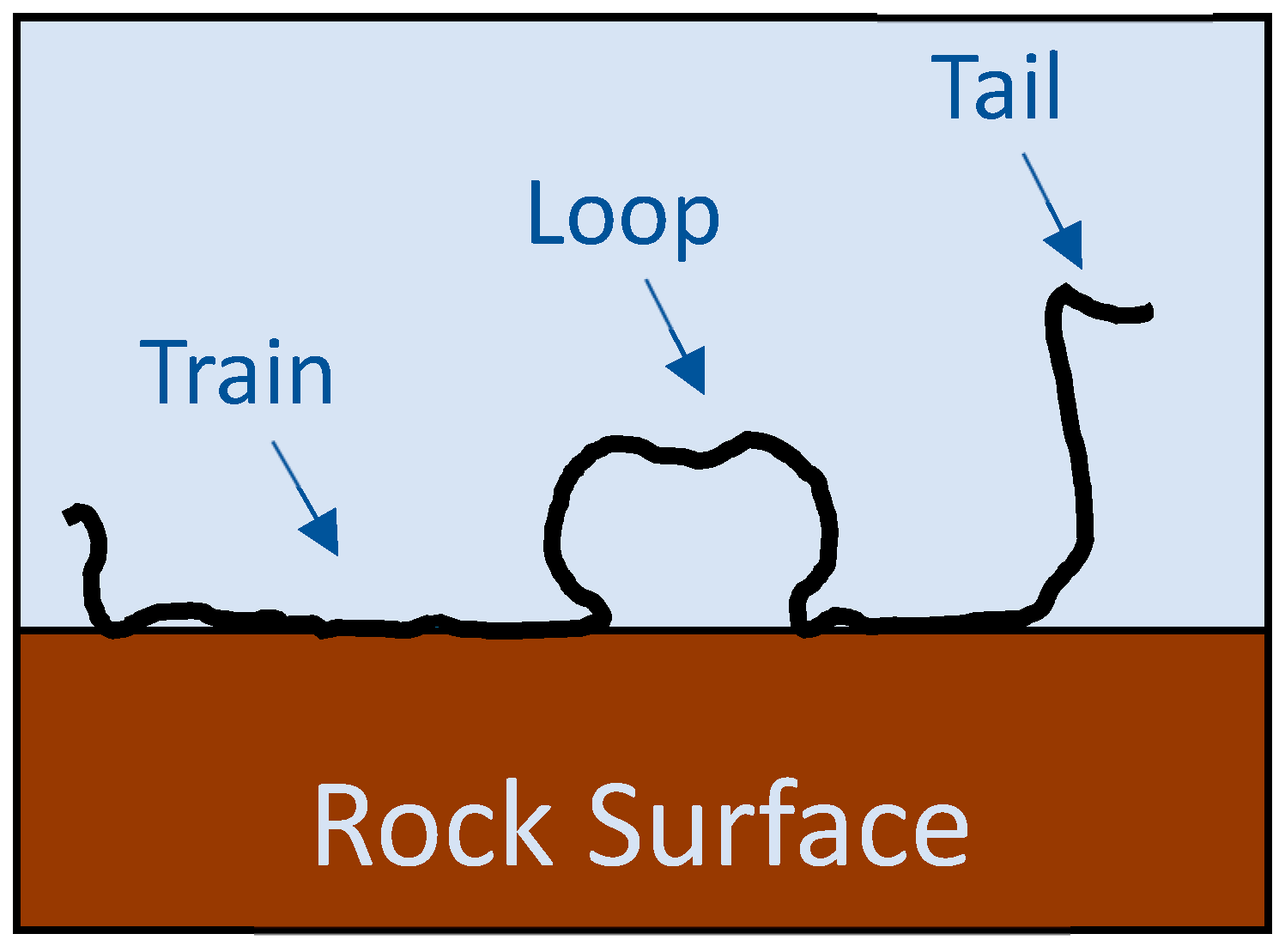
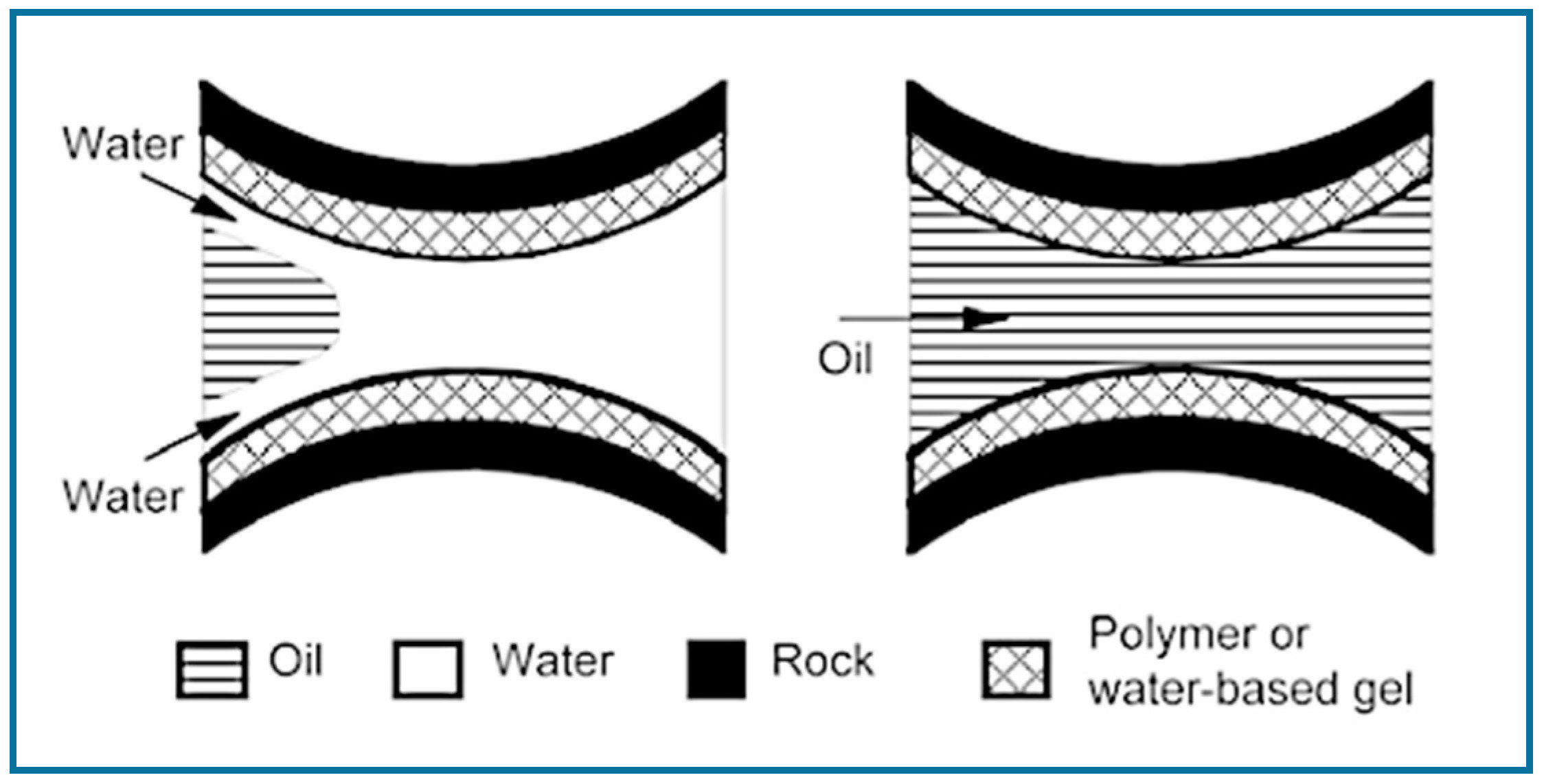
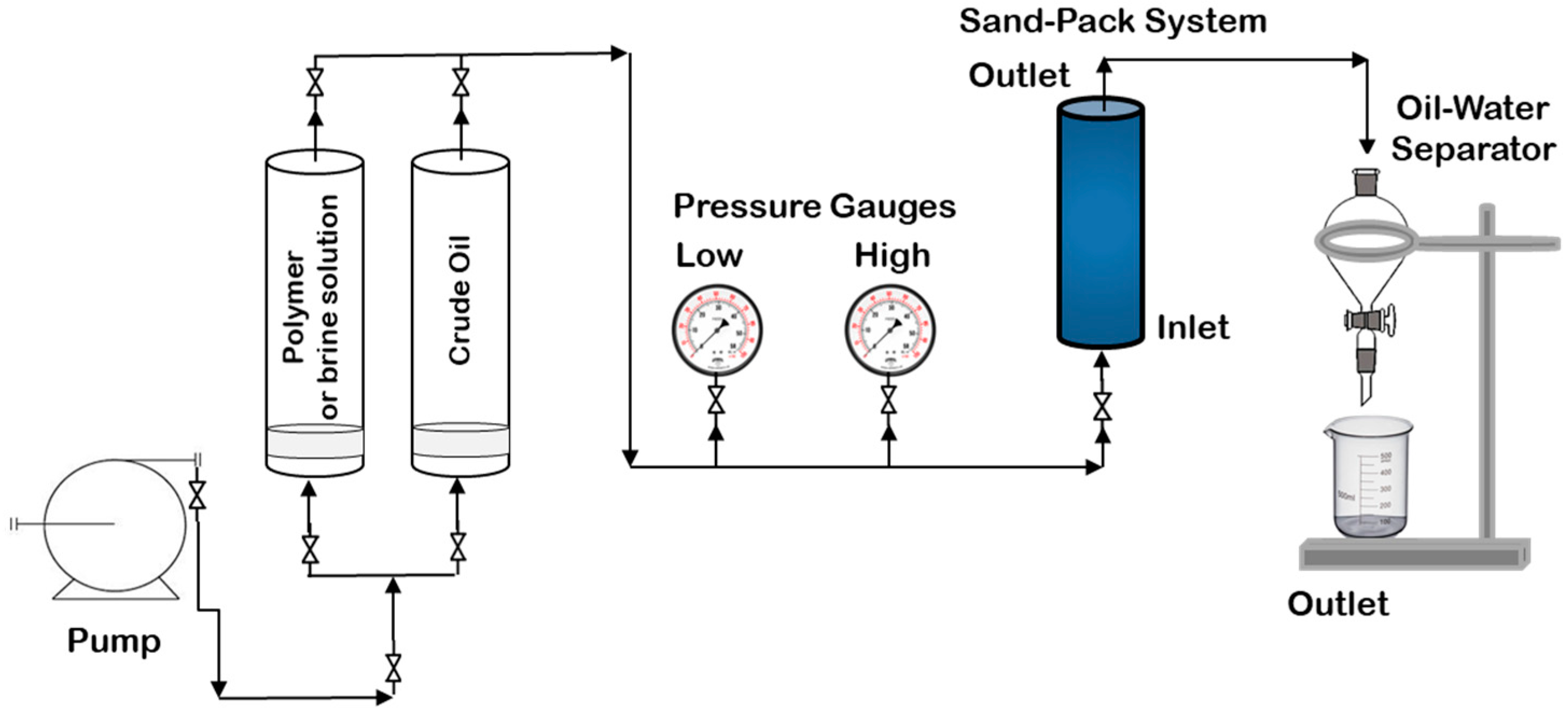
| Ref. | Polymeric Material | Polymer Properties | Polymer Performance |
|---|---|---|---|
| [12] | HPAM and proprietary hydrophobically modified AAm-based copolymer | HPAM: Degree of hydrolysis = 5% MW = 8 × 106 g/mol Proprietary copolymer: MW = 6×106 g/mol | --Improved performance for proprietary copolymer over HPAM (more elastic properties, reformability, and high mobility control) --Significant polymer retention in sand-pack tests (RRF = 165) |
| [24] | PAAm with controlled molecular architectures | Linear, star, and comb structures; MW ranging from 2.8 × 104 g/mol to 5.9 × 105 g/mol | --Molecular architecture impacts polymer solution viscosity --Comb-like structures have higher viscosity and more elasticity than linear or star-like equivalents |
| [25] | Salt-resistant HPAM derivatives with modified molecular architecture | Comb-shaped, branched, star-shaped, and hydrophobic-associating polymers Degree of hydrolysis ~25%; MW ranging from 10 × 106 g/mol to 25 × 106 g/mol | --Modified polymers have better salt tolerance (viscosifying ability, long term stability, and flow properties) than linear HPAM --Some materials have been used in EOR, but results are not reported |
| [26,28] | AAm copolymers and NNDAM copolymers with AAc or AMPS | or ~ 0.60; or ~ 0.40 | --NNDAM/AMPS had superior brine compatibility, shear stability, and thermal stability --Flooding with NNDAM/AMPS allowed for incremental oil recovery up to ~11% (using 2000 ppm polymer solution) |
| [27] | AAm copolymers with AAc, AMPS, or NVP | AAm/AAc copolymer: 0.72; MW = 18.5 × 106 g/mol 0.67; MW = 12 × 106 g/mol AAm/AMPS copolymers: 0.95; MW = 6 × 106 g/mol 0.75; MW = 8 × 106 g/mol AAm/NVP copolymer: 0.50; MW = 6–8 × 106 g/mol | --Higher molecular weight polymers were more shear-sensitive --AAm/AMPS had the highest shear stability (larger AMPS proportion improved shear stability) --AAm/NVP and AAm/AAc had similar shear stability (not as good as AAm/AMPS but better than PAAm) |
| [29] | AAm/AMPS/VN terpolymer | 0.89; 0.10 (low concentrations of VN); low MW | --Experimental evidence of temperature-thickening, pseudoplastic behavior, anti-shearing behavior, and brine compatibility |
| [30] | AAm/AMPS copolymer (AN125) | MW = 12 × 106 g/mol | --0.2 wt % polymer solution in seawater exhibited RRF values up to 2.2; no plugging observed |
| [32] | AAm/SAM/NABI terpolymer | AAm/NABI copolymer sulfonated with HCHO and NaHSO3; 0.95 | --Incremental oil recovery up to 10.6% in brine at 60 °C (using 7 g/L polymer solution) |
| [33] | PAAm and AAm/AH copolymer | PAAm: MW = 5.0 × 106 g/mol AAm/AH copolymer: 0.66; MW = 5.6 × 106 g/mol | --Incremental oil recovery up to 20.0% with 2000 ppm copolymer solution in water (at 30 °C); achieved 18.7% in brine (at 80 °C) --Incremental oil recovery up to 18.8% with 2000 ppm PAAm solution in water (at 30 °C); only 11.8% in brine (at 80 °C) |
| [34] | HPAM and AAm/AAc/NAE terpolymer | HPAM: MW = 5×106 g/mol AAm/AAc/NAE terpolymer synthesis conditions: (AAm) = 16 wt %, (AAc) = 4 wt %, and (NAE) = 0.3 wt % (balance water) | --Incremental oil recovery from 1 g/L polymer solution (in brine at 65 °C): HPAM: 4% Terpolymer: 7.6% |
| [35,36] | Crosslinked AAm/DBSV copolymer | MW = 1.2 × 106 g/mol~~ | --Incremental oil recovery up to 20.8% (using 2 g/L polymer solution) |
| [37] | Hydrophobically associating HPAM-based water-soluble polymer | AAm-rich multicomponent polymer containing functional monomers (ACMO, HDDE, AMPS, and IBOMA) | --Experimental evidence of heat resistance, salt tolerance, and good antimicrobial degradation performance |
| Biopolymer | Advantages | Disadvantages |
|---|---|---|
| Carboxymethylcellulose |
|
|
| Cellulose |
|
|
| Guar Gum |
|
|
| Hydroxyethylcellulose |
|
|
| Lignin |
|
|
| Schizophyllan |
|
|
| Scleroglucan |
|
|
| Welan Gum |
|
|
| Xanthan Gum |
|
|
| Parameter | HPAMs | Xanthan Gum | Associating Polymers |
|---|---|---|---|
| Porosity (%) | 10 to 44 | 10 to 47 | 21 to 40 |
| Permeability (mD) | 2.5 to 13,000 | 18 to 6000 | 300 to 12,600 |
| Temperature (°C) | 22 to 120 | 20 to 100 | 22 to 93 |
| Oil Viscosity (cP) | 1.7 to 5500 | 8 to 129 | 140 to 18,700 |
| Polymer MW (g/mol) | 1 × 106 to 25 × 106 | 1 × 106 to 20 × 106 | 1.3 × 106 to 20 × 106 |
| Polymer Concentration (ppm) | 50 to 10,000 | 30 to 2000 | 500 to 3000 |
| Water Salinity (ppm) | 250 to 133,470 | 661 to 350,000 | 5000 to 186,000 |
| Variable | Range |
|---|---|
| Porosity (%) | 11 to 34 |
| Permeability (mD) | 3.9 to 15,000 |
| Temperature (°C) | 22 to 90 |
| Oil Viscosity (cP) | 0.2 to 10,000 |
| Polymer MW (g/mol) | 5 × 106 to 37 × 106 |
| Polymer Concentration (ppm) | 200 to 2500 |
| Polymer Viscosity (cP) | 1.35 to 40 |
| Water Salinity (ppm) | 500 to 120,000 |
| Variable | Range |
|---|---|
| Porosity, φ (%) | 4.0 to 37 |
| Permeability, k (mD) | >50 |
| Temperature (°C) | <99 |
| Formation-water-salinity (ppm) | <50,000 |
| Concentration of divalent cations (ppm) | <100 |
| Reservoir lithology | Majority in sandstone reservoirs. Very few in carbonate reservoirs. |
| Reservoir water cuts to start polymer flooding | 95% |
| Clay content in reservoir rock | Low |
| Oil viscosity, μ (cP) | <5000 |
| Oil saturation, So (%) | >21 |
| Injection well completion | Large diameter holes, high density, and deep penetration. Hydraulic fracturing to reduce mechanical shearing of the polymer near the wellbore. |
| Aquifer | None—weak |
| Gas cap | None—weak |
| Polymer type | HPAMs and Xanthan Gum |
| Polymer MW (g/mol) Reservoir permeability, k (mD) | 100 mD 400 mD |
| Polymer injection rate (PV/year) Well spacing (m) | 0.14 to 0.16 PV/year—>250 m 0.16 to 0.20 PV/year—150 to 175 m |
| Addition of oxygen scavengers to polymer solutions | Alcohols, thiourea, sodium sulphite, and tri- or pentachlorophenol. |
| Addition of biocides to control biological degradation | Formaldehyde |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, A.J.; Romero-Zerón, L.; Penlidis, A. Evaluation of Polymeric Materials for Chemical Enhanced Oil Recovery. Processes 2020, 8, 361. https://doi.org/10.3390/pr8030361
Scott AJ, Romero-Zerón L, Penlidis A. Evaluation of Polymeric Materials for Chemical Enhanced Oil Recovery. Processes. 2020; 8(3):361. https://doi.org/10.3390/pr8030361
Chicago/Turabian StyleScott, Alison J., Laura Romero-Zerón, and Alexander Penlidis. 2020. "Evaluation of Polymeric Materials for Chemical Enhanced Oil Recovery" Processes 8, no. 3: 361. https://doi.org/10.3390/pr8030361
APA StyleScott, A. J., Romero-Zerón, L., & Penlidis, A. (2020). Evaluation of Polymeric Materials for Chemical Enhanced Oil Recovery. Processes, 8(3), 361. https://doi.org/10.3390/pr8030361






