Ketone Solvent to Reduce the Minimum Miscibility Pressure for CO2 Flooding at the South Sumatra Basin, Indonesia
Abstract
1. Introduction
2. Methodology
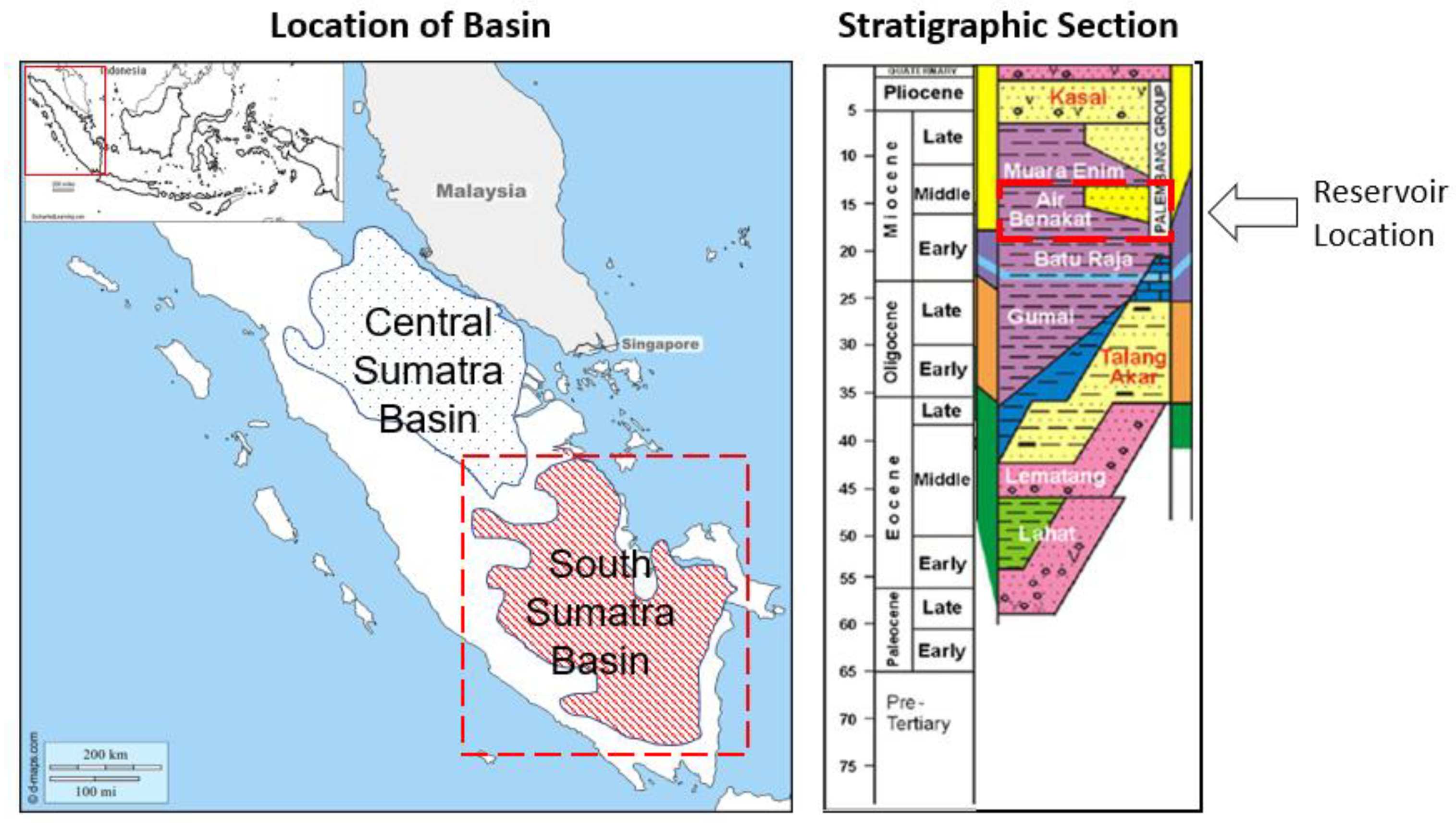
2.1. Properties of the Crude Oil Samples and the Chemical Additives
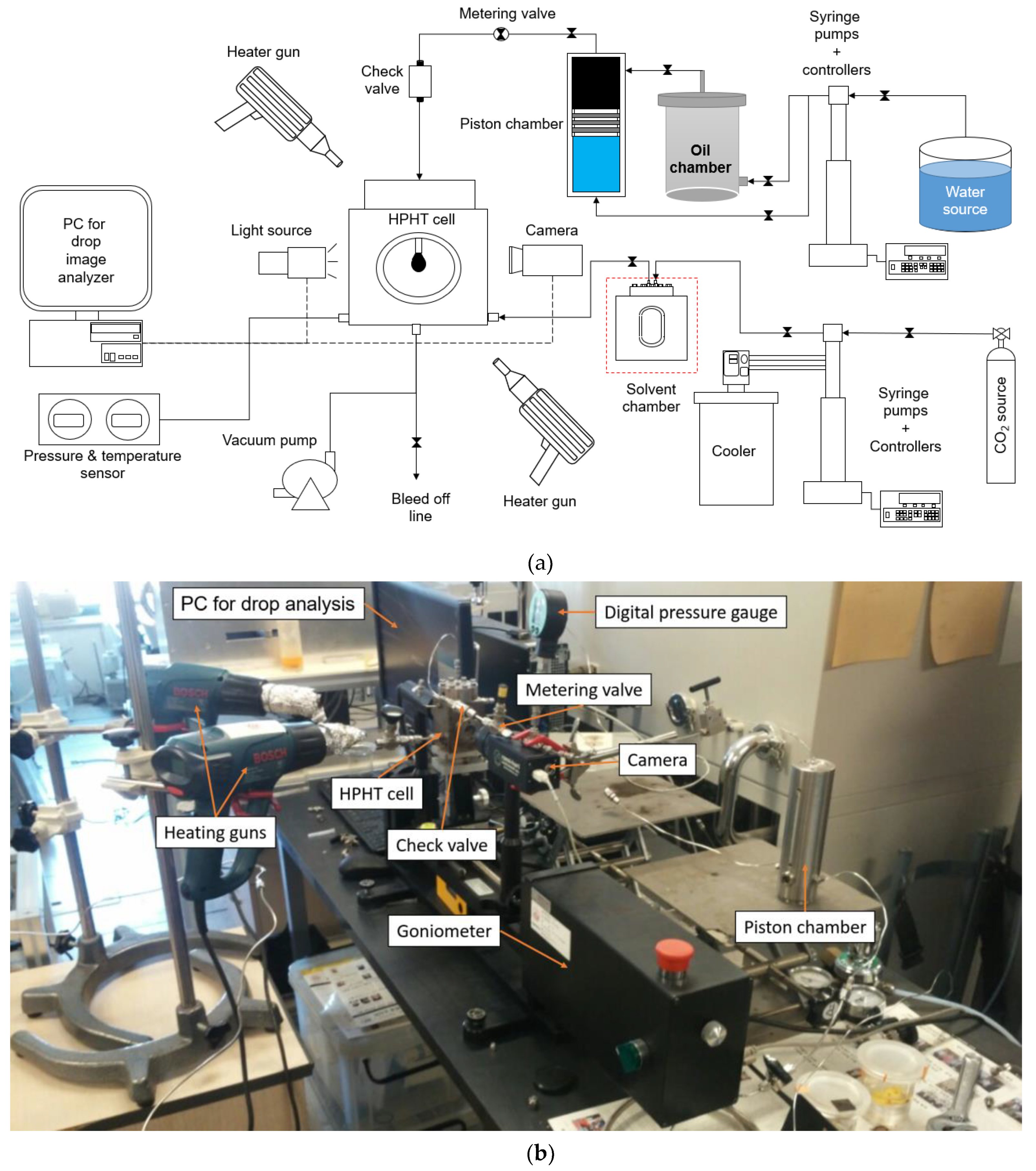
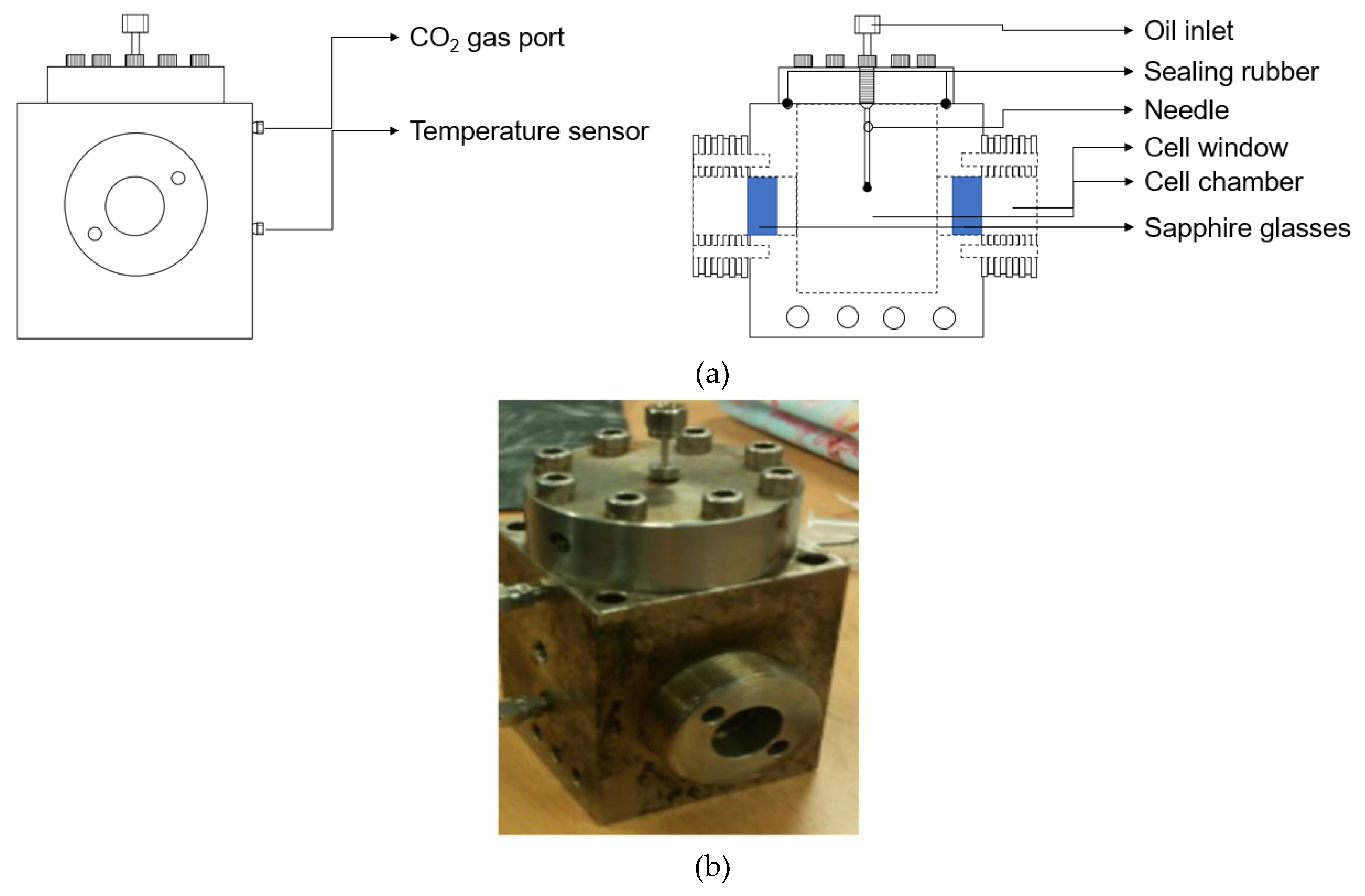
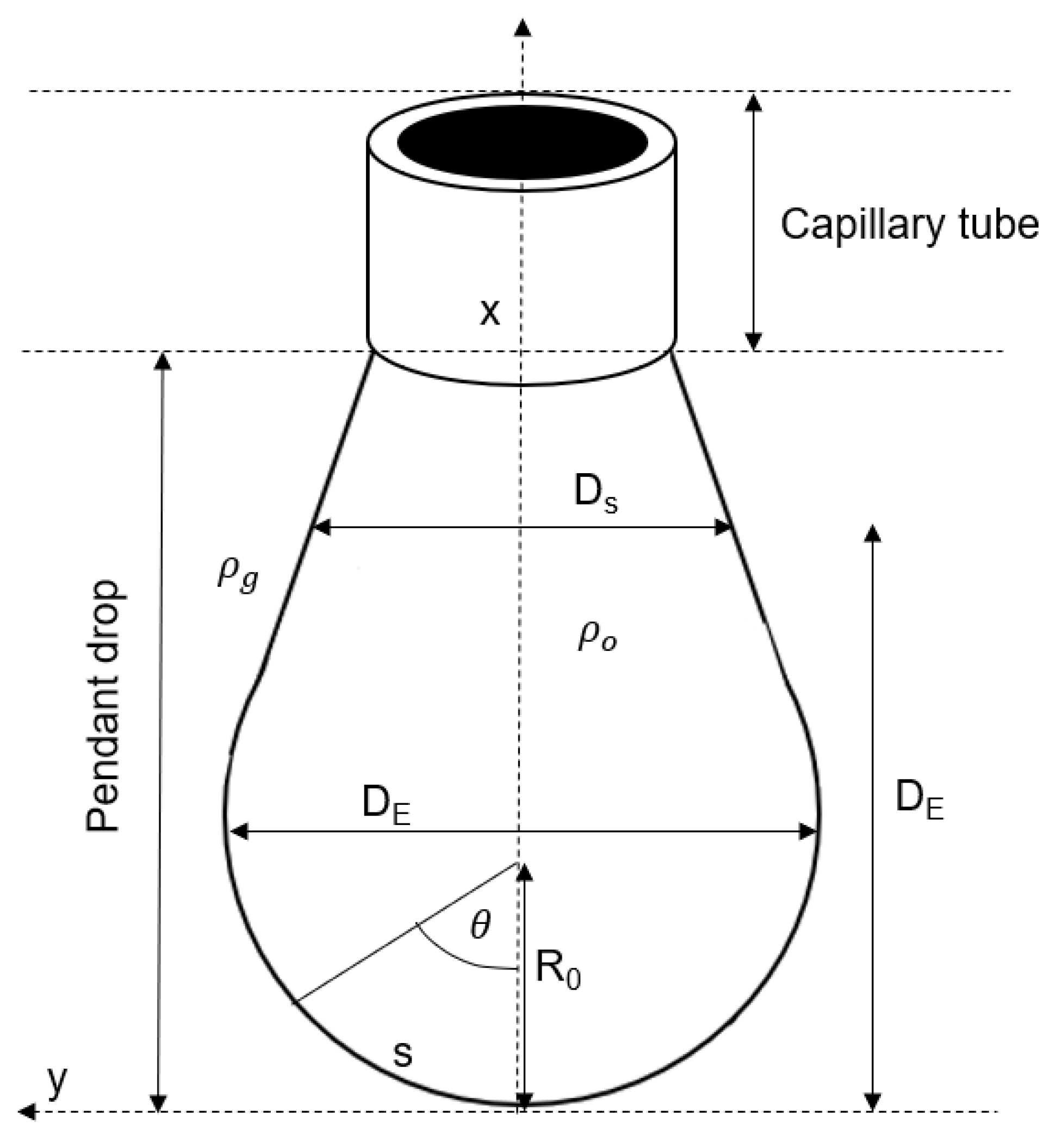
2.2. VIT Experiment
3. Results and Discussion
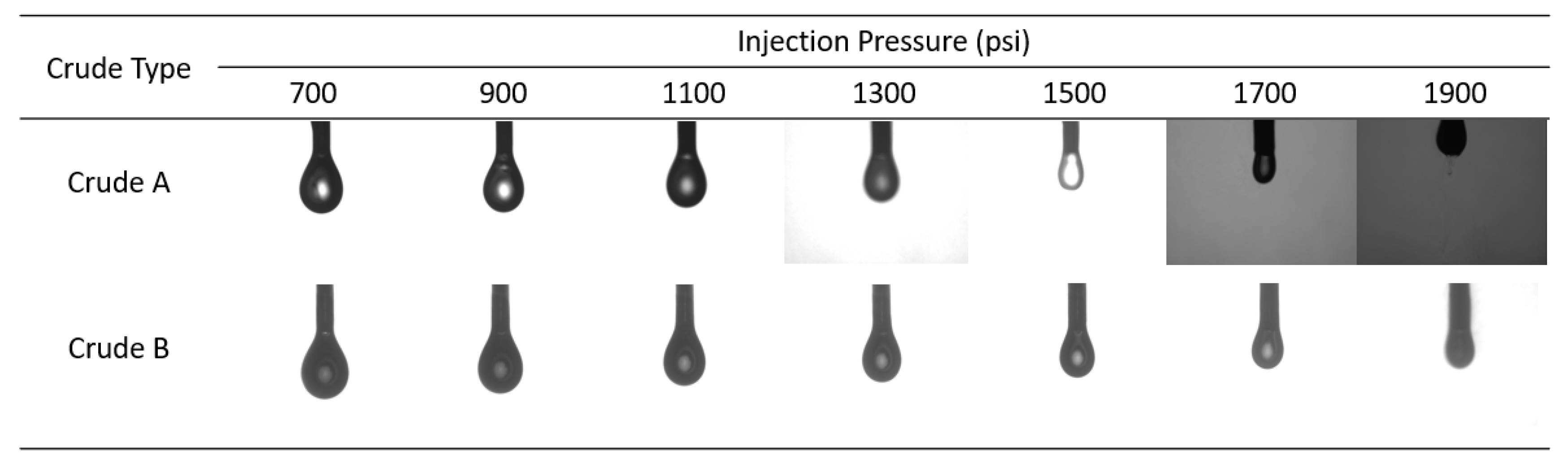
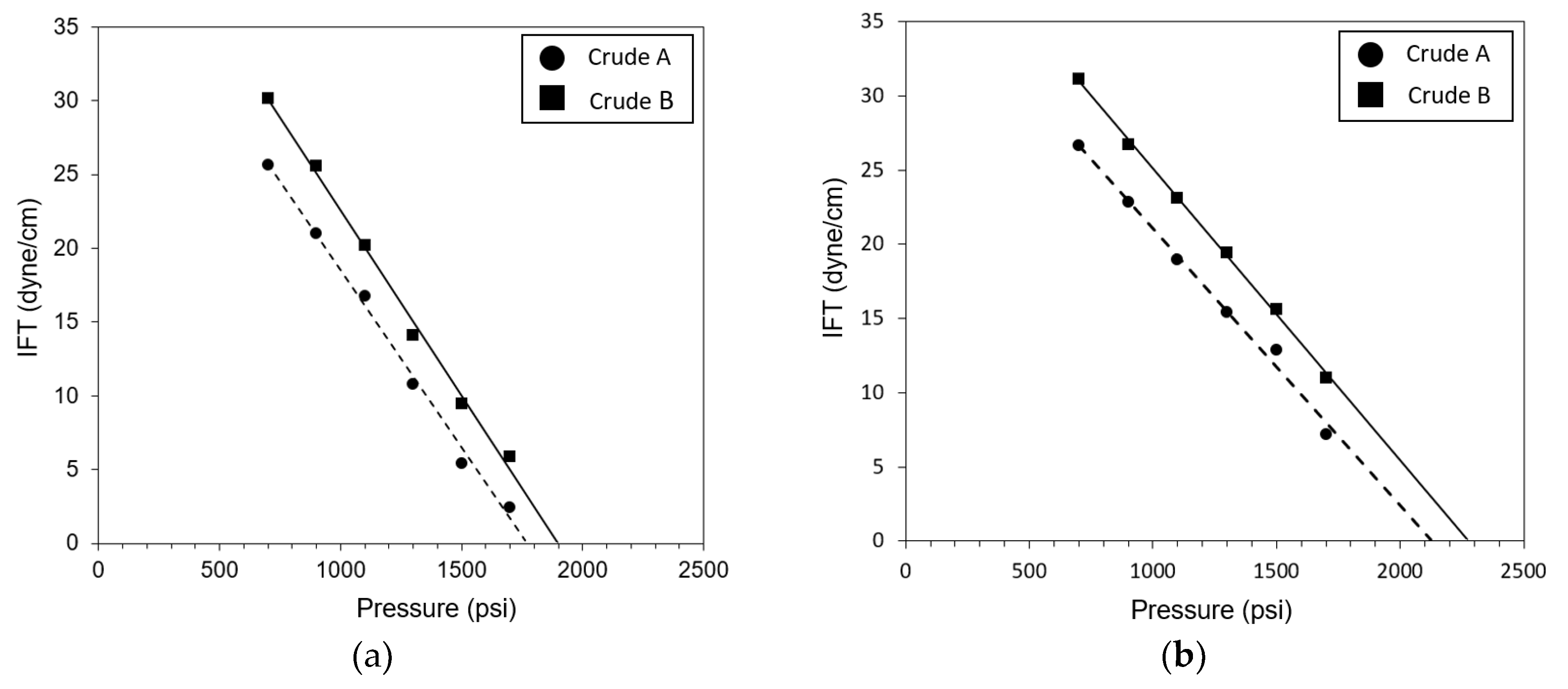
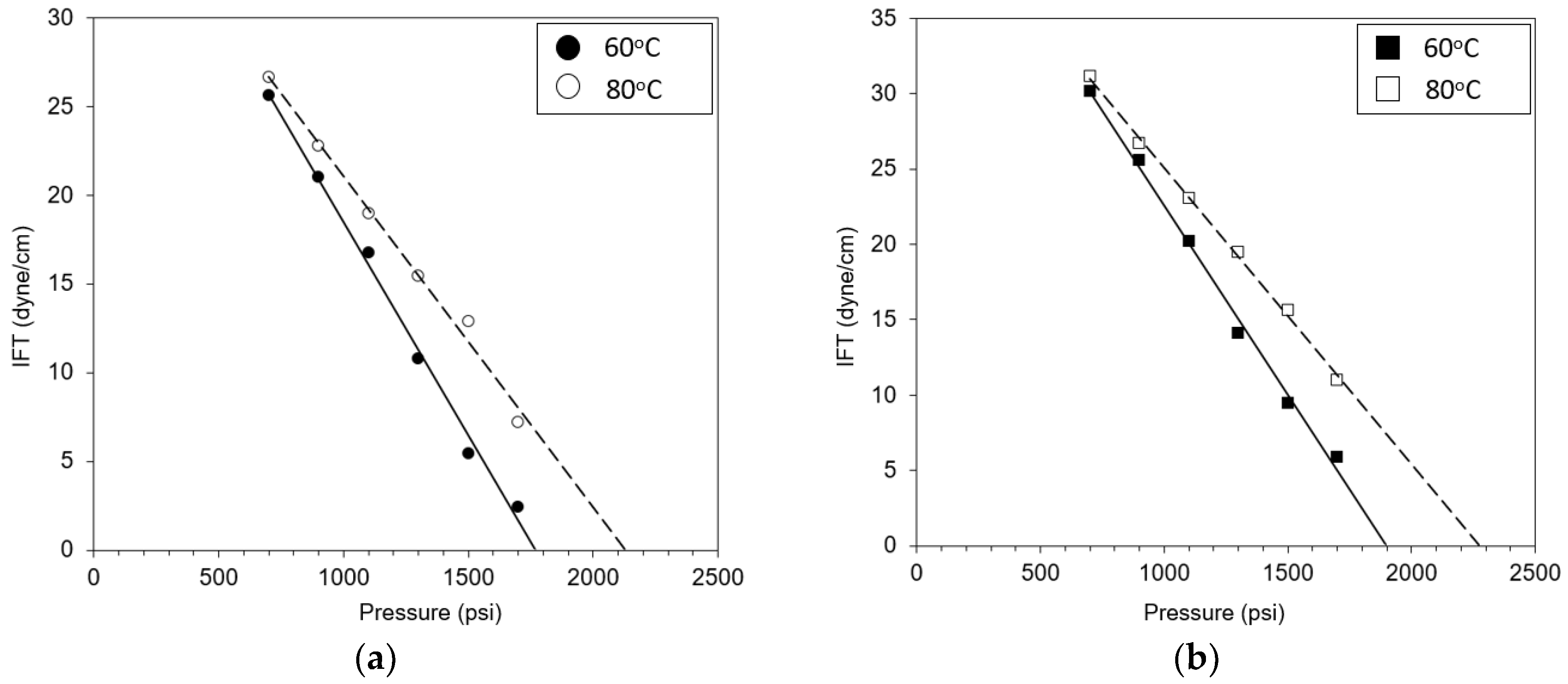
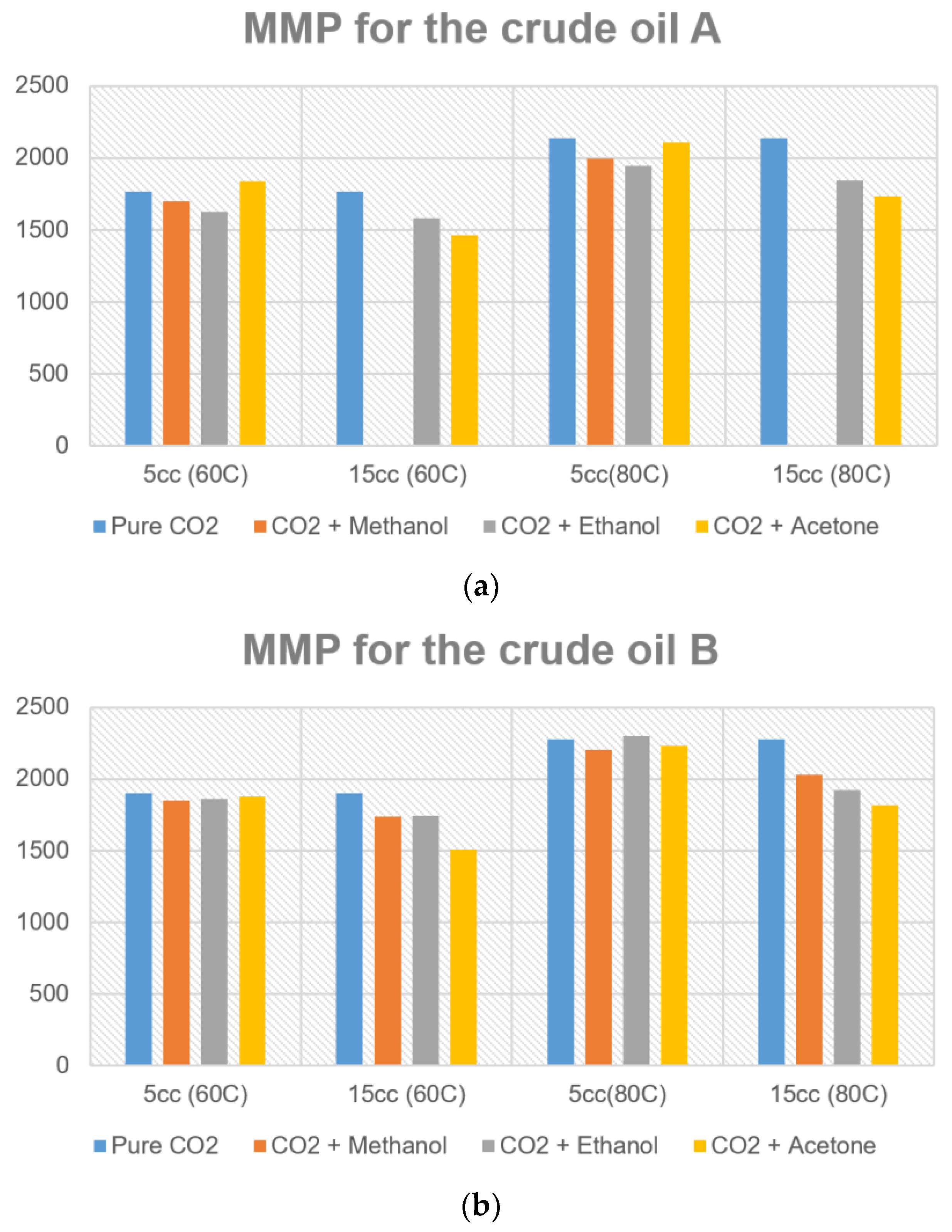
3.1. Effects of Temperature and Crude Oils on MMP
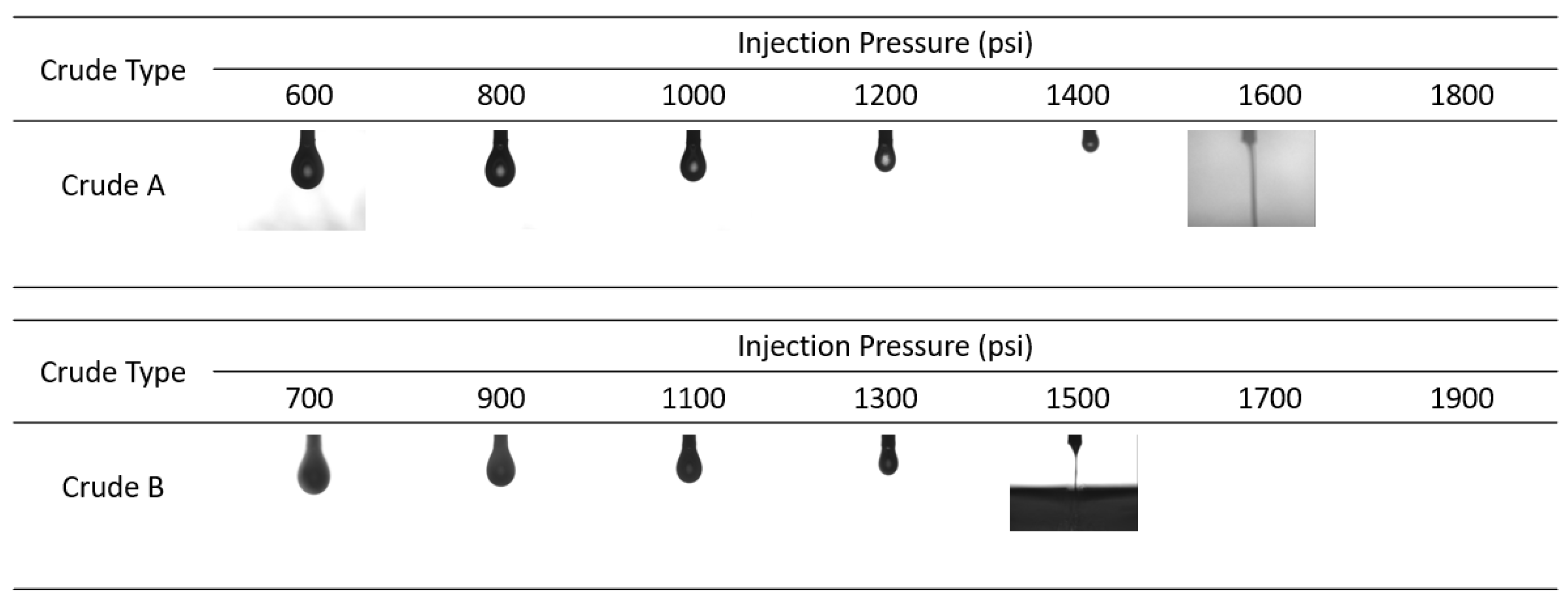
3.2. Mixture Design with Solvents to Reduce MMP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghorbani, M.; Momeni, A.; Gandomkar, A. Modified vanishing interfacial tension (VIT) test for CO2–oil minimum miscibility pressure (MMP) measurement. J. Nat. Gas Sci. Eng. 2014, 20, 92–98. [Google Scholar] [CrossRef]
- Hassan, A.; Elkatatny, S.; Abdulraheem, A. Intelligent prediction of minimum miscibility pressure (MMP) during CO2 flooding using artificial intelligence techniques. Sustainability 2019, 11, 7020. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Li, Z.; Wu, X. Experimental study on reducing CO2–oil minimum miscibility pressure with hydrocarbon agents. Energies 2019, 12, 1975. [Google Scholar] [CrossRef]
- Abdurrahman, M.; Permadi, A.K.; Bae, W.S.; Masduki, A. EOR in Indonesia: Past, present, and future. Int. J. Oil Gas Coal Technol. 2017, 16, 250–270. [Google Scholar] [CrossRef]
- Carbonbrief. Available online: https://www.carbonbrief.org/the-carbon-brief-profile-indonesia (accessed on 1 February 2020).
- Gozalpour, F.; Ren, S.R.; Tohidi, B. CO2 EOR and storage in oil reservoir. Oil Gas Sci. Technol. 2005, 60, 537–546. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Gamage, R.P.; Rathnaweera, T.D.; Ranathunga, A.S.; Koay, A.; Choi, X. A review of CO2–enhanced oil recovery with a simulated sensitivity analysis. Energies 2016, 9, 481. [Google Scholar] [CrossRef]
- Handayani, K.; Krozer, Y.; Filatova, T. Trade–offs between electrification and climate change mitigation: An analysis of the Java–Bali power system in Indonesia. Appl. Energy 2017, 208, 1020–1037. [Google Scholar] [CrossRef]
- Bahadori, A. Fundamentals of Enhanced Oil and Gas Recovery from Conventional and Unconventional Reservoirs; Gulf Professional Publishing: Cambridge, MA, USA, 2018; ISBN 9780128130278. [Google Scholar] [CrossRef]
- Moradi, B.; Awang, M.; Bashir, A.; Sabil, K.M. Effects of alcohols on interfacial tension between carbon dioxide and crude oil at elevated pressures and temperature. J. Pet. Sci. Eng. 2014, 121, 103–109. [Google Scholar] [CrossRef]
- Rommerskirchen, R.; Nijssen, P.; Bilgili, H.; Sottmann, T. Reducing the miscibility pressure in gas injection oil recovery processes. In Proceedings of the SPE Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 7–10 November 2016. [Google Scholar] [CrossRef]
- Choubineh, A.; Helalizadeh, A.; Wood, D.A. The impacts of gas impurities on the minimum miscibility pressure of injected CO2–rich gas–crude oil systems and enhanced oil recovery potential. Pet. Sci. 2019, 16, 117–126. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, W.; Dong, Z.; Lin, M.; Zhang, S.; Zhang, J. Reducing the minimum miscibility pressure of CO2 and crude oil using alcohols. Colloid Surf. A 2019, 568, 105–112. [Google Scholar] [CrossRef]
- Jödecke, M.; Kamps, Á.P.; Maurer, G. Experimental investigation of the solubility of CO2 in (acetone + water). J. Chem. Eng. Data 2007, 52, 1003–1009. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Utilization of methanol and acetone as mutual solvents to reduce interfacial tension (IFT) in enhance oil recovery process by carbonated smart water injection. J. Mol. Liq. 2020, 304, 112733. [Google Scholar] [CrossRef]
- Suntana, A.S.; Vogt, K.A.; Turnblom, E.C.; Upadhye, R. Bio–methanol potential in Indonesia: Forest biomass as a source of bio–energy that reduces carbon emissions. Appl. Energy 2009, 86, S215–S221. [Google Scholar] [CrossRef]
- Khatiwada, D.; Silveira, S. Scenarios for bioethanol production in Indonesia: How can we meet mandatory blending targets? Energy 2017, 119, 351–361. [Google Scholar] [CrossRef]
- Doust, H.; Noble, R.A. Petroleum systems of Indonesia. Mar. Pet. Geol. 2008, 25, 103–129. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Hua, Z.; Ling, Y.; Li, M.; Lin, M.; Dong, Z. Interfacial tension of CO2 and crude oils under high pressure and temperature. Colloid Surface A 2015, 482, 611–616. [Google Scholar] [CrossRef]
- Ahmad, W.; Vakili–Nezhaad, G.; Al–Bemani, A.S.; Al–Wahaibi, Y. Experimental determination of minimum miscibility pressure. Procedia Eng. 2016, 148, 1191–1198. [Google Scholar] [CrossRef]
- Naser, M.A.; Permadi, A.K.; Bae, W.S.; Al–Bemani, A.S.; Dang, S.T. A novel experimental method to generate steam within a small optical cell for measuring interfacial properties. Arab. J. Sci. Eng. 2015, 40, 2795–2804. [Google Scholar] [CrossRef]
- Hemmati–Sarapardeh, A.; Ayatollahi, S.; Ghazanfari, M.; Masihi, M. Experimental determination of interfacial tension and miscibility of the CO2–crude oil system; temperature, pressure, and composition effects. J. Chem. Eng. Data 2014, 59, 61–69. [Google Scholar] [CrossRef]
- Reichardl, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley–VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; ISBN 9783527324736. [Google Scholar]
- Ciborowski, P.; Silberring, J. Proteomic Profiling and Analytical Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780444636881. [Google Scholar]
- Miller, M.B.; Chen, D.; Luebke, D.R.; Johnson, J.K.; Enick, R.M. Critical assessment of CO2 solubility in volatile solvents at 298.15 K. J. Chem. Eng. Data 2011, 56, 1565–1572. [Google Scholar] [CrossRef]
| Component | Crude Oil A | Crude Oil B | ||
|---|---|---|---|---|
| Mole% | Weight% | Mole% | Weight% | |
| Hydrogen sulfide | 0.00 | 0.00 | 0.00 | 0.00 |
| Carbon dioxide | 0.09 | 0.03 | 0.38 | 0.10 |
| Nitrogen | 0.01 | 0.00 | 0.47 | 0.08 |
| Methane | 17.43 | 2.54 | 11.28 | 1.14 |
| Ethane | 2.50 | 0.68 | 0.16 | 0.03 |
| Propane | 2.83 | 1.13 | 0.25 | 0.07 |
| Iso-Butane | 1.20 | 0.63 | 0.18 | 0.07 |
| n-Butane | 1.96 | 1.03 | 0.44 | 0.16 |
| Iso-Pentane | 1.68 | 1.10 | 2.32 | 1.05 |
| n-Pentane | 1.32 | 0.86 | 2.63 | 1.19 |
| Hexanes | 2.72 | 2.12 | 13.27 | 7.17 |
| Heptane plus | 68.26 | 89.88 | 68.62 | 88.94 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Solvents | Alcohols | Ketone | |
|---|---|---|---|
| Methanol | Ethanol | Acetone | |
| Molecular weight (g/mol) | 32.042 | 46.069 | 58.079 |
| Boiling point (°C) 1 | 64.70 | 78.20 | 56.08 |
| Density (liquid; kg/m3) 1 | 786.3 | 785.3 | 784.5 |
| Critical pressure (bar) | 82.2 | 62.5 | 46.9 |
| Critical temperature (°C) | 240.2 | 240.8 | 235.0 |
| Triple point pressure (bar) | 1.86 × 10−6 | 4.3 × 10−9 | 2.33 × 10−5 |
| Triple point temperature (°C) | −97.7 | −123.15 | −94.7 |
| Parameter, Unit | Value |
|---|---|
| Cell inner diameter (cm) | 3.0 |
| Cell height (cm) | 6.0 |
| Wall thickness of cell (cm) | 1.6 |
| Needle inner diameter (cm) | approximately 0.9 |
| Needle length (cm) | 5 |
| Sapphire thickness (cm) | 1 |
| Sapphire diameter (cm) | 3 |
| Cell working pressure (psi) | 3000 |
| Cell working temperature (°C) | 300 |
| Crude Oil A | Additive Volume: 5 cm3 | Additive Volume: 15 cm3 | ||
|---|---|---|---|---|
| MMP at 60 °C (psi) | MMP at 80 °C (psi) | MMP at 60 °C (psi) | MMP at 80 °C (psi) | |
| Pure CO2 | 1767 | 2134 | 1767 | 2134 |
| CO2 + Methanol | 1699 | 1997 | N/A | N/A |
| CO2 + Ethanol | 1625 | 1943 | 1578 | 1841 |
| CO2 + Acetone | 1838 | 2108 | 1461 | 1732 |
| Crude Oil B | Additive Volume: 5 cm3 | Additive Volume: 15 cm3 | ||
|---|---|---|---|---|
| MMP at 60 °C (psi) | MMP at 80 °C (psi) | MMP at 60 °C (psi) | MMP at 80 °C (psi) | |
| Pure CO2 | 1901 | 2273 | 1901 | 2273 |
| CO2 + Methanol | 1851 | 2204 | 1737 | 2026 |
| CO2 + Ethanol | 1861 | 2297 | 1743 | 1922 |
| CO2 + Acetone | 1879 | 2232 | 1506 | 1818 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novriansyah, A.; Bae, W.; Park, C.; Permadi, A.K.; Sri Riswati, S. Ketone Solvent to Reduce the Minimum Miscibility Pressure for CO2 Flooding at the South Sumatra Basin, Indonesia. Processes 2020, 8, 360. https://doi.org/10.3390/pr8030360
Novriansyah A, Bae W, Park C, Permadi AK, Sri Riswati S. Ketone Solvent to Reduce the Minimum Miscibility Pressure for CO2 Flooding at the South Sumatra Basin, Indonesia. Processes. 2020; 8(3):360. https://doi.org/10.3390/pr8030360
Chicago/Turabian StyleNovriansyah, Adi, Wisup Bae, Changhyup Park, Asep K. Permadi, and Shabrina Sri Riswati. 2020. "Ketone Solvent to Reduce the Minimum Miscibility Pressure for CO2 Flooding at the South Sumatra Basin, Indonesia" Processes 8, no. 3: 360. https://doi.org/10.3390/pr8030360
APA StyleNovriansyah, A., Bae, W., Park, C., Permadi, A. K., & Sri Riswati, S. (2020). Ketone Solvent to Reduce the Minimum Miscibility Pressure for CO2 Flooding at the South Sumatra Basin, Indonesia. Processes, 8(3), 360. https://doi.org/10.3390/pr8030360






