Data-Driven Modelling of the Complex Interaction between Flocculant Properties and Floc Size and Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flocculation Process Monitoring
2.3. The Statistical Design of Experiments and Multivariate Data Analysis
3. Results and Discussion
- Case 1: The prediction of the SE for copolymers.
- Case 2: The prediction of the d0.5 for copolymers.
- Case 3: The prediction of the SE for terpolymers.
- Case 4: The prediction of the d0.5 for terpolymers.
- Case 5: The prediction of the SE for copolymers and terpolymers.
- Case 6: The prediction of the d0.5 for copolymers and terpolymers.
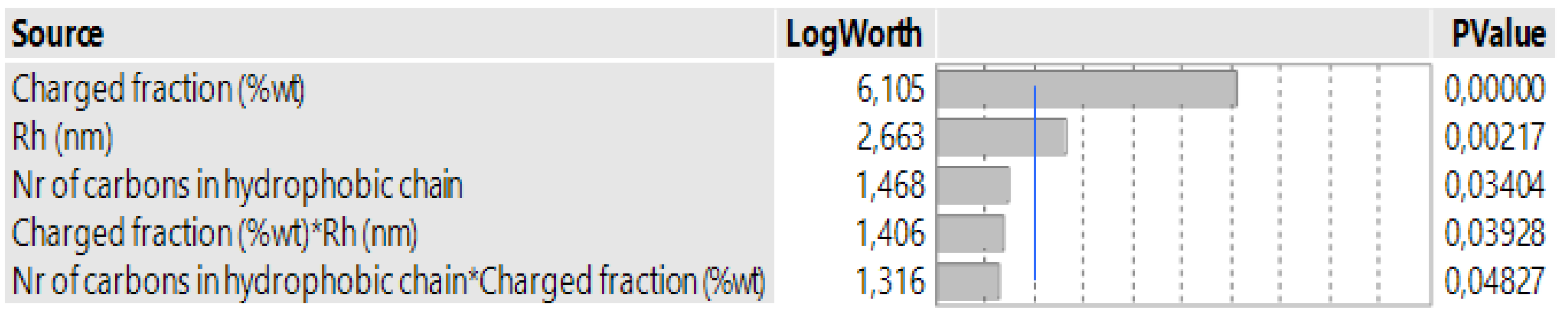
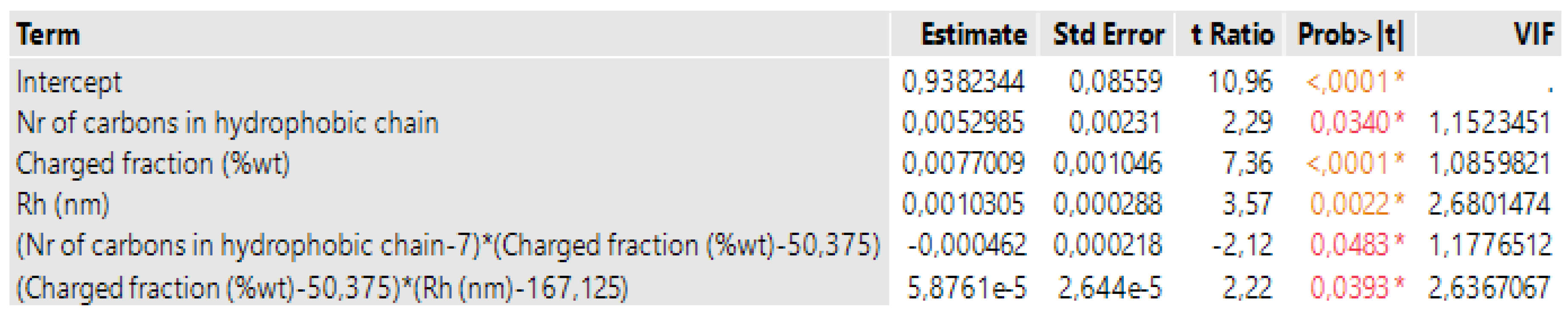
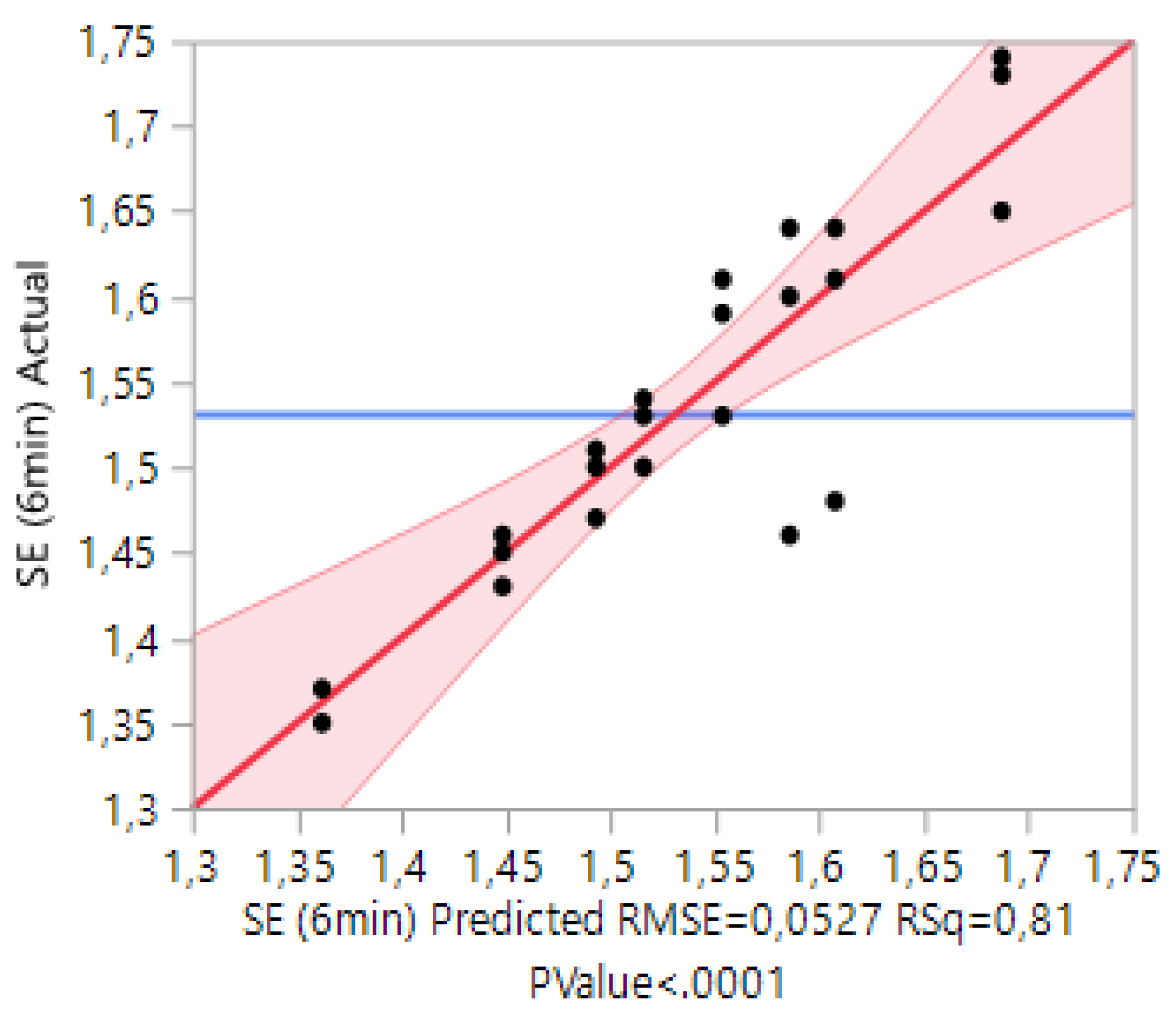
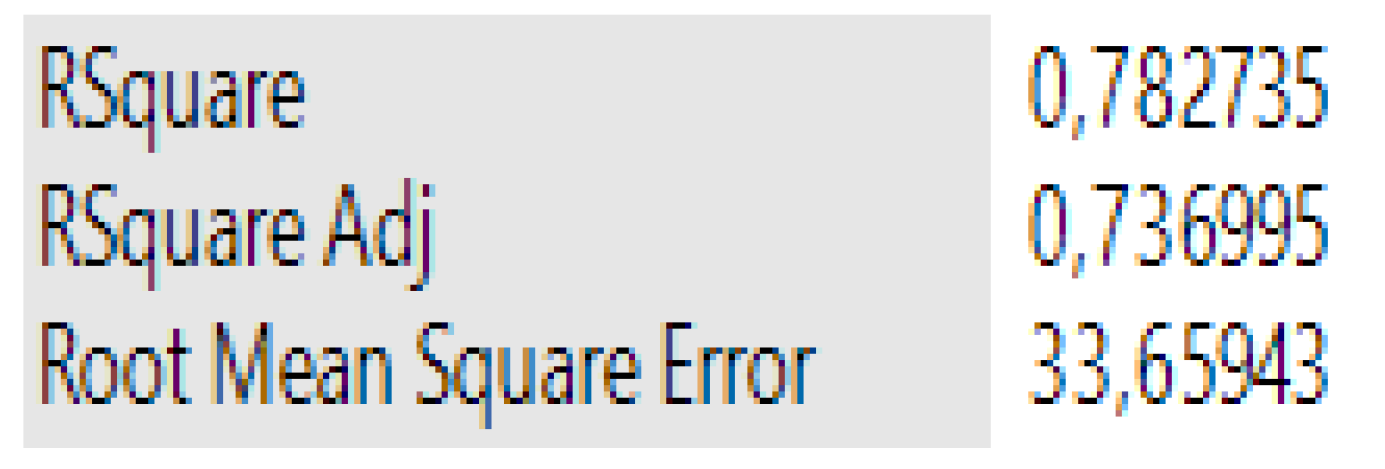
3.1. Case 1: The Prediction of the SE for Copolymers
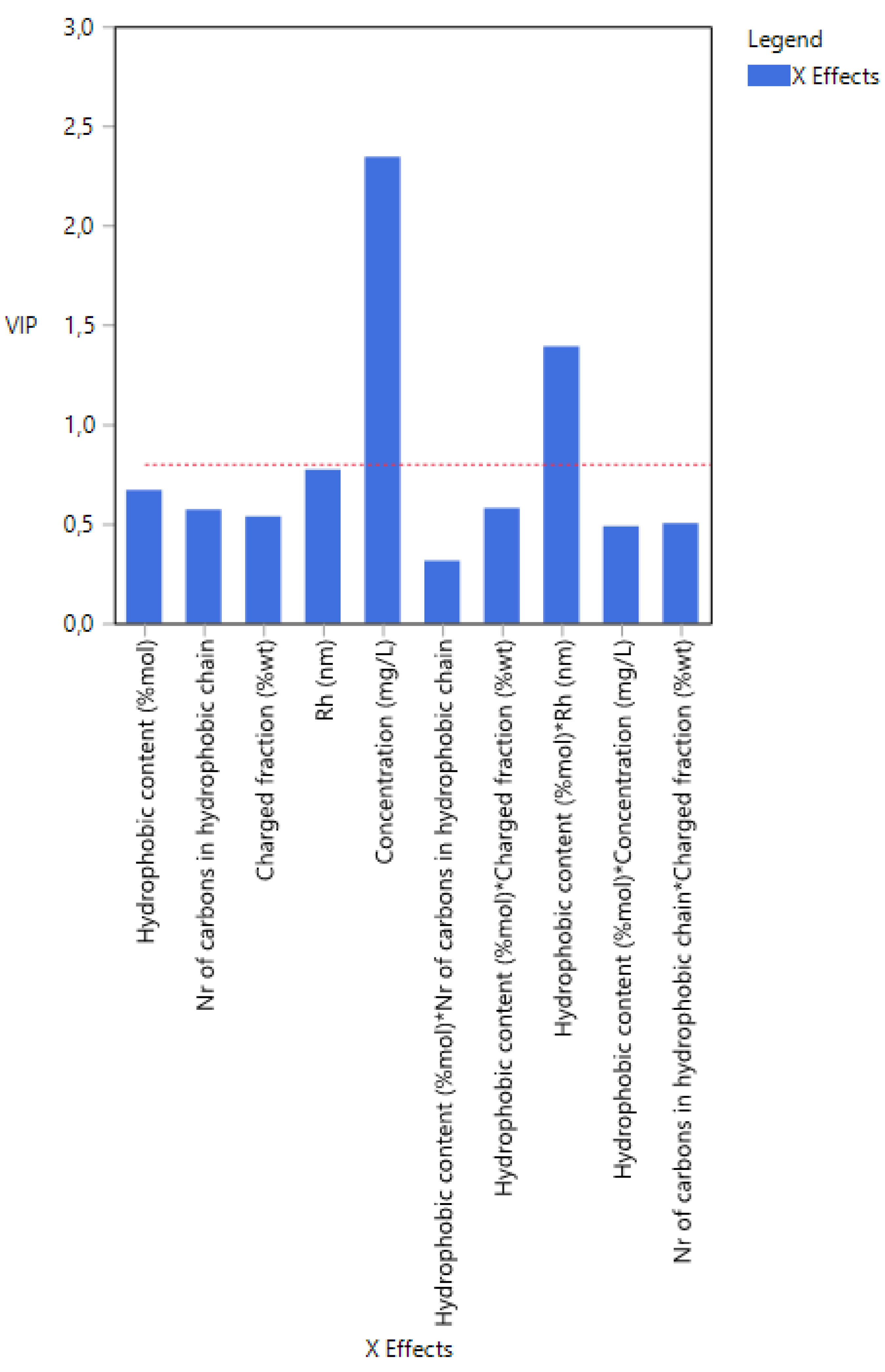
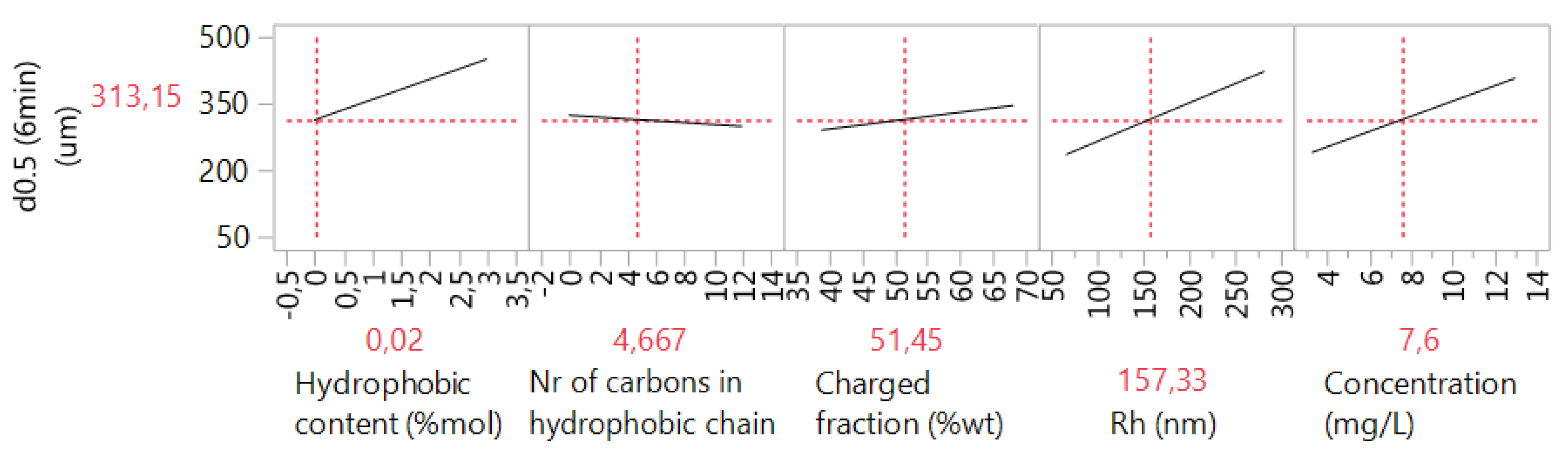
3.2. Case 2: The Prediction of the d0.5 for Copolymers
3.3. Case 3: The Prediction of the SE for Terpolymers
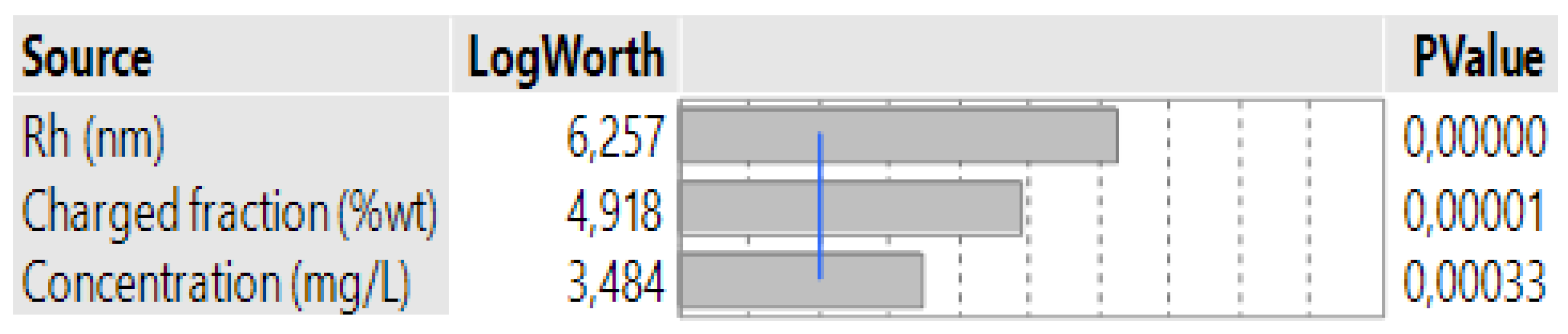
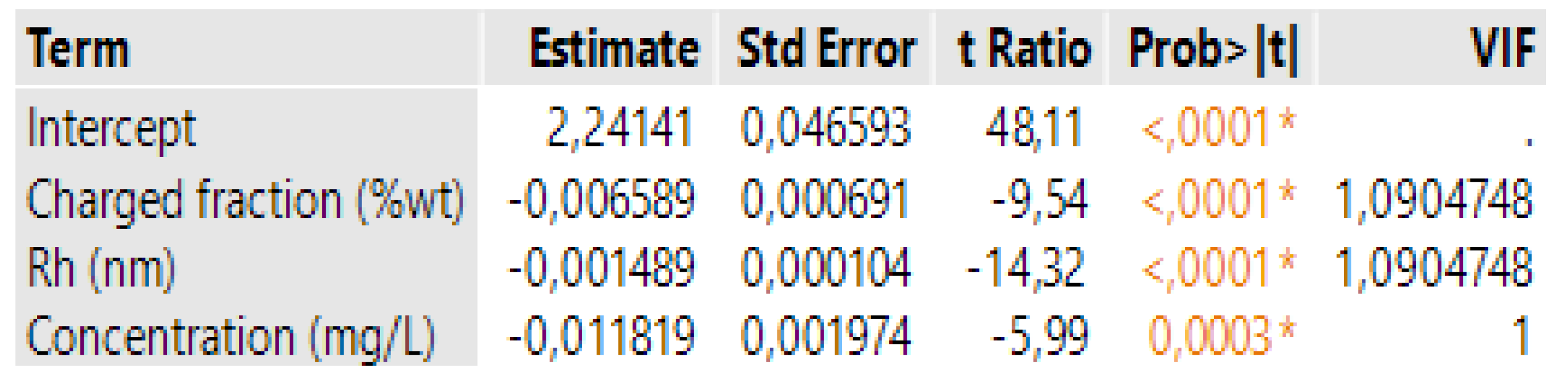
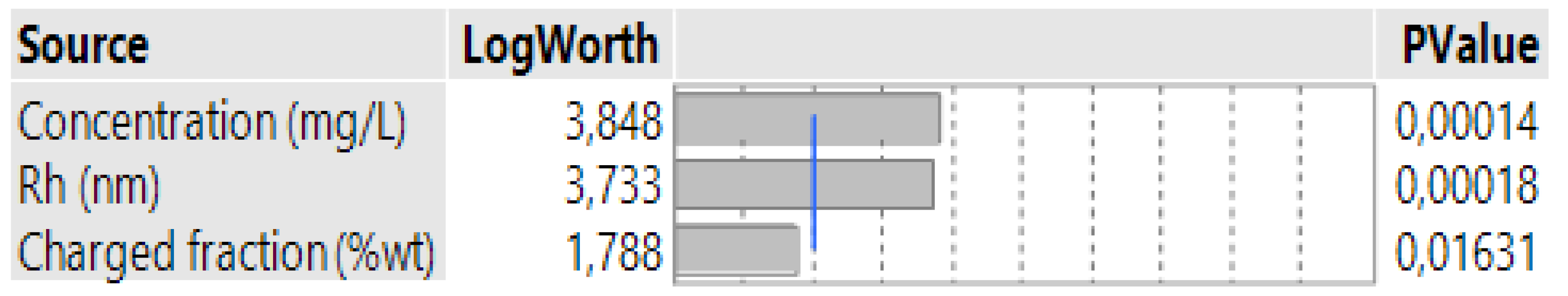
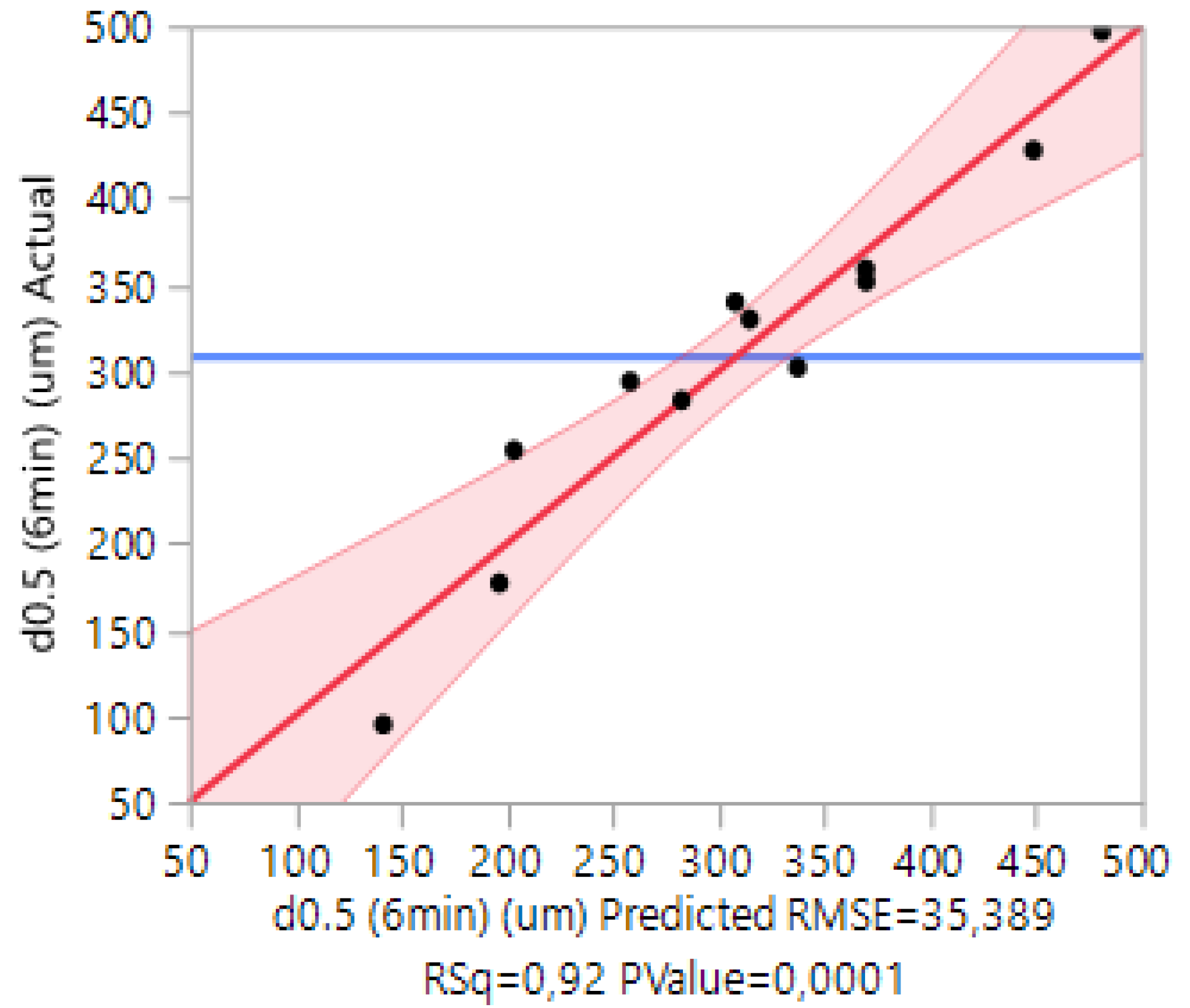
3.4. Case 4: The Prediction of the d0.5 for Terpolymers
3.5. Case 5: The Prediction of the SE for Copolymers and Terpolymers
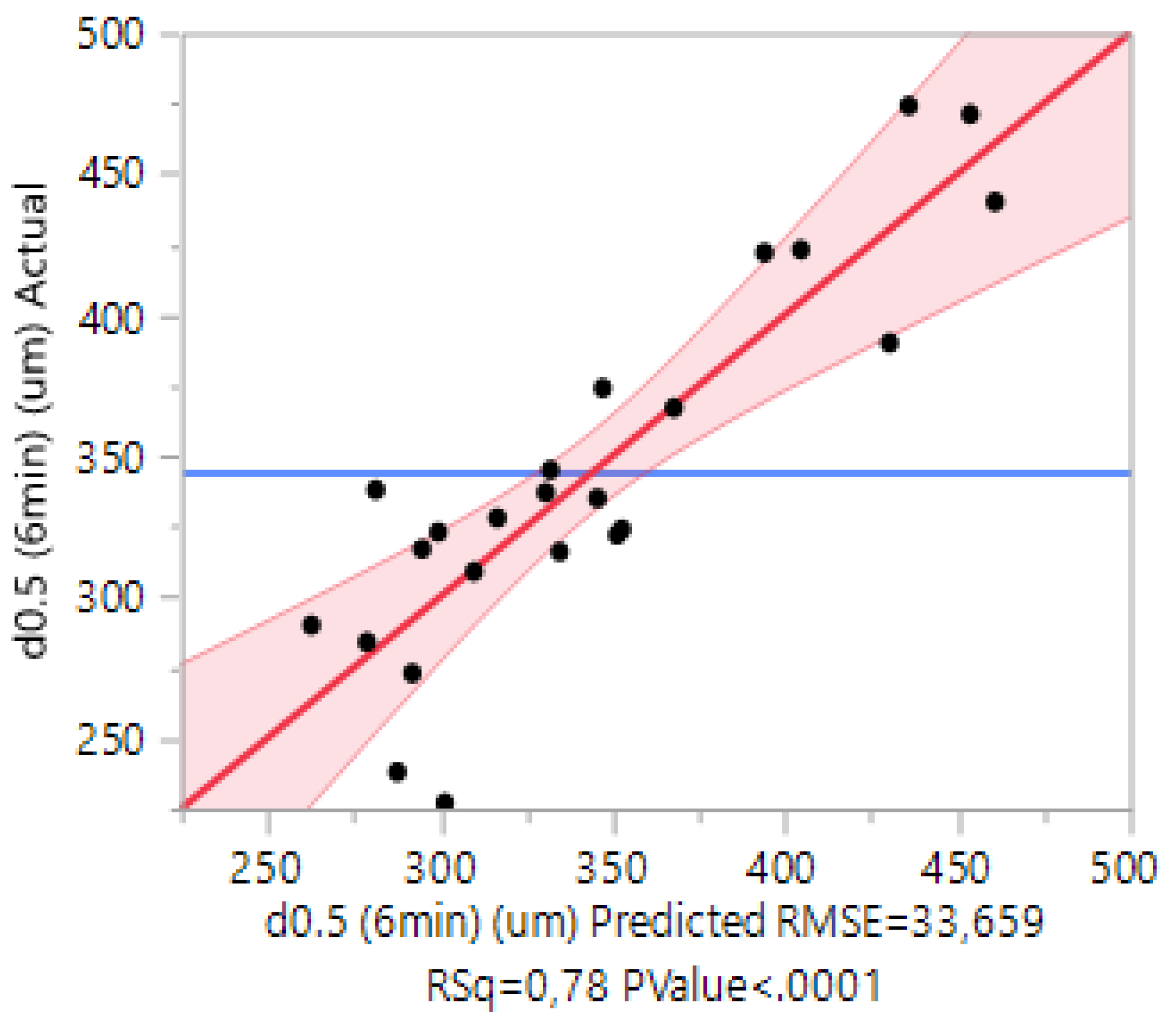
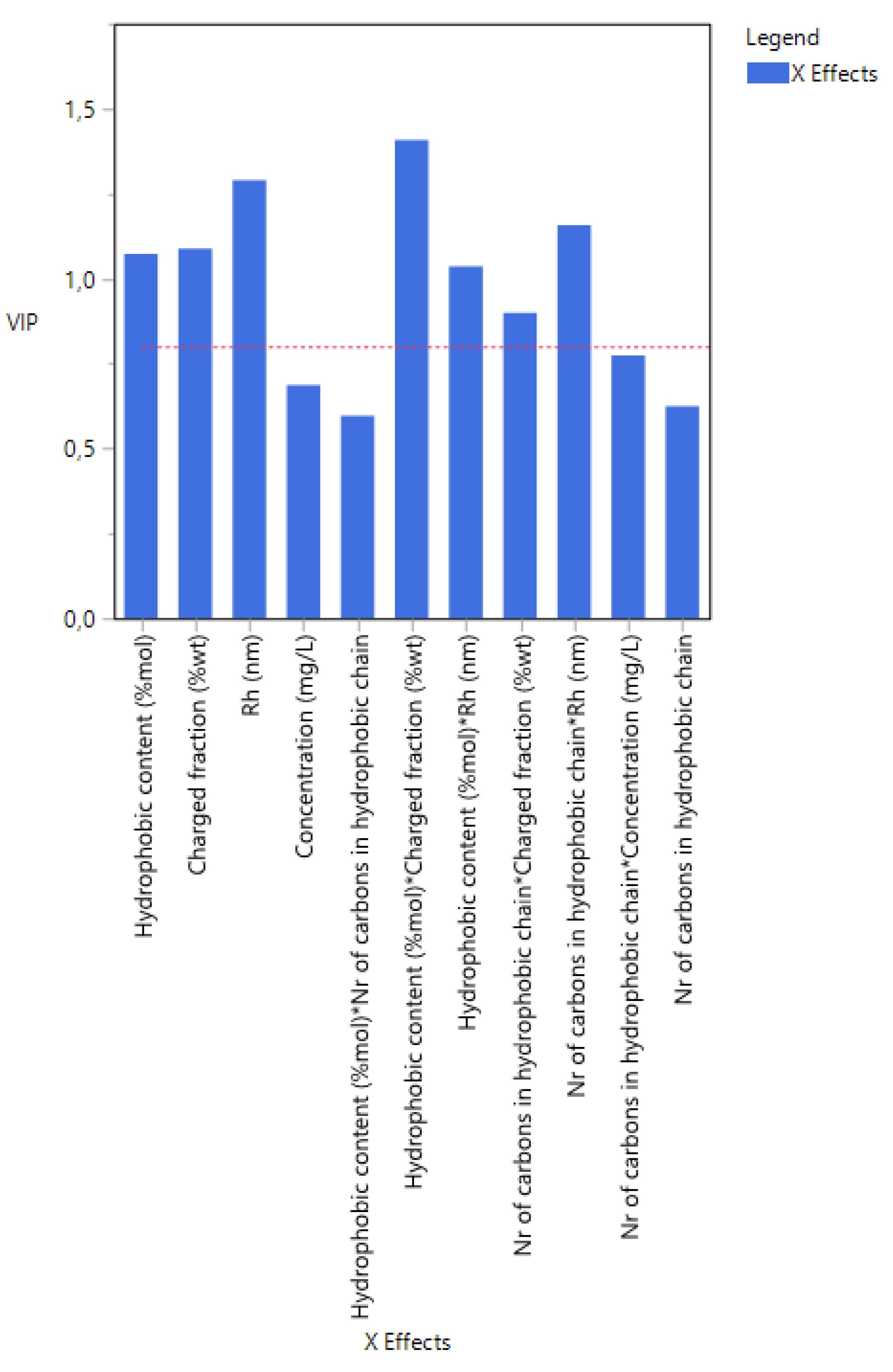
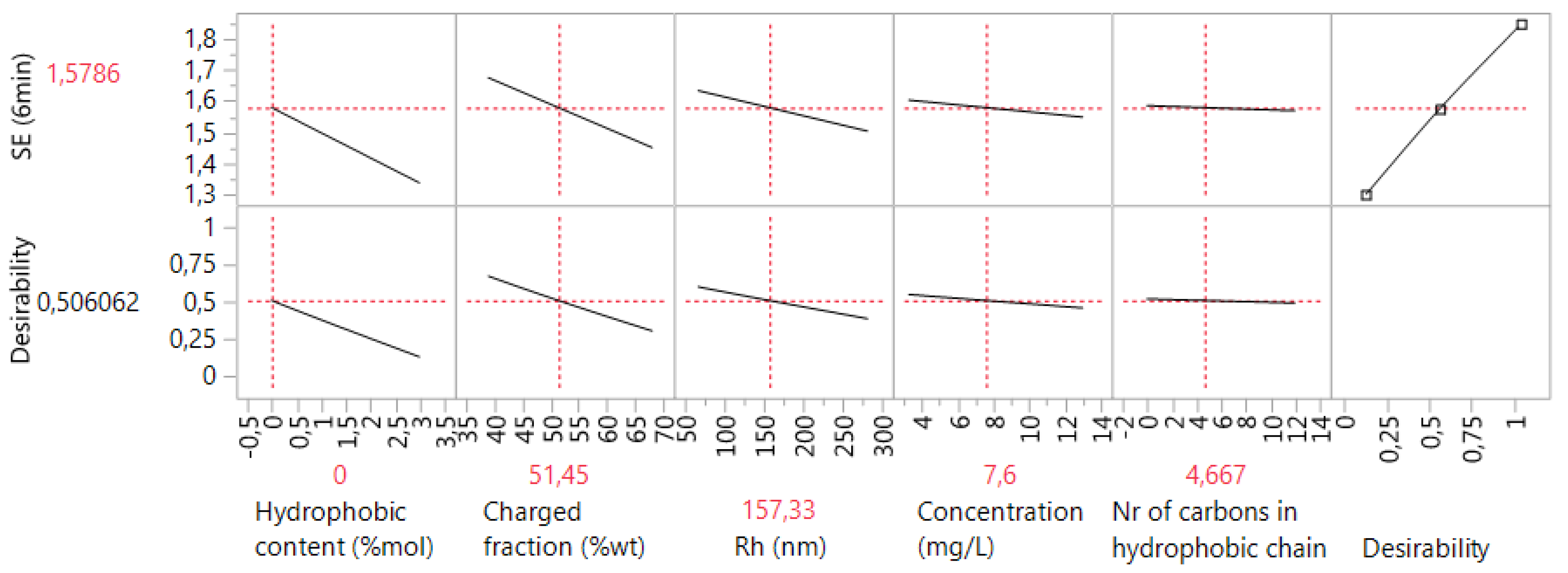
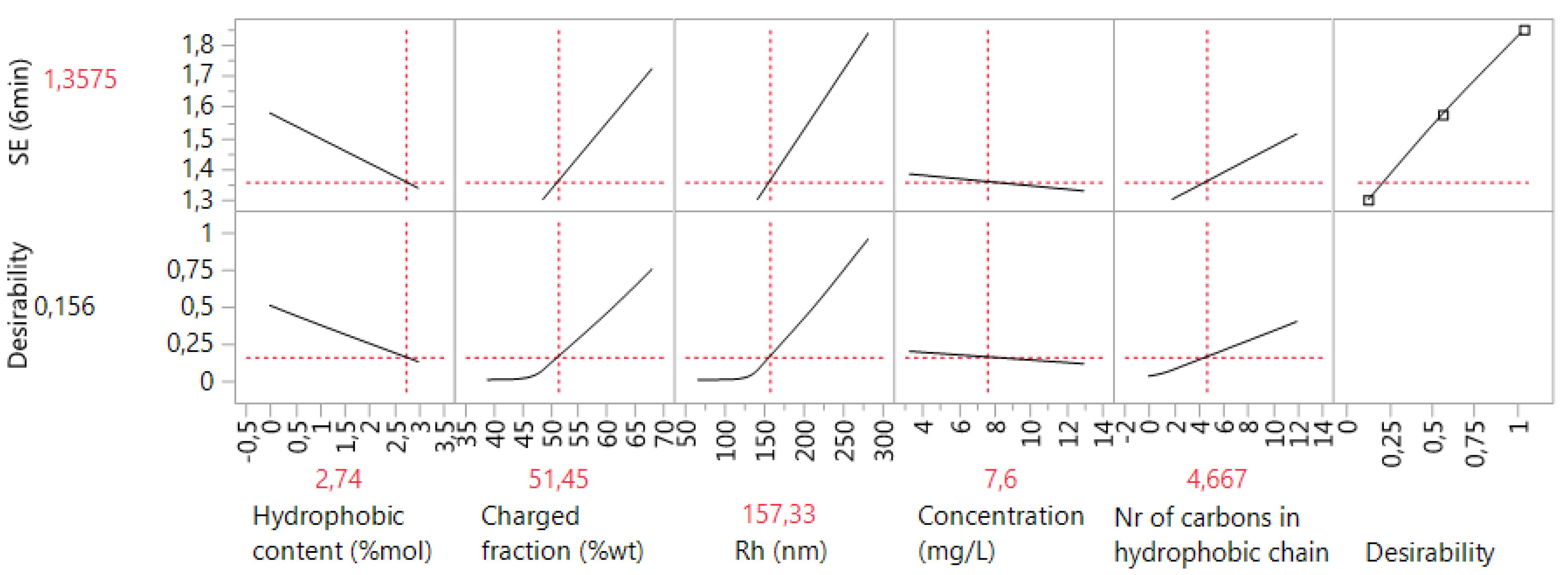
3.6. Case 6: The Prediction of the d0.5 for Copolymers and Terpolymers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jarvis, P.; Parsons, S.A.; Henderson, R.; Nixson, N.; Jefferson, B. The practical application of fractal dimension in water treatment practice–the impact of polymer dosing. Sep. Sci. Technol. 2008, 43, 1785–1797. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Kleimann, J.; Gehin-Delval, C.; Auweter, H.; Borkovec, M. Super-stoichiometric charge neutralization in particle-polyelectrolyte systems. Langmuir 2005, 21, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Biggs, S.; Habgood, M.; Jameson, G.J.; Yan, Y. Aggregate structures formed via a bridging flocculation mechanism. Chem. Eng. J. 2000, 80, 13–22. [Google Scholar] [CrossRef]
- Ahmad, A.; Wong, S.; Teng, T.; Zuhairi, A. Improvement of alum and PACl coagulation by polyacrylamides (PAMs) for the treatment of pulp and paper mill wastewater. Chem. Eng. J. 2008, 137, 510–517. [Google Scholar] [CrossRef]
- Caskey, J.A.; Primus, R.J. The effect of anionic polyacrylamide molecular conformation and configuration on flocculation effectiveness. Environ. Prog. 1986, 5, 98–103. [Google Scholar] [CrossRef]
- Blanco, A.; Fuente, E.; Negro, C.; Tijero, J. Flocculation monitoring: Focused beam reflectance measurement as a measurement tool. Can. J. Chem. Eng. 2008, 80, 1–7. [Google Scholar] [CrossRef]
- Eriksson, L.; Alm, B.; Stenius, P. Formation and structure of polystyrene latex aggregates obtained by flocculation with cationic polyelectrolytes. Colloids Surfaces A Physicochem. Eng. Asp. 1993, 70, 47–60. [Google Scholar] [CrossRef]
- Hadjivassilis, I.; Gajdos, S.; Vanco, D.; Nicolaou, M. Treatment of wastewater from the potato chips and snacks manufacturing industry. Water Sci. Technol. 1997, 36, 329–335. [Google Scholar] [CrossRef]
- Haydar, A.H.S.; Hussain, G.; Nadeem, O.; Haider, H.; Bari, A.J. Performance evaluation of anaerobic-aerobic treatment for the wastewater of potato processing industry: A case study of a local chips factory. Pakistan J. Eng. Appl. Sci. 2014, 14, 27–37. [Google Scholar]
- Kobya, M.; Hiz, H.; Senturk, E.; Aydiner, C.; Demirbas, E. Treatment of potato chips manufacturing wastewater by electrocoagulation. Desalination 2006, 190, 201–211. [Google Scholar] [CrossRef]
- Krzemińska, D.; Neczaj, E.; Borowski, G. Advanced oxidation processes for food industrial wastewater decontamination. J. Ecol. Eng. 2015, 16, 61–71. [Google Scholar] [CrossRef]
- Wu, D.; Yang, Z.; Wang, W.; Tian, G.; Xu, S.; Sims, A. Ozonation as an advanced oxidant in treatment of bamboo industry wastewater. Chemosphere 2012, 88, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Raman, A.A.A.; Wan Daud, W.M.A. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Lourenço, A.; Arnold, J.; Cayre, O.J.; Rasteiro, M.G. Flocculation Treatment of an Industrial Effluent: Performance Assessment by Laser Diffraction Spectroscopy. Ind. Eng. Chem. Res. 2018, 57, 2628–2637. [Google Scholar] [CrossRef]
- Liao, J.Y.H.; Selomulya, C.; Bushell, G.; Bickert, G.; Amal, R. On Different Approaches to Estimate the Mass Fractal Dimension of Coal Aggregates. Part. Part. Syst. Charact. 2005, 22, 299–309. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: Hoboken, NY, USA, 2012. [Google Scholar]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hamilton, J. Time Series Analysis; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Warne, M.A.; Lenz, E.M.; Osborn, D.; Weeks, J.M.; Nicholson, J.K. Comparative biochemistry and short-term starvation effects on the earthworms Eisenia veneta and Lumbricus terrestris studied by 1H NMR spectroscopy and pattern recognition. Soil Biol. Biochem. 2001, 33, 1171–1180. [Google Scholar] [CrossRef]
- Lourenço, A.; Arnold, J.; Gamelas, J.A.F.; Cayre, O.J.; Rasteiro, M.G. Anionic Polyelectrolytes Synthesized in an Aromatic-Free-Oils Process for Application as Flocculants in Dairy-Industry-Effluent Treatment. Ind. Eng. Chem. Res. 2018, 57, 16884–16896. [Google Scholar] [CrossRef]








| Polymer Code | AAm Ratio (wt %)/(mol %) | Na-AMPS Ratio (wt %)/(mol %) | Hydro-Phobic Monomer | Ratio (wt %) | Ratio (mol %) | Organic Phase for Polymerization | ||
|---|---|---|---|---|---|---|---|---|
| 50AC | 50.0 | 74.0 | 50.0 | 26.0 | - | - | - | Carnation |
| 80AC | 20.0 | 42.0 | 80.0 | 58.0 | - | - | - | Carnation |
| 50AP | 50.0 | 74.0 | 50.0 | 26.0 | - | - | - | Puresyn4 |
| 80AP | 20.0 | 42.0 | 80.0 | 58.0 | - | - | - | Puresyn4 |
| 50A1EC | 49.4 | 74.0 | 49.4 | 25.0 | EA | 1.2 | 1.0 | Carnation |
| 50A3EC | 48.5 | 72.0 | 48.5 | 25.0 | EA | 3.0 | 3.0 | Carnation |
| 80A1EC | 19.7 | 42.0 | 79.7 | 57.0 | EA | 0.6 | 1.0 | Carnation |
| 80A3EC | 19.0 | 40.0 | 79.0 | 57.0 | EA | 2.0 | 3.0 | Carnation |
| 50A1LC | 48.5 | 74.0 | 48.5 | 25.0 | LMA | 3.0 | 1.0 | Carnation |
| 50A3LC | 47.0 | 73.0 | 47.0 | 25.0 | LMA | 6.0 | 3.0 | Carnation |
| 80A1LC | 19.0 | 41.0 | 79.0 | 58.0 | LMA | 2.0 | 1.0 | Carnation |
| 80A3LC | 17.5 | 39.0 | 77.5 | 58.0 | LMA | 5.0 | 3.0 | Carnation |
| 50A1SC | 48.0 | 74.0 | 48.0 | 25.0 | SMA | 4.0 | 1.0 | Carnation |
| 50A3SC | 46.0 | 72.0 | 46.0 | 25.0 | SMA | 8.0 | 3.0 | Carnation |
| 80A1SC | 19.0 | 41.0 | 79.0 | 58.0 | SMA | 2.0 | 1.0 | Carnation |
| 80A3SC | 17.0 | 38.0 | 77.0 | 59.0 | SMA | 6.0 | 3.0 | Carnation |
| Polymer Code | Hydrophobic Content (mol %) | Nr of Carbons in Hydrophobic Chain | Measured Charged Fraction (wt %) | Rh (nm) | Concentration (mg/L) | SE | d(0.5) (µm) |
|---|---|---|---|---|---|---|---|
| 50AC | 0 | - | 41.5 | 67 ± 2 | 3.3 | 1.81 | 95 |
| 50AC | 0 | - | 41.5 | 67 ± 2 | 6.5 | 1.84 | 177 |
| 50AC | 0 | - | 41.5 | 67 ± 2 | 13 | 1.69 | 340 |
| 80AC | 0 | - | 62.9 | 72 ± 1 | 3.3 | 1.68 | 254 |
| 80AC | 0 | - | 62.9 | 72 ± 1 | 6.5 | 1.62 | 294 |
| 80AC | 0 | - | 62.9 | 72 ± 1 | 13 | 1.58 | 352 |
| 50AP | 0 | - | 41.9 | 265 ± 37 | 3.3 | 1.53 | 330 |
| 50AP | 0 | - | 41.9 | 265 ± 37 | 6.5 | 1.52 | 359 |
| 50AP | 0 | - | 41.9 | 265 ± 37 | 13 | 1.39 | 497 |
| 80AP | 0 | - | 68.1 | 147 ± 4 | 3.3 | 1.52 | 283 |
| 80AP | 0 | - | 68.1 | 147 ± 4 | 6.5 | 1.5 | 302 |
| 80AP | 0 | - | 68.1 | 147 ± 4 | 13 | 1.44 | 428 |
| 50A1EC | 1 | 2 | 39.5 | 70 ± 1 | 3.3 | 1.37 | 328 |
| 50A1EC | 1 | 2 | 39.5 | 70 ± 1 | 6.5 | 1.37 | 335 |
| 50A1EC | 1 | 2 | 39.5 | 70 ± 1 | 13 | 1.35 | 423 |
| 50A3EC | 3 | 2 | 39.7 | 282 ± 32 | 3.3 | 1.46 | 290 |
| 50A3EC | 3 | 2 | 39.7 | 282 ± 32 | 6.5 | 1.45 | 273 |
| 50A3EC | 3 | 2 | 39.7 | 282 ± 32 | 13 | 1.43 | 322 |
| 80A1EC | 1 | 2 | 62.2 | 143 ± 10 | 3.3 | 1.6 | 317 |
| 80A1EC | 1 | 2 | 62.2 | 143 ± 10 | 6.5 | 1.64 | 374 |
| 80A1EC | 1 | 2 | 62.2 | 143 ± 10 | 13 | 1.46 | 471 |
| 80A3EC | 3 | 2 | 61.6 | 206 ± 22 | 3.3 | 1.73 | 284 |
| 80A3EC | 3 | 2 | 61.6 | 206 ± 22 | 6.5 | 1.74 | 337 |
| 80A3EC | 3 | 2 | 61.6 | 206 ± 22 | 13 | 1.65 | 474 |
| 50A1LC | 1 | 12 | 41 | 129 ± 10 | 3.3 | 1.51 | 227 |
| 50A1LC | 1 | 12 | 41 | 129 ± 10 | 6.5 | 1.47 | 345 |
| 50A1LC | 1 | 12 | 41 | 129 ± 10 | 13 | 1.5 | 422 |
| 50A3LC | 3 | 12 | 39 | 209 ± 28 | 3.3 | 1.5 | 338 |
| 50A3LC | 3 | 12 | 39 | 209 ± 28 | 6.5 | 1.54 | 309 |
| 50A3LC | 3 | 12 | 39 | 209 ± 28 | 13 | 1.53 | 367 |
| 80A1LC | 1 | 12 | 57 | 174 ± 28 | 3.3 | 1.61 | 238 |
| 80A1LC | 1 | 12 | 57 | 174 ± 28 | 6.5 | 1.48 | 316 |
| 80A1LC | 1 | 12 | 57 | 174 ± 28 | 13 | 1.64 | 390 |
| 80A3LC | 3 | 12 | 63 | 124 ± 13 | 3.3 | 1.59 | 323 |
| 80A3LC | 3 | 12 | 63 | 124 ± 13 | 6.5 | 1.53 | 324 |
| 80A3LC | 3 | 12 | 63 | 124 ± 13 | 13 | 1.61 | 440 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço, A.; Reis, M.S.; Arnold, J.; Rasteiro, M.G. Data-Driven Modelling of the Complex Interaction between Flocculant Properties and Floc Size and Structure. Processes 2020, 8, 349. https://doi.org/10.3390/pr8030349
Lourenço A, Reis MS, Arnold J, Rasteiro MG. Data-Driven Modelling of the Complex Interaction between Flocculant Properties and Floc Size and Structure. Processes. 2020; 8(3):349. https://doi.org/10.3390/pr8030349
Chicago/Turabian StyleLourenço, Anita, Marco S. Reis, Julien Arnold, and Maria Graca Rasteiro. 2020. "Data-Driven Modelling of the Complex Interaction between Flocculant Properties and Floc Size and Structure" Processes 8, no. 3: 349. https://doi.org/10.3390/pr8030349
APA StyleLourenço, A., Reis, M. S., Arnold, J., & Rasteiro, M. G. (2020). Data-Driven Modelling of the Complex Interaction between Flocculant Properties and Floc Size and Structure. Processes, 8(3), 349. https://doi.org/10.3390/pr8030349







