Analysis of the Degradation Process of Alginate-Based Hydrogels in Artificial Urine for Use as a Bioresorbable Material in the Treatment of Urethral Injuries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
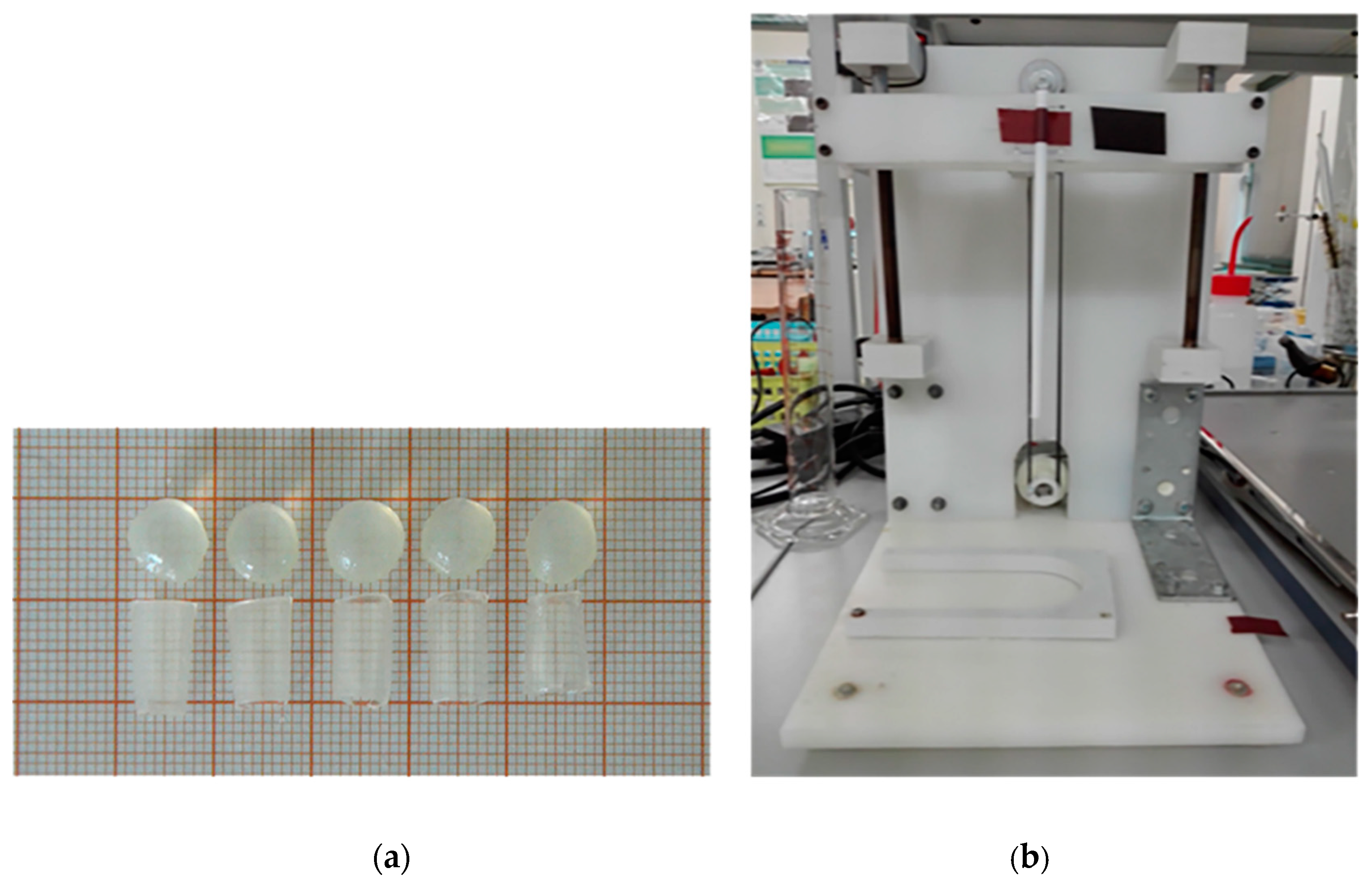
2.2. Samples Preparation
2.3. Degradation, Swelling, and Water Loss
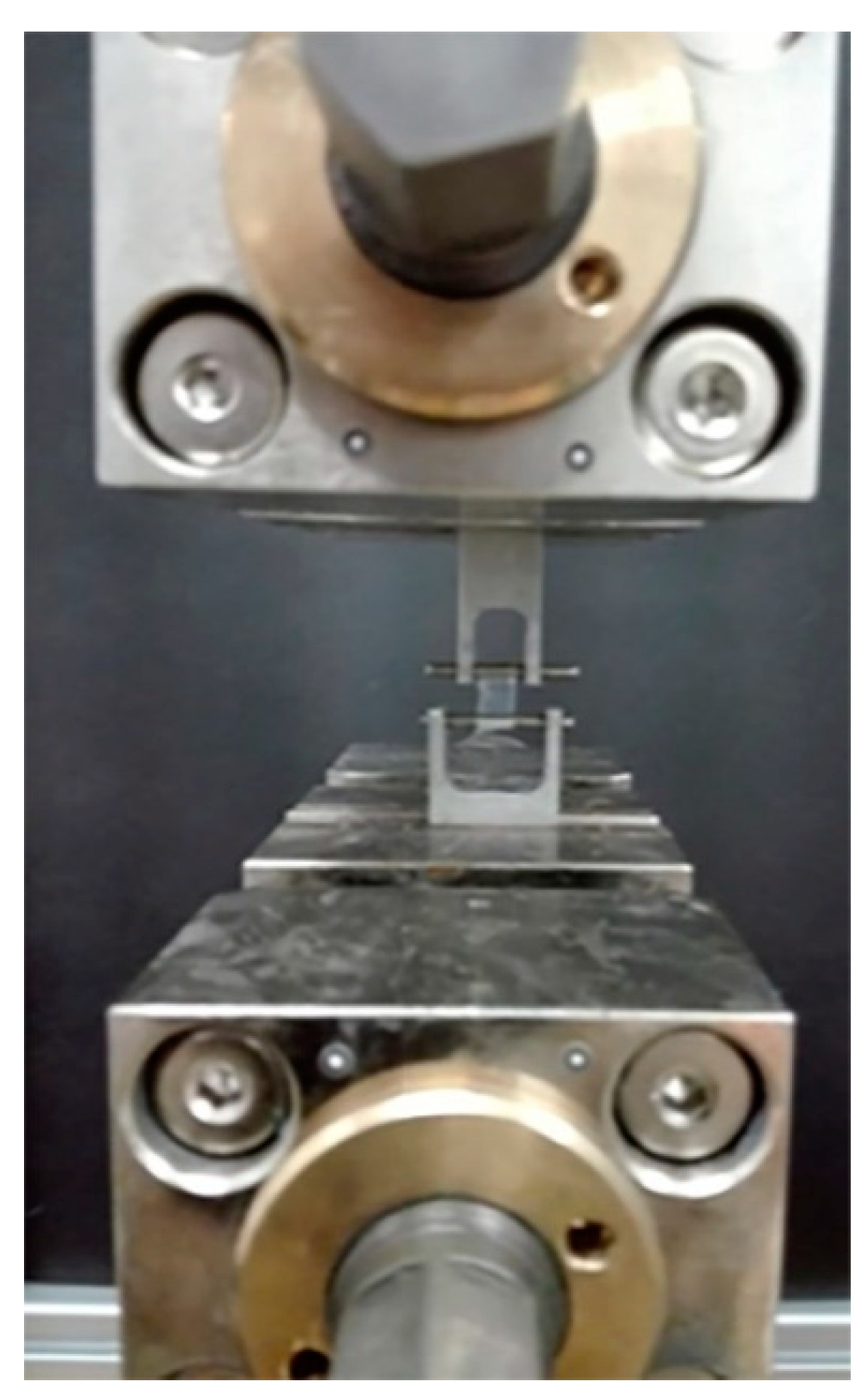
2.4. Examination of Mechanical Properties
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
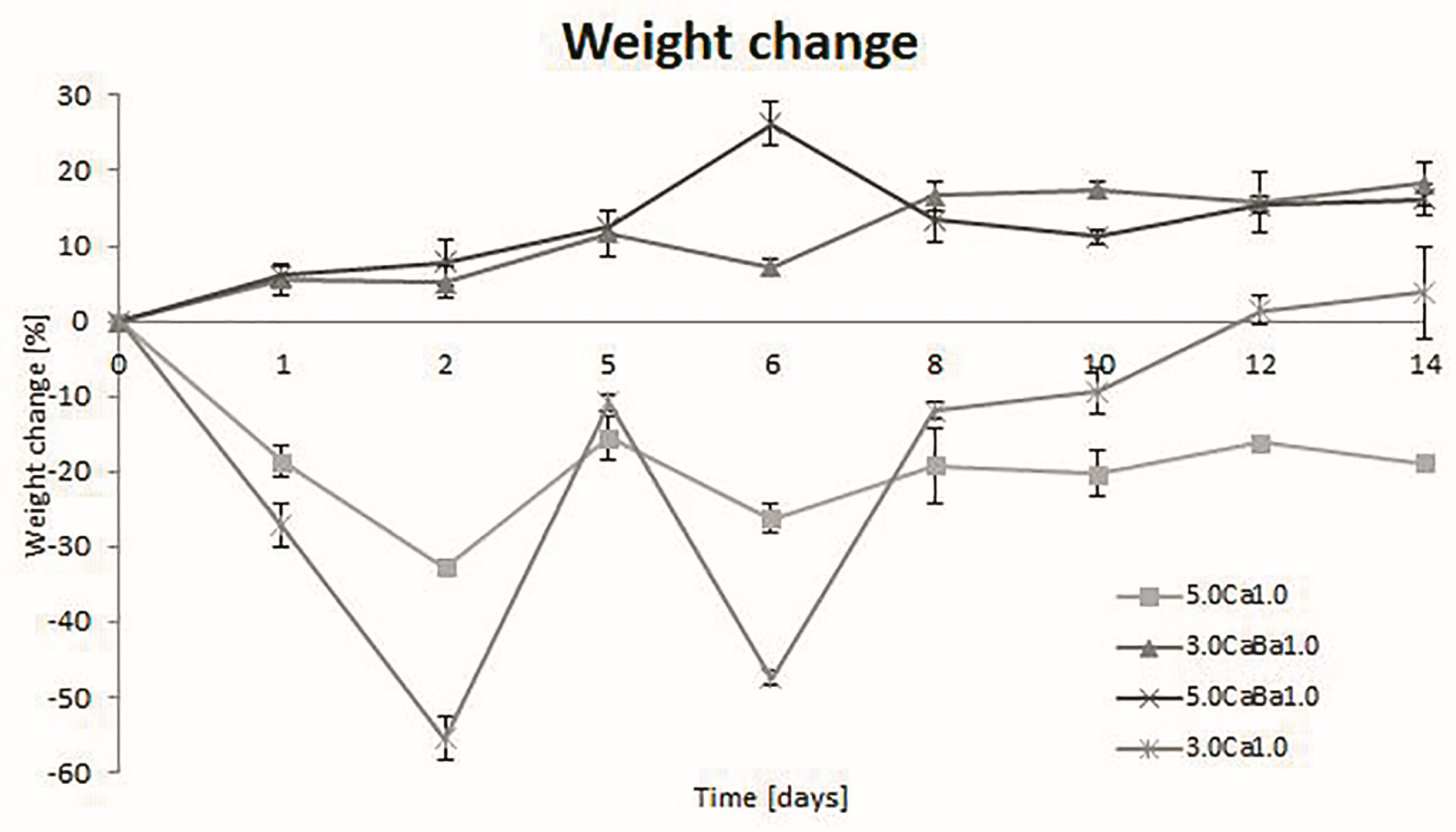
3.1. Degradation, Swelling, and Water Loss
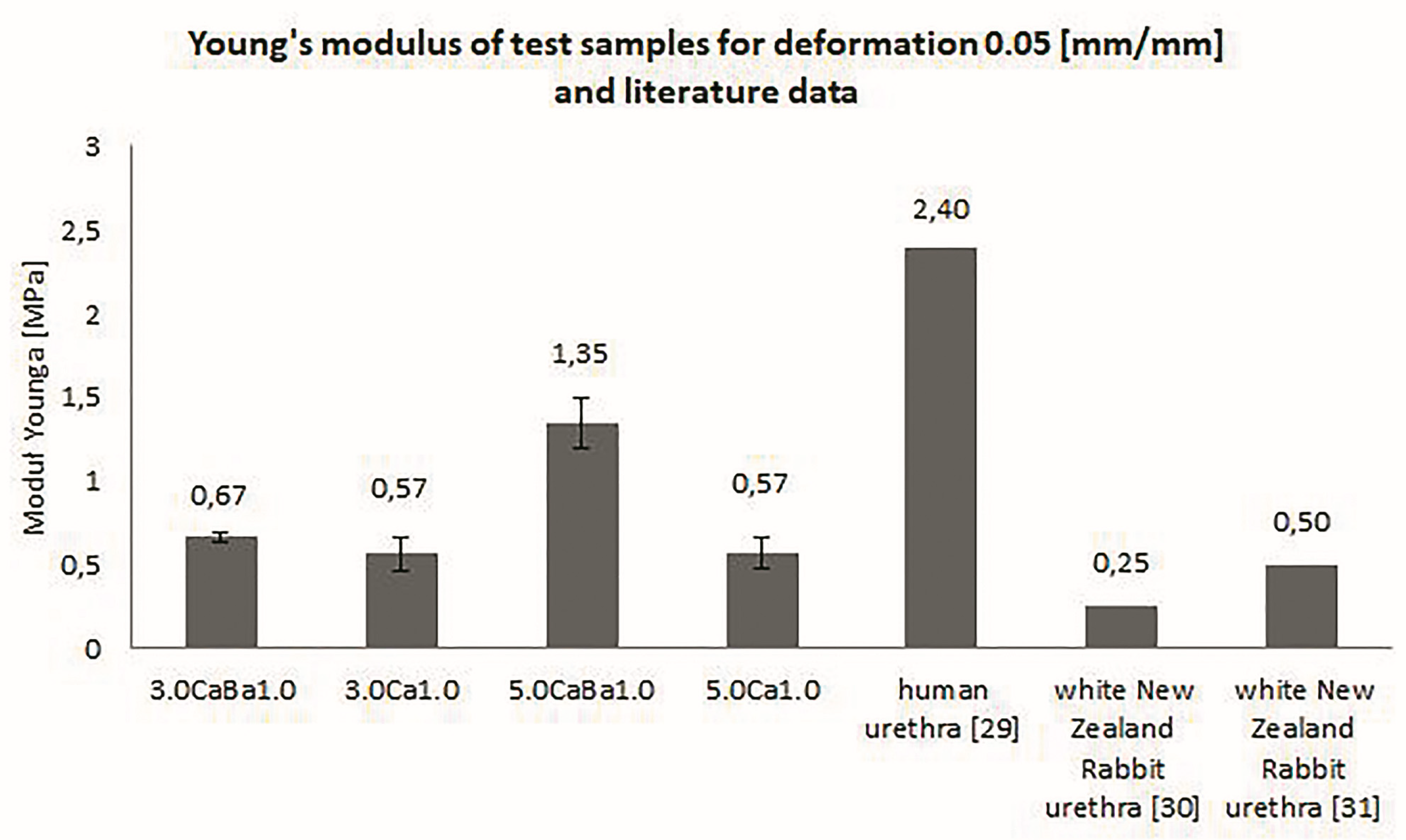
3.2. Mechanical Properties of Alginate Hydrogels
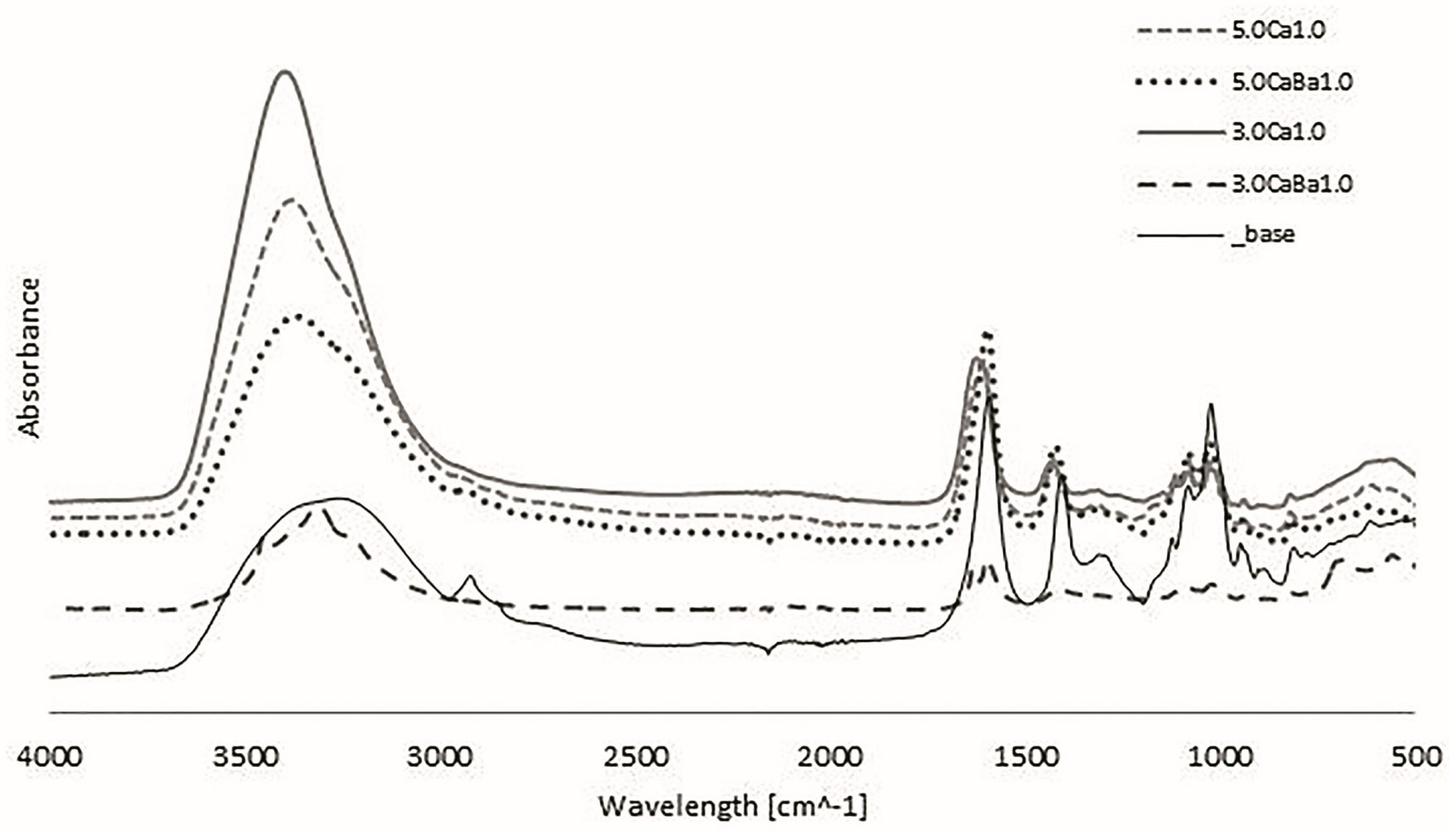
3.3. Fourier Transform Infrared Spectroscopy (FTIR-ATR)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saul, J.M.; Williams, D.F. 12-Hydrogels in Regenerative Medicine. In Handbook of Polymer Applications in Medicine and Medical Devices, 2nd ed.; Modjarrad, K., Ebnesajjad, S., Eds.; Elsevier Inc. William Andrew: San Diego, CA, USA, 2013; pp. 279–302. [Google Scholar]
- Aljohani, W.J.; Wenchao, L.; Ullah, M.W.; Zhang, X.; Yang, G. Application of Sodium Alginate Hydrogel. J. Biotech. Biochem. 2017, 3, 19–31. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Budama-Kilinc, Y.; Çakır-Koç, R.; Aslan, B.; Özkan, B.; Mutlu, H.; Üstün, E. Hydrogels in Regenerative Medicine. In Biomaterials in Regenerative Medicine; Dobrzanski, L.A., Ed.; IntechOpen Limited: London, UK, 2018; pp. 277–301. [Google Scholar]
- Sinno, H.; Prakash, S. Complements and wound healing cascade: An updated review. Plast. Surg. Int. 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Naseer, I.; Khan, A.; Asif, A.; Yar, M.; Haycock, J.; Rehman, I. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Inter. Mater. Rev. 2018, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019, 146, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, M.; Ramezani, M. Mechanical and tribological assessment of silica nanoparticle-alginate-polyacrylamide nanocomposite hydrogels as a cartilage replacement. J. Mech. Behav. Biomed. Mater. 2019, 95, 196–204. [Google Scholar] [CrossRef]
- Swieszkowski, W.; Ku, D.N.; Bersee, H.E.; Kurzydlowski, K.J. An elastic material for cartilage replacement in an arthritic shoulder joint. Biomaterials 2006, 27, 1534–1541. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 1–20. [Google Scholar] [CrossRef]
- Chen, J.; Irianto, J.; Inamdar, S.; Pravincumar, P.; Lee, D.A.; Bader, D.L.; Knight, M.M. Cell mechanics, structure, and function are regulated by the stiffness of the three-dimensional microenvironment. Biophys. J. 2012, 103, 1188–1197. [Google Scholar] [CrossRef] [Green Version]
- Summa, M.; Russo, D.; Penna, J.; Marganoli, N.; Bayer, J.S.; Bandlera, T.; Athanassion, A.; Bertorelli, R. A biocompatible sodium alginate/povidine iodine film enhances wound healing. Eur. J. Pharm Biopharm. 2018, 122, 17–24. [Google Scholar] [CrossRef]
- Marković, D.; Zarubica, A.; Stojković, N.; Vasić, M.; Cakić, M.; Nikolić, G. Alginates and similar exopolysaccharides in biomedical application and pharmacy: Controled delivery of drugs. Adv. Technol. 2016, 5, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Pawelska, A. Alginate-Based Hydrogels in Regenerative Medicine. In Alginates–Recent Uses of This Natural Polymer; Pereira, L., Ed.; IntechOpen Limited: London, UK, 2019; pp. 1–12. [Google Scholar]
- Bartkowiak-Jowsa, M.; Będziński, R.; Kozłowska, A.; Filipiak, J.; Pezowicz, C. Mechanical, rheological, fatigue, and degradation behavior of PLLA, PGLA and PDGLA as materials for vascular implants. Meccanica 2013, 48, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Gibas, I.; Janik, H. Review: Synthetic polymer hydrogels for biomedical applications. Chem. Chem. Technol. 2010, 4, 297–304. [Google Scholar]
- Barros, A.A.; Rita, A.; Duarte, C.; Pires, R.A.; Sampaio-Marques, B.; Ludovico, P.; Lima, E.; Mano, J.F.; Reis, R.L. Bioresorbable ureteral stents from natural origin polymers. J. Biomed. Mater. Res. Part B 2014, 1–10. [Google Scholar] [CrossRef]
- Barros, A.A.; Oliveira, C.; Lima, E.; Rita, A.; Duarte, C.; Reis, R.L. Gelatin-based biodegradable ureteral stents with enhanced mechanical properties. Appl. Mater. Today 2016, 5, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Bartkowiak-Jowsa, M.; Będziński, R.; Chłopek, J.; Filipiak, J.; Szaraniec, B. Comparative analysis of the deformation characteristics of biodegradable polymers considered as a material for vascular stents. Polymers 2010, 56, 224–231. [Google Scholar] [CrossRef]
- Kaczmarek-Pawelska, A.; Winiarczyk, K.; Mazurek, J. Alginate based hydrogel for tissue regeneration: Optimization, antibacterial activity and mechanical properties. J. Achiev. Mater. Manuf. Eng. 2017, 81, 35–40. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skijåk-Break, G. Effect of Ca2+, Ba2+, and Sr2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Thongboonkerd, V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal. Biochem. 2010, 402, 110–112. [Google Scholar] [CrossRef]
- Straccia, M.C.; Gomez d’Ayala, G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate Hydrogels Coated with Chitosan for Wound Dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, J.S.; Jorge, R.M.; Martins, P.S.; Soldi Mda, S.; Alves, O.L.; Patricio, B.; Mascarenhas, T.; Sartori, M.G.; Girao, M.J. Structural and thermal properties of polypropylene mesh used in treatment of stress urinary incontinence. Acta Bioeng. Biomech. 2009, 11, 27–33. [Google Scholar] [PubMed]
- Chen, L.; Shen, R.; Komasa, S.; Xue, Y.; Jin, B.; Hou, Y.; Okazaki, J.; Gao, J. Drug-Loadable Calcium Alginate Hydrogel System for Use in Oral Bone Tissue Repair. Int. J. Mol. Sci. 2017, 18, 989. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Laudano, M.A.; Seklehner, S.; Chughtai, B.; Lee, R.K. Image-based simulation of urethral distensibility and flow resistance as a function of pelvic floor anatomy. Neurol. Urodyn. 2015, 34, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Q.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Feng, C.; Xu, Y.M.; Fu, Q.; Zhu, W.D.; Cui, L.; Chen, J. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J. Biomed. Mater. Res. A 2010, 94A, 317–325. [Google Scholar] [CrossRef]
- Nastaj, J.; Przewlocka, A.; Rajkowska-Mysliwiec, M. Biosorption of Ni(II), Pb(II) and Zn(II) on calcium alginate beads: Equilibrium, kinetic and mechanism studies. Pol. J. Chem. Tech. 2016, 18, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, A.; Choubey, A.; Bajpai, A.K. Biosorption of chromium ions by calcium alginate nanoparticles. J. Chil. Chem. Soc. 2018, 63, 4077–4081. [Google Scholar] [CrossRef] [Green Version]
| Source | Chemical Reagent | Quantity in Grams |
|---|---|---|
| Sigma-Aldrich | Urea | 124.9 |
| Sigma-Aldrich | NaCl | 45.0 |
| CHEMPUR | NH4Cl | 12.85 |
| Avantor Performance Materials Poland S.A. | Na2SO4 | 15.0 |
| Avantor Performance Materials Poland S.A. | NaH2PO4 | 13.7 |
| Type of Crosslinker | Concentration of Crosslinker | 30 mg/mL Sodium Alginate | 50 mg/mL Sodium Alginate |
|---|---|---|---|
| CaCl2 | 1.0 M | 3.0Ca1.0 | 5.0Ca1.0 |
| CaCl2/BaCl2 | 3.0CaBa1.0 | 5.0CaBa1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurowiak, J.; Kaczmarek-Pawelska, A.; Mackiewicz, A.G.; Bedzinski, R. Analysis of the Degradation Process of Alginate-Based Hydrogels in Artificial Urine for Use as a Bioresorbable Material in the Treatment of Urethral Injuries. Processes 2020, 8, 304. https://doi.org/10.3390/pr8030304
Kurowiak J, Kaczmarek-Pawelska A, Mackiewicz AG, Bedzinski R. Analysis of the Degradation Process of Alginate-Based Hydrogels in Artificial Urine for Use as a Bioresorbable Material in the Treatment of Urethral Injuries. Processes. 2020; 8(3):304. https://doi.org/10.3390/pr8030304
Chicago/Turabian StyleKurowiak, Jagoda, Agnieszka Kaczmarek-Pawelska, Agnieszka G. Mackiewicz, and Romuald Bedzinski. 2020. "Analysis of the Degradation Process of Alginate-Based Hydrogels in Artificial Urine for Use as a Bioresorbable Material in the Treatment of Urethral Injuries" Processes 8, no. 3: 304. https://doi.org/10.3390/pr8030304
APA StyleKurowiak, J., Kaczmarek-Pawelska, A., Mackiewicz, A. G., & Bedzinski, R. (2020). Analysis of the Degradation Process of Alginate-Based Hydrogels in Artificial Urine for Use as a Bioresorbable Material in the Treatment of Urethral Injuries. Processes, 8(3), 304. https://doi.org/10.3390/pr8030304





