Abstract
Centella asiatica has been included in Thai traditional medicinal plants and recipes, as a well-established historical use as a vegetable and tonic. However, when applied in modern formulations, the progressive degradation of the plant pigments occurs, causing color-fading and color variation in the products. Depigmentation of the comminuted sample using supercritical carbon dioxide (scCO2) fluid extraction with a cosolvent was introduced as a pretreatment to solve the color-fading problem. The contents of compounds with known biological activities and the wound healing activities (antioxidant screening by DPPH and ABTS+ scavenging activities; cell migration assay; matrix metallopeptidase [MMP]-2 inhibition on human skin fibroblast; endothelial cell tube formation assay) of the C. asiatica leaf extracts obtained by conventional ethanolic extraction (CV) and pretreatment using scCO2 extraction, were determined. Total triterpenoids (madecassoside, asiaticoside B, asiaticoside, madecassic acid, terminolic acid and asiatic acid) and total triterpenoid glucosides (madecassoside, asiaticoside B and asiaticoside) were notably more abundant in the extract that had been pretreated using scCO2 than the extract obtained by CV. Moreover, the scCO2 pretreatment not only caused greater relative MMP-2 inhibition (58.48 ± 7.50% of the control), but also exhibited a higher cell migration (59.83 ± 1.85% of the initial) and number of vessels (18.25 ± 4.58) of angiogenesis in the wound healing process. Additionally, positive correlations were observed between the DPPH antioxidant activity and madecassoside content (r = 0.914, p < 0.01), as well as between the cell migration activity and asiaticoside content (r = 0.854, p < 0.05). It can be concluded that the scCO2 pretreatment of C. asiatica can eliminate color pigments from the extract and improve its in vitro wound healing activity.
1. Introduction
Wound healing is critical in maintaining the physical barrier function of the skin. It plays a pivotal role to protect the body from pathogens when the skin is damaged. The wound healing process involves three overlapping stages: inflammation, proliferation and remodeling [1]. During the inflammatory stage, edema and erythema result from the activation of inflammatory cytokines and the action of the immune cells, which are necessary for angiogenesis and fibroplasia. Cell migration occurs to protect against environmental pathogens, and local hemorrhaging is stopped by coagulation factors. In the second or proliferation stage, cells contract to close the wound, and angiogenesis is initiated, forming new blood vessels to deliver nutrients, fluid and oxygen to the cells in the wound area. Finally, the remodeling stage is the last recovery process. The stimulation of collagen fiber production in the cells is required to remodel the tissue for full recovery [2].
It has been evident for several years that matrix metallopeptidases (MMPs) are expressed throughout the wound healing process, although the mechanisms through which these proteinases function are only now being discovered. To date, it appears that almost all MMPs are positive regulators of these stages. However, it has been suggested that both MMP-2 and MMP-9 may have inhibitory effects upon cell proliferation [3], and so natural sources of bioactive compounds that can inhibit MMP-2 activity provide positive feedback regulation to induce cell proliferation.
Centella asiatica is a famous Thai traditional herb. In Thailand, C. asiatica aqueous leaf extract is popular as an everyday beverage because of its longevity effect. Several biological activities of the C. asiatica ethanolic extract have been reported, such as in vitro anticancer [4], antioxidant [5] and antimicrobial [6], besides in vitro and in vivo wound healing [7,8]. The C. asiatica leaf extract contains four triterpenes, including madecassoside, asiaticoside, madecassic acid and asiatic acid, which impact on wound healing activity [9]. An investigation into the effects of asiaticoside on human periodontal ligament cell proliferation, protein synthesis and osteogenic differentiation, revealed periodontal wound healing activity [10]. Moreover, C. asiatica extract can promote epithelium wound healing in rabbit corneal epithelial cells [11].
Conventional maceration is the most common approach to extract the compounds with known biological activities from medicinal plants. There is no prior report of the wound healing activity of the extract by the supercritical carbon dioxide (scCO2) extraction method. Supercritical fluids have been introduced as an alternative extraction method at low temperature for the preparation of plant extracts. At the critical point, supercritical fluids have the density of a liquid, but low viscosity with better flow property like a gas. Carbon dioxide (CO2) is a gas widely used to produce supercritical fluid because of its low critical temperature (Tc = 31.1 °C) and pressure (Pc = 73.8 bar), and provides high solvating power at the near-critical point [12].
Notably, scCO2 has been used for the substitution of organic solvent in extracting thermal-sensitive constituents, with the advantages of not only being environmentally friendly, nontoxic and nonflammable, but also inexpensive [13]. Although the maceration method is simple and low-cost, it requires a large amount and high purity of the organic solvents, which are usually hazardous and flammable solvent wastes, and are generally cumbersome [14]. Moreover, extraction by maceration is nonselective and time-consuming, in comparison to scCO2 extraction.
An important disadvantage of plant extracts in topical products is the plant pigments, which produce vibrant plant extracts that naturally fade with time, resulting in color variation in the same product [15]. To overcome this issue, we introduced scCO2 with the cosolvent to extract the pigments from C. asiatica, and thereby eliminate the dark green appearance of the extract before ethanolic maceration.
This present study aimed to decrease the presence of plant pigments, and to compare the composition of the bioactive compounds and their biological activities involved in wound healing activity (antioxidant screening by 2,2-diphenyl-1-picrylhydrazyl radical [DPPH] and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) [ABTS+] radical scavenging activities, cell migration assay, MMP-2 inhibition on human skin fibroblast and endothelial cell tube formation assay) of C. asiatica leaf extracts obtained by ethanolic maceration with and without pretreatment using scCO2 extraction.
2. Materials and Methods
2.1. Chemicals
Trolox, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) diammonium salt and L-ascorbic acid standard were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other chemical substances were of analytical grade.
2.2. Preparation of Centella asiatica Sample
The leaves of C. asiatica were gathered from Nampu, Ratchaburi, Thailand, during January to June 2018, by botanists from the Pharmaceutical and Natural Products Research and Development Unit (PNPRDU), Chiang Mai University, Chiang Mai, Thailand. A herbarium voucher specimen (PNPRDU004/62) was deposited in the PNPRDU, Faculty of Pharmacy, Chiang Mai University. The dried leaves were ground into small pieces and kept at 4 °C until use.
2.3. Extraction Method
2.3.1. Conventional Extraction
For conventional extract preparation, the powder of C. asiatica (2 kg) was macerated with 70% ethanol (1:4.5, w/w) at room temperature for 48 h, following the extraction protocol of Thai National List of Essential Medicines [16]. Then, the extract was filtered through Whatman paper No. 1, and the filtrate was evaporated at 50 °C to remove the solvent using a rotary evaporator (Hei-VAP Precision, Heidolph, Schwabach, Germany) until it was completely dry. The sample was called CV.
2.3.2. Pretreatment Using scCO2
For the pretreatment using scCO2 extraction, the powder of C. asiatica (3.6 kg) was placed into the supercritical fluid extractor (Guangzhou Gongcheng Digital Science Technology, Guangzhou, China) at 35 MPa, 60 °C for 3 h, with 95% ethanol (2 kg) as the cosolvent. The volume of the extractor was 24 L. The ratio of ethanol to CO2 was 1:25 by weight. This process eliminated the color pigments from the C. asiatica powder. Then, the processed powder of C. asiatica was macerated with 85% ethanol (1:4.5, w/w) at room temperature for 24 and 48 h to obtain scCO2 extract 1 (SCE1) and 2 (SCE2), respectively. The 85% ethanol was used in the maceration process, since it gave the highest total triterpene and triterpenoid glycoside in our preliminary study. Each extract was filtered and evaporated to dryness, as described in Section 2.3.1.
2.4. Identification of the Active Compounds by High-Performance Thin-Layer Chromatography (HPTLC)
2.4.1. Preparation of Crude Extract and Standard Solution
The C. asiatica extracts (CV, SCE1 and SCE2) were dispersed in 5 mL methanol at 160 µL/mL. Each sample was sonicated for 10 min and centrifuged to collect the supernatant. The asiaticoside United States Pharmacopoeia reference standard (USP RS) was dissolved in methanol at 0.5 mg/mL for standard solution A. The USP RS of powdered C. asiatica extract was dispersed in methanol at 10 mg/mL, sonicated for 10 min and centrifuged. The supernatant was collected and used as standard solution B.
2.4.2. HPTLC
A Camag high-performance thin-layer chromatography (HPTLC) system (Muttenz, Switzerland) equipped with an automatic thin-layer chromatography (TLC) sampler ATS 4, thin-layer chromatography (TLC) scanner 3 and integrated software winCATS version 1.2.3 was used for the analysis. HPTLC was performed on an HPTLC plate (20 cm × 10 cm) precoated with silica gel 60F254 (layer thickness 0.20 mm).
The samples (6 µL) and standards (10 µL) were automatically dispensed (TLC sampler ATS 4) on the plate under a flow of N2 gas, as 10-mm wide bands, positioned 10 mm from the bottom and 10 mm from the side, at an inter-band distance of 35 mm. Linear ascending development was carried out at room temperature (25 ± 2 °C) and 50 ± 5% relative humidity for 30 min in a Camag twin trough chamber (20 cm × 10 cm) previously saturated with 10 mL of dichloromethane:methanol:water (14:6:1, v/v/v). The migration distance was 7.5 cm. The developed TLC plates were air-dried using an air dryer in a wooden chamber with adequate ventilation. The air-flow in the laboratory was maintained unidirectional (laminar flow, towards exhaust). The spray reagent was 10% (v/v) sulfuric acid in methanol. The plate was then oven-heated at 120 °C for 4 min and observed under white light. The Camag TLC scanner with D2&W lamp at the plate scanning wavelength of 500 nm with the absorption measurement mode and winCATS software was used for imaging and archiving the thin-layer chromatograms at the following settings: detector mode, automatic; slit dimensions, 4.0 mm × 0.3 mm; data resolution, 100 µm/step; scanning speed, 20 mm/s. Each sample was analyzed in triplicate.
2.5. Determination of the Active Compounds by High-Performance Liquid Chromatography (HPLC)
2.5.1. Preparation of Crude Extract and Standard Solutions
According to USP 40-NF 35, the asiaticoside USP RS was dissolved in methanol at 0.05 mg/mL (standard solution A). The USP RS of powdered C. asiatica extract was dispersed and sonicated in methanol at 5.0 mg/mL (standard solution B). The 1 g of C. asiatica extracts (CV and SCE1) and methanol (100 mL) was transferred to a Soxhlet apparatus (GLASSCO, Ambala, India), extracted for 8 h, and diluted with methanol to 100 mL (sample stock solution). The sample solution was prepared by diluting sample stock solution (5 mL) with methanol to 10 mL. Before injection, the solutions were filtered (0.45 µm pore size), discarding the first few milliliters of the filtrate.
2.5.2. HPLC
The total triterpenoids and total triterpenoid glycosides in the standard and C. asiatica extracts were determined by high performance liquid chromatography (HPLC) using a Luna® C18 column (250 mm × 4.60 mm, 5 µm; Phenomenex, Torrance, CA, USA), LC1200UV/VIS detector and LC1100HPLC pump. The chromatographic separation was achieved using a binary mobile phase system of 0.3% (v/v) phosphoric acid and acetonitrile under gradient elution, according to the composition of the triterpene derivatives of C. asiatica in the USP 40-NF35 monograph. The injection volume and flow rate were, respectively, 10 µL and 1 mL/min, and the UV detector was set at 200 nm. The content of total triterpenoids (madecassoside, asiaticoside B, asiaticoside, madecassic acid, terminolic acid and asiatic acid) and total triterpenoid glucosides (madecassoside, asiaticoside B, asiaticoside) were calculated by Equation (1):
| ru | peak areas of the triterpene derivatives in the sample solution |
| rs | peak areas of asiaticoside in the standard solution A |
| Cs | concentration of USP RS asiaticoside in the standard solution A (mg/mL) |
| V | final volume of sample stock solution (mL) |
| W | weight of C. asiatica used to prepare the sample stock solution (mg) |
| D | dilution factor in preparing the sample solution from the sample stock solution |
| F | conversion factors for analytes: 1.00 for asiaticoside, 1.017 for the sum of madecassoside and asiaticoside B, 0.526 for the sum of madecassic acid and terminolic acid, and 0.509 for asiatic acid. |
2.6. Determination of Biological Activities of Centella asiatica Extracts
2.6.1. Antioxidant Screening by DPPH Radical Scavenging Activity
The DPPH radical scavenging activity of CV and SCE1 was determined using a previously described method [17] with slight modifications. Briefly, five serially-diluted concentrations of each sample, along with 50 µL of distilled water and 50 µL of 0.1 mM ethanolic DPPH solution, were added to the wells of a 96-well plate (Nalge Nunc International, Rochester, NY, USA). The reaction mixture was incubated at 25 ± 2 °C for 30 min, then the solution was separated, transferred to a 96-well microplate, and the absorbance was measured at 515 nm against a blank (ethanol) using a plate reader (EZ Read 400 Flexi, Biochrom, Cambridge, UK). Ascorbic acid standard was the positive control. All experiments were performed in triplicate. The percentages of the DPPH radical scavenging activity were calculated by Equation (2):
Here Asample is the absorbance of the DPPH radical with the extract, and Acontrol is the absorbance of the DPPH radical alone. The DPPH scavenging activity (%) of five serially-diluted concentrations were determined.
2.6.2. Antioxidant Screening by ABTS+ Scavenging Activity
For the ABTS+ assay, the method was used with some modifications [18]. The stock solutions included ABTS+ solution and potassium persulfate solution at the 1:0.5 molar ratio. To prepare the working solution, these two stock solutions were mixed in equal quantities and reacted at room temperature in the dark for 12 h. The solution was then diluted by mixing 1 mL of ABTS+ solution with distilled water to obtain a spectrophotometric absorbance of 0.7–0.9 at 734 nm. Fresh ABTS+ solution was prepared for each assay. The extracts (150 μL) were reacted with 2850 μL of ABTS+ solution for 2 h in the dark, and the absorbance was recorded at 734 nm using a 96-well plate reader (EZ Read 400 Flexi, Biochrom, Cambridge, UK). The standard curve was linear between 0.001–1 mg/mL Trolox. Results are expressed in milligrams of Trolox equivalent antioxidant capacity (TEAC) per gram of extract. Additional dilution was needed if the ABTS+ value measured was outside the linear range of the standard curve.
2.7. Wound Healing Activity Assay
2.7.1. Cell Culture
The normal human telomerase reverse transcriptase (hTERT) human fibroblasts (OUMS-36T-2) and immortalized human umbilical vein endothelial cells (HUEhT-2) were provided by the JCRB Cell Bank (Osaka, Japan). The normal hTERT human fibroblasts were cultured in complete culture medium containing Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin (100 U/mL) and streptomycin (100 mg/mL). HUEhT-2 cells were cultured in incomplete MCDB 131 medium supplemented with 10% (v/v) FBS, glutamine, endothelial cell growth supplement (ECGS) and heparin, according to the manufacturer’s instructions. All cells were incubated in a temperature-controlled and humidified incubator (CCL-050B-8, Esco®, Singapore) with 5% CO2 at 37 °C.
2.7.2. Determination of Non-Cytotoxic Concentration by the Sulforhodamine B (SRB) Assay
The samples were tested for non-cytotoxic concentration on normal hTERT human fibroblasts and HUEhT-2 by the SRB assay, as previously described [19]. L-Ascorbic acid (0.00001–0.1 mg/mL) was used as a positive control for normal hTERT human fibroblasts. The cells were plated at a density of 1.0 × 105 cells/well in 96-well plates, and left overnight for cell attachment in 5% CO2 atmosphere at 37 °C.
Cells were then exposed to ten serial concentrations of the extracts (in the range of 0.00001–0.1 mg/mL for hTERT human fibroblasts and 0.0005–0.5 mg/mL for HUEhT-2) for 24 h. After incubation, the adherent cells were fixed in situ, washed, and dyed with SRB. The bound dye was solubilized, and the absorbance was measured at 540 nm using a 96-well plate reader (EZ Read 400 Flexi, Biochrom). The experiments were conducted in triplicate. The percentages of cell viability were calculated by Equation (3), where OD denotes optical density:
2.7.3. Gelatinolytic Activity (Zymography) of MMP-2 Inhibition on Human Skin Fibroblast
The samples (CV and SCE1) were investigated for the gelatinolytic activity of MMP-2 inhibition in comparison to L-ascorbic acid as the standard. A monolayer of 5 × 105 cells of normal human skin fibroblasts was maintained in the culture medium without FBS for 24 h, treated with the samples and L-ascorbic acid standard, respectively, at 0.1 mg/mL, and incubated for 48 h. The culture supernatants were collected. To assess the gelatinolytic activities of MMP-2 in the culture media, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) zymography was performed using gelatin as a substrate. Briefly, 20 µL of the cell culture supernatant was suspended in the loading buffer (0.125 M Tris pH 6.8, 4% SDS and 0.04% bromophenol blue), without prior denaturation and run on 10% SDS polyacrylamide gel containing 1 mg/mL gelatin. After electrophoresis, gels were washed to remove SDS and incubated for 20 min in the renaturing buffer (50 mM Tris, 5 mM CaCl2, 0.02% NaN3, 2.5% Triton X-100), followed by incubation in developing buffer (50 mM Tris pH 7.5, 5 mM CaCl2, 0.02% NaN3 and 1% Triton X-100) at 37 °C for 24 h to allow gelatin degradation by MMPs. Gels were subsequently stained with 0.5% Coomassie brilliant blue G-250 and de-stained in 30% methanol and 10% acetic acid (v/v) to detect gelatinolytic activity [20]. Gelatinase activity was detected as a white band on a blue background. The gel was scanned using a gel documentation system (Gel DocTM EZ Gel, Bio-Rad Laboratories, Hercules, CA, USA) and analyzed by Image LabTM software 5.1 (Bio-Rad Laboratories, USA). The volume (intensity) multiplied by the number of pixels of the bands on the gel was determined as the relative MMP-2 content (intensity unit) [21]. The percentages of MMP-2 inhibition relative to the control (the untreated systems) were calculated by Equation (4):
The assays were done in three independent experiments. The potency of MMP-2 inhibition of the samples was compared with the positive control (L-ascorbic acid).
2.7.4. Cell Migration Assay
The normal hTERT human fibroblasts were plated in 24-well plates at 1 × 106 cells/well and left overnight for cell attachment in 5% CO2 atmosphere at 37 °C, as described previously [22,23]. A sterile, 10-μL plastic pipette tip was used to create a linear scratch on the cell monolayer. The cells were washed once with phosphate-buffered saline (pH 7.4) to remove the cell debris and replaced with the sample in the incomplete medium. The cell migration was photographed under an inverted microscope at 0 and 12 h. The cell migration area was determined using ImageJ software version 1.52a (NIH, Bethesda, MD, USA). The percentage of wound closure and the rate of cell migration were calculated according to Equations (5) and (6), respectively.
2.7.5. Endothelial Cell Tube Formation Assay
An ice-cold 24-well plate was coated with 150 μL/well of Matrigel® Matrix (Discovery Labware, Inc. Bedford, MA, USA), which was allowed to solidify at 37 °C for 60 min, according to the manufacturer’s instructions and a previous study [24]. Then, 2 × 105 cells/well of HUEhT-2 were seeded into 24-well plates, as previously described [24,25]. Incomplete MCDB 131 was used as a culture medium and negative control. Samples were treated in triplicate in each well, and compared with the positive control (incomplete MCDB 131 supplemented with glutamine, ECGS, heparin and FBS, according to the manufacturer’s instructions and a previous study [24]). After incubation at 37 °C and 5% CO2 for 24 h, tube formation was examined under an inverted phase-contrast microscope. Three different phase-contrast photomicrographs (10× objective) per well were taken to quantify the length of capillaries by ImageJ software. The enclosed capillary networks of tube formation were expressed as a fold increase relative to the cells cultured in the negative control.
2.8. Statistical Analysis
The results were presented as the mean of three independent experiments and analyzed by SPSS version 17.0. Analysis of Variance (ANOVA) was used for the analysis of the test results (LSD test) at p < 0.05. The bivariant correlation coefficient (r) was calculated to determine the relationship between the variables.
3. Results and Discussion
3.1. Physical Characteristic of Centella asiatica Extracts
The C. asiatica extracts (CV, SCE1, SCE2) were green–brown (CV) and yellow–brown (SCE1 and SCE2) liquids with the density of 1.156, 1.036 and 1.033 g/mL, respectively. The extraction yield of CV was 34.15% (dry weight basis), while those of SCE1 and SCE2 were 41.67% and 42.87% (dry weight basis), respectively. The increased yield obtained by 24 and 48 h pretreatment using scCO2 extraction is possibly because CO2 is a nonpolar molecule, resulting in the enhanced solubility of nonpolar compounds [26], and the use of ethanol as a cosolvent also allowed to extract polar components [27]. Furthermore, organic solvents that are used at high pressures could enhance the interaction between the solvent and the matrix, as well as increase the solvent power. Consequently, the mass transfer of the solutes to the solvent was increased, and in turn, the extraction yields of C. asiatica extracts were improved [26,27,28]. Notably, the pretreatment scCO2 extraction with ethanol as a cosolvent destabilized the dark pigments from the extract, resulting in the yellow–brown SCE extracts [15,29]. A previous study reported that the ethanol addition to scCO2 promoted certainly the reduction in a- and b-value in CIE L*, a* and b* (CIE-LAB) values. The reduction of b-value resulted in less yellow product in the extracts of Pisum sativum L. [30]. The color pigments (e.g., lutein and ß-carotene) might be removed by ethanol. Due to ethanol as a cosolvent, this might remove polar compounds and cause changes for the protein structure in plants to release more pigments. Ethanol might promote the affinity between CO2 and pigment molecules by the association pigment–ethanol, decreasing the polarity and increasing the local density near solute molecules [31].
The scCO2 is a green technology. However, it is not perfectly suitable for the extraction of polar organic compounds including alcohols and/or high molecular components, such as saponins, phenolic compounds and terpenoids from plants [32]. The scCO2 and ethanol as a cosolvent used in the pretreatment allowed us to extract both moderately polar and nonpolar compounds, combined with short extraction times and high penetrating of the solvent in the extraction [33], which compared to conventional extraction for producing a variety of natural product extracts [30].
3.2. Identification of the Bioactive Compounds by HPTLC
The composition of the biologically-active compounds was compared among the C. asiatica extracts (CV, SCE1 and SCE2) by using HPTLC. Figure 1A shows the chromatogram of the USP RS asiaticoside (track 1), SCE1 (track 2), SCE2 (track 3), CV (track 4) and the USP RS powdered C. asiatica extract (track 5). Asiaticoside was the major component in all extracts, but presented in different amounts. It had the same retention factor (Rf) in the TLC fingerprint of each extract (see Figure 1B), and no unique peaks occurred in SCE1 and SCE2 when compared with CV.
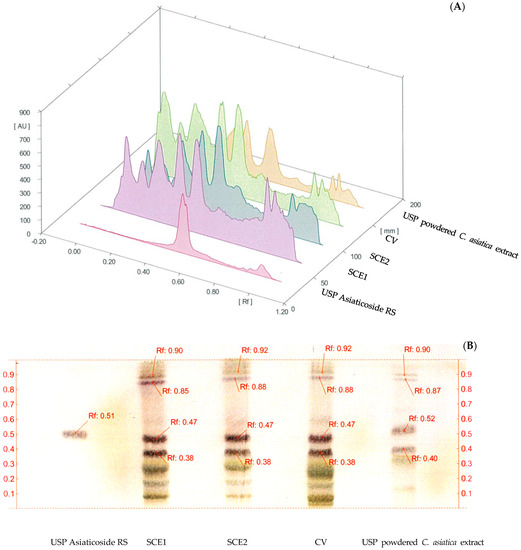
Figure 1.
(A) High-Performance Thin-Layer Chromatography (HPTLC) chromatogram and (B) HPTLC fingerprint of Centella asiatica extracts, USP Asiaticoside RS (track 1), C. asiatica extracts from the pretreatment of scCO2 method, SCE1 (track 2), SCE2 (track 3), C. asiatica extracts from conventional, CV (track 4) and the USP powdered C. asiatica extract (track 5).
In this study, C. asiatica was pretreated using scCO2 extraction with ethanol as a cosolvent, followed by ethanolic maceration for 24 and 48 h to obtain SCE1 and SCE2, respectively. Several reports showed that the ethanolic extraction by maceration produced the same type of biologically-active compounds, but in different amounts relative to the pretreatment using scCO2 extraction [34,35,36,37]. Here, SCE1 was selected for further analysis because of its greater yield than SCE2, and the same profile of compounds between SCE1 and SCE2 was found in the HPTLC chromatogram.
3.3. HPLC Profile of Bioactive Compounds
The compounds with known biological activities in the C. asiatica extracts (CV and SCE1) are shown in Table 1. The contents of madecassoside, asiaticoside, madecassic acid, terminolic acid and asiatic acid in SCE1 were different from those in CV, resulting in noticeably higher total triterpenoid glycosides and total triterpenes when the samples were pretreated using scCO2 (p < 0.05). This enriched amount of active compounds from C. asiatica extracted with scCO2 might be explained by the increased contact surface associated with the degradation of the plant cell wall during comminution of the sample, which promoted the solvent–solute interactions during scCO2 extraction [26]. Besides the increased content of total triterpenes in the C. asiatica extracts, extraction with scCO2 eliminated the pigments. A previous study reported that the approximate 30 MPa pressure of scCO2 was incapable of extracting the pigments from the seed tissues. High pressure of approximately 50 MPa, and the use of a low polar modifier, was necessary to dissociate chlorophylls from marine algae [38,39]. At the pressure of 35 MPa used in the current study, the decreased solvent power and increased diffusivity of CO2 lowered the content of pigments in the extracts.

Table 1.
Biological compound contents of Centella asiatica extracts from conventional (CV) and the pretreatment of scCO2 method (SCE1).
3.4. Biological Activity of Centella asiatica Extracts
Table 2 shows the biological activities (DPPH and ABTS+ radical scavenging activities) were higher for CV (46.85 ± 2.31% and 0.017 ± 0.004 mg TEAC/g) than SCE1, which might be due to the high solubilizing power of scCO2, which was applied 3 h prior to the ethanolic maceration process in the C. asiatica extraction (SCE1). The Trolox standard curve in the ABTS+ scavenging assay was y = −7.547x + 1.4813, r2 = 0.995.

Table 2.
Biological activities of Centella asiatica extracts from conventional and scCO2 method (SCE1).
Moreover, the total phenolic content was greater for CV (1.391 ± 0.044 µg gallic acid equivalents [GAE]/g extract) than SCE1 (0.953 ± 0.031 µg GAE/g extract). The scCO2 extraction method with ethanol as a cosolvent could have facilitated dissolution of the polar compounds, such as those containing phenolic groups, in the SCE1 extract, leading to the relatively lower total phenolic content, and antioxidant activity (DPPH and ABTS+). The trisaccharides, namely asiaticoside and madecassoside, have higher polarities than the aglycones, which include madecassic acid and asiatic acid, leading to the lower abundance of these compounds in SCE1 than CV [40].
3.5. Wound Healing Activity
The non-cytotoxic concentration of C. asiatica extracts (CV and SCE1), as assessed by the SRB assay, was 0.01 mg/mL in both the normal hTERT human fibroblasts and HUEhT-2 cell line. This concentration, which provided more than 90% cell viability, was selected for further wound healing analyses.
3.5.1. MMP-2 Inhibition Activity
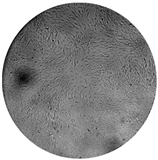
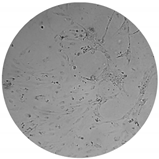
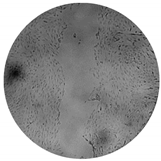
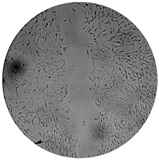
The inhibitory effects of the gelatinolytic activity on MMP-2 expression found for the C. asiatica extracts (CV and SCE1) and the ascorbic acid standard, are illustrated in Figure 2. The faded band of MMP-2 inhibition was not due to cell cytotoxicity, because all samples were non-cytotoxic to human skin fibroblasts by the SRB assay. In comparison to the control, SCE1 exhibited the highest MMP-2 inhibition of 58.48 ± 7.50%, followed by CV (50.74 ± 7.31%) and then the ascorbic acid standard (31.20 ± 2.18%).
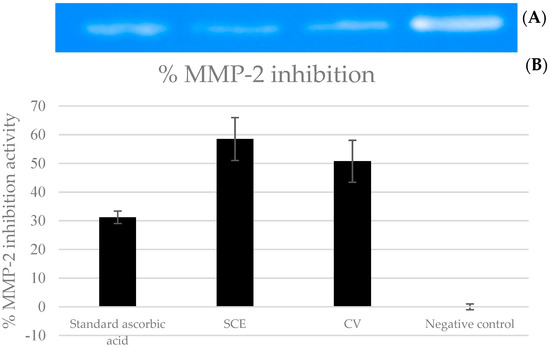
Figure 2.
Matrix metallopeptidases 2 (MMP-2) inhibition activity of Centella asiatica extracts from the pretreatment of scCO2 (SCE1) and conventional method (CV) compared to standard ascorbic acid. (A) Zymograms and (B) the percentages of MMP-2 inhibition.
MMPs may also have negative effects on cell proliferation in the wound healing process [9]. The extracts that presented wound healing activity should show high inhibition of MMP-2 expression. As mentioned above (Section 1), the normal wound healing is a complex process involving inflammation, proliferation and remodeling/maturation phases. During the maturation of the wound, the extracellular matrix undergoes certain changes, followed by scar formation, which marks the physiological endpoint of healing. The endpoint is also directly proportional to the extent of inflammatory processes occurring throughout the healing. The MMPs, which have an important role in tissue remodeling, eliminate damaged protein, destroy the provisional extracellular matrix, facilitate its migration to the center of the wound, remodel the granulation tissue, regulate the activity of some growth factors and probably control angiogenesis [3]. In earlier research, MMP-2 and MMP-9 decreased in the wound tissue treated with the triterpenoid saponin-rich fraction of C. asiatica. MMP-2 and MMP-9 regulate the turnover of the matrix proteins by degrading basement membrane proteins, which include collagen IV and V, gelatin, elastin and fibronectin [41]. Other work demonstrated that asiatic acid and triterpene compounds reduced the UVA-induced activation and expression of MMP-2, using HaCaT human keratinocytes as a model cell system [42].
3.5.2. Cell Migration and Angiogenesis Activity
Table 3 shows the cell migration and angiogenesis of C. asiatica extracts relative to the positive and negative controls. Consistent with the trend of MMP-2 inhibition, SCE1 induced higher cell migration (59.83 ± 1.85% of initial) and the number of vessels (18.25 ± 4.58) in angiogenesis activities compared with CV (50.43 ± 6.22% of initial and 16.50 ± 3.51, respectively). This observation might be due to the higher content of asiaticoside, total triterpenoid glycosides, madecassic acid, terminolic acid, asiatic acid and total triterpenes in SCE1 than CV. These active compounds in C. asiatica play an important role in the wound healing process, especially in cell migration [41,43,44] and angiogenesis [7,45]. Moreover, the synergistic effect of these active compounds in C. asiatica could be observed in the wound healing activity. Lu et al. (2004) reported that asiaticoside could stimulate the mRNA levels and increase the protein production of certain genes responsible for extracellular matrix synthesis encoding type I and type III collagen proteins in human fibroblasts. Furthermore, asiaticoside may control the cell-cycle progression and cell proliferation that enhance cell migration and angiogenesis activity [46].

Table 3.
Cell migration and angiogenesis of Centella asiatica extracts from conventional and scCO2 method (SCE1).
3.6. Relationship between the Biological Activities and Bioactive Compound Contents in Centella asiatica Extracts
Pearson’s correlation coefficients (r) between the biological activities (the cell migration, number of vessels and angiogenesis) and the content of bioactive compounds in the C. asiatica extracts (SCE1) are presented in Table 4. The correlations based on Akoglu’s (2018) classification of the correlation coefficient as particularly high (r > 0.9), high (0.9 > r > 0.7), moderate (0.7 > r > 0.5) and poor (r < 0.5) [47]. There was a strong and significant linear relationship between the DPPH radical scavenging activity and the content of madecassoside (r = 0.914, p < 0.01). This significant positive correlation implied that the antioxidant activity of SCE1 varied proportionally to change of concentration of madecassoside. Numerous studies also reported that madecassoside from other plant has been shown to exert various pharmacological activities, including antioxidative properties [48,49]. Asiaticoside and cell migration were strongly correlated (r = 0.854, p < 0.05). This result agreed with a previous study, showing that asiaticoside enhanced the migration rate of normal human dermal fibroblasts. Furthermore, the initial skin cell adhesion and cell proliferation were also increased by asiaticoside [50]. Therefore, the yield of madecassoside and asiaticoside can be used to predict the antioxidant activity of SCE1 due to their good correlation with antioxidant activity, whereas asiaticoside might be attributed to predict the cell migration potential.

Table 4.
Pearson’s correlation coefficients between the biological activities and the content of bioactive compounds in Centella asiatica extracts (SCE1).
4. Conclusions
Pretreatment of C. asiatica using scCO2 extraction with ethanol as the cosolvent prior to ethanolic maceration can eliminate the plant pigments, avoiding color fading and color variation in the products, and provide an extract with greater in vitro wound healing activity than the conventional method.
Author Contributions
Conceptualization, W.R.; methodology, W.R.; software, C.K.; validation, K.S.; formal analysis, P.J.; investigation, C.K.; resources, W.R.; data curation, S.S.; writing—original draft preparation, W.R. and C.K.; writing—review and editing, W.R. and C.K.; supervision, W.R.; project administration, W.R.; funding acquisition, W.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by Chiang Mai University, Thailand, with a grant number 6/2562.
Acknowledgments
This study was partially supported by Chiang Mai University, Thailand. We are very thankful to Bangkok Lab and Cosmetic Co., Ltd. for C. asiatica powder.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gonzalez, A.C.D.O.; Costa, T.F.; Andrade, Z.D.A.; Medrado, A.R.A.P. Wound healing-a literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Babykutty, S.; Padikkala, J.; Sathiadevan, P.; Vijayakurup, V.; Azis, T.; Srinivas, P.; Gopala, S. Apoptosis induction of Centella asiatica on human breast cancer cells. Afr. J. Tradit. Complement. Altern. Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Pittella, F.; Dutra, R.; Junior, D.; Lopes, M.T.; Barbosa, N. Antioxidant and cytotoxic activities of Centella asiatica (L) Urb. Int. J. Mol. Sci. 2009, 10, 3713–3721. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, N.S.; Khadabadi, S.S.; Ghorpade, D.S.; Banarase, N.B.; Naphade, S.S. Antimicrobial and antifungal activity of Centella asiatica (L.) Urban, Umbelliferae. Int. J. Pharm. Technol. Res. 2009, 2, 328–330. [Google Scholar]
- Shukla, A.; Rasik, A.M.; Jain, G.K.; Shankar, R.; Kulshrestha, D.K.; Dhawan, B.N. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J. Ethnopharmacol. 1999, 65, 1–11. [Google Scholar] [CrossRef]
- Suguna, L.; Sivakumar, P.; Chandrakasan, G. Effects of Centella asiatica extract on dermal wound healing in rats. Indian J. Exp. Biol. 1996, 34, 1208–1211. [Google Scholar]
- Sigurdson, L.; Sen, T.; Hall, L.; Rubenfeld, A.; Hard, R.; Gardella, J.; Bright, F.; Hicks, W.L. Possible impedance of luminal reepithelialization by tracheal cartilage metalloproteinases. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 197–200. [Google Scholar] [CrossRef][Green Version]
- Nowwarote, N.; Osathanon, T.; Jitjaturunt, P.; Manopattanasoontorn, S.; Pavasant, P. Asiaticoside induces type I collagen synthesis and osteogenic differentiation in human periodontal ligament cells. Phytother. Res. 2013, 27, 457–462. [Google Scholar] [CrossRef]
- Ruszymah, B.H.I.; Chowdhury, S.R.; Manan, N.A.B.A.; Fong, O.S.; Adenan, M.I.; Saim, A.B. Aqueous extract of Centella asiatica promotes corneal epithelium wound healing in vitro. J. Ethnopharmacol. 2012, 140, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Manosroi, J.; Abe, M.; Manosroi, W.; Manosroi, A. 5α-Reductase type 1 inhibition of Oryza sativa bran extract prepared by supercritical carbon dioxide fluid. J. Supercrit. Fluids. 2011, 59, 61–71. [Google Scholar] [CrossRef]
- Manosroi, A.; Chutoprapat, R.; Abe, M.; Manosroi, J. Characteristics of niosomes prepared by supercritical carbon dioxide (scCO2) fluid. Int. J. Pharm. Sci. 2008, 352, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of supercritical CO2 in lipid extraction–a review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell Serrano, C.; Rodríguez Rodríguez, M.; Martínez de la Ossa, E.; Lubián, L.M.; Montero, O. Extraction of carotenoids and chlorophyll from microalgae with supercritical carbon dioxide and ethanol as cosolvent. J. Sep. Sci. 2008, 31, 1352–1362. [Google Scholar] [CrossRef]
- FDA. Thai National List of Essential Medicines; Ministry of Public Health: Bangkok, Thailand, 2018; p. 265. [Google Scholar]
- Tachibana, Y.; Kikuzaki, H.; Lajis, N.H.; Nakatani, N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J. Agric. Food Chem. 2001, 49, 5589–5594. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernandez, R.J.; Garcıa, C.F.; Acosta, M. Inhibition byl-ascorbic acid and other antioxidants of the 2, 2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) oxidation catalyzed by peroxidase: A new approach for determining total antioxidant status of foods. Anal. Biochem. 1996, 236, 255–261. [Google Scholar] [CrossRef]
- Papazisis, K.T.; Geromichalos, G.D.; Dimitriadis, K.A.; Kortsaris, A.H. Optimization of the sulforhodamine B colorimetric assay. J. Immunol. Methods 1997, 208, 151–158. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Kim, J.E.; Cho, K.H.; Chung, J.H. Berberine inhibits TPA-induced MMP-9 and IL-6 expression in normal human keratinocytes. Phytomedicine 2008, 15, 340–347. [Google Scholar] [CrossRef]
- Manosroi, A.; Jantrawut, P.; Akihisa, T.; Manosroi, W.; Manosroi, J. In vitro anti-aging activities of Terminalia chebula gall extract. Pharm. Biol. 2010, 48, 469–481. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Dwivedi, A.; Preez, J.L.; Plessis, J. In vitro wound healing and cytotoxic effects of sinigrin–phytosome complex. Int. J. Pharm. Sci. 2016, 498, 283–293. [Google Scholar] [CrossRef] [PubMed]
- DeCicco Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J. Vis. Exp. 2014, 91, 51312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Wang, Q.L.; Su, J.F.; Deng, B.; Cai, G.; Li, Y. Angiogenesis effects of Xuefu zhuyu decoction and VEGF protein expression of rats with acute myocardial ischemia. Chin. J. Inf. Tradit. Chin. Med. 2011, 18, 53–54. [Google Scholar]
- Garcia, M.M.P.; Paula, J.T.; Paviani, L.C.; Cabral, F.A.; Martinez, C.H.A. Extracts from mango peel by-product obtained by supercritical CO2 and pressurized solvent processes. LWT Food Sci. Technol. 2015, 62, 131–137. [Google Scholar] [CrossRef]
- Martinez-Correa, H.A.; Magalhaes, P.M.; Queiroga, C.L.; Peixoto, C.A.; Oliveira, A.L.; Cabral, F.A. Extracts from pitanga (Eugenia uniflora L.) leaves: Influence of extraction process on antioxidant properties and yield of phenolic compounds. J. Supercrit. Fluids 2011, 55, 998–1006. [Google Scholar] [CrossRef]
- Luthria, D.L. Optimization of extraction of phenolic acids from a vegetable waste product using a pressurized liquid extractor. J. Funct. Foods 2012, 4, 842–850. [Google Scholar] [CrossRef]
- Gracia, I.; Rodriguez, J.F.; de Lucas, A.; Fernandez, R.M.P.; García, M.T. Optimization of supercritical CO2 process for the concentration of tocopherol, carotenoids and chlorophylls from residual olive husk. J. Supercrit. Fluids 2011, 59, 72–77. [Google Scholar] [CrossRef]
- Vatansever, S.; Hall, C. Flavor modification of yellow pea flour using supercritical carbon dioxide+ ethanol extraction and response surface methodology. J. Supercrit. Fluids 2020, 156, 104659. [Google Scholar] [CrossRef]
- Cobb, B.F.; Kallenbach, J.; Hall, C.A.; Pryor, S.W. Optimizing the supercritical fluid extraction of lutein from corn gluten meal. Food Bioprocess Technol. 2018, 11, 757–764. [Google Scholar] [CrossRef]
- Şanal, İ.S.; Bayraktar, E.; Mehmetoğlu, Ü.; Çalımlı, A. Determination of optimum conditions for SC-(CO2 + ethanol) extraction of β-carotene from apricot pomace using response surface methodology. J. Supercrit. Fluids 2005, 34, 331–338. [Google Scholar] [CrossRef]
- Pasquel Reátegui, J.L.; Machado, A.P.d.F.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids 2014, 94, 223–233. [Google Scholar] [CrossRef]
- Sondari, D.; Irawadi, T.T.; Setyaningsih, D.; Tursiloadi, S. Effect of Pressure on the Supercritical CO2 Extraction of Asiaticoside from Centella asiatica. Adv. Sci. Lett. 2017, 23, 11828–11833. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Abe, M.; Sakai, H.; Manosroi, W.; Manosroi, J. Biological activities of the rice bran extract and physical characteristics of its entrapment in niosomes by supercritical carbon dioxide fluid. J. Supercrit. Fluids 2010, 54, 137–144. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martinez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Sayyad, R. Antioxidant activity and total phenolic content of Capsicum frutescens extracted by supercritical CO2, ultrasound and traditional solvent extraction methods. J. Essent. Oil Bear. Plants 2017, 20, 196–204. [Google Scholar] [CrossRef]
- Hamdan, S.; Daood, H.G.; Toth, M.M.; Illes, V. Extraction of cardamom oil by supercritical carbon dioxide and sub-critical propane. J. Supercrit. Fluids 2008, 44, 25–30. [Google Scholar] [CrossRef]
- Subra, P.; Tufeu, R. Solubility of chlorophyllian pigments in supercritical carbon dioxide and ethane. J. Supercrit. Fluids 1990, 3, 20–22. [Google Scholar] [CrossRef]
- Schaneberg, B.T.; Mikell, J.R.; Bedir, E.; Khan, I.A.; Nachname, V. An improved HPLC method for quantitative determination of six triterpenes in Centella asiatica extracts and commercial products. Int. J. Pharm. Sci. 2003, 58, 381–384. [Google Scholar]
- Mahmood, A.; Tiwaril, A.; Sahin, K.; Kucuk, O.; Ali, S. Triterpenoid saponin-rich fraction of Centella asiatica decreases IL-1ß andNF-kB, and augments tissue regeneration and excision wound repair. Turk. J. Biol. 2016, 40, 399–409. [Google Scholar] [CrossRef]
- Soo, L.Y.; Jin, D.Q.; Beak, S.M.; Lee, E.S.; Kim, J.A. Inhibition of ultraviolet-A-modulated signaling pathways by asiatic acid and ursolic acid in HaCaT human keratinocytes. Eur. J. Pharmacol. 2003, 476, 173–178. [Google Scholar] [CrossRef]
- Liu, M.; Dai, Y.; Li, Y.; Luo, Y.; Huang, F.; Gong, Z.; Meng, Q. Madecassoside isolated from Centella asiatica herbs facilitates burn wound healing in mice. Planta Med. 2008, 74, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Chastang, F.; Simeon, A.; Birembaut, P.H.; Gillery, P.H.; Wegrowski, Y. Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. Eur. J. Dermatol. 1999, 9, 289–296. [Google Scholar] [PubMed]
- Cheng, C.L.; Guo, J.S.; Luk, J.; Koo, M.W.L. The healing effects of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sci. 2004, 74, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ying, K.; Wei, S.; Fang, Y.; Liu, Y.; Lin, H.; Ma, L.; Mao, Y. Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. Int. J. Dermatol. 2004, 43, 801–807. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Su, Z.; Ye, J.; Qin, Z.; Ding, X. Protective effects of madecassoside against doxorubicin induced nephrotoxicity in vivo and in vitro. Sci. Rep. 2015, 5, 18314. [Google Scholar] [CrossRef]
- Bian, D.; Liu, M.; Li, Y.; Xia, Y.; Gong, Z.; Dai, Y. Madecassoside, a triterpenoid saponin isolated from Centella asiatica herbs, protects endothelial cells against oxidative stress. J. Biochem. Mol. Toxicol. 2012, 26, 399–406. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.L.; Lee, M.H.; You, K.E.; Kwon, B.J.; Seo, H.J.; Park, J.C. Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine 2012, 19, 1223–1227. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).