Novel Concept of Cogeneration-Integrated Heat Pump-Assisted Fractionation of Alkylation Reactor Effluent for Increased Power Production and Overall CO2 Emissions Decrease
Abstract
1. Introduction
2. Materials and Methods
2.1. General Problem Superstructure and Assessment
2.1.1. General Process Model
- Feedstock quality and temperature remain constant throughout the evaluated time interval.
- Ideal gas and ideal liquid behavior is assumed.
- Pressure drop on each tray is equal, thus resulting in a linear pressure profile.
- There is no radial temperature or pressure gradient.
- The column reboiler operates at thermodynamic equilibrium.
- Column bottoms leave the reboiler at boiling point.
- Murphree tray efficiency is the same for each component.
- Stage efficiencies along the column remain unchanged.
- The column condenser operates as a total condenser; column distillate leaves the column at boiling point.
- Vapor and liquid phases leaving each stage have the same temperature.
- Pressure and heat losses in the system are negligible.
- Column bottoms leave the reboiler at boiling point.
- material balance, Equation (1):
- phase equilibrium, Equation (2):
- summation, Equations (3) and (4):
- heat balance, Equation (5):
2.1.2. Steam Consumption and Condensates Management
2.1.3. Heating Steam Pressure Reduction by Steam Turbine
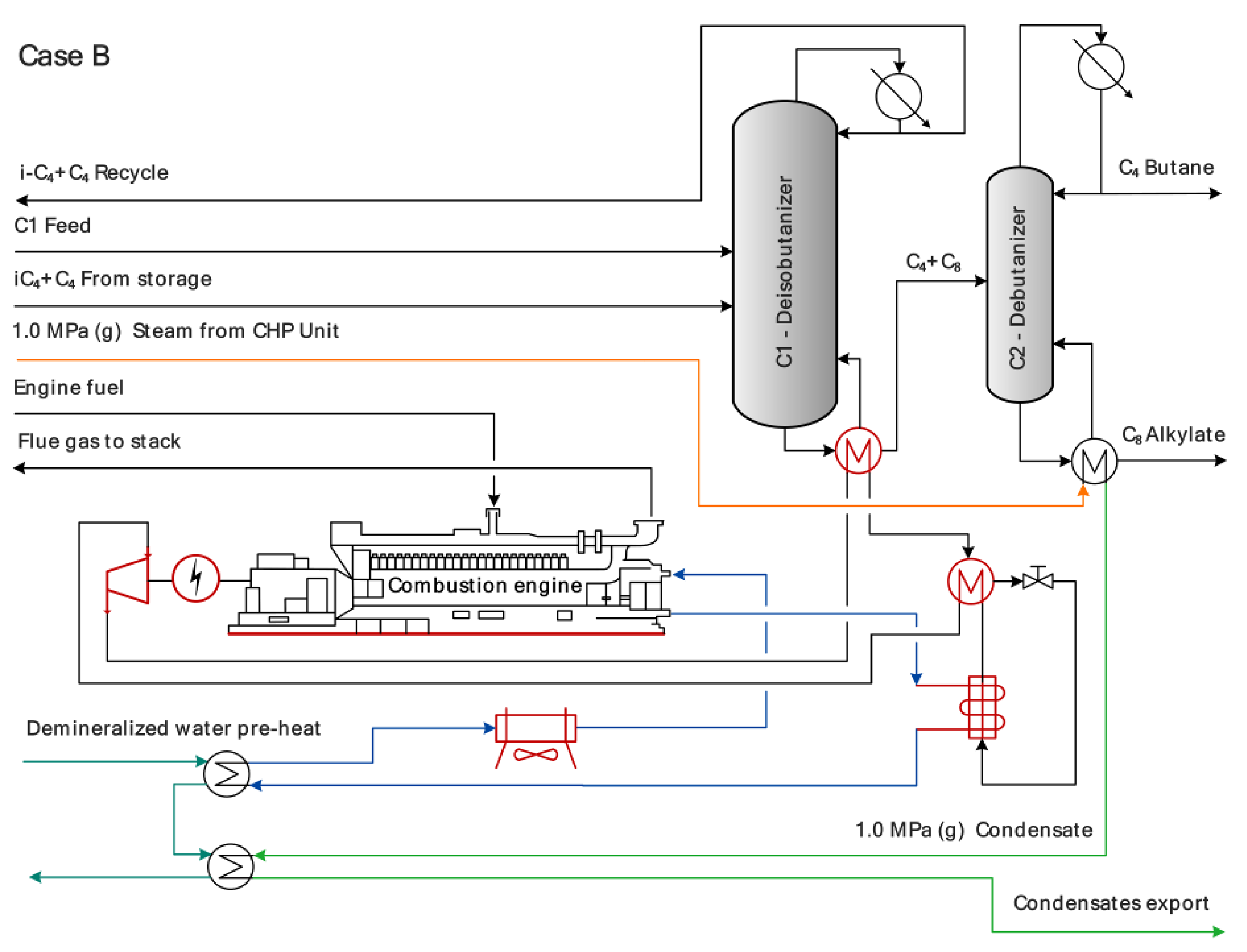
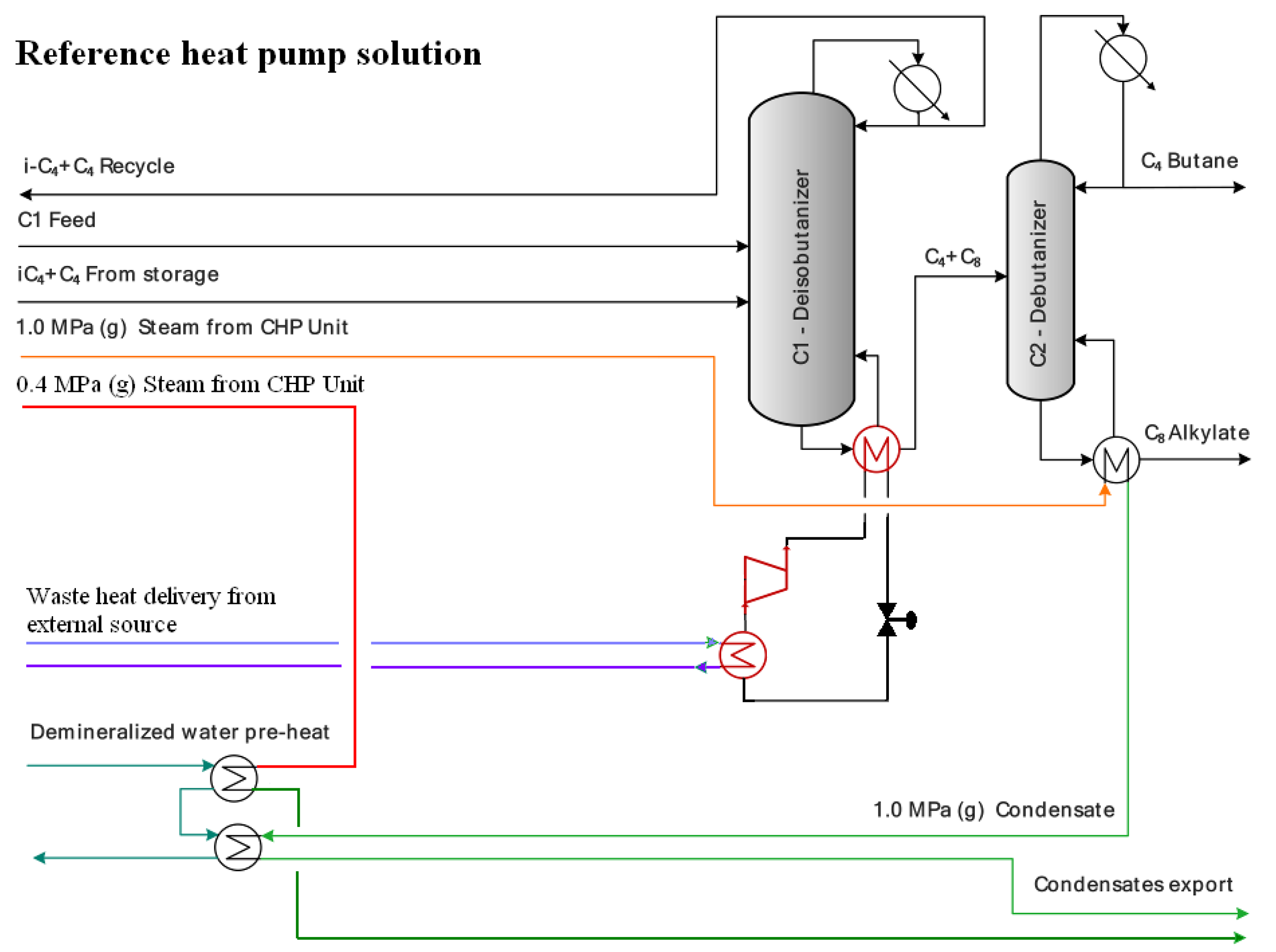
2.1.4. Combustion Engine-Driven Heat Pump
2.1.5. Combined Heat and Power Unit (CHP) and Refinery’s Electric Energy Balance
2.1.6. CO2 Emissions from Combustion Processes and External Power Production
2.1.7. Economic Assessment
2.2. Calculations and Methods
2.2.1. Process Model
2.2.2. CHP Unit and CO2 Emissions Assessment
2.2.3. Total Investment Cost Estimation
3. Results
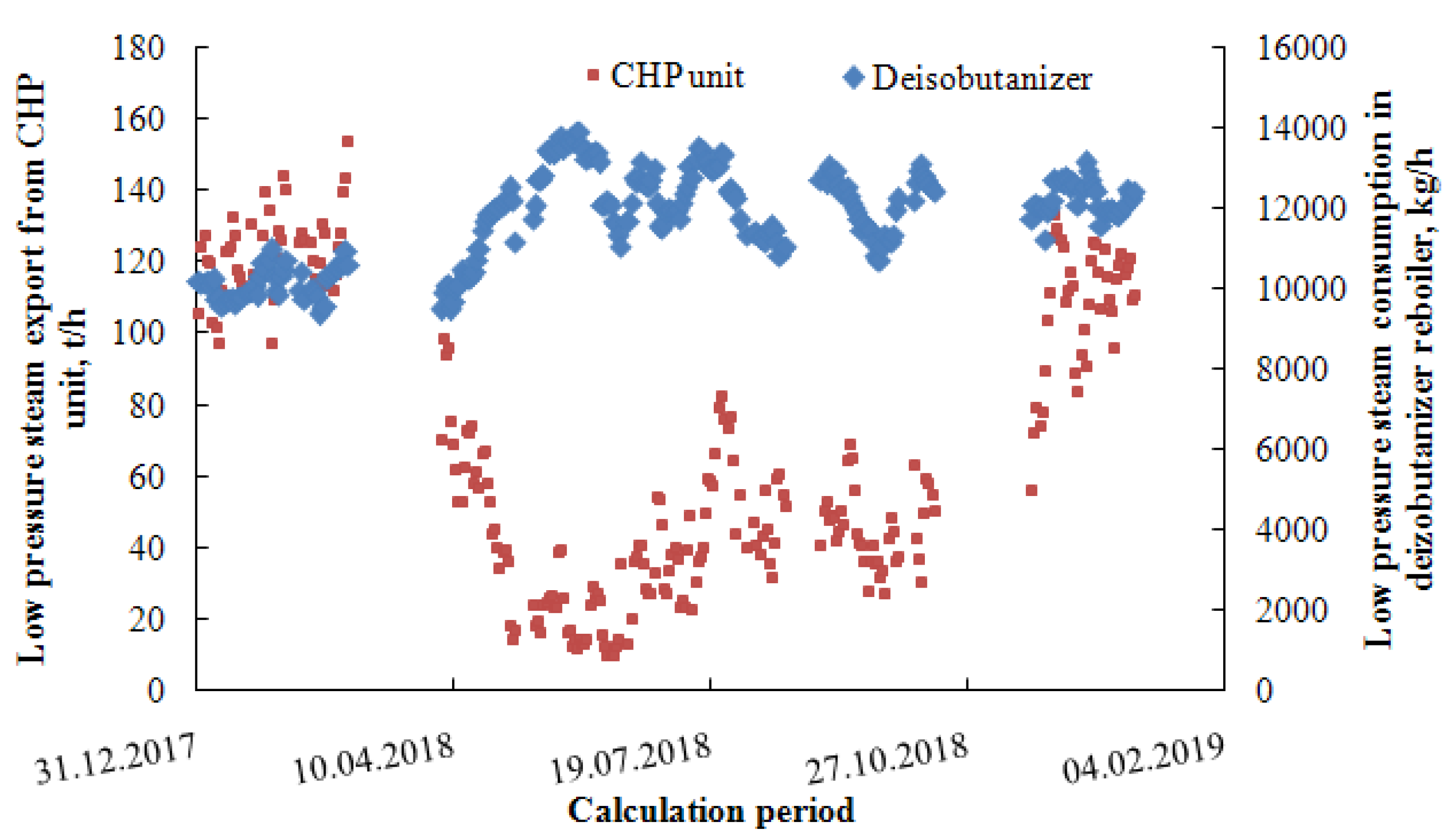
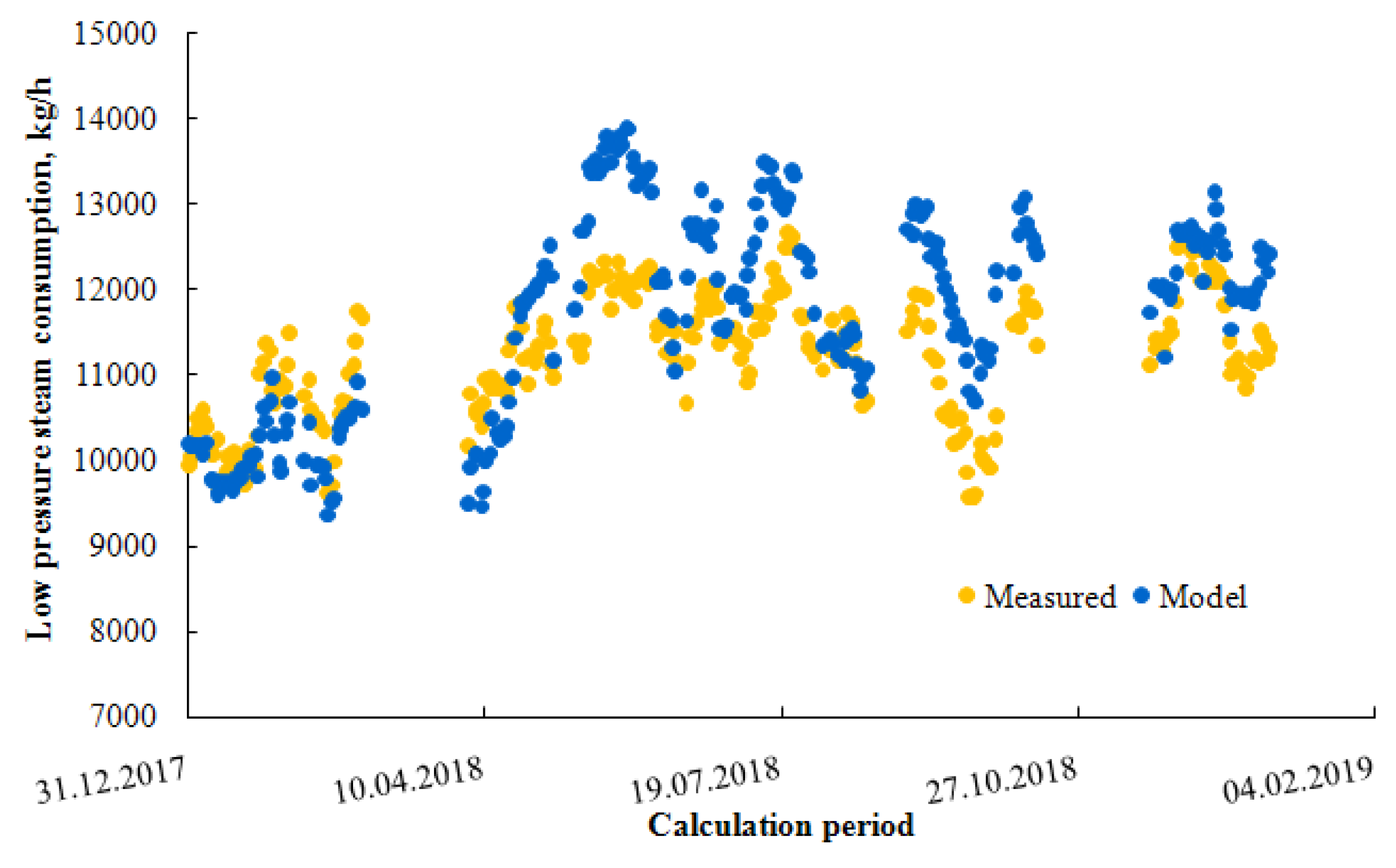
3.1. Model Verification
3.2. Investment Scope and Cost
3.3. CO2 Balances
3.4. Economics and Sensitivity Analysis
- The whole refinery, including its CHP unit, has to be included in the balancing scheme when evaluating the energetic, ecological, and economic performance of any process revamp.
- System specificities and seasonal operation variations must be incorporated in the calculation model to yield a realistic assessment of proposed revamp alternatives. Technical and operational constraints resulting from these system features affect their economics significantly, leading to e.g., periods with economically infeasible operation during summer.
- CO2 balancing on the refinery level is insufficient to assess the ecological footprint of the individual revamp alternatives. CO2 emissions change resulting from the refinery’s electric energy balance can be comparable or even larger than that of the refinery itself.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | |
| a | year, Equation (39) |
| area, m2 | |
| Equation (8) parameters | |
| benefit over time, € hour−1, € year−1 | |
| Equation (11) parameters | |
| cost, € | |
| cost over time, € h−1 | |
| Equation (12) parameters | |
| coefficient of performance | |
| isobaric molar heat capacity, kmol kg−1 K−1 | |
| d | discount rate |
| electric energy production rate, kJ h−1 | |
| mechanical energy consumption, kJ h−1 | |
| Murphree stage efficiency | |
| emission factor | |
| feedstock molar flow rate, kmol h−1 | |
| molar enthalpy, kJ kmol−1 | |
| heat of vaporization, kJ kmol−1 | |
| vapor-liquid equilibrium constant | |
| liquid phase molar flow rate, kmol h−1 | |
| lower heating value, kJ kg−1 | |
| mass flow rate, kg h−1 | |
| molar mass, kg kmol−1 | |
| number of components | |
| NPV | Net Present Value, € |
| pressure, kPa | |
| saturated vapor pressure, kPa | |
| power production rate, kJ h−1 | |
| payback period, year | |
| heat flux, kJ h−1 | |
| universal gas constant, J mol−1 K−1 | |
| temperature, °C | |
| thermodynamic temperature, K | |
| total investment cost, € | |
| overall heat transfer coefficient, W m−2 K−1 | |
| vapor phase molar flow rate, kmol h−1 | |
| component mass fraction | |
| liquid molar fraction | |
| vapor molar fraction | |
| compressibility factor | |
| Superscripts | |
| disch | discharge conditions |
| F | feedstock conditions |
| in | inlet conditions |
| IS | isoentropic conditions |
| L | liquid phase |
| max | maximal |
| min | minimal |
| net | netto value |
| out | outlet conditions |
| V | vapor phase |
| Subscripts | |
| add | additional |
| bp | backpressure |
| CHP | referring to Combined Heat and Power Unit |
| cond | referring to condensate |
| demi | demineralized (water) |
| DF | degrees of freedom |
| el | electric (efficiency) |
| eng | referring to engine |
| evap | referring to evaporator |
| ext | external |
| f | fuel numbering |
| h | time period numbering (hour) |
| heat | referring to waste heat |
| HEX | referring to heat exchanger |
| hp | referring to heat pump |
| i | component numbering |
| imp | import |
| IS | isoentropic (efficiency) |
| j | stage numbering |
| LPS | referring to low pressure steam |
| marg | marginal |
| mech | mechanical (efficiency) |
| r | reference |
| R | reduced |
| reb | referring to reboiler |
| rec | recuperated |
| ref | referring to refinery |
| s | pressure level numbering |
| st | referring to steam turbine |
| th | thermal (efficiency) |
| Greek symbols | |
| Poisson’s coefficient | |
| efficiency | |
Abbreviations
| C4 | hydrocarbons with four carbon atoms in the molecule |
| CHP | combined heat and power unit |
| MESH | mass, equilibrium, summation and heat balances |
| SC | simultaneous correction |
References
- Energy Information Administration. International Energy Outlook 2016; Energy Information Administration, U.S. Department of Energy: WA, USA, 2016. Available online: https://www.eia.gov/outlooks/ieo/pdf/0484(2016).pdf (accessed on 15 January 2019).
- Morrow, W.R., III; Marano, J.; Hasanbeigi, A.; Masanet, E.; Sathaye, J. Efficiency improvement and CO2 emission reduction potentials in the United States petroleum refining industry. Energy 2015, 93, 95–105. [Google Scholar] [CrossRef]
- Gorak, A.; Sorensen, E. Distillation: Fundamentals and Principles; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-386547-2. [Google Scholar]
- Worell, E.; Corsten, M.; Galitsky, C.H. Energy Efficiency Improvement and Cost Saving Opportunities for Petroleum Refineries. In An ENERGY STAR® Guide for Energy and Plant Managers; A Document Prepared for The United States Environmental Protection Agency; 2015. Available online: www.energystar.gov/industry (accessed on 4 June 2018).
- García, N.; Fernández-Torres, M.J.; Caballero, J.A. Simultaneous environmental and economic process synthesis for isobutene alkylation. J. Clean. Prod. 2014, 81, 270–280. [Google Scholar] [CrossRef]
- Kumar, P.; Vermeiren, W.; Dath, J.P.; Hoelderich, W.F. Production of alkylated gasoline using ionic liquids and immobilized ionic liquids. Appl. Catal. A 2006, 304, 131–141. [Google Scholar] [CrossRef]
- Hiladgo, J.M.; Zbuzek, M.; Černý, R.; Jíša, P. Current uses and trends in isomerization, alkylation and etherification processes to improve gasoline quality. Cent. Eur. J. Chem. 2014, 12, 1–13. [Google Scholar]
- Di Girolamo, M.; Tagliabue, L. MTBE and alkylate co-production: Fundamentals and operating experience. Catal. Today 1999, 52, 307–319. [Google Scholar] [CrossRef]
- Khvostenko, N.N.; Lagutenko, N.M.; Kurylev, V.D.; Kirillov, D.V.; Esipko, B.A. Modernization of a sulfuric acid alkylation unit. Chem. Technol. Fuels Oils 2000, 36, 18–20. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, T.; Yang, Y.; Tang, S. Simulation and design microreactor configured with micromixers to intensify the isobutane/2-butene alkylation process. J. Taiwan Inst. Chem. Eng. 2019, 98, 53–62. [Google Scholar] [CrossRef]
- Tsadkin, M.A.; Badikova, A.D.; Mortikov, E.S. The formation of emulsions during the jet mixing of reagent flows as applied to the sulfuric acid alkylation of iso-butane with olefins. Theor. Found. Chem. Eng. 2016, 50, 165–170. [Google Scholar] [CrossRef]
- Tsadkin, M.A.; Badikova, A.D. Industrial trials of a new generation contactor for the process of the sulfuric-acid alkylation of isobutene with olefins. Theor. Found. Chem. Eng. 2018, 52, 246–257. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Zhao, G.; Zhou, Z.; Zhang, J.; Shi, C.H.; Liu, X.; Zhang, X.; Zhang, S. Simulation and optimization of fixed bed solid acid catalyzed isobutene/2-butene alkylation process. Fuel 2018, 216, 686–696. [Google Scholar] [CrossRef]
- Ivanchina, E.D.; Kirgina, M.V.; Chekantsev, N.V.; Sakhnevich, B.V.; Sviridova, E.V.; Romanovskiy, R.V. Complex modeling system for optimization of compounding process in gasoline pool to produce high-octane finished gasoline fuel. Chem. Eng. J. 2015, 282, 194–205. [Google Scholar] [CrossRef]
- Nurmakanova, A.E.; Ivashkina, E.N.; Ivanchina, E.D.; Dolganov, I.A.; Boychenko, S.S. Predicting alkylate yield and its hydrocarbon composition for sulfuric acid catalyzed isobutane alkylation with olefins using the method of mathematical modeling. Procedia Chem. 2015, 15, 54–64. [Google Scholar] [CrossRef]
- Kiss, A.A.; Ferreira, C.A.I. Chapter 10. Case studies. In Heat Pumps in Chemical Process Industry; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 9781315371030. [Google Scholar]
- Liu, L.; Zhu, L.; Sun, L.; Zhu, M.; Liu, G. Simulation and optimization of different pressure thermally coupled distillation for separating a close-boiling mixture of n-butanol and iso-butanol. Appl. Petrochem. Res. 2017, 7, 143–150. [Google Scholar] [CrossRef]
- Demirel, Y. Thermodynamic Analysis of Separation Systems. Papers Therm. Mech. 2004, 39, 3897–3942. [Google Scholar] [CrossRef]
- Lee, J.; Son, Y.; Lee, K.S.; Won, W. Economic analysis and environmental impact assessment of heat pump-assisted distillation in a gas fractionation unit. Energies 2019, 12, 852. [Google Scholar] [CrossRef]
- Danilov, Y.; Sinkevich, I.; Lavrova, I.; Mardupenko, A.; Tulskaya, A. Energy saving technologies in the petroleum refining processes. Pet. Coal 2018, 60, 128–133. [Google Scholar]
- Kazemi, A.; Hosseini, M.; Mehrabani-Zeinabad, A.; Faizi, V. Evaluation of different vapor recompression distillation configurations based on energy requirements and associated costs. Appl. Therm. Eng. 2016, 94, 305–313. [Google Scholar] [CrossRef]
- Variny, M.; Furda, P.; Kováč, N.; Mierka, O. Analysis of C3 fraction splitting system performance by mathematical modeling in MATLAB environment. Acta Chim. Slovaca 2019, 12, 127–135. [Google Scholar] [CrossRef]
- Long, N.V.D.; Lee, M.Y. Design and optimization of heat integrated dividing wall columns for improved debutanizing and deisobutanizing fractionation of NLG. Korean J. Chem. Eng. 2013, 30, 286–294. [Google Scholar] [CrossRef]
- You, X.; Rodriguez-Donis, I.; Gerbaud, V. Reducing process cost and CO2 emissions for extractive distillation by double-effect heat integration and mechanical heat pump. Appl. Energy 2016, 166, 128–140. [Google Scholar] [CrossRef]
- Song, D.; Yoon, Y.-G.; Seo, S.-K.; Lee, C.H.-J. Improvement of 1,3-butadiene separation in 2,3-butanediol dehydration using extractive distillation. Processes 2019, 7, 410. [Google Scholar] [CrossRef]
- Asprion, N.; Rumpf, B.; Gritsch, A. Work flow in process development for energy efficient processes. Appl. Therm. Eng. 2011, 31, 2067–2072. [Google Scholar] [CrossRef]
- Yang, M.; Feng, X.; Liu, G. Heat integration of heat pump assisted distillation into the overall process. Appl. Energy 2016, 162, 1–10. [Google Scholar] [CrossRef]
- van de Boer, D.M.; Ferreira, C.A.I.; Kiss, A.A. Low grade waste heat recovery using heat pumps and power cycles. Energy 2015, 89, 864–873. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Industrial Heat Pumps for Steam and Fuel Savings. A Best Practice Steam Technical Brief. 2003. Available online: https://www.energy.gov/sites/prod/files/2014/05/f15/heatpump.pdf (accessed on 12 December 2019).
- Bütün, H.; Kantor, I.; Maréchal, F. Incorporating location aspects in process integration methodology. Energies 2019, 12, 3338. [Google Scholar] [CrossRef]
- Rimár, M.; Fedák, M.; Abraham, M.; Kulikov, A.; Váhovský, J. The efficiency of the cogeneration unit implemented in the CHS system in terms of heat generation. Acta Tecnol. Int. Sci. J. Technol. 2017, 3, 9–13. [Google Scholar] [CrossRef]
- Variny, M.; Blahušiak, M.; Mierka, O.; Godó, Š.; Margetíny, T. Energy saving measures from their cradle to full adoption with verified, monitored, and targeted performance: A look back at energy audit at Catalytic Naphtha Reforming Unit (CCR). Energy Eff. 2019, 12, 1771–1793. [Google Scholar] [CrossRef]
- Sarco, S. The steam and condensate loop. In Effective Steam Engineering for Today; Spirax-Sarco Limited: Cheltenham, UK, 2011; ISBN 978-0-9550691-5-4. [Google Scholar]
- INNIO. Product Flyer, Jenbacher j920 Flextra Combustion Engine. 2019. Available online: https://www.innio.com/en/products/jenbacher/j920-flextra#brochures/j920-flextra-brochure (accessed on 11 October 2019).
- Rimár, M.; Abraham, M.; Fedák, M.; Kulikov, A.; Oravec, P.; Váhovský, J. Methods for increasing the efficiency of cogeneration based energy equipment. Modern Mach. Sci. J. 2019, 12, 2935–2938. [Google Scholar] [CrossRef]
- Flimel, M. Potential Optimisation of Heat Pump Placement in Terms of Environmental Noise Levels. In Heat Pumps. Performance and Applications; Espenson, T., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2018; ISBN 978-1-53614-341-6. [Google Scholar]
- Bejan, A. Advanced Engineering Thermodynamics, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1119245964. Available online: https://app.knovel.com/hotlink/toc/id:kpAETE0003/advanced-engineering/advanced-engineering (accessed on 5 October 2019).
- Yaws, C.L. Yaws’ Critical Property Data for Chemical Engineers and Chemists; Knovel, 2014; ISBN 978-1-61344-932-5. Available online: https://app.knovel.com/hotlink/toc/id:kpYCPDCECD/yaws-critical-property/yaws-critical-property (accessed on 6 October 2019).
- Variny, M.; Jediná, D.; Kizek, J.; Illés, P.; Lukáč, L.; Janošovský, J.; Lesný, M. An Investigation of the Techno-Economic and Environmental Aspects of Process Heat Source Change in a Refinery. Processes 2019, 7, 776. [Google Scholar] [CrossRef]
- Smith, C.N.; Hittinger, E. Using marginal emission factors to improve estimates of emission benefits from appliance efficiency upgrades. Energy Eff. 2019, 12, 585–600. [Google Scholar] [CrossRef]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; International Edition; McGraw-Hill: New York, NY, USA, 2003; ISBN 0-07-239266-5. [Google Scholar]
- Holland, F.A. Process Economics. In Perry’s Chemical Engineers’ Handbook, 7th ed.; Perry, R.H., Ed.; McGraw-Hill Professional: London, UK, 1997; ISBN 0-07-049841-5. [Google Scholar]
- Variny, M.; Blahušiak, M.; Janošovský, J.; Hruška, M.; Mierka, O. Optimization study on a modern regeneration boiler cold end operation and its feedwater system integration into energy system of a paper mill. Energy Eff. 2019, 12, 1595–1617. [Google Scholar] [CrossRef]
- Boonekamp, P.G.M. Evaluation of methods used to determine realized energy savings. Energy Policy 2006, 34, 3977–3992. [Google Scholar] [CrossRef]
- El-Temtamy, S.A.; Gendi, T.S. Economic evaluation and sensitivity analysis of some fuel oil upgrading processes. Egypt. J. Pet. 2014, 23, 397–407. [Google Scholar] [CrossRef]
- Eurostat. Annual Average Change of Harmonized Index of Consumer Prices in the 2007–2018 Period. 2019. Available online: https://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&language=en&pcode=tec00118&plugin=1 (accessed on 10 January 2020).
- Seader, J.D.; Henley, E.J.; Roper, D.K. Separation Process Principles with Applications using Process Simulators, 3rd ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0470481837. [Google Scholar]
- Towler, G.; Sinnot, R. Chemical Engineering Design—Principles, Practice and Economics of Plant and Process Design, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0080966601. Available online: https://app.knovel.com/hotlink/toc/id:kpCEDPPEP4/chemical-engineering/chemical-engineering (accessed on 5 October 2019).
- Friend, D.G. Physical and Chemical Data. In Perry’s Chemical Engineers’ Handbook, 7th Ed.; Perry, R.H., Ed.; McGraw-Hill Professional: London, UK, 1997; ISBN 0-07-049841-5. [Google Scholar]
- Yaws, C.L. Yaws’ Handbook of Thermodynamic Properties for Hydrocarbons and Chemicals; Knovel, 2009; ISBN 978-1-60119-797-9. Available online: https://app.knovel.com/hotlink/toc/id:kpYHTPHC09/yaws-handbook-thermodynamic/yaws-handbook-thermodynamic (accessed on 5 October 2019).
- Coker, A.K. Ludwig’s Applied Process Design for Chemical and Petrochemical Plants, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, ISBN 978-0080942094. Available online: https://app.knovel.com/hotlink/toc/id:kpLAPDCP02/ludwigs-applied-process/ludwigs-applied-process (accessed on 6 October 2019).
- CHP Unit Scheme. Available online: http://www.cmep.sk/index.php/sk/teplaren (accessed on 11 March 2019). (In Slovak).
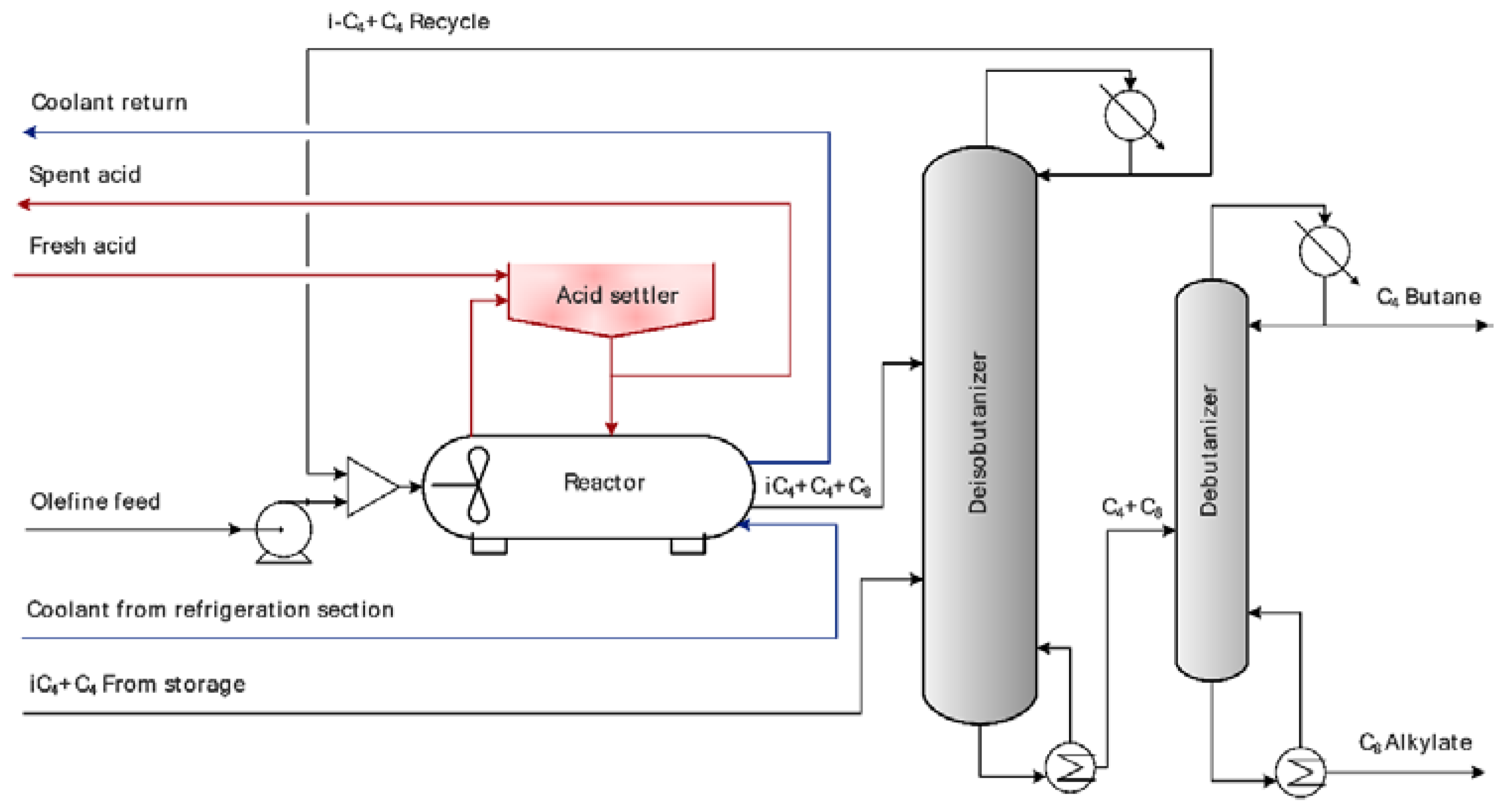

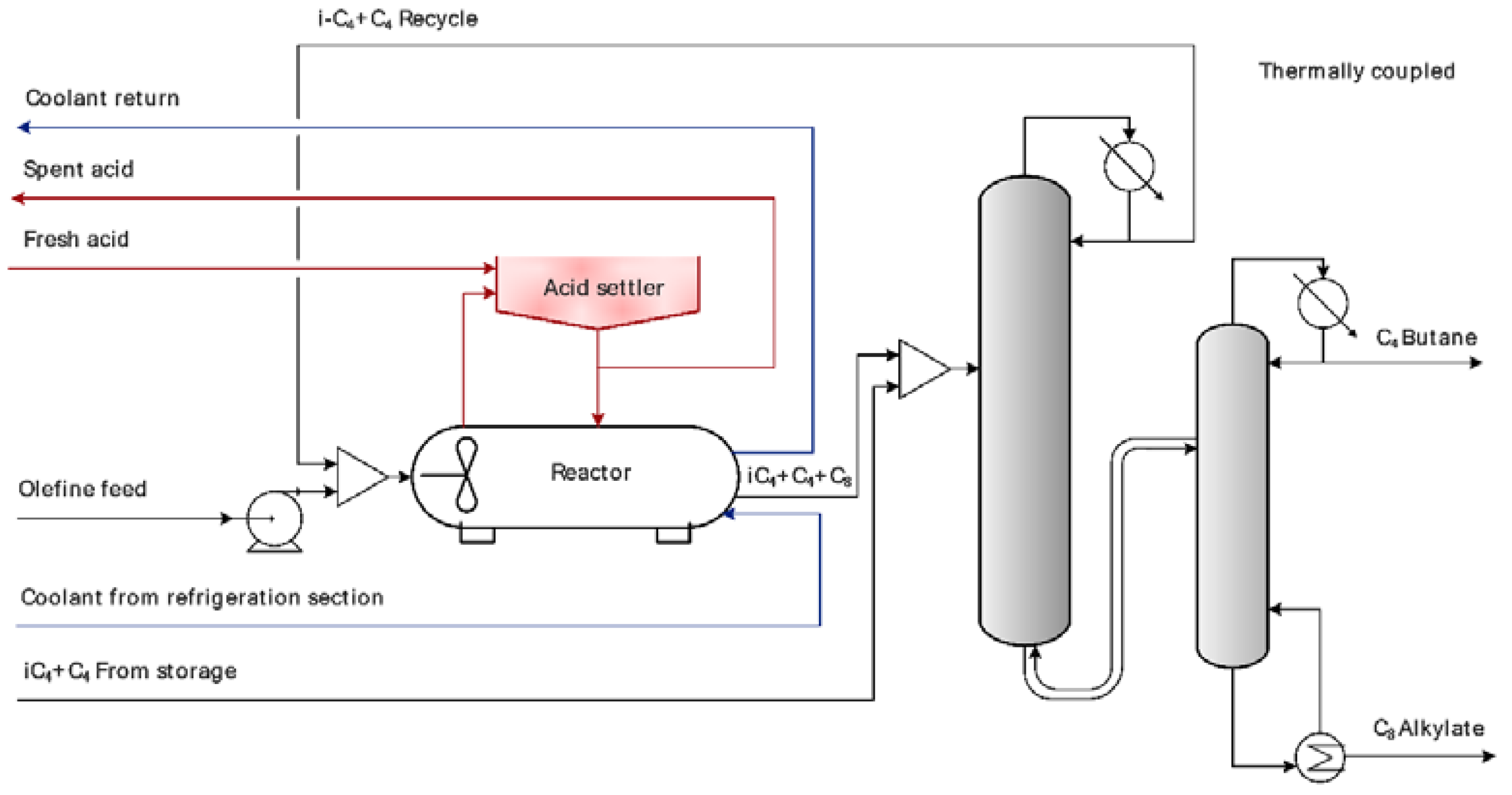
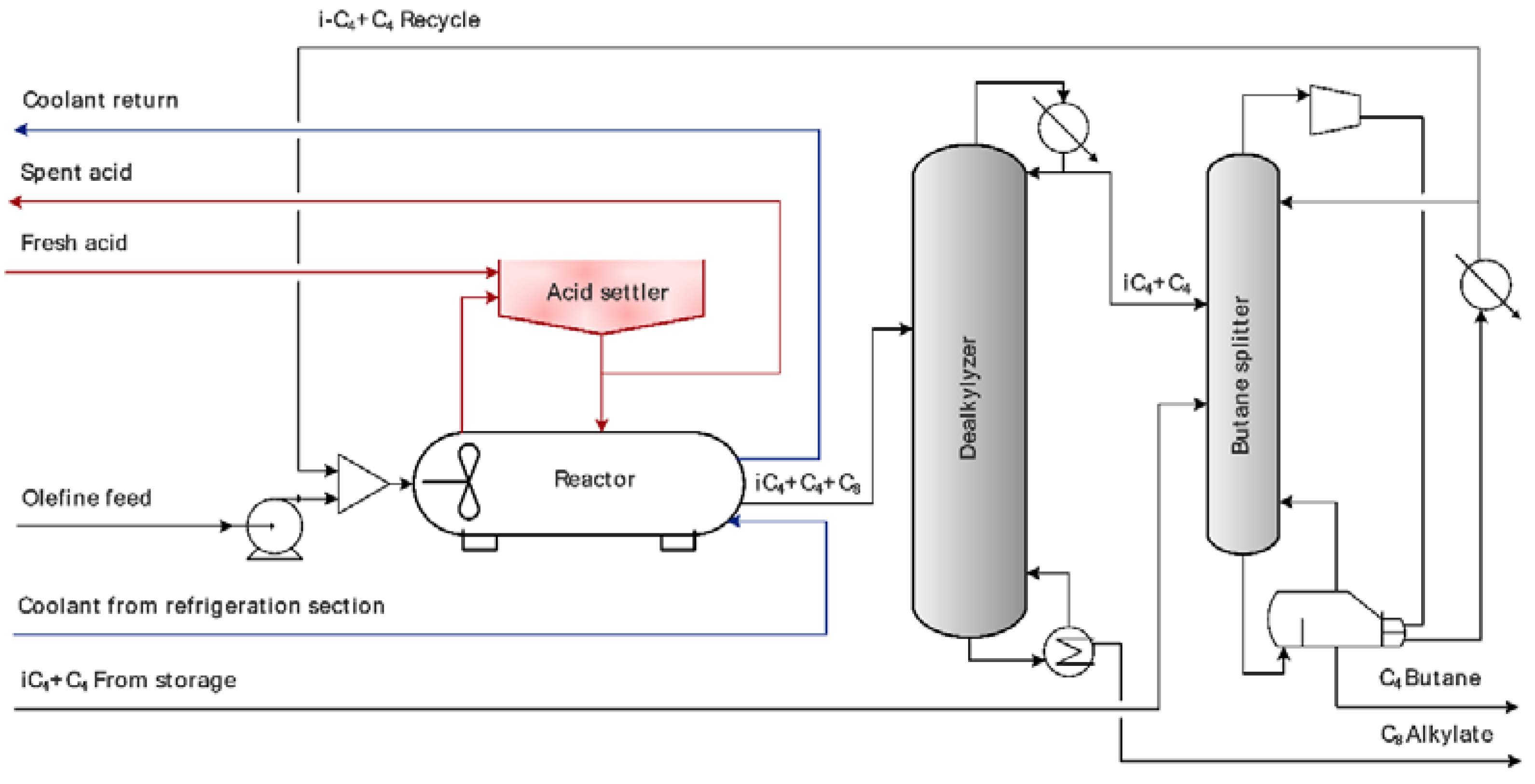

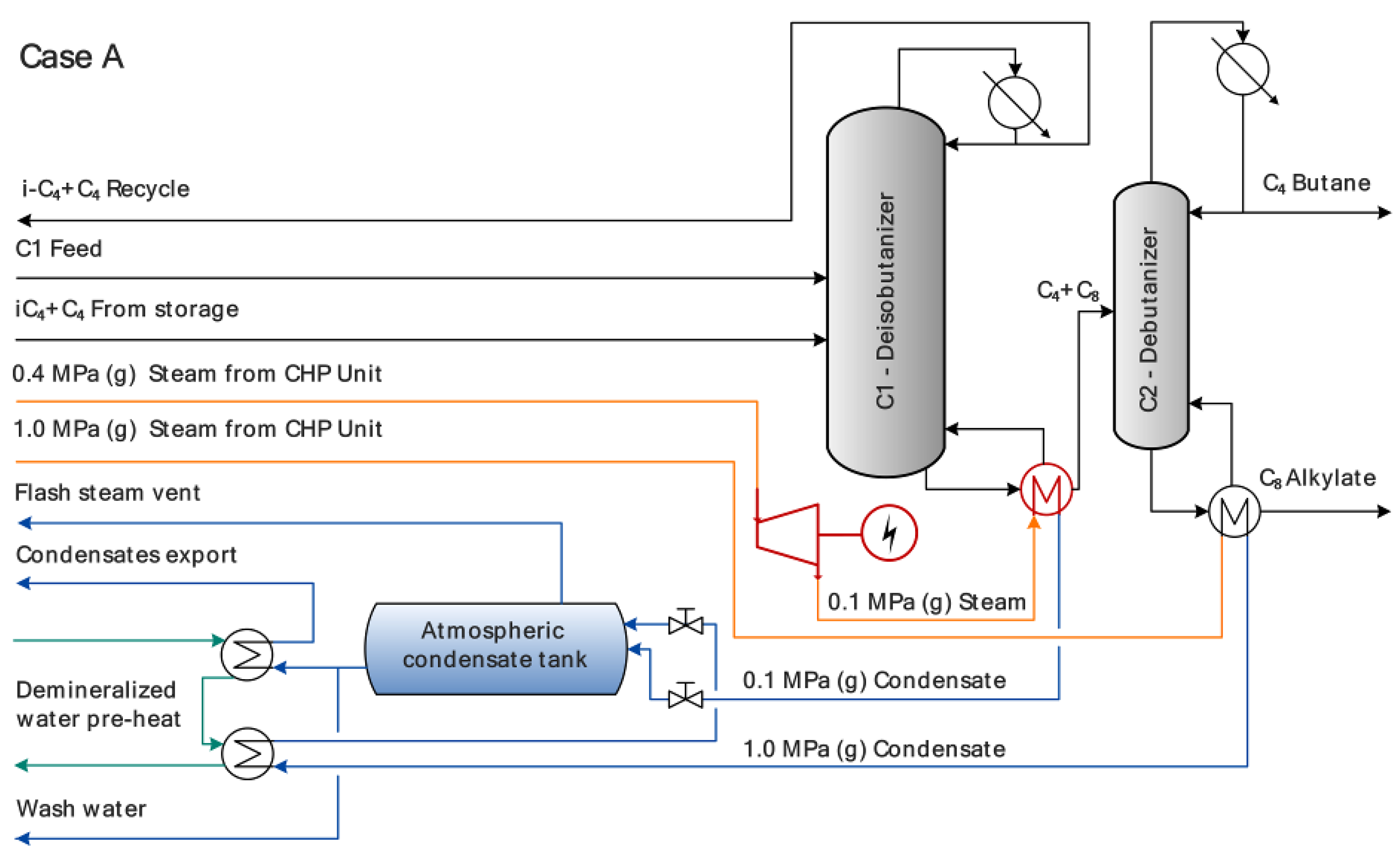
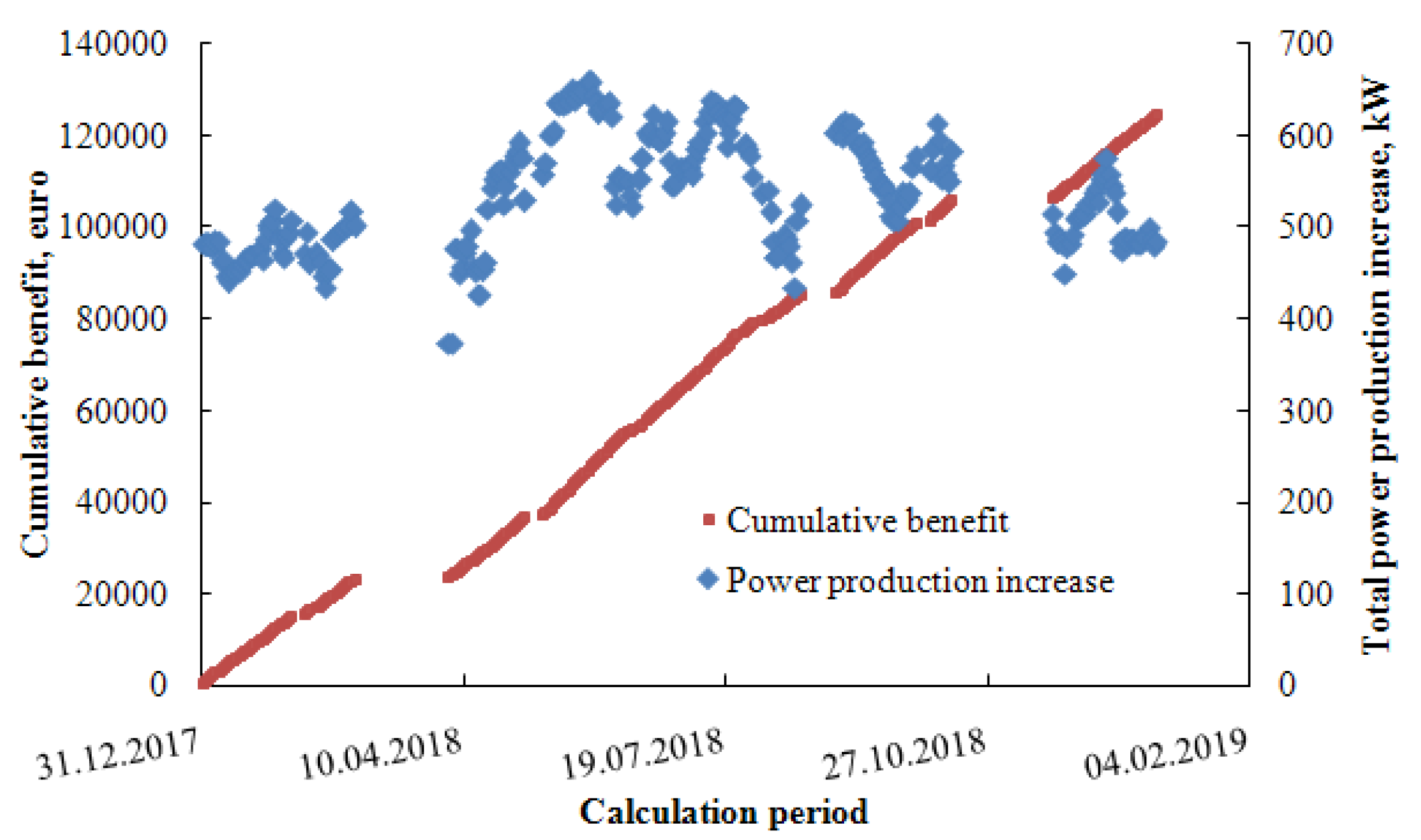
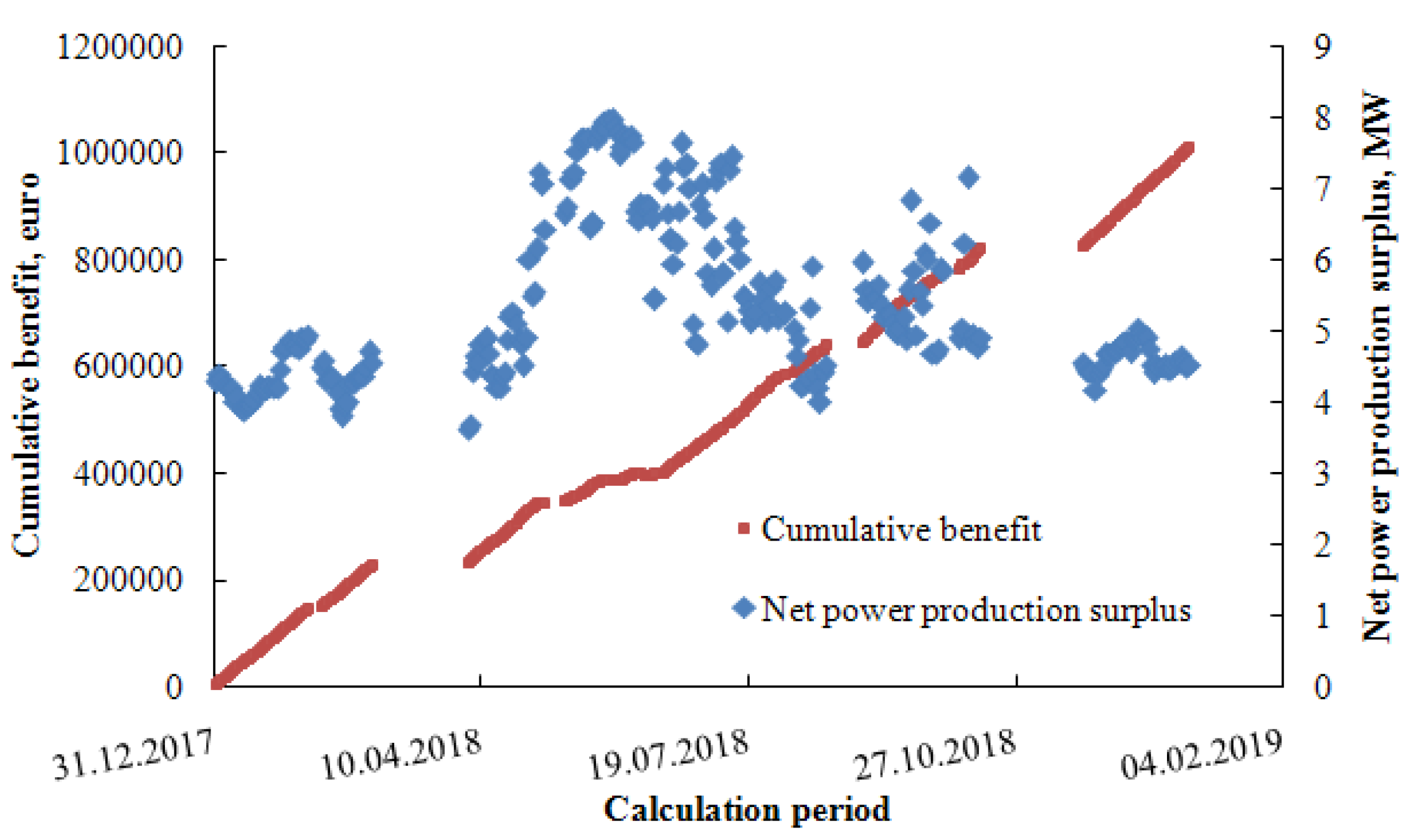
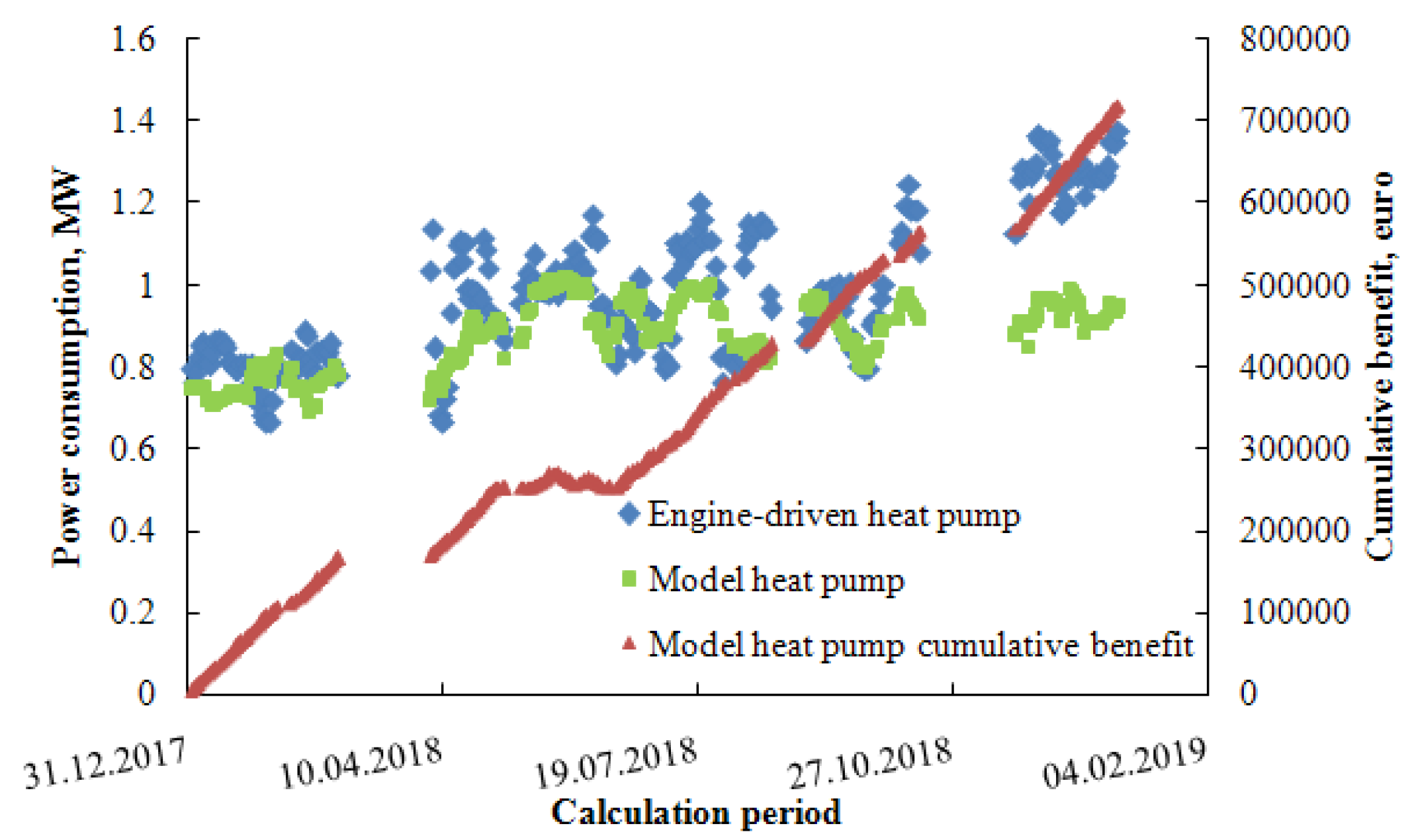
| Parameter | Numerical Value |
|---|---|
| Number of Stages | 70 |
| Alkylate Feed Stage Number | 8 |
| Butane Fraction Feed Stage Number | 29 |
| Column Head/Bottom Pressure *, bar | 6/6.5 |
| Alkylate Feed Temperature *, °C | 35 |
| Butane Fraction Feed Temperature *, °C | 48 |
| Alkylate Feed Vapor Fraction * | 0 |
| Butane Fraction Feed Vapor Fraction * | 0 |
| Alkylate Feed Composition, mol % | |
| Alkylate * | 17.00 |
| i-Butane * | 63.70 |
| n-Butane * | 15.90 |
| Propane * | 1.70 |
| n-Pentane | 1.70 |
| Butane Fraction Feed Composition, mol % | |
| Alkylate * | 0 |
| i-Butane * | 37.78 |
| n-Butane * | 58.22 |
| Propane * | 2.00 |
| n-Pentane | 2.00 |
| Column Bottoms’ Quality Requirement | |
| Content of i-Butane *, mol % | 0.70 |
| Component | A1 | A2.10−3 | A3 | A4 | A5.106 | A6 |
|---|---|---|---|---|---|---|
| Alkylate (Isooctane) | 77.81 | −6.805 | 0 | −9.439 | 6.755 | 2 |
| Isobutane | 58.78 | −4.317 | 0 | −7.017 | 10.37 | 2 |
| n-Butane | 66.94 | −4.604 | 0 | −8.255 | 11.57 | 2 |
| Propane | 52.38 | −3.491 | 0 | −6.109 | 11.19 | 2 |
| n-Pentane | 63.33 | −5.118 | 0 | −7.483 | 7.766 | 2 |
| Component | B1 | B2.10 | B3.103 | B4.106 | B5.108 |
|---|---|---|---|---|---|
| Alkylate (isooctane) | 95 275 | 6 967 | −1 376.5 | 2 173.4 | 0 |
| Isobutane | 71.791 | 4.8472 | −2.0519 | 4.0634 | 0 |
| n-Butane | 62.873 | 5.8913 | −2.3588 | 4.2257 | 0 |
| Propane | 121.525 | −10.9821 | 10.9178 | −43.0167 | 6.28137 |
| n-Pentane | 80.641 | 6.2195 | −2.2682 | 3.7423 | 0 |
| Component | C1.10-7 | C2 | C3 | C4 |
|---|---|---|---|---|
| Alkylate | 4.7721 | 0.37992 | 0 | 0 |
| Isobutane | 3.1667 | 0.38550 | 0 | 0 |
| n-Butane | 3.6238 | 0.83370 | −0.82274 | 0.39613 |
| Propane | 2.9209 | 0.78237 | −0.77319 | 0.39246 |
| n-Pentane | 3.9109 | 0.38681 | 0 | 0 |
| Model Parameter | Equation Number | Numeric Value |
|---|---|---|
| , % | 17 | 97 |
| , % | 18 | 75 |
| , % | 20 | 40 |
| , % | 21 | 47 |
| , % | 22 | 75 |
| , % | 22 | 97 |
| ,°C | 25 | 80 |
| ,°C | 25 | 10 |
| ,°C | 26 | 10 |
| Live Steam from Boilers → High Pressure Steam | Live Steam from Boilers → Middle Pressure Steam | Live Steam from Boilers → Low Pressure Steam |
|---|---|---|
| 50 | 105 | 160 |
| Model Parameter | Equation Number | Numeric Value |
|---|---|---|
| for heavy fuel oil, % | 31 | 0.873 |
| for natural gas, % | 31 | 0.748 |
| average, kgCO2/MWh | 32 | 135.5 |
| marginal, kgCO2/MWh | 32 | 900 |
| LHV for heavy fuel oil, MJ/kg | 35 | 40.5 |
| LHV for natural gas, MJ/kg | 35 | 48.9 |
| Parameter | Deisobutanizer Feed | Distillate | Bottoms |
|---|---|---|---|
| Molar flow, kmol/h | 805.25 | 571.09 | 234.16 |
| Composition, % mol | |||
| Isooctane | 14.21 | 0.00 | 48.87 |
| n-pentane | 1.84 | 0.12 | 6.03 |
| n-butane | 22.84 | 13.92 | 44.59 |
| i-butane | 59.36 | 83.49 | 0.51 |
| propane | 1.75 | 2.47 | 0.00 |
| Reflux ratio | 0.91 | ||
| Column head temperature, °C | 48.4 | ||
| Column bottom temperature, °C | 93.4 | ||
| Heat duty reboiler, kW | 6210 | ||
| Steam consumed in reboiler, calculated, t/h | 10.92 | ||
| Steam consumed in reboiler, measured, t/h | 11.38 | ||
| Equipment | Design Heat Duty, MW | Heat Exchange Surface Needed, m2 | Purchased Cost Estimate (Year 2002), k€ | Purchased Cost Estimate (Year 2020) Corrected by Pressure Factors and Materials, k€ |
|---|---|---|---|---|
| Reboiler for C1 | 10 | 2 HXs, 650 each | 130 | 190 |
| Heat pump evaporator | 10 | 2 HXs, 650 each | 130 | 190 |
| Total purchased heat exchangers cost estimate, 103 €, year 2020 | 380 = 100% | |||
| Purchased equipment installation | 50% | |||
| Combustion engine installed cost estimate, 103 €, year 2020 | 2000 | |||
| Instrumentation and controls | 45% | |||
| Piping installed | 60% | |||
| Electrical systems | 20% | |||
| Buildings | 5% | |||
| Yard improvements | 15% | |||
| Service facilities installed | 15% | |||
| Engineering and supervision | 45% | |||
| Construction expenses | 40% | |||
| Legal expenses | 5% | |||
| Contractor’s fee | 20% | |||
| Contingency | 30% | |||
| TIC estimate, excl. heat pump compressor part, millions of €, year 2020 | 7.6 | |||
| Heat pump compressor TIC estimate, millions of €, year 2020 | 1.5 | |||
| TIC estimate, millions of €, year 2020 | 9.1 | |||
| Case | A | B | Model Heat Pump * |
|---|---|---|---|
| Estimated TIC, millions of €, year 2020 | 1.15 | 9.1 | 4.6 |
| Classification of Emissions | Case A | Case B | Model Heat Pump |
|---|---|---|---|
| Refinery only, kton/year | 0.342 | 3.551 | −15.507 |
| External CO2 emissions, emission factor SE, a.s. | −0.449 | −4.489 | 1.795 |
| Overall CO2 emissions | −0.107 | −0.938 | −13.712 |
| External CO2 emissions, marginal factor coal power plant | −2.972 | −29.653 | 11.877 |
| Overall CO2 emissions | −2.630 | −26.102 | −3.630 |
| Cumulative Benefit, Thousands of € Per Year/Simple Payback Period, Years/NPV, Thousands of € | Case A | Case B | Model Heat Pump * |
|---|---|---|---|
| Base scenario | 124.6/9.22/62.6 | 1011.0/9.00/739.1 | 718.7/6.40/2394.4 |
| Fuel price + 10% | 116.3/9.89/−18.2 | 905.0/10.06/−292.5 | 856.1/5.84/3731.6 |
| Produced electricity price + 10% | 146.1/7.87/271.8 | 1225.1/7.43/2822.7 | 633.0/7.90/1560.4 |
| CO2 price + 100% | 117.8/9.76/−3.6 | 940.0/9.68/48.1 | 1028.9/4.86/5413.3 |
| Heat price €2 per GJ for model heat pump | - | - | 421.7/11.86/−496.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Variny, M.; Furda, P.; Švistun, L.; Rimár, M.; Kizek, J.; Kováč, N.; Illés, P.; Janošovský, J.; Váhovský, J.; Mierka, O. Novel Concept of Cogeneration-Integrated Heat Pump-Assisted Fractionation of Alkylation Reactor Effluent for Increased Power Production and Overall CO2 Emissions Decrease. Processes 2020, 8, 183. https://doi.org/10.3390/pr8020183
Variny M, Furda P, Švistun L, Rimár M, Kizek J, Kováč N, Illés P, Janošovský J, Váhovský J, Mierka O. Novel Concept of Cogeneration-Integrated Heat Pump-Assisted Fractionation of Alkylation Reactor Effluent for Increased Power Production and Overall CO2 Emissions Decrease. Processes. 2020; 8(2):183. https://doi.org/10.3390/pr8020183
Chicago/Turabian StyleVariny, Miroslav, Patrik Furda, Ladislav Švistun, Miroslav Rimár, Ján Kizek, Norbert Kováč, Peter Illés, Ján Janošovský, Jakub Váhovský, and Otto Mierka. 2020. "Novel Concept of Cogeneration-Integrated Heat Pump-Assisted Fractionation of Alkylation Reactor Effluent for Increased Power Production and Overall CO2 Emissions Decrease" Processes 8, no. 2: 183. https://doi.org/10.3390/pr8020183
APA StyleVariny, M., Furda, P., Švistun, L., Rimár, M., Kizek, J., Kováč, N., Illés, P., Janošovský, J., Váhovský, J., & Mierka, O. (2020). Novel Concept of Cogeneration-Integrated Heat Pump-Assisted Fractionation of Alkylation Reactor Effluent for Increased Power Production and Overall CO2 Emissions Decrease. Processes, 8(2), 183. https://doi.org/10.3390/pr8020183








