Abstract
Lactobacillus johnsonii and Lactobacillus zeae are among the lactobacilli with probiotic properties, which occur in sour milk products, cheeses, and to a lesser extent in raw milk. Recently, resistant strains have been detected in various species of lactobacilli. The aim of the study was to determine the incidence of resistant Lactobacillus johnsonii and Lactobacillus zeae strains in various types of raw milk. A total of 245 isolates were identified by matrix-assisted laser desorption/ionization mass spectrometry and polymerase chain reaction methods as Lactobacillus sp., of which 23 isolates of Lactobacillus johnsonii and 18 isolates of Lactobacillus zeae were confirmed. Determination of susceptibility to selected antibiotics was performed using the E-test and broth dilution method, where 7.3% of lactobacilli strains were evaluated as ampicillin-resistant, 14.7% of isolates as erythromycin-resistant, and 4.9% of isolates as clindamycin-resistant. The genus Lactobacillus johnsonii had the highest resistance to erythromycin (34.8%), similar to Lactobacillus zeae (33.3%). Of the 41 isolates, the presence of the gene was confirmed in five Lactobacillus johnsonii strains and in two strains of Lactobacillus zeae. The presence of resistant strains of Lactobacillus johnsonii and Lactobacillus zeae is a potential risk in terms of spreading antimicrobial resistance through the food chain.
1. Introduction
Lactobacilli belonging to the family Lactobacillaceae are one of the most important bacteria found in the dairy industry. Due to their beneficial effects, they are often used for the production of fermented foods or as probiotics [1,2]. Among them we include the so-called wild lactobacilli, such as Lactobacillus (Lb.) paracasei, Lb. praplantarum, Lb. plantarum, and Lb. johnsonii, which have antimicrobial as well as probiotic properties. The most common lactobacillus in fermented milk is Lb. johnsonii [3,4]. Lb. johnsonii is characterized by processing and protective properties in the production of cheese and beverages. Lb. johnsonii also have good potential probiotic properties, especially in terms of lysozyme resistance and simulated gastric juice environment [5]. Another important species of lactobacilli is Lactobacillus zeae, which is responsible for the hydrolysis of a cow’s milk protein [6]. It has a strong inhibitory effect on angiotensin converting enzyme (ACE) [7]. These species are found to a lesser extent in raw milk. Their number increases mainly during the production of dairy products. We rank these lactobacilli among non-starting lactic acid bacteria (LAB) [8].
Recently, lactobacilli, as well as other LAB, have been detected as a potential reservoir of antimicrobial resistance in the food chain [9], which has also been confirmed by the presence of resistant LAB strains (including lactobacilli) in the most-consumed fermented foods [10,11]. Lactobacilli are naturally resistant to most nucleic acid inhibitors and to antibiotics (aminoglycosides and vancomycin) [12]. Antimicrobial resistance to other groups of antibiotics is different in different species of lactobacilli. High antimicrobial resistance to lincosamides, macrolides, and streptogramin was confirmed in lactobacilli (42–70%) [13,14]. At the same time, resistance to erythromycin, encoded by erm genes, which are often considered to be potentially transmissible genetic determinants, has recently been detected [15,16].
There are more than 30 erm genes [17]. The ermB gene, which encodes an rRNA methylase acting on the 23S ribosomal subunit, is very often detected in erythromycin-resistant lactobacilli. Other erm genes, such as ermA, ermC or ermT, have been confirmed in lactobacilli [18,19]. These erm genes can be found in transposons and plasmids and are propagated by conjugation mechanisms. Their nucleotide sequence and analysis of adjacent regions in the gene can give important information about their origin and the process of obtaining and transferring these determinants [20]. The transfer of some genes between Lactobacillus strains, and also from lactobacilli to Gram-positive bacteria and vice versa, has been found [21].
Detection of the phenotypic manifestation of antimicrobial resistance in lactobacilli alone is difficult, due to the absence of standards for antibiotic susceptibility testing. Existing limit values have been developed only for some antibiotics and the most commonly used probiotic species of lactobacilli [22]. In addition, the recently detected minimum inhibitor concentration (MIC) values for lactobacilli indicate acquired resistance, but it is not entirely clear whether the resistance in lactobacilli is caused by chromosomal mutations or by the acquisition of genetic factors. For this reason, it is still necessary to analyze mentioned bacteria with acquired resistance [23].
Based on these facts, isolates of the genus Lactobacillus will be identified in the study using matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF-MS) and polymerase chain reaction (PCR) methods. Subsequently, antimicrobial resistance of Lb. johnsonii and Lb. zeae against selected antibiotics (ampicillin, clindamycin and erythromycin) will be detected by E-test and the broth microdilution method (BMM). At the same time, we want to confirm the presence of the ermB gene, which most often encodes erythromycin resistance in lactobacilli.
2. Materials and Methods
2.1. Isolation of Strains
In the study, samples of raw cows’, sheep’s, and goats’ milk were used, which came from production farms located in eastern Slovakia. Samples of freshly milked milk were taken into sterile sample boxes (approximately 10 mL), according to the the principles of milk sampling STN EN ISO 6887-5 (2010) (Slovak Standards Institute, Bratislava, Slovakia) [24]. Fresh milk samples were tested during storage on the first, third, and seventh days. In order to research Lactobacillus sp., a total of 60 milk samples were analyzed. Of these, 20 samples in the number were cows’, sheep´s, and goats’ milk, which were subjected to further examination.
Lactobacillus strains were isolated from the samples taken, according to the instructions of STN EN ISO 6887-1 (2017) (Slovak Standards Institute, Bratislava, Slovakia) [25]. From three successive dilutions, 0.1 mL of inoculum was inoculated by spreading on the surface of de Man, Rogosa, and Sharpe agar (MRS) selective diagnostic medium (Oxoid, Basingstoke, United Kingdom). Subsequent incubation was performed under anaerobic conditions at 37 °C for 24–72 h using AnaeroGen, which creates an anaerobic atmosphere (Oxoid, Basingstoke, United Kingdom).
For a more accurate identification of the genus Lactobacillus sp., suspected colonies were used, i.e., round cream–white convex colonies with a glossy surface of 2–5 mm. Selected colonies were inoculated into liquid BHI broth medium (Oxoid, Basingstoke, United Kingdom), using a sterile bacterial loop, and were incubated in anaerostat at 37 °C for 24–72 h under anaerobic conditions. After incubation, the propagated strains in liquid medium were used for genus and species identification by MALDI-TOF-MS and PCR.
2.2. Identification of Isolates
An extraction procedure using ethanol and formic acid was used to prepare samples for MALDI-TOF-MS identification. Analysis of the results was performed on an Ultraflex III instrument and Flex Analysis software, version 3.0. The results were evaluated using BioTyper software, version 1.1 (Bruker Daltonics, Massachusetts, United States), where the similarity between the mass spectra of the isolates and the reference mass spectrum of MALDI-TOF was expressed by scoring. A score greater than 2.30 represents a highly reliable identification at the species level; a score value between 2.00 and 2.29 means a highly reliable identification at the genus level, and a probable identification at the species level; a score value between 1.70 and 1.99 represents a reliable identification at the genus level; and a score value below 1.70 represents an unreliable identification [26]. The obtained identification results by MALDI-TOF-MS were confirmed by PCR method.
DNA from multiplied isolates was isolated using chelating agent Chelex 100 (Bio-Rad, Hertfordshire, United Kingdom), where 1.5 mL of bacterial culture was transferred to microtubes with 1 mL of sterile saline. The microtubes thus prepared were centrifuged at 10,000 g/10 min. The obtained sediment was resuspended in 200 mL of Chelex 100 chemical reagent and incubated at 95.5 °C/10 min. At the end of the incubation period, centrifugation was performed at 4 °C (13,000 g/3 min). The obtained supernatant was used as a source of DNA in PCR reactions.
Primers LbLMA (CTC AAA ACT AAA CAA AGT TCC) and R16 (ATG CGA TGC GAA TTT CTA ATT T) (AMPLIA s.r.o., Bratislava, Slovakia) [27] were used for genus identification of isolated strains by PCR method. The synthesized primers delimit the specific DNA sequence characteristic of the genus Lactobacillus spp., and where the size of the amplified fragment was 250 bp.
For species identification, we used ZeaI (TGT TTA GTT TTG AGG GGA CG) and ZeaII (CGT AAT GAG ATT TCA GTA GAT AAT ACA ACA) primers specific for Lb. zeae strains where the size of the amplified fragment was 185 bp. To identify Lb. johnsonii, JohSI (GAC CTT GCC TAG ATG ATT TTA) and 16SII (ACT ACC AGG GTA TCT AAT CC) primers were used, which delimited a specific sequence of 750 bp [11]. The Firepol Master Mix (Amplia s.r.o., Bratislava, Slovakia) was used in the PCR reactions.
Initiation denaturation was at 95 °C/3 min, followed by 30 amplification cycles (denaturation at 95 °C for 20 s; annealing at 55 °C/30 s for LbLMA/R16, and at 57 °C/30 s for ZeaI/ZeaII and JohSI/16SII; extension at 72 °C/2 min) was used to amplify specific sequences. The last cycle was followed by a final extension at 72 °C/10 min. Reference strains—Lb. zeae CCM 7069 and Lb. johnsonii CCM 2935 and Lb. paracasei CCM 4649 (Czech Collection of Microorganisms, Brno, Czech Republic)—were used to verify the specificity of the PCR reaction. The amplified PCR product in an amount of 5 μL was analyzed on a 1.5% agarose gel in Tris-Borate-EDTA (TBE) solution. We added Goldview Nucleic (Beijing SBS Genetech, Beijing, China) to the agarose gels for DNA visualization. Electrophoresis was performed for 1 h at 120 V. Individual PCR fragments were visualized using a Mini Bis Pro reader (DNR Bio-Imaging systems Ltd., Neve Yamin, Israel). The sizes of the resulting PCR products were determined based on their mobility in agarose gels compared to a 100 bp standard (Sigma-Aldrich, United States).
The identity of the PCR products with the selected primers was confirmed by a commercial company (GATC Biotech AG, Cologne, Germany). The DNA sequences obtained from strains were searched for homology to those available at the GenBank–EMBL (The European Molecular Biology Laboratory) database using the BLAST program (NCBI software package).
2.3. Antimicrobial Resistance
The phenotypic manifestation of antimicrobial resistance in strains of Lactobacillus sp. against ampicillin (AMP), erythromycin (ERY), and clindamycin (CLI) was determined using a semi-quantitative E-test method [28]. To determine antibiotic susceptibility by E-test, an inoculum adjusted to a standard density of 0.5 McF° (degree of McFarland’s turbidity standard) was inoculated onto the surface of Müller–Hinton agar medium enriched with 10% MRS agar (Oxford, United Kingdom). After soaking the inoculum, a test strip with a well-defined antibiotic content was applied sterile to the inoculated surface of the agar medium. The test plates thus prepared were incubated at 37 °C for 18–24 h. After the determined incubation, a teardrop-shaped zone of inhibition (drops) formed on the surface of the Petri dishes. The minimum inhibitor concentration (MIC) value was determined according to the manufacturer´s instructions, so that the resulting MIC value corresponded to the concentration of antibiotics on the strip where the edges of the inhibition zone converged. The E-test was read according to the manufacturer´s instructions, which specified reading at the point of complete inhibition of all growth.
Subsequently, antimicrobial resistance was determined using the broth microdilution method (BMM) [28]. Mueller–Hinton broth plates supplemented with lysed horse blood (Oxoid, Basingstoke, United Kingdom) were used for testing. The test plates contained concentrations of 0.125, 0.250, 0.500, 1.000, 2.000, 4.000, 8.000, 16.000, and 32.000 mg/L of each tested antibiotic. Streptococcus pneumoniae CCM 4501 (Czech collection of microorganisms, Brno, Czech Republic) was used as a reference strain. After incubation, the lowest concentrations of antibiotics that inhibited the visible growth of the test strains were determined. The results were evaluated according to document CLSI (Clinical laboratory Standards Institute, Wayne, Pennsylvania, US) [28].
After evaluating the phenotypic properties of antimicrobial resistance, the genetic determinant of erythromycin resistance of the ermB gene was detected using primers ermB2-F (GAAAAGGTACTCAACCAAATA) and ermB2-R (AGTAACGGTACTTAAATTGTTTAC), as well as the FIREpol Master Mix (Amplia s.r.o., Slovakia) [29]. The PCR reaction was performed as for genus and species identification of bacterial isolates, except for annealing, where the temperature was adjusted to 59 °C. The final PCR product was 639 bp in size. DNA sequences obtained from strains were searched for homology to those available from the GenBank–EMBL database using the BLAST program (NCBI software package). Staphylococcus aureus CCM 4223 showing the presence of the ermB gene was used as a reference strain [30].
2.4. Statistical Analyses
For statistical analyses, one-way analysis of variance (ANOVA) was used, along with a Tukey test for multiple comparison of means, with a confidence interval set at 95%, which was conducted with statistics software GraphPad Prism 8.3.0.538 (GraphPad Software, San Diego, CA, United States). The various types of milk were set as main factor.
3. Results
The occurrence of lactobacili in raw milk is well-known. They play an important role in the gastrointestinal tract of the consumer and in the production of fermented dairy products. Several types of lactobacili are present in individual kinds of milk. This study points on the possible occurrence of species Lb. johnsonii and Lb. zeae in raw milk and not only in fermented dairy products and cheeses.
During the cultivation of milk samples during the first, third, and seventh days, the lowest number of lactobacilli colonies per day was recorded during the seventh day, at 3.7 ± 0.1 log CFU/mL in cows’ milk and 1.8 ± 0.1 log CFU/mL in goats’ milk. The highest number of lactobacilli colonies in goats’ milk (2.0 ± 0.1 log CFU/mL) was measured on the first day of measurement. In cows’ milk, the number of colonies during the first and third days was the same (3.8 ± 0.1 log CFU/mL). In sheep’s milk, the number of colonies counted during the first, third, and seventh days did not change, and remained the same at 3.2 ± 0.1 log CFU/mL (Table 1).

Table 1.
Number of lactobacilli colonies (log CFU/mL) obtained by culture microbiological examination of milk samples using de Man, Rogosa, and Sharpe (MRS) agar medium (mean ± SD).
In our study, a total of 60 individual milk samples were examined, of which one-third was represented by raw cows’ milk, one-third by raw sheep’s milk, and one-third by raw goats’ milk. Table 1 shows the average values numbers of lactobacilli colonies (log CFU/mL) from all analyzed milk samples during the tested period. Microbial quality parameters represented by numbers of lactobacilli colonies (log CFU/mL) in different types of milk were changed during storage (Table 1). There was a statistically significant change (p < 0.05) in the goats’ milk in the number of lactobacilli between the first day (2.0 ± 0.1 log CFU/mL) and the seventh day (1.8 ± 0.1 log CFU/mL) of milk storage. No statistically significant change in the number of lactobacilli in cows’ and sheep’s milk was detected during the storage. However, on the first, third, and seventh measured days of the study, the number of lactobacilli colonies showed a statistically significant difference (p < 0.001) between all types of tested milk (cows’, sheep’s, and goats’).
By microbiological examination of the culture samples of individual types of raw milk (cows’, sheep’s, and goats’ milk), a total of 300 strains were isolated using the selective diagnostic medium MRS agar, which formed typical colonies according to their phenotypic growth characteristics.
For genus and species identification of individual isolated strains from all types of milk, the MALDI-TOF-MS method was used, which identified 252 isolates from 300 strains as Lactobacillus sp. with score values of 1.658–2.258. Subsequently, for further identification we selected only strains that were identified as Lb. johnsonii (26 isolates; 10.3%) and Lb. zeae (19 isolates; 7.5%) (Table 2). The score value ranged from 1.698 to 2.176 with Lb. johnsonii and from 1.734 to 2.246 for Lb. zeae. The score value of the isolates was below 2.300, which means that the identification of the isolates at the species level was not sufficiently reliable; therefore, the PCR method was used to confirm the results obtained by MALDI-TOF-MS (Table 2).

Table 2.
The number of identified strains of Lactobacillus sp. and Lb. johnsonii and Lb. zeae species in milk samples.
Subsequently, the molecular PCR method was used for accurate identification at the genus and species level [27].
In Figure 1 a PCR product of 250 bp in length was detected using specific primers for the genus Lactobacillus. Based on the visualized fragments and their subsequent sequencing, the genus Lactobacillus sp. confirmed in 245 strains isolated from samples of all types of raw milk. The species identification of isolates was performed by using specific synthesized primers [15]. Lb. johnsonii (Figure 2) and Lb. zeae (Figure 3) have been identified.
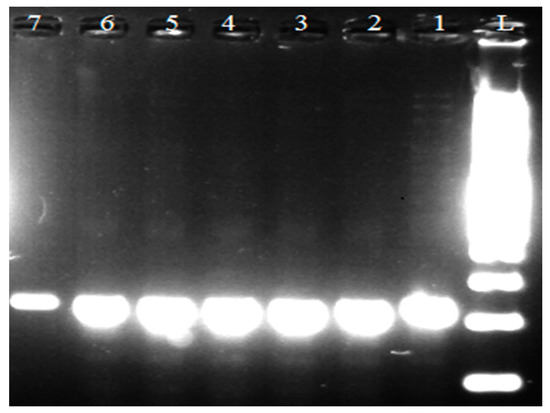
Figure 1.
Identification of genus Lactobacillus sp. L: 100 bp ladder standard; lines 1, 2, 3, and 4: isolates of Lactobacillius sp. (250 bp); line 5: Lb. johnsonii reference strain CCM 2935; line 6: Lb. zeae reference strain CCM 7069; line 7: Lb. paracasei reference strain CCM 4649.
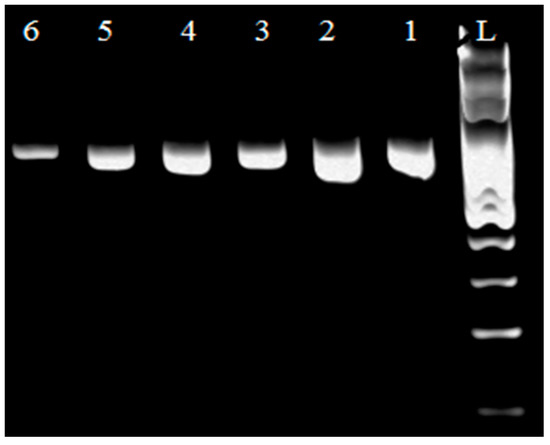
Figure 2.
Identification of genus Lb. johnsonii. L: 100 bp ladder standard; lines 1, 2, 3, 4, and 5: Lb. johnsonii isolates (750 bp); line 6: Lb. johnsonii reference strain CCM 2935.
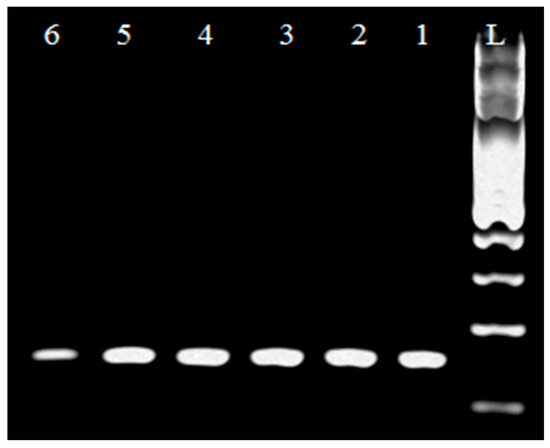
Figure 3.
Identification of genus Lb. zeae. L: 100 bp standard; line 1: Lb. zeae reference strain CCM 7069 (750 bp); lines 2, 3, 4, and 5: Lb. zeae isolates.
When comparing the representation of Lactobacillus sp. for various types of milk, the highest incidence of this genus, confirmed by PCR, was in cows’ raw milk (128 isolates), and the lowest was in goats’ raw milk (40 isolates). From strains of the genus Lactobacillus sp., the species Lb. johnsonii was, among the selected samples of raw milk, the most represented in goats’ milk (15.0%) and the least in cows’ milk (7.8%). Lb. zeae was also predominantly isolated from goats’ milk (12.5%), while in sheep’s (6.5%) and cows’ milk (6.3%) it had a similar proportion of isolates. These results correspond to the results of the MALDI-TOF-MS identification, despite that the results of the identification of both methods differing in some isolates. Conflicting identification occurred in three Lb. johnsonii strains and one Lb. zeae strain. However, these isolates showed a low score value between 1.698–1.792 when identified, indicating the reliability of identification only at the genus level.
In the next part of the study, only strains identified as Lb. johnsonii (23 isolates) and Lb. zeae (18 isolates) by MALDI-TOF-MS and PCR were used to determine antimicrobial resistance.
When determining susceptibility by E-test, the MIC for each identified antibiotic tested was detected in the range of 0.125–16.000 μg/mL for species-identified isolates (41 isolates) (Table 3). As there are no uniform standards for determining antimicrobial resistance in the lactobacilli species we tested, we proceeded to evaluate the obtained MIC values [28]. Strains whose MIC for ampicillin was >8 μg/mL and for erythromycim was ≥8 μg/mL were considered resistant. For clindamycin, resistant strains were considered to be those with an MIC ≥ 2 μg/mL. Based on these criteria, 7.3% of lactobacilli strains were evaluated as ampicillin-resistant, 14.7% of isolates as erythromycin-resistant, and 4.9% of isolates as clindamycin-resistant. The genus Lb. johnsonii had the highest resistance to erythromycin (34.8%), but no strain was resistant to clindamycin. The genus Lb. zeae also showed the greatest resistance to erythromycin (33.3%), and this species was also confirmed to be resistant to clindamycin (Table 3).

Table 3.
MIC determination of test isolates by E-test.
As the determination of susceptibility to individual antibiotics in lactobacilli is difficult to test, we also proceeded with antimicrobial resistance testing using the broth microdilution method (BMM).
As shown in Table 4, resistant strains were detected for all antibiotics tested. The highest number of resistant strains was, similarly to the E-test, confirmed against erythromycin (14.7%), and the smallest against clindamycin (7.3%). Minor discrepancies in the results of both methods occurred in the detection of susceptibility to ampicillin and clindamycin.

Table 4.
MIC determination of Lb. johnsonii and Lb. zeae isolates using the broth dilution method.
When determining the susceptibility to ampicillin, one strain of Lb. zeae was marked as susceptible based on the determined MIC (8 μg/mL) by E-test, but with the BMM method, the MIC was found to be 16 μg/mL, and therefore was marked as resistant. One Lb. johnsonii strain was evaluated as intermediate sensitive by E-test (MIC was 1.0 μg/mL). However, it was assessed as resistant by BMM, as the MIC was determined to be 2.0 μg/mL.
In one Lb. johnsonii isolate, resistance to two antibiotics was confirmed simultaneously by both methods; namely, it was resistance to ampicillin and erythromycin. When comparing the resistance of tested lactobacilli from the different types of examined milk, most resistant strains occurred in lactobacilli isolated from cows’ milk (Figure 4).
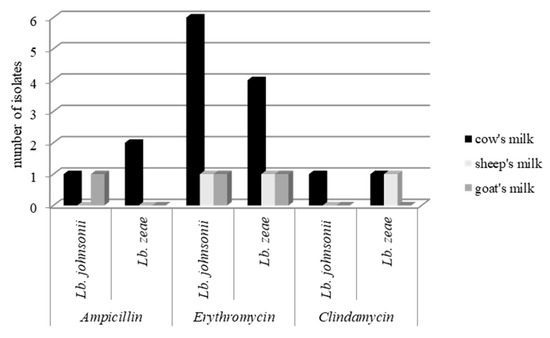
Figure 4.
Number of resistant strains in each type of milk.
As erythromycin resistance predominated in the determination of the phenotypic manifestation of antimicrobial resistance, we proceeded to detect the ermB gene, which according to previous studies, is often detected in erythromycin-resistant lactobacilli.
The PCR method confirmed the presence of the ermB gene in 7 of 41 isolates of species-identified lactobacilli (Figure 5), namely the five strains of Lb. johnsonii (four isolates of cows’ milk and one isolate of goats’ milk) and two strains of Lb. zeae (one isolate of cows’ milk and one isolate of sheep’s milk). Isolates in which the presence of the ermB gene was confirmed had a phenotypic manifestation of erythromycin resistance.
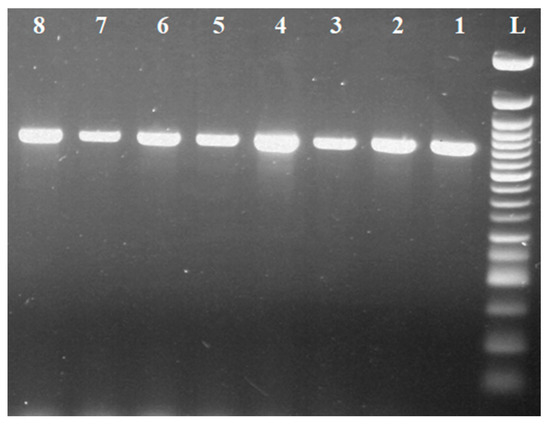
Figure 5.
Identification of the ermB gene in Lactobacillus sp. L: 50 bp standard; line 1: Staphylococcus aureus reference strain CCM 4223 (639 bp); lines 2–3: isolates of Lb. zeae with ermB gene; lines 4-8: isolates of Lb. johnsonii with ermB gene.
4. Discussion
Lactobacilli form a significant microbial population of raw milk are widely present in various fermented dairy products. Their presence in several types of raw milk was also confirmed in this study, where the number of bacteria of the genus Lactobacillus sp. was in fresh cows’ milk (3.8 ± 0.1 log CFU/mL), sheep’s milk (3.2 ± 0.1 log CFU/mL), and goats’ milk (2.0 ± 0.1 log CFU/mL). The presence of lactobacilli in raw milk has been confirmed by several studies, including Vataščinová et al., where in 23 geographical areas of Eastern Slovakia, 43 strains have been identified as Lactobacillus sp. [31]. However, the high nutrient content of raw milk during storage makes it a suitable medium for a large number of other microorganisms, such as C. perfringens, S. aureus, E. coli, L. monocytogenes, Salmonella spp., Bacillus cereus, Streptococcus sp., etc., which develop rapidly in it and cause its deterioration [32].
This leads to a decrease in quality due to changes in its organoleptic, technological, and other properties. At the same time, the microflora of lactobacilli is suppressed, and undesirable microorganisms develop in samples of stored raw milk [33]. The suppression of lactobacilli microflora during storage of all types of milk is also indicated by the results of this study. In addition, differences in lactobacilli counts were observed between milk species. These differences are given by the differential chemical composition of cows’, sheep’s, and goats milk, as well as geographical areas [34]. Many studies have addressed the species identification of lactobacilli, but few studies indicate the detection of Lb. johnsonii and Lb. zeae species from various types of raw milk [34]. Lb. zeae is described as a closely related species of Lb. casei [10]. A group of species Lb. casei includes Lb. casei, Lb. paracasei subsp. Paracasei, Lb. paracasei subsp. Tolerans, Lb. rhamnosus, and Lb. zeae. The first three species are commonly isolated from milk and dairy products, and play an important role in human and animal nutrition as a probiotic [35]. Lb. johnsonii, in turn, is largely involved in the colonization of the gastrointestinal tract and its functional properties related to its ability to adhere to the intestinal mucosa, resist bile and acids, or colonize the intestines and produce bacteriocin [36]. Due to the positive properties and low uptake in fresh milk, another part of this study focused on the detection of the above-mentioned types of lactobacilli.
Identification was performed by MALDI-TOF-MS and PCR method. Using MALDI-TOF-MS, from 300 isolated strains, 252 isolates were identified as Lactobacillus sp. with the score value 1658–2258. A total of 26 isolates were confirmed as Lb. johnsonii species, and 19 isolates as Lb. zeae. The score value ranged from 1.698 to 2.176 for Lb. johnsonii, and from 1.734 to 2.246 for species Lb. zeae. Dec et al. identified four species of lactobacilli—Lb. casei, Lb. johnsonii, Lb. kitasatonis, and Lb. zeae—in foods of animal origin, but with low score values comparable to our study. He also described the lack of discriminatory ability to distinguish closely related species, such as Lb. johnsonii, Lb. gasseri, Lb. crispatus, Lb. ultunensis, Lb. oris, and Lb. antri by MALDI-TOF-MS [14].
In a presented study, there was no score value for confirmation of a species more than 2.30. We approached the detection using PCR method, which is considered to be one of the foundation stones of modern microbial taxonomy [26].
PCR is a standard method for identifying bacteria and determining microbial diversity, and is widely applied to the Lactobacillus genus, which is the result of the high specificity of the gene sequence 16s rDNA for each bacterial species [37]. Using specific primers, we verified the presence of the genus Lactobacillus sp. in 245 isolated strains [27]. Subsequent species-level identification detected a species-specific PCR product Lb. zeae (7.5%) in 19 isolates, and Lb. johnsonii in 26 isolates (representing 10.3% of identified lactobacilli) [11]. The other studies reported that of the 47 strains of Lactobacillus sp. isolated from different media, 16 strains were identified as Lb. johnsonii by multiplex PCR [36]. Delaveane et al. research antifungal strains of Lactobacillus sp. (including Lb. zeae) previously isolated from cows’ and goats’ milk. All showed different acidification and growth capacities, which makes Lb. zeae a potential candidate for yogurt biopreservation [38].
Microorganism resistance to antibiotics is a current global problem. A major role in the spread of lactobacillus resistance in the food chain is the ill-considered choice of antibiotics in the treatment of food animals [39]. The new findings also point to the presence of resistant strains in probiotic bacteria (including the genus Lactobacillus), the use of which has a positive effect on the intestinal microflora in humans and animals [40,41]. Antibiotic resistance of probiotic bacteria has recently been perceived as a risk to the consumer. Bacteria belonging to the group of probiotics show a so-called natural resistance to metronidazole, colistin, and vancomycin [42].
In our study, the antimicrobial resistance was detected by two methods: E-test and broth microdilution method to ampicillin, clindamycin, and, to a greater extent, erythromycin (Lb. johnsonii = 34.8% and Lb. zeae = 33.3%). The highest agreement when comparing the MIC determination by E-test and BMM was detected for erythromycin. A lower level of agreement was detected for ampicillin and clindamycin, where some isolates showed lower MICs in the E-test. Similarly, Mayrhofer et al. detected the levels of agreement between these methods that were high for the antimicrobial agents ampicillin, gentamicin, streptomycin, and vancomycin (90%) [43]. Lower levels of agreement were obtained for clindamycin (71%), erythromycin (80%), and especially tetracycline (34%). In general, lower MICs of ampicillin, clindamycin, erythromycin, and streptomycin were obtained by E-test. Discrepancies in the determination of MIC by the E-test and BMM method have also been pointed out by Kushiro et al. for only 5% of cases with three antimicrobials (tetracycline, rifampicin, and kanamycin), and a more than four-fold difference between the two methods has been observed [44]. The biggest differences were shown with Lb. delbrueckii for rifampicin. The BMM method also confirmed the resistance of several antibiotics in lactobacilli [14].
Resistance to at least three groups of antimicrobials was observed in 64.5% of lactobacilli, and 43.5% of isolates showed cross-resistance between erythromycin and lincomycin. Resistance to streptomycin and gentamicin was recorded simultaneously for 6% of isolates. Acquired resistance was observed for erythromycin, lincomycin, and tetracycline. Sharma et al. detected multiresistance and polyresistance in lactobacilli strains, as well as other lactobacilli. Multiple resistance to the most commonly used antibiotics, such as norfloxacin, teicoplanin, cefepime, and amikacin, has been confirmed in most isolates. Lactobacilli has shown low levels of resistance to, for example, ampicillin, cefaclor, gentamicin, oxacillin, tetracycline, and novobiocin [45]. Recently, erythromycin resistance has also been increasingly detected in lactobacilli. Major genes responsible for erythromycin resistance are erm (A), erm(C), erm(T), and erm(B), which encode rRNA methylase acting on the 23 S subunit, which is predominate in Lactobacillus genus [45,46,47]. This is also confirmed by our study, where this gene was confirmed in 17% of lactobacilli isolates isolated from raw milk. The phenotypic manifestation of antimicrobial resistance of the tested lactobacilli was associated with the presence of the ermB gene. Drago et al. also confirmed erythromycin resistance in more than one-third of 40 lactobacillus isolates. Again, macrolide resistance was mostly associated with the presence of the ermB gene [48].
This study confirmed the presence of resistant strains Lb. zeae and Lb. johnsonii in various types of raw milk. At the same time, the ermB gene, which is responsible for encoding erythromycin resistance, was detected in these strains. The presence of these resistant strains, even in raw milk, indicates an increased risk of the possibility of the spread of resistance across the food chain.
Author Contributions
Conceptualization, J.V.; methodology, J.V. and I.R.; software, M.K.; formal analysis, E.D. and S.M.; investigation and data curation, J.V., M.K., and J.M.; writing—original draft preparation, J.V.; writing—review and editing, I.R. and E.D.; supervision, I.R. and J.V.; project administration and funding acquisition, J.M. and I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the following grants: APVV-19-0234, “Development of probiotic preparation based on autochthonous lactobacilli for salmonids intended to improve fish health and production of quality food”; and KEGA 007UVLF-4/2020, “Innovation of milk and milk products hygiene and technology education” at the University of Veterinary Medicine and Pharmacy in Košice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, H.; Cai, Y. Lactic Acid Bacteria: Fundamentals and Practice; Springer: Berlin/Heidelberg, Germany, 2014; p. 535. ISBN 978-94-017-8841-0. [Google Scholar]
- Mozzi, F.; Raya, R.R.; Vignolo, G.M.; Love, J.C. Biotechnology of Lactic Acid Bacteria: Novel Applications; John Wiley and Sons: Hoboken, NJ, USA, 2010; p. 352. ISBN 978-0-8138-1583-1. [Google Scholar]
- Baruzzi, F.; Poltronieri, P.; Quero, G.M.; Morea, M.; Morelli, L. An in vitro protocol for direct isolation of potential probiotic Lactobacilli from raw bovine milk and traditional fermented milks. Appl. Microbiol. Biotechnol. 2011, 90, 331–342. [Google Scholar] [CrossRef]
- Kološta, M.; Slottová, A.; Drončovský, M.; Klapáčová, L.; Kmeť, V.; Bujňáková, D.; Lauková, A.; Grief, G.; Griefová, M.; Tomáška, M. Characterisation of Lactobacilli from eweʼs and goatʼs milk for their further processing re-utilisation. Potravin. Slovak J. Food Sci. 2014, 8, 130–134. [Google Scholar]
- Tomáška, M.; Drončovský, M.; Klapáčová, L.; Slottová, A.; Kološta, M. Potential probiotic properties of Lactobacilli isolated from goat´ s milk. Potravin. Slovak J. Food Sci. 2015, 9, 66–71. [Google Scholar] [CrossRef]
- Vukotić, G.; Strahinić, I.; Begović, J.; Lukić, J.; Kojić, M.; Fira, D. Survey on proteolytic activity and diversity of proteinase genes in mesophilic lactobacilli. Microbiology 2016, 85, 33–41. [Google Scholar] [CrossRef]
- Lim, S.D.; Kim, K.S.; Do, J.R. Physiological characteristics and ACE inhibitory activity of Lactobacillus zeae RMK354 isolated from raw milk. Korean. J. Food Sci. Anl. Resour. 2008, 28, 587–595. [Google Scholar] [CrossRef][Green Version]
- Henri-Dubernet, S.; Desmasures, N.; Gueguen, M. Diversity and dynamics of lactobacilli populations during ripening of RDO Camembert cheese. Can. J. Microbiol. 2008, 54, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Salyers, A.A.; Shoemaker, N.B.; Stevens, A.M.; Li, L.Y. Conjugative transposons: An unusual and diverse set of integrated gene transfer elements. Microbiol. Rev. 1995, 59, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.; El Jakee, J.; Rashidy, A.; Asfour, H.; Omara, S.; Kandil, M.M.I.; Mahmood, Z.; Hahne, J.; Seida, A.A. Potential antimicrobial activities of probiotic Lactobacillus strains isolated from raw milk. J. Prob. Health 2016, 4. [Google Scholar] [CrossRef]
- Walter, J.; Tannock, G.W.; Tilasala-Timisjarvi, A.; Rodtong, S.; Loach, D.M.; Munro, K.; Alatossava, T. Detection and Identification of Gastrointestinal Lactobacillus Species by Using Denaturing Gradient Gel Electrophoresis and Species-Specific PCR Primers. Appl. Environ. Microbiol. 2000, 66, 297–303. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Klare, I.; Konstabel, C.; Müller-Bertling, S.; Reissbrodt, R.; Huys, G.; Vancanneyt, M.; Witte, W. Evaluation of new broth media for microdilution antibiotic susceptibility testing of lactobacilli, pediococci, lactococci, and bifidobacteria. Appl. Environ. Microbiol. 2005, 71, 8982–8986. [Google Scholar] [CrossRef] [PubMed]
- Dec, M.; Nowaczek, A.; Stępień-Pyśniak, D.; Wawrzykowski, J.; Urban-Chmiel, R. Identification and antibiotic susceptibility of lactobacilli isolated from turkeys. BMC Microbiol. 2018, 18, 168. [Google Scholar] [CrossRef]
- Roberts, M.C. Update on acquired tetracycline resistance genes. FEMS Microbiol. Lett. 2005, 245, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol. Lett. 2008, 282, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Sutcliffe, J.; Courvalin, P.; Jensen, L.B.; Rood, J.; Seppala, H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 1999, 43, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.H.A.M.; Margolles, A.; Damig, K.J.; Korhonen, J.M.; ZyckaKrzesinka, J.; Bardowsky, J.; Danielsen, M.; Huys, G.; Morelli, L.; Aarts, H.J.M. Molecular assessment of erythromycin and tetracycline resistance genes in lactic acid bacteria and bifidobacteria and their relation to the phenotypic resistance. Int. J. Probiotics Prebiotics 2008, 3, 271–280. [Google Scholar]
- Mayrhofer, S.; van Hoek, A.H.; Mair, C.; Huys, G.; Aarts, H.J.; Kneifel, W.; Domig, K.J. Antibiotic susceptibility of members of the Lactobacillus acidophilus group using broth microdilution and molecular identification of their resistance determinants. Int. J. Food Microbiol. 2010, 144, 81–87. [Google Scholar] [CrossRef]
- Aquilanti, L.; Garofalo, C.; Osimani, A.; Silvestri, G.; Vignaroli, C.; Clementi, F. Isolation and molecular characterization of antibiotic-resistant lactic acid bacteria from poultry and swine meat products. J. Food Prot. 2007, 70, 557–565. [Google Scholar] [CrossRef]
- Tannock, G.W.; Luchansky, J.B.; Miller, L.; Connell, H.; Thode-Andersen, S.; Mercer, A.A.; Klaenhammer, T.R. Molecular characterization of a plasmid-borne (pGT633) erythromycin resistance determinant (ermGT) from Lactobacillus reuteri 100–63. Plasmid 1994, 31, 60–71. [Google Scholar] [CrossRef]
- EFSA. Technical guidance prepared by the panel on additives and Products or substances used in animal feed (FEEDAP) on the update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance (question no. EFSA-Q-2008-004). EFSA J. 2008, 732, 1–15. [Google Scholar]
- Cauwerts, K.; Pasmans, F.; Devriese, L.A.; Martel, A.; Haesebrouck, F.; Decostere, A. Cloacal Lactobacillus isolates from broilers show high prevalence of resistance towards macrolide and lincosamide antibiotics. Avian Pathol. 2006, 35, 160–164. [Google Scholar] [CrossRef] [PubMed]
- STN EN ISO 6887-5. Microbiology of Food and Animal Feeding Stuffs. Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. Part 5: Specific Rules for the Preparation of Milk and Milk Products (ISO 6887-5:2010); Slovak Standards Institute: Bratislava, Slovak, 2010. [Google Scholar]
- STN EN ISO 6887-1. Microbiology of the Food Chain-Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination-Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions (ISO 6887-1:2017); Slovak Standards Institute: Bratislava, Slovak, 2017. [Google Scholar]
- Bruker Daltonics. MALDI Biotyper 2.0. Software for Microorganism Identification and Classification User Manual; Bruker Corporation: Billerica, MA, USA, 2008. [Google Scholar]
- Dubernet, S.; Desmasures, N.; Guéguen, M.A. PCR-based method for identification of Lactobacilli at the genus level. FEMS Microbiol. Lett. 2002, 214, 271–275. [Google Scholar] [CrossRef] [PubMed]
- CLSI document M–45. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Sutcliffe, J.; Grebe, T.; Tait-Kamradt, A.; Wondrack, L. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 1996, 40, 2562–2566. [Google Scholar] [CrossRef]
- Anisimova, E.; Yarullina, D. Characterization of erythromycin and tetracycline resistance in Lactobacillus fermentum strains. Int. J. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vataščinová, T.; Pipová, M.; Fraqueza, M.J.R.; Mala, P.; Dudriková, E.; Drážovská, M.; Lauková, A. Antimicrobial potential of Lactobacillus plantarum strains isolated from Slovak raw sheep milk cheeses. J. Dairy Sci. 2019, 103, 6900–6903. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.F.; Chin, N.L.; Tee, T.P.; Chooi, S.K. Physico-Chemical Changes, Microbiological Properties, and Storage Shelf Life of Cow and Goat Milk from Industrial High-Pressure Processing. Processes 2020, 8, 697. [Google Scholar] [CrossRef]
- Ebringer, L.; Soják, L. Mlieko ako multifunkčná potravina. Interná Med. 2007, 7–8, 423–427. [Google Scholar]
- Mahdavi, S.; Isazadeh, A.; Azimian, S.H.; Bonab, N.M.; Shekar, F.; Asgharian, A. Isolation of Lactobacillus Species from Domestic Dairy Products of Mahabad City. Int. J. Infect. 2018, 5, 62152. [Google Scholar] [CrossRef]
- Pantoflickova, D.; Corthésy-Theulaz, I.; Stolte, M.; Isler, P.; Rochat, F.; Enslen, M.; Blum, A.L. Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Aliment. Pharmacol. Ther. 2003, 18, 805–813. [Google Scholar] [CrossRef]
- Ventura, M.; Zink, R. Specific identification and molecular typing analysis of Lactobacillus johnsonii by using PCR-based methods and pulsed-field gel electrophoresis. FEMS Microbiol. Lett. 2002, 17, 141–154. [Google Scholar] [CrossRef]
- Nemska, V.; Lazarova, N.; Georgieva, N.; Danova, S. Lactobacillus spp. From traditional Bulgarian dairy products. J. Chem. Technol. Met. 2016, 51, 693–704. [Google Scholar]
- Delavenne, E.; Ismail, R.; Pawtowski, A.; Mounier, J.; Barbier, G.; Le Blay, G. Assessment of lactobacilli strains as yogurt bioprotective cultures. Food Control. 2013, 30, 206–213. [Google Scholar] [CrossRef]
- Collingnon, P. Antibiotic resistance: Are we all doomed. Intern. Med. J. 2015, 45, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Borriello, S.P.; Hammes, W.P.; Holzapfel, W.; Marteau, P.; Schrezenmeir, J.; Vaara, M.; Valtonen, V. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin. Infect. Dis. 2003, 36, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Capurso, L.; Collins, K.; Cummings, J.; Delzenne, N.; Goulet, O.; Guarner, F.; Marteau, P.; Meier, R. Current level of consensus on probiotic science. Report of an expert meeting London 23 November 2009. Gut Microbes 2010, 1, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, O. Effect of Probiotics in the Mitigation of Clostridium difficile Associated Disease. Diploma Thesis, Georgia State University, Atlanta, GA, USA, 2015. [Google Scholar]
- Mayrhofer, S.; Domig, K.J.; Mair, C.; Zitz, U.; Huys, G.; Kneifel, W. Comparison of Broth Microdilution, Etest, and Agar Disk Diffusion Methods for Antimicrobial Susceptibility Testing of Lactobacillus acidophilus Group Members. Appl. Environ. Microbiol. 2008, 12, 3745–3748. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, A.; Chervaux, C.; Cools-Portier, S.; Perony, A.; Legrain-Raspaud, S.; Obis, D.; Onoue, M.; van de Moer, A. Antimicrobial susceptibility testing of lactic acid bacteria and bifidobacteria by broth microdilution method and Etest. Int. J. Food Microbiol. 2009, 132, 54–58. [Google Scholar] [CrossRef]
- Sharma, P.; Tomar, S.; Sangwan, V.; Goswami, P.; Singh, R. Antibiotic Resistance of Lactobacillus sp. isolated from commercial probiotic preparations. J. Food Saf. 2016, 36, 38–51. [Google Scholar] [CrossRef]
- Cataloluk, O.; Gogebakan, B. Presence of drug resistance in intestinal lactobacilli of dairy and human origin in Turkey. FEMS Microbiol. Lett. 2004, 236, 7–12. [Google Scholar] [CrossRef]
- Gad, G.F.; Abdel-Hamid, A.M.; Farag, Z.S. Antibiotic resistance in lactic acid bacteria isolated from some pharmaceutical and dairy products. Braz. J. Microbiol. 2014, 45, 25–33. [Google Scholar] [CrossRef]
- Drago, L.; Mattina, R.; Nicola, L.; Rodighiero, V.; De Vecchi, E. Macrolide resistance and in vitro selection of resistance to antibiotics in Lactobacillus isolates. J. Microbiol. 2011, 49, 651. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).