Shear-Thinning Effect of the Spinning Disc Mixer on Starch Nanoparticle Precipitation
Abstract
1. Introduction
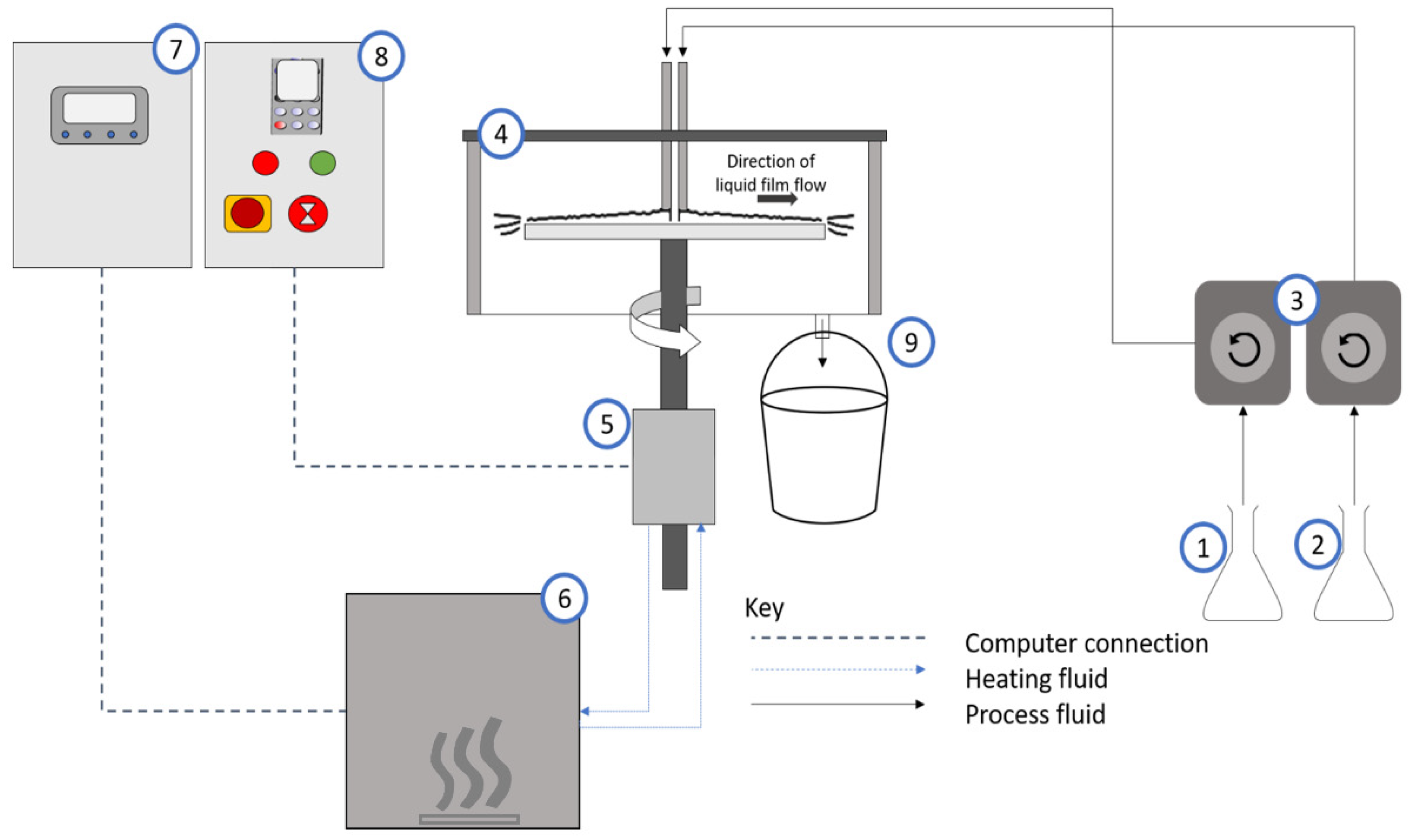
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Starch Concentration on Viscosity
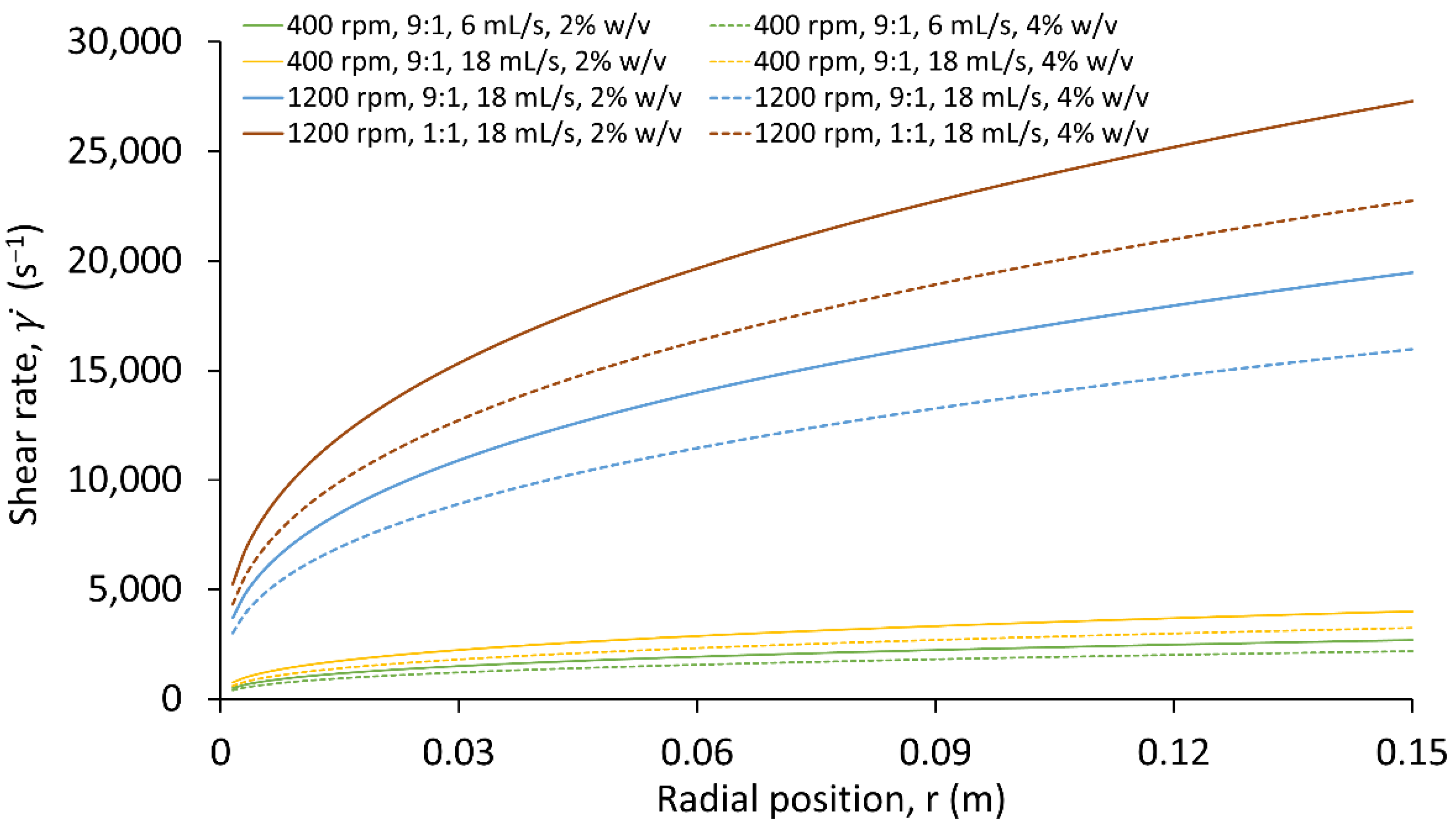
3.2. Rheological Characterisation of Starch
3.3. Effect of Starch Concentration on Nanoparticle Precipitation
3.3.1. Semi-Batch Reactor
3.3.2. Spinning Disc Reactor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Power law index (-) | |
| Consistency index (mPa) | |
| Radial position (m) | |
| Flow rate (m3 s−1) | |
| Micromixing time (s) | |
| Residence time (s) | |
| Vertical distance from disc surface (along z-axis) (m) | |
| Greek symbols | |
| Local shear rate at a given radial position (s−1) | |
| Average shear rate across whole disc (s−1) | |
| Liquid density (kg m−3) | |
| Film thickness (m) | |
| Specific dissipation rate (W kg−1) | |
| Angular velocity (rad s−1) | |
| Dynamic viscosity (Pa s or mPa s) | |
| Kinematic viscosity (m2 s−1) |
References
- Dufresne, A. Starch and nanoparticle. Polysaccharides 2014, 1–28. [Google Scholar] [CrossRef]
- Le Corre, D.; Bras, J.; Dufresne, A. Starch nanoparticles: A review. Biomacromolecules 2010, 11, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Nath, L.K.; Singh, A. Pharmaceutical, food and non-food applications of modified starches: A critical review. Electron. J. Environ. Agric. Food Chem. 2010, 9, 1214–1221. [Google Scholar]
- Ahmad, M.; Gani, A.; Hassan, I.; Huang, Q.; Shabbir, H. Production and characterization of starch nanoparticles by mild alkali hydrolysis and ultra-sonication process. Sci. Rep. 2020, 10, 3533. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, G.; Sharma, S.; Jeet, K. Cereal starch nanoparticles—A prospective food additive: A review. Crit. Rev. Food. Sci Nutr. 2017, 58, 1097–1107. [Google Scholar] [CrossRef]
- Le Corre, D.; Angellier-Coussy, H. Preparation and application of starch nanoparticles for nanocomposites: A review. React. Funct. Polym. 2014, 85, 97–120. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Nain, V. Starch nanoparticles: Their preparation and applications. In Plant Biotechnology: Recent Advancements and Developments; Gahlawat, S.K., Salar, R.K., Siwach, P., Duhan, J.S., Kumar, S., Kaur, P., Eds.; Springer: Singapore, 2017; pp. 213–232. [Google Scholar] [CrossRef]
- Sun, Q.; Li, G.; Dai, L.; Ji, N.; Xiong, L. Green preparation and characterisation of waxy maize starch nanoparticles through enzymolysis and recrystallisation. Food Chem. 2014, 162, 223–228. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Rafie, M.; EL-Sheikh, M.; El-Naggar, M. Ultra-fine characteristics of starch nanoparticles prepared using native starch with and without surfactant. J. Inorg. Organomet. Polym. Mater. 2014, 24, 515–524. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.S.; Lim, S. Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Thielemans, W.; Magnin, A.; Boufi, S. Starch nanocrystals and starch nanoparticles from waxy maize as nanoreinforcement: A comparative study. Carbohydr. Polym. 2016, 143, 310–317. [Google Scholar] [CrossRef]
- Wu, X.; Chang, Y.; Fu, Y.; Ren, L.; Tong, J.; Zhou, J. Effects of non-solvent and starch solution on formation of starch nanoparticles by nanoprecipitation. Starch Stärke 2016, 68, 258–263. [Google Scholar] [CrossRef]
- Chin, S.F.; Azman, A.; Pang, S.C. Size Controlled synthesis of starch nanoparticles by a microemulsion method. J. Nanomater. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Sana, S.; Boodhoo, K.; Zivkovic, V. Production of starch nanoparticles through solvent-antisolvent precipitation in a spinning disc reactor. Green Process. Synth. 2019, 8, 507–515. [Google Scholar] [CrossRef]
- Hedayati, S.; Niakousari, M.; Mohsenpour, Z. Production of tapioca starch nanoparticles by nanoprecipitation-sonication treatment. Int. J. Biol. Macromol. 2020, 143, 136–142. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, K.; Li, L.; Liu, C.; Song, C.; Wang, P. Fabrication of size-controlled starch-based nanospheres by nanoprecipitation. Acs Appl. Mater. Interfaces 2009, 1, 956–959. [Google Scholar] [CrossRef]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and characterization of citric acid-modified starch nanoparticles/plasticized-starch composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, C.; Jiang, S.; Xiong, L.; Sun, Q. Characterization of starch nanoparticles prepared by nanoprecipitation: Influence of amylose content and starch type. Ind. Crops Prod. 2016, 87, 182–190. [Google Scholar] [CrossRef]
- Dalvi, S.V.; Dave, R.N. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind. Eng. Chem. Res. 2009, 48, 7581–7593. [Google Scholar] [CrossRef]
- Boodhoo, K. Spinning disc reactor for green processing and synthesis. In Process Intensification for Green Chemistry; Boodhoo, K., Harvey, A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 59–90. [Google Scholar]
- Mohammadi, S.; Boodhoo, K.V.K. Online conductivity measurement of residence time distribution of thin film flow in the spinning disc reactor. Chem. Eng. J. 2012, 207, 885–894. [Google Scholar] [CrossRef]
- Mohammadi, S.; Harvey, A.; Boodhoo, K.V.K. Synthesis of TiO2 nanoparticles in a spinning disc reactor. Chem. Eng. J. 2014, 258, 171–184. [Google Scholar] [CrossRef]
- Cafiero, L.M.; Baffi, G.; Chianese, A.; Jachuck, R.J.J. Process intensification: Precipitation of barium sulfate using a spinning disk reactor. Ind. Eng. Chem. Res. 2002, 41, 5240–5246. [Google Scholar] [CrossRef]
- Tai, C.Y.; Tai, C.; Chang, M.; Liu, H. Synthesis of magnesium hydroxide and oxide nanoparticles using a spinning disk reactor. Ind. Eng. Chem. Res. 2007, 46, 5536–5541. [Google Scholar] [CrossRef]
- Chin, S.F.; Iyer, K.S.; Raston, C.L.; Saunders, M. Size selective synthesis of superparamagnetic nanoparticles in thin fluids under continuous flow conditions. Adv. Funct. Mater. 2008, 18, 922–927. [Google Scholar] [CrossRef]
- Ahoba-Sam, C.; Boodhoo, K.V.K.; Olsbye, U.; Jens, K. Tailoring Cu nanoparticle catalyst for methanol synthesis using the spinning disk reactor. Materials 2018, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- D’Intino, A.F.; de Caprariis, B.; Santarelli, M.L.; Verdone, N.; Chianese, A. Best operating conditions to produce hydroxyapatite nanoparticles by means of a spinning disc reactor. Front. Chem. Sci. Eng. 2014, 8, 156–160. [Google Scholar] [CrossRef]
- Moharir, R.G.; Gogate, P.R.; Rathod, V.K. Process intensification of synthesis of magnetite using spinning disc reactor. Can. J. Chem. Eng. 2012, 90, 996–1005. [Google Scholar] [CrossRef]
- Khan, W.H.; Rathod, V.K. Process intensification approach for preparation of curcumin nanoparticles via solvent–nonsolvent nanoprecipitation using spinning disc reactor. Chem. Eng. Process. Process Intensif. 2014, 80, 1–10. [Google Scholar] [CrossRef]
- Oxley, P.; Brechtelsbauer, C.; Ricard, F.; Lewis, N.; Ramshaw, C. Evaluation of spinning disk reactor technology for the manufacture of pharmaceuticals. Ind. Eng. Chem. Res. 2000, 39, 2175–2182. [Google Scholar] [CrossRef]
- Rathod, W.R.; Rathod, V.K. Continuous preparation of nimesulide nanoparticles by liquid antisolvent precipitation using spinning disc reactor. J. Chem. Technol. Biotechnol. 2019, 94, 919–926. [Google Scholar] [CrossRef]
- Vicevic, M.; Boodhoo, K.V.K.; Scott, K. Catalytic isomerisation of α-pinene oxide to campholenic aldehyde using silica-supported zinc triflate catalysts. Chem. Eng. J. 2007, 133, 43–57. [Google Scholar] [CrossRef]
- Ghiasy, D.; Tham, M.T.; Boodhoo, K.V.K. Control of a spinning disc reactor: An experimental study. Ind. Eng. Chem. Res. 2013, 52, 16832–16841. [Google Scholar] [CrossRef]
- Boodhoo, K.V.K.; Jachuck, R.J. Process intensification: Spinning disk reactor for styrene polymerisation. Appl. Therm. Eng. 2000, 20, 1127–1146. [Google Scholar] [CrossRef]
- Boodhoo, K.V.K.; Jachuck, R.J. Process intensification: Spinning disc reactor for condensation polymerisation. Green Chem. 2000, 2, 235–244. [Google Scholar] [CrossRef]
- Dobie, C.G.; Vicevic, M.; Boodhoo, K.V.K. An evaluation of the effectiveness of continuous thin film processing in a spinning disc reactor for bulk free-radical photo-copolymerisation. Chem. Eng. Process. Process Intensif. 2013, 71, 97–106. [Google Scholar] [CrossRef]
- Boodhoo, K.V.K. Chapter 11. Higee technologies and their applications to green intensified processing. Altern. Energy Sources Green Chem. 2016, 339–359. [Google Scholar] [CrossRef]
- Boodhoo, K.V.K.; Dunk, W.A.E.; Jachuck, R.J. Influence of centrifugal field on free-radical polymerization kinetics. J. Appl. Polym. Sci. 2002, 85, 2283–2286. [Google Scholar] [CrossRef]
- Sana, S.; Zivkovic, V.; Boodhoo, K. Empirical modelling of hydrodynamic effects on starch nanoparticles precipitation in a spinning disc reactor. Nanomaterials 2020, 10, 2202. [Google Scholar] [CrossRef]
- Shin, J.; Jones, N.; Ik Lee, D.; Fleming, P.; Joyce, M.; Dejong, R.; Bloembergen, S. Rheological properties of starch latex dispersions and starch latex-containing coating colors. In Technical Association of the Pulp and Paper Industry, Proceedings of the Papercon 2012 Conference Proceedings, New Orleans, LA, USA, 22–25 April 2012; TAPPI Press: Peachtree Corners, GA, USA, 2012; pp. 382–406. [Google Scholar]
- Roberts, S.A.; Cameron, R.E. The effects of concentration and sodium hydroxide on the rheological properties of potato starch gelatinisation. Carbohydr. Polym. 2002, 50, 133–143. [Google Scholar] [CrossRef]
- Che, L.; Li, D.; Wang, L.; Özkan, N.; Chen, X.D.; Mao, Z. Rheological properties of dilute aqueous solutions of cassava starch. Carbohydr. Polym. 2008, 74, 385–389. [Google Scholar] [CrossRef]
- Boodhoo, K. Process Intensification: Spinning Disc Reactor for the Polymerisation of Styrene. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 1999. [Google Scholar]
- Hu, J.; Cheng, F.; Lin, Y.; Zhao, K.; Zhu, P. Dissolution of starch in urea/NaOH aqueous solutions. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Sánchez Pérez, J.A.; Rodríguez Porcel, E.M.; Casas López, J.L.; Fernández Sevilla, J.M.; Chisti, Y. Shear rate in stirred tank and bubble column bioreactors. Chem. Eng. J. 2006, 124, 1–5. [Google Scholar] [CrossRef]
- Mersmann, A. Crystallization and precipitation. Chem. Eng. Process. Process Intensif. 1999, 38, 345–353. [Google Scholar] [CrossRef]
- Matteucci, M.E.; Hotze, M.A.; Johnston, K.P.; Williams, R.O. Drug nanoparticles by antisolvent precipitation: Mixing energy versus surfactant stabilization. Langmuir 2006, 22, 8951–8959. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ng, W.K.; Hu, J.; Shen, S.; Tan, R.B.H. A continuous and highly effective static mixing process for antisolvent precipitation of nanoparticles of poorly water-soluble drugs. Int. J. Pharm. 2010, 386, 256–261. [Google Scholar] [CrossRef]
- D’Addio, S.M.; Prud’homme, R.K. Controlling drug nanoparticle formation by rapid precipitation. Adv. Drug Deliv. Rev. 2011, 63, 417–426. [Google Scholar] [CrossRef]
- Valente, I.; Celasco, E.; Marchisio, D.L.; Barresi, A.A. Nanoprecipitation in confined impinging jets mixers: Production, characterization and scale-up of pegylated nanospheres and nanocapsules for pharmaceutical use. Chem. Eng. Sci. 2012, 77, 217–227. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, M.; Kuang, Y.; Wang, J.; Le, Y.; Zeng, X.; Chen, J. Preparation of amorphous drug nanoparticles by high-gravity reactive precipitation technique. Chem. Eng. Process. Process Intensif. 2016, 104, 253–261. [Google Scholar] [CrossRef]
- Dong, Y.; Ng, W.K.; Shen, S.; Kim, S.; Tan, R.B.H. Controlled antisolvent precipitation of spironolactone nanoparticles by impingement mixing. Int. J. Pharm. 2011, 410, 175–179. [Google Scholar] [CrossRef]
- Kakran, M.; Sahoo, N.G.; Tan, I.; Li, L. Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Ozar, B.; Cetegen, B.M.; Faghri, A. Experiments on the flow of a thin liquid film over a horizontal stationary and rotating disk surface. Exp. Fluids 2003, 34, 556–565. [Google Scholar] [CrossRef]
- Baldyga, J.; Podgórska, W.; Pohorecki, R. Mixing-precipitation model with application to double feed semibatch precipitation. Chem. Eng. Sci. 1995, 50, 1281–1300. [Google Scholar] [CrossRef]
- Sana, S. Experimental and Modelling Studies of Process Intensification for the Solvent-Antisolvent Precipitation of Nanoparticles in a Spinning Disc Reactor. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2020. [Google Scholar]












| Starch Concentration (% w/v) | Consistency Index, k (mPa) | Power Law Index, n | Source |
|---|---|---|---|
| 2 | 21.8 ± 2.4 | 0.911 ± 0.010 | This work |
| 4 | 31.5 ± 1.2 | 0.897 ± 0.006 | This work |
| 21 | 102.6 | 0.852 | [40] |
| 1 (tapioca starch) | 15.2 | 0.858 | [42] |
| Flow Rate (mL/s) | Rotational Speed (rpm) | Antisolvent to Solvent Ratio | at 2% w/v (s−1) | at 4% w/v (s−1) |
|---|---|---|---|---|
| 6 | 400 | 9 | 2000 | 1600 |
| 18 | 400 | 9 | 3000 | 2400 |
| 18 | 1200 | 9 | 14,000 | 12,000 |
| 18 | 1200 | 1 | 20,000 | 17,000 |
| Flow Rate (mL/s) | Rotational Speed (rpm) | Antisolvent to Solvent Ratio | Concentration (% w/v) | Average Shear Rate, (s−1) | Micromixing Time (s) |
|---|---|---|---|---|---|
| 6 | 400 | 1 | 2 | 2000 | |
| 6 | 400 | 9:1 | 4 | 1600 | |
| 18 | 1200 | 1:1 | 2 | 20,000 | |
| 18 | 1200 | 1:1 | 4 | 17,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sana, S.; Zivkovic, V.; Boodhoo, K. Shear-Thinning Effect of the Spinning Disc Mixer on Starch Nanoparticle Precipitation. Processes 2020, 8, 1622. https://doi.org/10.3390/pr8121622
Sana S, Zivkovic V, Boodhoo K. Shear-Thinning Effect of the Spinning Disc Mixer on Starch Nanoparticle Precipitation. Processes. 2020; 8(12):1622. https://doi.org/10.3390/pr8121622
Chicago/Turabian StyleSana, Sahr, Vladimir Zivkovic, and Kamelia Boodhoo. 2020. "Shear-Thinning Effect of the Spinning Disc Mixer on Starch Nanoparticle Precipitation" Processes 8, no. 12: 1622. https://doi.org/10.3390/pr8121622
APA StyleSana, S., Zivkovic, V., & Boodhoo, K. (2020). Shear-Thinning Effect of the Spinning Disc Mixer on Starch Nanoparticle Precipitation. Processes, 8(12), 1622. https://doi.org/10.3390/pr8121622





